Human Brain Lipidomics: Utilities of Chloride Adducts in Flow Injection Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Brain Samples
2.2. Sample Preparation
2.3. Lipidomics Analysis
3. Results
3.1. Chloride Adducts: Assay Validation
3.2. Chloride Adducts: Specificity
3.3. Ceramide Families
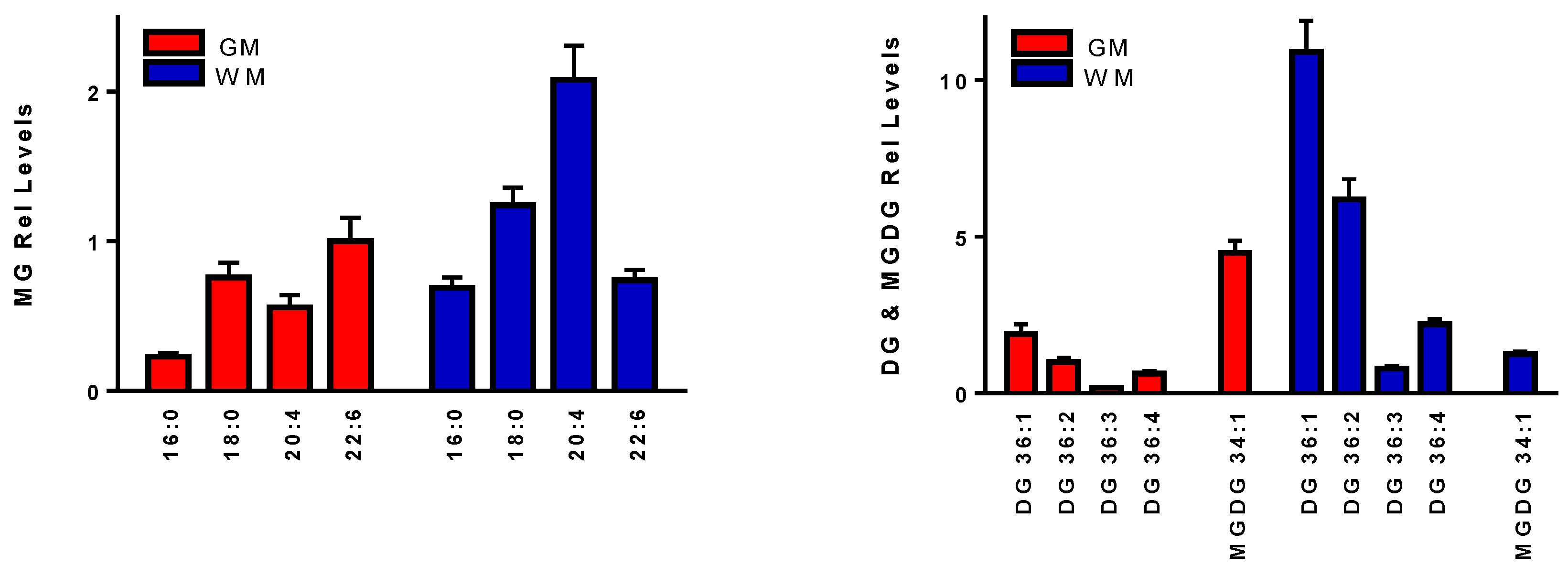
3.4. Neutral Lipids
3.5. Glucosides (Glc)
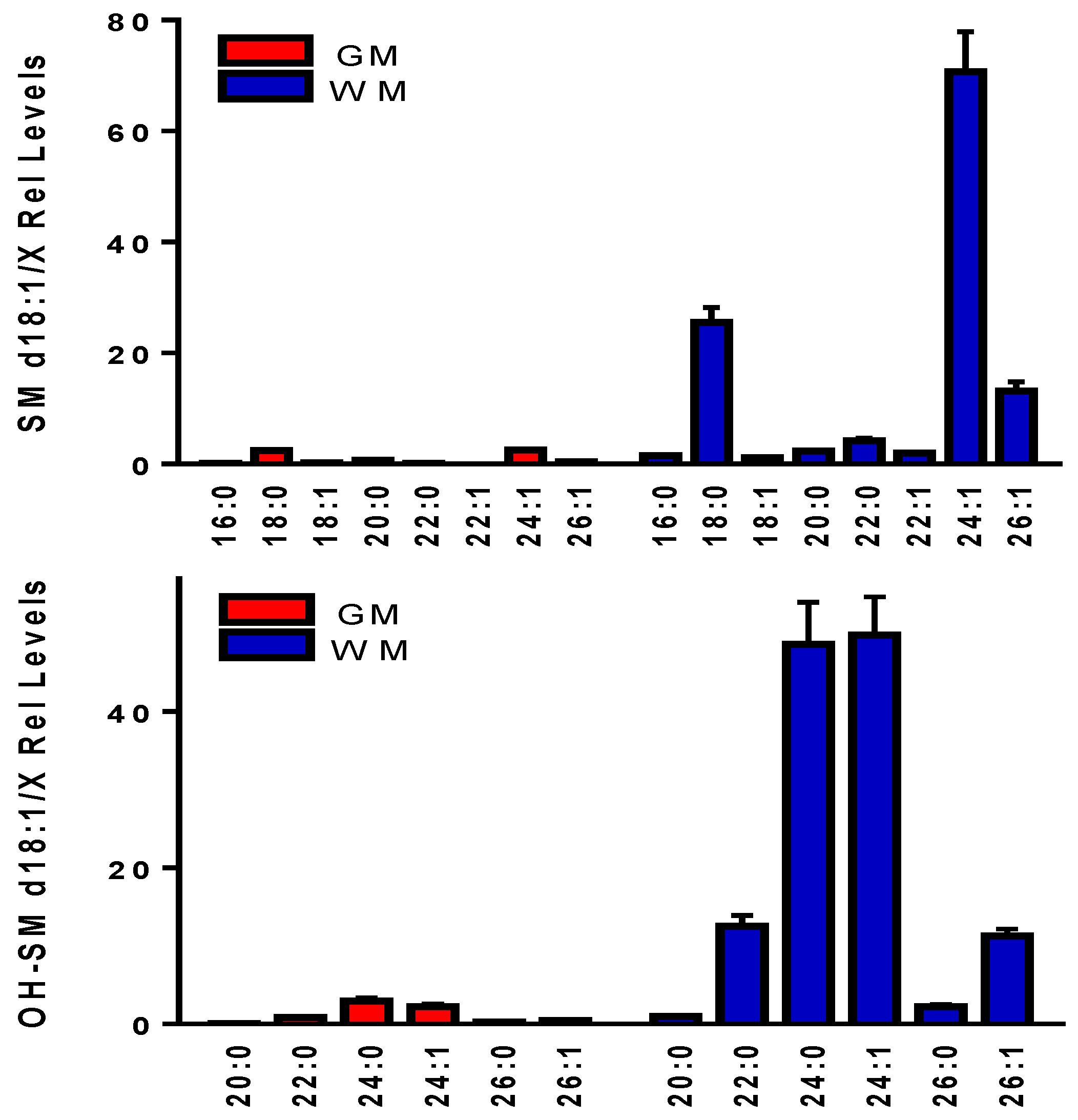
3.6. Sphingomyelins (SM) and Hydroxysphingomyelins (OH-SM)
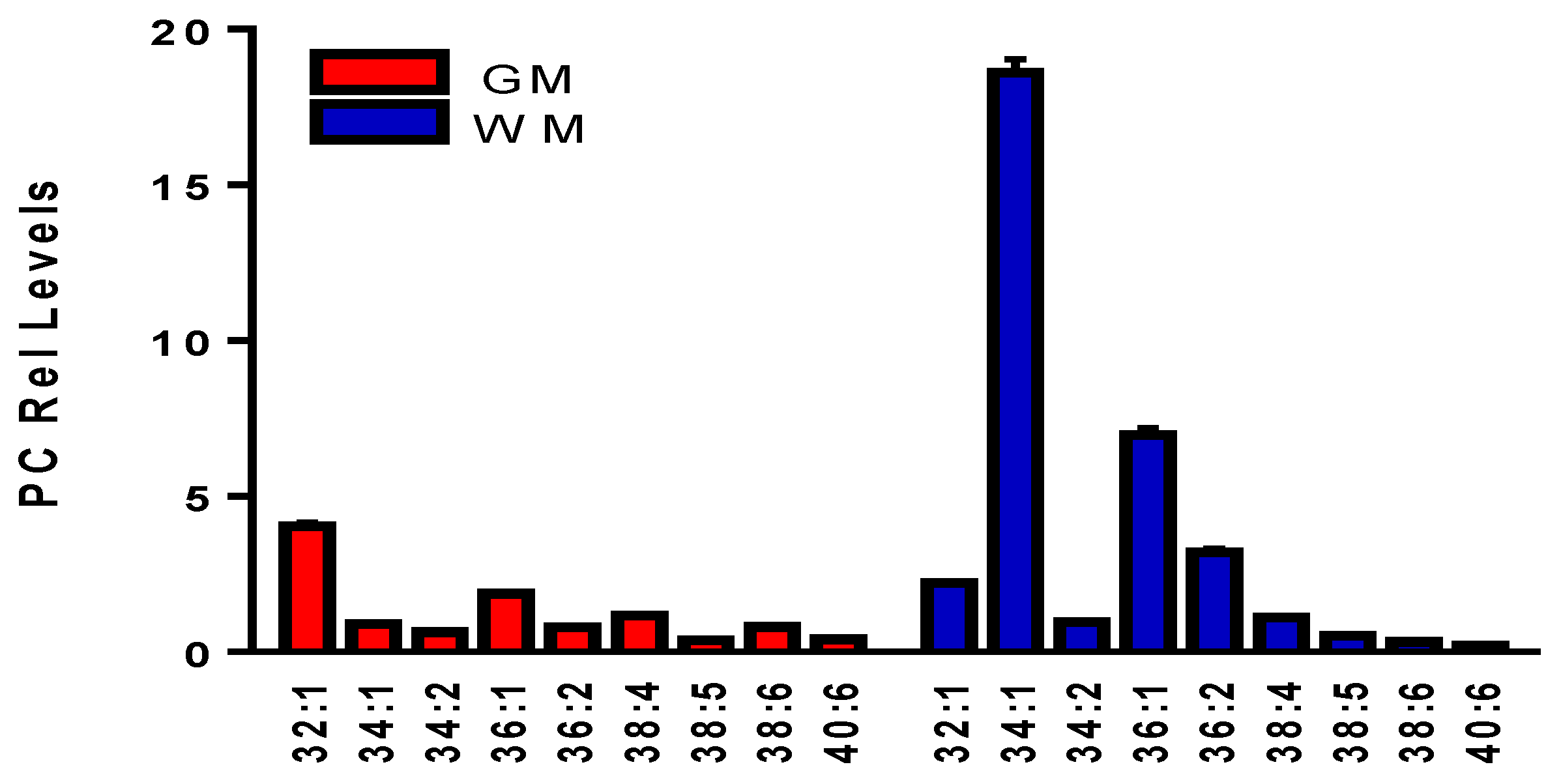
3.7. Phosphatidylcholines (PC)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurz, J.; Parnham, M.J.; Geisslinger, G.; Schiffmann, S. Trends Ceramides as novel disease biomarkers. Mol. Med. 2019, 25, 20–32. [Google Scholar]
- Cutler, R.G.; Kelly, J.; Storie, K.; Pedersen, W.A.; Tammara, A.; Hatanpaa, K.; Troncoso, J.C.; Mattson, M.P. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 2070–2075. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Holtzman, D.M.; McKeel, D.W., Jr.; Kelley, J.; Morris, J.C. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: Potential role in disease pathogenesis. J. Neurochem. 2002, 82, 809–818. [Google Scholar] [CrossRef]
- Chan, R.B.; Oliveira, T.G.; Cortes, E.P.; Honig, L.S.; Duff, K.E.; Small, S.A.; Wenk, M.R.; Shui, G.; Di Paolo, G. Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J. Biol. Chem. 2012, 287, 2678–2688. [Google Scholar] [CrossRef]
- Wood, P.L.; Barnette, B.L.; Kaye, J.A.; Quinn, J.F.; Woltjer, R.L. Non-targeted lipidomics of CSF and frontal cortex gray and white matter in control, mild cognitive impairment, and Alzheimer’s disease subjects. Acta Neuropsychiatr. 2015, 27, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Filippov, V.; Song, M.A.; Zhang, K.; Vinters, H.V.; Tung, S.; Kirsch, W.M.; Yang, J.; Duerksen-Hughes, P.J. Increased ceramide in brains with Alzheimer’s and other neurodegenerative diseases. Alzheimers Dis. 2012, 29, 537–547. [Google Scholar] [CrossRef]
- Hejazi, L.; Wong, J.W.; Cheng, D.; Proschogo, N.; Ebrahimi, D.; Garner, B.; Don, A.S.; Biochem, J. Mass and relative elution time profiling: Two-dimensional analysis of sphingolipids in Alzheimer’s disease brains. Biochem. J. 2011, 438, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L. Non-targeted lipidomics utilizing constant infusion high resolution ESI mass spectrometry. In Springer Protocols, Neuromethods: Lipidomics; Humana Press: New York, NY, USA, 2017; Volume 125, pp. 13–19. [Google Scholar]
- Wood, P.L.; Woltjer, R.L. Electrospray ionization high resolution mass spectrometry of the chloride adducts of steroids, mono- and oligo-saccharides, xyloglucans, ceramides, gangliosides, and phenols. In Springer Protocols, Neuromethods: Metabolomics; Human Press: New York, NY, USA, 2020; Volume 159, pp. 69–76. [Google Scholar]
- Han, X.; Yang, K.; Gross, R.W. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom. Rev. 2012, 31, 134–178. [Google Scholar] [CrossRef]
- Ostrinskaya, A.; Kelley, J.A.; Kunz, R.R. Characterization of nitrated sugar alcohols by atmospheric-pressure chemical-ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 333–343. [Google Scholar] [CrossRef]
- Zhu, J.; Cole, R.B. Formation and decompositions of chloride adduct ions. J. Am. Soc. Mass Spectrom. 2000, 11, 932–941. [Google Scholar] [CrossRef]
- Leaptrot, K.L.; May, J.C.; Dodds, J.N.; McLean, J.A. Ion mobility conformational lipid atlas for high confidence lipidomics. Nat. Commun. 2019, 10, 985. [Google Scholar] [CrossRef]
- Hsu, F.F.; Bohrer, A.; Turk, J.J. Formation of lithiated adducts of glycerophosphocholine lipids facilitates their identification by electrospray ionization tandem mass spectrometry. Am. Soc. Mass Spectrom. 1998, 9, 516–526. [Google Scholar] [CrossRef]
- Wood, P.L.; Tippireddy, S.; Feriante, J.; Woltjer, R.L. Augmented frontal cortex diacylglycerol levels in Parkinson’s disease and Lewy Body Disease. PLoS ONE 2018, 13, 0191815. [Google Scholar] [CrossRef]
- Wenger, D.A.; Petitpas, J.W.; Pieringer, R.A. The metabolism of glyceride glycolipids. II. Biosynthesis of monogalactosyl diglyceride from uridine diphosphate galactose and diglyceride in brain. Biochemistry 1968, 7, 3700–3707. [Google Scholar] [CrossRef]
- Wenger, D.A.; Subba Rao, K.; Pieringer, R.A. The metabolism of glyceride glycolipids. 3. Biosynthesis of digalactosyl diglyceride by galactosyl transferase pathways. J. Biol. Chem. 1970, 245, 2513–2519. [Google Scholar] [CrossRef]
- Deshmukh, D.S.; Flynn, T.J.; Pieringer, R.A. The biosynthesis and concentration of galactosyl diglyceride in glial and neuronal enriched fractions of actively myelinating rat brain. J. Neurochem. 1974, 22, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Schultz, T.; Althaus, H.H. Monogalactosyl diglyceride, a marker for myelination, activates oligodendroglial protein kinase C. J. Neurochem. 1994, 62, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Kohyama-Koganeya, A.; Hirabayashi, Y. New insights on glucosylated lipids: Metabolism and functions. Biochim. Biophys. Acta. 2013, 1831, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, Y. A world of sphingolipids and glycolipids in the brain--novel functions of simple lipids modified with glucose. Proc. Jpn. Acad Ser. B Phys. Biol. Sci. 2012, 88, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Misialek, J.R.; Huang, F.; Windham, G.B.; Yu, F.; Alonso, A.J. Independent association of plasma hydroxysphingomyelins with physical function in the atherosclerosis risk in communities (ARIC) study. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1103–1110. [Google Scholar] [CrossRef]
- Ding, T.; Kabir, I.; Li, Y.; Lou, C.; Yazdanyar, A.; Xu, J.; Dong, J.; Zhou, H.; Park, T.; Boutjdir, M.; et al. All members in the sphingomyelin synthase gene family have ceramide phosphoethanolamine synthase activity. J. Lipid Res. 2015, 56, 537–545. [Google Scholar] [CrossRef]
- Maurice, A.; Malgat, M. Evidence for the biosynthesis of ceramide-phosphoethanolamine in brain synaptic plasma membrane vesicles and in sciatic nerve microsomes from normal and Trembler mice. Neurosci. Lett. 1990, 118, 177–180. [Google Scholar] [CrossRef]
- Alderson, N.L.; Rembiesa, B.M.; Walla, M.D.; Bielawska, A.; Bielawski, J.; Hama, H. The human FA2H gene encodes a fatty acid 2-hydroxylase. J. Biol. Chem. 2004, 279, 48562–48568. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, K.; Prinetti, A.; Sonnino, S.; Mauri, L.; Kobayashi, T.; Ishii, K.; Kaga, N.; Murayama, K.; Kurihara, H.; Nakayama, H.; et al. Involvement of very long fatty acid-containing lactosylceramide in lactosylceramide-mediated superoxide generation and migration in neutrophils. Glycoconj. J. 2008, 25, 357–374. [Google Scholar] [CrossRef]
- Sikora, J.; Dworski, S.; Jones, E.E.; Kamani, M.A.; Micsenyi, M.C.; Sawada, T.; Le Faouder, P.; Bertrand-Michel, J.; Dupuy, A.; Dunn, C.K.; et al. Acid ceramidase deficiency in mice results in a broad range of Central Nervous System Abnormalities. Am. J. Pathol. 2017, 187, 864–883. [Google Scholar] [CrossRef] [PubMed]
- Bodas, M.; Min, T.; Vij, N. Lactosylceramide-accumulation in lipid-rafts mediate aberrant-autophagy, inflammation and apoptosis in cigarette smoke induced emphysema. Apoptosis 2015, 20, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Jang, J.Y.; Yoo, H.S.; Seong, Y.H. Neuroprotective effect of phytoceramide against transient focal ischemia-induced brain damage in rats. Arch. Pharm. Res. 2015, 38, 2241–2250. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, H.K.; Yoo, H.S.; Seong, Y.H. Phytoceramide ameliorates ß-amyloid protein-induced memory impairment and neuronal death in mice. Arch. Pharm. Res. 2017, 40, 760–771. [Google Scholar] [CrossRef]
- Moreau, G.B.; Ramakrishnan, G.; Cook, H.L.; Fox, T.E.; Nayak, U.; Ma, J.Z.; Colgate, E.R.; Kirkpatrick, B.D.; Haque, R.; Petri, W.A., Jr. Childhood growth and neurocognition are associated with distinct sets of metabolites. EBioMedicine 2019, 44, 597–606. [Google Scholar] [CrossRef]
- Varma, V.R.; Oommen, A.M.; Varma, S.; Casanova, R.; An, Y.; Andrews, R.M.; O’Brien, R.; Pletnikova, O.; Troncoso, J.C.; Toledo, J.; et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study. PLoS Med. 2018, 15, e1002482. [Google Scholar] [CrossRef]
- Benvenutto, A.; Giusiano, B.; Koric, L.; Gueriot, C.; Didic, M.; Felician, O.; Guye, M.; Guedj, E.; Ceccaldi, M. Imaging Biomarkers of Neurodegeneration in Alzheimer’s Disease: Distinct Contributions of Cortical MRI Atrophy and FDG-PET Hypometabolism. J. Alzheimers Dis. 2018, 65, 1147–1157. [Google Scholar] [CrossRef]
- Riederer, I.; Bohn, K.P.; Preibisch, C.; Wiedemann, E.; Zimmer, C.; Alexopoulos, P.; Förster, S. Alzheimer disease and mild cognitive impairment: Integrated pulsed arterial spin-labeling MRI and 18F-FDG PET. Radiology 2018, 288, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Van der Bijl, P.; Strous, G.J.; Lopes-Cardozo, M.; Thomas-Oates, J.; van Meer, G. Synthesis of non-hydroxy-galactosylceramides and galactosyldiglycerides by hydroxy-ceramide galactosyltransferase. Biochem. J. 1996, 317 Pt 2, 589–597. [Google Scholar] [CrossRef]
- Han, X.; Gross, R.W. Structural determination of picomole amounts of phospholipids via electrospray ionization tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 1995, 6, 1202–1210. [Google Scholar] [CrossRef]

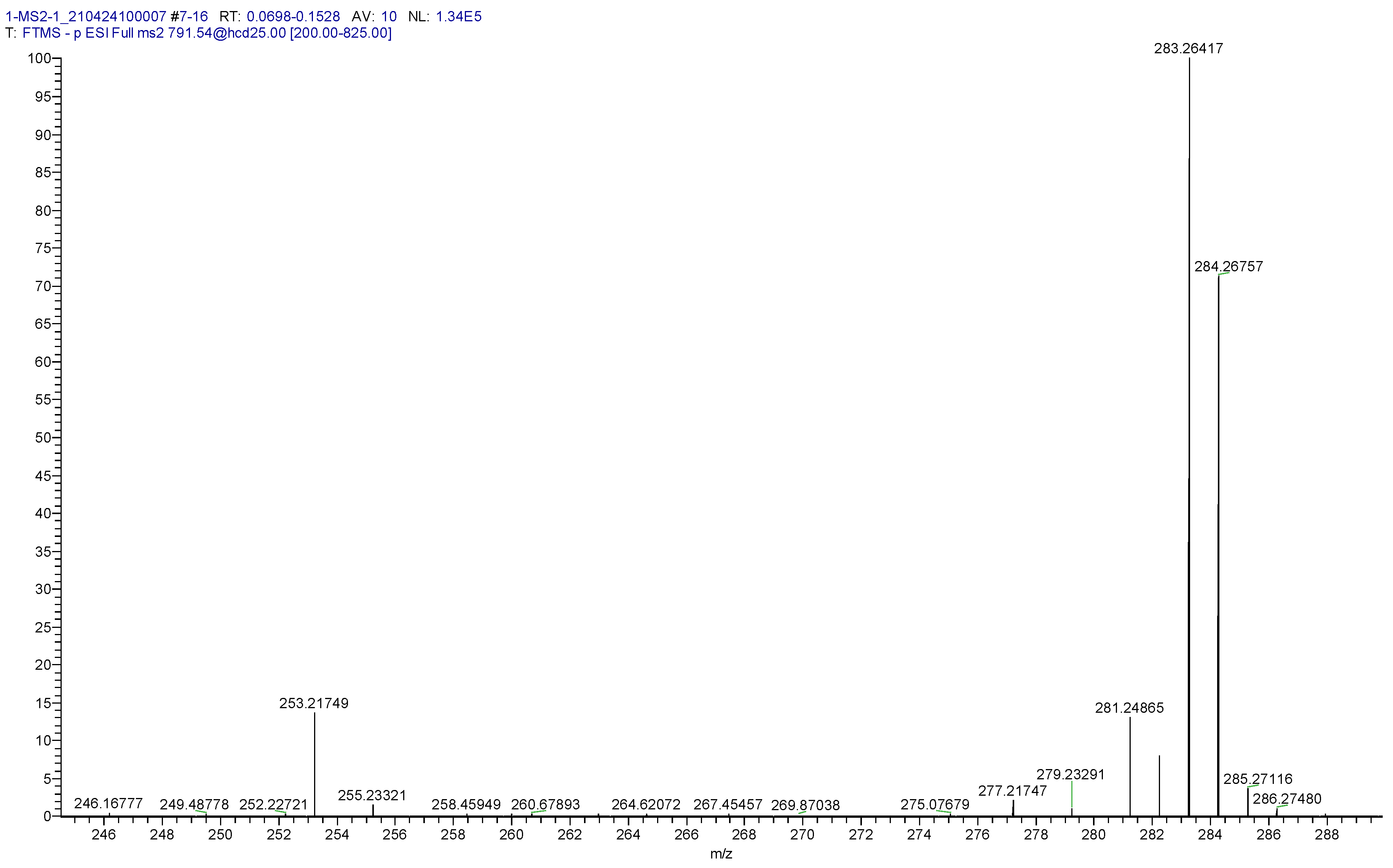
| Cer d18:1/x | Exact | [M+Cl]− | Error (ppm) | MS2 Product | MS2 Anion | Error (ppm) |
| 16:0 [13C16] | 553.5657 | 588.5356 | 0.35 | [M-H]− | 552.5584 | 0.67 |
| 16:0 | 537.5121 | 572.4819 | 0.24 | [M-H]− | 536.5048 | 1.18 |
| 18:0 | 565.5434 | 600.5132 | 1.1 | [M-H]− | 564.5361 | 0.64 |
| 20:0 | 593.5747 | 628.5445 | 0.53 | [M-H]− | 592.5674 | 0.75 |
| 20:1 | 591.5591 | 626.5289 | 0.23 | [M-H]− | 590.5518 | 0.68 |
| 22:0 | 621.6060 | 656.5758 | 0.18 | [M-H]− | 620.5987 | 1.05 |
| 22:1 | 619.5904 | 654.5602 | 0.31 | [M-H]− | 618.5831 | 0.91 |
| 24:0 | 649.6373 | 684.6071 | 0.32 | [M-H]− | 648.6300 | 0.30 |
| 24:1 | 647.6217 | 682.5915 | 0.15 | [M-H]− | 646.6144 | 0.45 |
| OH-Cer d18:1/x | Exact | [M+Cl]− | Error (ppm) | MS2 Product | MS2 Anion | Error (ppm) |
| 18:0 | 581.5383 | 616.5082 | 0.47 | [M-H]− | 580.5310 | 0.34 |
| 22:0 | 637.6009 | 672.5708 | 0.018 | [M-H]− | 636.5936 | 0.45 |
| 24:0 | 665.6322 | 700.6021 | 0.11 | [M-H]− | 664.6249 | 0.26 |
| 24:1 | 663.6166 | 698.5864 | 0.25 | [M-H]− | 662.6093 | 0.051 |
| Phy-Cer t18:0/x | Exact | [M+Cl]− | Error (ppm) | MS2 Product | MS2 Anion | Error (ppm) |
| 16:0 | 555.5226 | 590.4925 | 0.84 | [M-H]− | 554.5153 | 0.36 |
| 18:0 | 583.55396 | 618.5238 | 0.77 | [M-H]− | 582.5467 | 0.44 |
| 18:0(OH) | 599.5489 | 634.5187 | 0.016 | [M-H]− | 598.5416 | 0.39 |
| 22:0 | 639.6166 | 674.5864 | 0.37 | [M-H]− | 638.6093 | 0.78 |
| 22:0(OH) | 655.6115 | 690.5813 | 0.29 | [M-H]− | 654.6042 | 0.52 |
| Hex-Cer | Exact | [M+Cl]− | Error (ppm) | MS2 Product | MS2 Anion | Error (ppm) |
| [D7] | 664.5619 | 699.5317 | 0.42 | |||
| 18:0 | 727.5962 | 762.5660 | 1.1 | [M-(Hex-H2O)]− | 564.5361 | 0.28 |
| 18:1 | 725.5806 | 762.5661 | 1.1 | [M-(Hex-H2O)]− | 562.5205 | 0.45 |
| 20:0 | 755.6275 | 790.5973 | 0.98 | [M-(Hex-H2O)]− | 592.5674 | 0.87 |
| 24:0 | 811.6901 | 846.6599 | 0.14 | [M-(Hex-H2O)]− | 648.6299 | 0.94 |
| Hex-OH-Cer | Exact | [M+Cl]− | Error (ppm) | MS2 Product | MS2 Anion | Error (ppm) |
| 24:0 | 827.6850 | 862.6548 | 0.29 | [M-(Hex-H2O)]− | 664.6249 | 1.43 |
| [M-Hex]− | 646.61436 | 1.22 | ||||
| 24:1 | 825.6693 | 860.6392 | 1.0 | [M-(Hex-H2O)]− | 662.6092 | 0.40 |
| [M-Hex]− | 644.5987 | 0.24 | ||||
| 26:0 | 855.7163 | 890.6861 | 0.42 | [M-(Hex-H2O)]− | 692.6562 | 0.83 |
| [M-Hex]− | 674.6456 | 0.95 | ||||
| 26:1 | 853.7006 | 888.6705 | 0.72 | [M-(Hex-H2O)]− | 690.6405 | 0.65 |
| [M-Hex]− | 672.6300 | 0.50 | ||||
| Cer-PE | Exact | [M+Cl]− | Error (ppm) | MS2 Product | MS2 Anion | Error (ppm) |
| 24:0 | 772.6458 | 807.6156 | 1.3 | PE | 140.0118 | 1.1 |
| 24:1 | 770.6302 | 805.5999 | 1.2 | PE | 140.0118 | 1.0 |
| 25:0 | 786.6614 | 821.6313 | 0.10 | PE | 140.0118 | 1.3 |
| 25:1 | 784.6458 | 819.6156 | 0.72 | PE | 140.0118 | 1.2 |
| 26:0 | 800.6771 | 835.6469 | 0.024 | PE | 140.0118 | 1.1 |
| 26:1 | 798.6615 | 833.6313 | 0.26 | PE | 140.0118 | 1.3 |
| MG | Exact | [M+Cl]− | Error (ppm) | DG | Exact | [M+Cl]− | Error (ppm) |
| 18:1 [D5] | 361.3240 | 396.2939 | 0.73 | DG 36:2 [13C3] | 623.5480 | 658.5179 | 1.1 |
| 16:0 | 330.2770 | 365.2469 | 0.52 | 36:1 | 622.5536 | 657.5235 | 0.89 |
| 18:0 | 358.3083 | 393.2782 | 0.13 | 36:2 | 620.5379 | 655.5078 | 0.83 |
| 20:4 | 378.2770 | 413.2469 | 0.10 | 36:3 | 618.5223 | 653.4922 | 1.1 |
| 22:6 | 402.2770 | 437.2469 | 0.13 | 36:4 | 616.4910 | 651.4765 | 0.66 |
| MGDG | Exact | [M+Cl]− | Error (ppm) | MS2 Product | MS2 Anion | Error (ppm) | |
| 34:1 | 756.5751 | 791.5450 | 0.76 | 16:1 | 253.2173 | 1.02 | |
| 18:0 | 283.2642 | 0.38 | |||||
| 36:1 | 784.6064 | 819.5763 | 0.69 | 18:0 | 283.2642 | 0.60 | |
| 18:1 | 281.2486 | 0.12 | |||||
| 36:2 | 782.5908 | 817.5606 | 0.77 | 18:1 | 281.2486 | 0.12 | |
| SM d18:1/x | Exact | [M+Cl]− | Error (ppm) | MS2 Product | MS2 Anion | Error (ppm) |
| 16:0 [D31] | 733.7621 | 768.7320 | 0.70 | [M-CH3]− | 718.7386 | 0.87 |
| 16:0 | 702.5676 | 737.5374 | 0.33 | [M-CH3]− | 687.5441 | 0.53 |
| 18:0 | 730.5988 | 765.5687 | 0.71 | [M-CH3]− | 715.5753 | 0.55 |
| 18:1 | 728.5832 | 763.5531 | 0.94 | [M-CH3]− | 713.5597 | 0.31 |
| 20:0 | 760.6458 | 795.6157 | 0.32 | [M-CH3]− | 745.6223 | 0.82 |
| 22:0 | 786.6615 | 821.6313 | 0.83 | [M-CH3]− | 771.6380 | 1.1 |
| 24:0 | 814.6928 | 849.6626 | 0.99 | [M-CH3]− | 799.6693 | 0.24 |
| 24:1 | 812.6772 | 847.6469 | 0.32 | [M-CH3]− | 797.6537 | 0.72 |
| 26:1 | 840.7085 | 875.6783 | 0.63 | [M-CH3]− | 825.6850 | 0.96 |
| OH-SM d18:1/x | Exact | [M+Cl]− | Error (ppm) | MS2 Product | MS2 Anion | Error (ppm) |
| 18:0 | 746.5938 | 781.5636 | 0.87 | [M-CH3]− | 563.5242 | 1.3 |
| 20:0 | 774.6251 | 809.5949 | 0.70 | [M-CH3]− | 591.5555 | 0.88 |
| 22:0 | 802.6564 | 837.6262 | 0.77 | [M-CH3]− | 619.5868 | 0.50 |
| 22:1 | 800.6408 | 835.6106 | 0.76 | [M-CH3]− | 617.5712 | 0.70 |
| 24:0 | 830.6877 | 865.6575 | 0.98 | [M-CH3]− | 647.6180 | 0.10 |
| 24:1 | 828.6721 | 863.6419 | 0.73 | [M-CH3]− | 645.6025 | 0.34 |
| 26:0 | 858.7190 | 893.6888 | 0.84 | [M-CH3]− | 675.6494 | 0.56 |
| 26:1 | 856.7034 | 891.6732 | 0.86 | [M-CH3]− | 673.6338 | 0.48 |
| PC | Exact | [M+H] | Error (ppm) | [M+Cl]− | Error (ppm) | MS2 Product | MS2 Anion | Error (ppm) |
| 28:0 [D54] | 729.8257 | 730.8330 | 0.78 | 764.7955 | 0.40 | 14:0 [D26] | 253.3649 | 0.61 |
| 34:1 [D31] | 789.7661 | 790.7734 | 0.86 | 824.7360 | 0.52 | 16:0 [D31] | 285.4213 | 0.54 |
| 32:1 | 731.5465 | 732.5537 | 0.10 | 766.5163 | 1.3 | 14:0 | 227.2016 | 0.17 |
| 18:1 | 281.2486 | 0.98 | ||||||
| 16:0 | 255.2329 | 0.39 | ||||||
| 16:1 | 253.2173 | 0.67 | ||||||
| 34:1 | 759.5778 | 760.5850 | 0.64 | 794.5476 | 1.3 | 16:0 | 255.2329 | 0.66 |
| 18:1 | 281.2486 | 0.67 | ||||||
| 36:1 | 787.6091 | 788.6163 | 0.94 | 822.5789 | 1.4 | 18:0 | 283.2643 | 0.44 |
| 18:1 | 281.2486 | 0.10 | ||||||
| 36:2 | 785.5931 | 786.6007 | 0.47 | 820.5633 | 1.1 | 18:1 | 281.2486 | 0.11 |
| 38:4 | 809.5931 | 810.6007 | 1.1 | 844.5633 | 1.2 | 18:0 | 283.2643 | 0.70 |
| 20:4 | 303.2329 | 0.82 | ||||||
| 16:0 | 255.2329 | 0.39 | ||||||
| 22:4 | 331.2642 | 0.36 | ||||||
| 38:5 | 807.5778 | 808.5851 | 0.85 | 842.5477 | 1.1 | 18:1 | 281.2486 | 0.53 |
| 20:4 | 303.2329 | 0.56 | ||||||
| 38:6 | 806.5622 | 806.5694 | 0.43 | 840.5320 | 1.3 | 16:0 | 255.2329 | 0.10 |
| 22:6 | 327.2329 | 0.27 | ||||||
| 40:6 | 833.5931 | 834.6007 | 0.12 | 868.5633 | 1.4 | 18:0 | 283.2643 | 0.10 |
| 22:6 | 327.2329 | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wood, P.L.; Hauther, K.A.; Scarborough, J.H.; Craney, D.J.; Dudzik, B.; Cebak, J.E.; Woltjer, R.L. Human Brain Lipidomics: Utilities of Chloride Adducts in Flow Injection Analysis. Life 2021, 11, 403. https://doi.org/10.3390/life11050403
Wood PL, Hauther KA, Scarborough JH, Craney DJ, Dudzik B, Cebak JE, Woltjer RL. Human Brain Lipidomics: Utilities of Chloride Adducts in Flow Injection Analysis. Life. 2021; 11(5):403. https://doi.org/10.3390/life11050403
Chicago/Turabian StyleWood, Paul L., Kathleen A. Hauther, Jon H. Scarborough, Dustin J. Craney, Beatrix Dudzik, John E. Cebak, and Randall L. Woltjer. 2021. "Human Brain Lipidomics: Utilities of Chloride Adducts in Flow Injection Analysis" Life 11, no. 5: 403. https://doi.org/10.3390/life11050403
APA StyleWood, P. L., Hauther, K. A., Scarborough, J. H., Craney, D. J., Dudzik, B., Cebak, J. E., & Woltjer, R. L. (2021). Human Brain Lipidomics: Utilities of Chloride Adducts in Flow Injection Analysis. Life, 11(5), 403. https://doi.org/10.3390/life11050403





