PD-L1 Expression in Muscle Invasive Urothelial Carcinomas as Assessed via Immunohistochemistry: Correlations with Specific Clinical and Pathological Features, with Emphasis on Prognosis after Radical Cystectomy
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic and Tumor Characteristics
3.2. Association of PD-L1 Expression with Clinical and Tumor Features
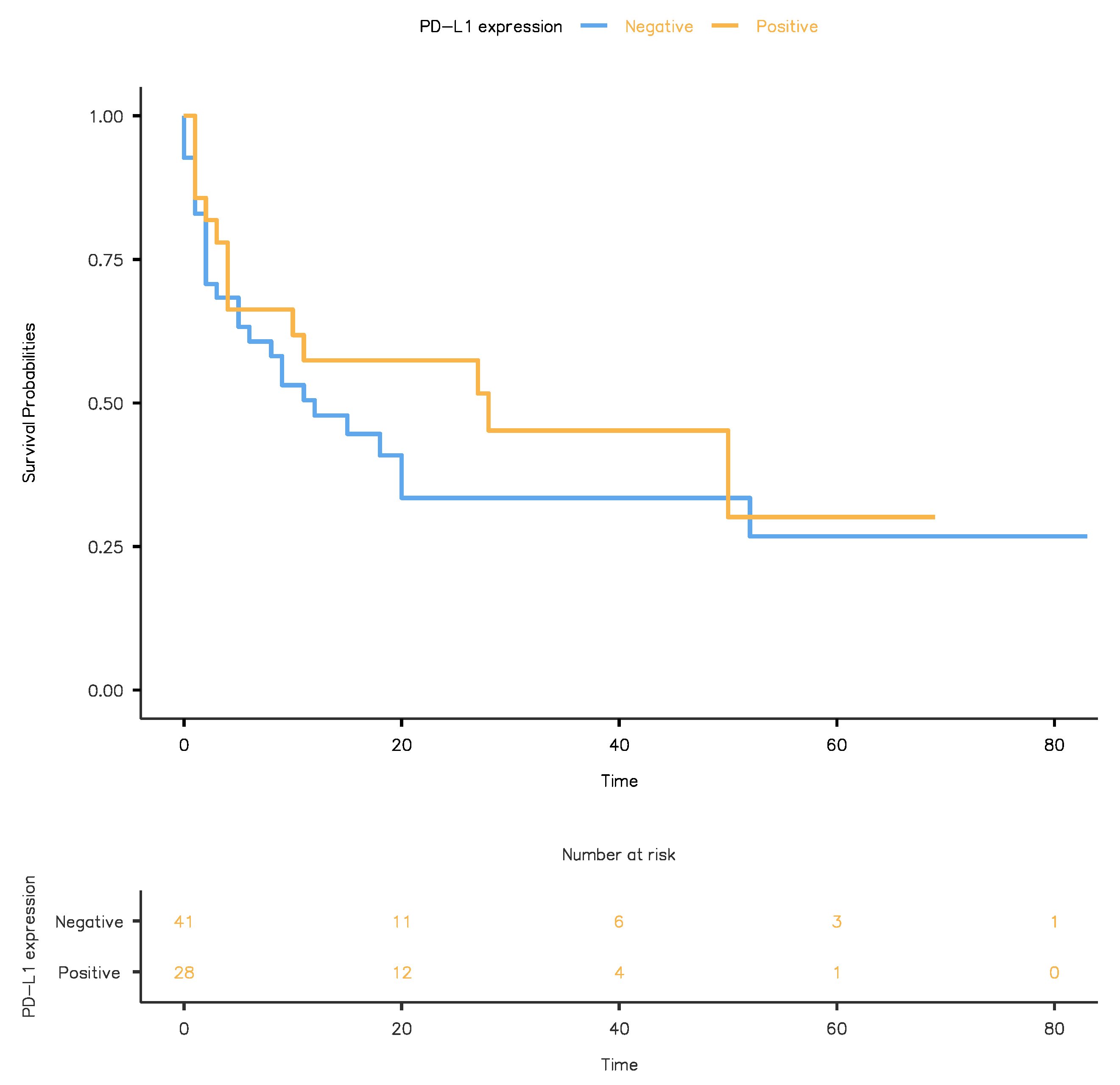
3.3. Association of PD-L1 Expression with Clinical Outcome Following RC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA J. Clin. 2018, 68, 1–31. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, M.; Cathomas, R.; Compérat, E.; Cowan, N.C.; Gakis, G.; Hernández, V.; Lorch, A.; Ribal, M.J.; Thalmann, G.N.; et al. EAU Guidelines on Muscle-Invasive and Metastatic Bladder Cancer; Witjes, J.A., Bruins, M., Cathomas, R., Compérat, E., Cowan, N.C., Gakis, G., Hernández, V., Lorch, A., Ribal, M.J., Thalmann, G.N., et al., Eds.; EAU Guidelines Office: Arnhem, The Netherlands, 2020. [Google Scholar]
- Reis, H.; Serrette, R.; Posada, J.; Lu, V.; Chen, Y.-B.; Gopalan, A.; Fine, S.W.; Tickoo, S.K.; Sirintrapun, S.J.; Iyer, G.; et al. PD-L1 Expression in Urothelial Carcinoma with Predominant or Pure Variant Histology: Concordance Among 3 Commonly Used and Commercially Available Antibodies. Am. J. Surg. Pathol. 2019, 43, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Mullane, S.A.; Werner, L.; Fay, A.P.; Callea, M.; Leow, J.J.; Taplin, M.E.; Choueiri, T.K.; Hodi, F.S.; Freeman, G.J.; et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann. Oncol. 2015, 26, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Tretiakova, M.; Fulton, R.; Kocherginsky, M.; Long, T.; Ussakli, C.; Antic, T.; Gown, A. Concordance study of PD-L1 expression in primary and metastatic bladder carcinomas: Comparison of four commonly used antibodies and RNA expression. Mod. Pathol. 2018, 31, 623–632. [Google Scholar] [CrossRef]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Hahn, A.W.; Sirohi, D.; Agarwal, N. The Role of PD-L1 Testing in Advanced Genitourinary Malignancies. Eur. Urol. Focus 2020, 6, 11–13. [Google Scholar] [CrossRef]
- European Medicines Agency. Keytruda (pembrolizumab): An overview of Keytruda and why it is authorised in the EU. Available online: https://www.ema.europa.eu/en/documents/overview/keytruda-epar-medicine-overview_en.pdf (accessed on 27 April 2021).
- Savic Prince, S.; Bubendorf, L. Predictive potential and need for standardization of PD-L1 immunohistochemistry. Virchows Arch. 2019, 474, 475–484. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Cimadamore, A.; Blanca, A.; Massari, F.; Vau, N.; Scarpelli, M.; Cheng, L.; Montironi, R. Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer. Cancers 2021, 13, 131. [Google Scholar] [CrossRef]
- Pichler, R.; Heidegger, I.; Fritz, J.; Danzl, M.; Sprung, S.; Zelger, B.; Brunner, A.; Pircher, A. PD-L1 expression in bladder cancer and metastasis and its influence on oncologic outcome after cystectomy. Oncotarget 2017, 8, 66849–66864. [Google Scholar] [CrossRef]
- Pichler, R.; Fritz, J.; Lackner, F.; Sprung, S.; Brunner, A.; Horninger, W.; Loidl, W.; Pircher, A.; Heidegger, I. Prognostic Value of Testing PD-L1 Expression After Radical Cystectomy in High-risk Patients. Clin. Genitourin. Cancer 2018, 16, e1015–e1024. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.M.; Ng, T.L.; De Jong, F.C.; Zuiverloon, T.C.M.; Fazzari, F.G.T.; Theodorescu, D. Molecular Biomarkers of Response to PD-1/ PD-L1 Immune Checkpoint Blockade in Advanced Bladder Cancer. Bladder Cancer 2019, 5, 131–145. [Google Scholar] [CrossRef]
- Moch, H.; Humphrey, P.A.; Ulbright, T.M.; Reuter, V.E. Tumours of the urinary tract. In WHO Classification of Tumors of the Urinary System and Male Genital Organs; Moch, H., Humphrey, P.A., Ulbright, T.M., Reuter, V.E., Eds.; International Agency for Research on Cancer (IARC): Lyon, France, 2016; pp. 81–99. [Google Scholar]
- Moschini, M.; D’Andrea, D.; Korn, S.; Irmak, Y.; Soria, F.; Compérat, E.; Shariat, S.F. Characteristics and clinical significance of histological variants of bladder cancer. Nat. Rev. Urol. 2017, 14, 651–668. [Google Scholar] [CrossRef] [PubMed]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Edge, S.B., Greene, F.L., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., et al., Eds.; Spriger: Berlin/Heidelberg, Germany, 2017; ISBN 978-3319406176. [Google Scholar]
- Loghin, A.; Chibelean, C.; Orsolya, M.; Nechifor-Boilă, A.; Nechifor-Boilă, A.; Borda, A. Micropapillary urothelial carcinoma: An aggressive variant of urothelial carcinoma. Rom. J. Morphol. Embryol. 2014, 55, 939–945. [Google Scholar]
- Cseke, A. Privind Aprobarea Ghidurilor de Practică Medicală Pentru Specialitatea Urologie; Ordinul nr. 1323/2010; Ministerul Sănătăţii: Bucharest, Romania, 18 October 2010. [Google Scholar]
- Dako/Agilent. PD-L1 IHC 22C3 pharmDx Interpretation Manual – Urothelial Carcinoma. Online Progr. Educ. 2016, 1–76. Available online: https://www.agilent.com/cs/library/usermanuals/public/29276_22C3_pharmdx_uc_interpretation_manual_us.pdf (accessed on 28 April 2021).
- Holland, B.C.; Sood, A.; Delfino, K.; Dynda, D.I.; Ran, S.; Freed, N.; Alanee, S. Age and sex have no impact on expression levels of markers of immune cell infiltration and immune checkpoint pathways in patients with muscle-invasive urothelial carcinoma of the bladder treated with radical cystectomy. Cancer Immunol. Immunother. 2019, 68, 991–997. [Google Scholar] [CrossRef]
- Ding, X.; Chen, Q.; Yang, Z.; Li, J.; Zhan, H.; Lu, N.; Chen, M.; Yang, Y.; Wang, J.; Yang, D. Clinicopathological and prognostic value of PD-L1 in urothelial carcinoma: A meta-analysis. Cancer Manag. Res. 2019, 11, 4171–4184. [Google Scholar] [CrossRef]
- Xylinas, E.; Robinson, B.D.; Kluth, L.A.; Volkmer, B.G.; Hautmann, R.; Kufer, R.; Zerbib, M.; Kwon, E.; Thompson, R.H.; Boorjian, S.A.; et al. Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur. J. Surg. Oncol. 2014, 40, 121–127. [Google Scholar] [CrossRef]
- Madersbacher, S.; Hochreiter, W.; Burkhard, F.; Thalmann, G.N.; Danuser, H.; Markwalder, R.; Studer, U.E. Radical cystectomy for bladder cancer today—A homogeneous series without neoadjuvant therapy. J. Clin. Oncol. 2003, 21, 690–696. [Google Scholar] [CrossRef]
- Marks, P.; Gild, P.; Soave, A.; Janisch, F.; Minner, S.; Engel, O.; Vetterlein, M.W.; Shariat, S.F.; Sauter, G.; Dahlem, R.; et al. The impact of variant histological differentiation on extranodal extension and survival in node positive bladder cancer treated with radical cystectomy. Surg. Oncol. 2019, 28, 208–213. [Google Scholar] [CrossRef]
- Torlakovic, E.; Lim, H.J.; Adam, J.; Barnes, P.; Bigras, G.; Chan, A.W.H.; Cheung, C.C.; Chung, J.-H.; Couture, C.; Fiset, P.O.; et al. “Interchangeability” of PD-L1 immunohistochemistry assays: A meta-analysis of diagnostic accuracy. Mod. Pathol. 2020, 33, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Zavalishina, L.; Tsimafeyeu, I.; Povilaitite, P.; Raskin, G.; Andreeva, Y.; Petrov, A.; Kharitonova, E.; Rumyantsev, A. RUSSCO-RSP comparative study of immunohistochemistry diagnostic assays for PD-L1 expression in urothelial bladder cancer. Virchows Arch. 2018, 719–724. [Google Scholar] [CrossRef]
- Zajac, M.; Scott, M.; Ratcliffe, M.; Scorer, P.; Barker, C.; Al-Masri, H.; Rebelatto, M.C.; Walker, J. Concordance among four commercially available, validated programmed cell death ligand-1 assays in urothelial carcinoma. Diagn. Pathol. 2019, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paver, E.C.; Cooper, W.A.; Colebatch, A.J.; Ferguson, P.M.; Hill, S.K.; Lum, T.; Shin, J.S.; O’Toole, S.; Anderson, L.; Scolyer, R.A.; et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: A guide to immunohistochemistry implementation and interpretation. Pathology 2021, 53, 141–156. [Google Scholar] [CrossRef] [PubMed]
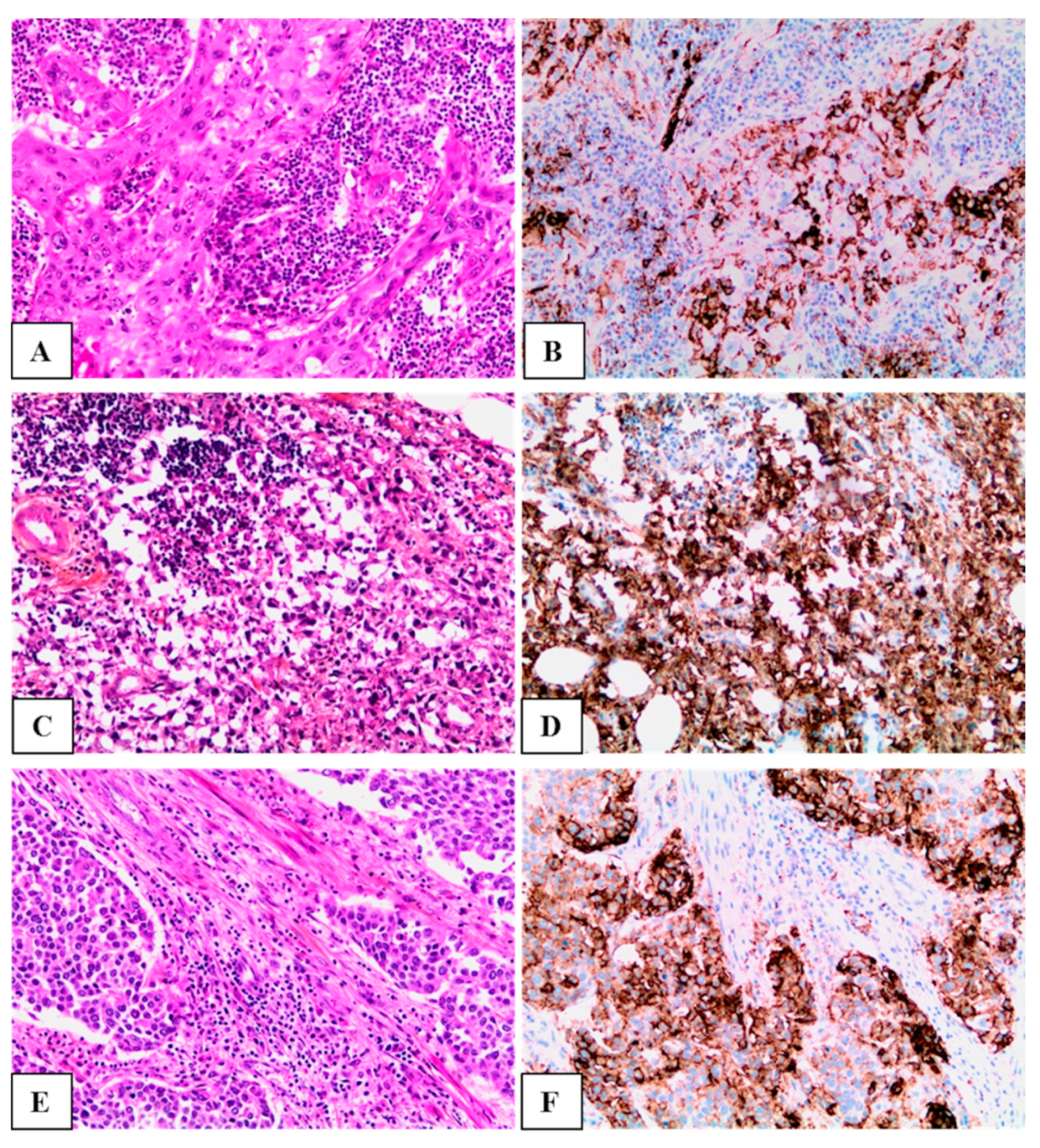
| Characteristic | Total (n = 69) | PD-L1 Positive (CPS ≥ 10) n = 28 (40.6%) | PD-L1 Negative (CPS < 10) n = 41 (59.4%) | p |
|---|---|---|---|---|
| Age (mean ± SD) | 67.35 ± 9.98 | 69.11 ± 9.25 | 66.15 ± 10.4 | 0.22 ‡ |
| Gender (n (%)) | ||||
| Male | 57 (82.6) | 25 (89.3) | 32 (78.0) | 0.33 † |
| Female | 12 (17.4) | 3 (10.7) | 9 (22.0) | |
| Histology. n (%) | ||||
| UC conventional | 36 (52.2) | 11 (39.3) | 25(61.0) | |
| UC nonconventional (variants): | ||||
| Poorly differentiated | 11 (15.9) | 4 (14.3) | 7 (17.1) | |
| Micropapillary | 5 (7.2) | 2 (7.1) | 3 (7.3) | |
| Squamous differentiation | 5 (7.2) | 5 (17.9) | 0 | |
| Glandular differentiation | 1 (1.4) | 1 (3.6) | 0 | |
| Plasmocitoid | 3 (4.3) | 0 | 3 (7.3) | |
| Sarcomatoid | 4 (5.8) | 3 (10.7) | 2 (4.8) | |
| Nested | 1 (1.4) | 0 | 1 (2.4) | |
| Others (mixed) | 3 (4.3) | 2 (7.1) | 1 (2.4) | |
| Assoc. papillary component (n (%)) | ||||
| Absent | 49 (71.0) | 22 (78.6) | 27 (65.9) | 0.29 † |
| Present | 20 (29.0) | 6 (21.4) | 14 (34.1) | |
| Concomitant CIS (n (%)) | ||||
| Absent | 45 (65.2) | 16 (57.1) | 29 (70.7) | 0.30 † |
| Present | 24 (34.8) | 12 (42.9) | 12 (29.3) | |
| Surgical margins status (n (%)) | ||||
| Negative | 61 (88.4) | 27 (96.4) | 34 (82.9) | 0.13 † |
| Positive | 8 (11.6) | 1 (3.6) | 7 (17.1) | |
| Primary tumour (pT) (n (%)) | ||||
| pT2 | 15 (21.7) | 5 (17.9) | 10 (24.4) | |
| pT3 | 34 (49.3) | 15 (53.6) | 19 (46.3) | 0.72 † |
| pT4 | 20 (29.0) | 8 (28.6) | 12(29.3) | |
| Lymph node involvement (n (%)) | ||||
| Nx | 20 (29.0) | 7 (25.0) | 13 (31.7) | |
| N0 | 25 (36.2) | 12 (42.9) | 13 (31.7) | 0.67 † |
| N+ (including N1, N2, N3) | 24 (34.8) | 9 (32.1) | 15 (36.6) | |
| Distant metastasis (n (%)) | ||||
| M0 | 56 (81.2) | 23 (82.1) | 33 (80.5) | 0.86 † |
| M1 | 13 (18.8) | 5 (17.9) | 8 (19.5) | |
| Follow-up data (median months) | 10 (0–83) | 10.5 (1–69) | 9 (0–83) | 0.68 ^ |
| Tumor recurrence (n (%)) | ||||
| Absent | 66 (95.7) | 27 (96.4) | 39 (95.1) | 0.79 † |
| Present | 3 (4.3) | 1 (3.6) | 2 (4.9) | |
| Death (n (%)) | ||||
| No | 29 (42.0) | 14 (50.0) | 15 (36.6) | 0.26 † |
| Yes | 40 (58.0) | 14 (50.0) | 26 (63.4) |
| PD-L1-Positive Group (CPS ≥ 10) | PD-L1-Negative Group (CPS < 10) | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical Factors | p-Value | Hazard Ratio (HR) | 95.0% CI for HR | p-Value | Hazard Ratio (HR) | 95.0% CI for HR | ||
| Lower | Upper | Lower | Upper | |||||
| Gender | 0.699 | 0.666 | 0.085 | 5.217 | 0.246 | 1.679 | 0.700 | 4.028 |
| Age | 0.924 | 0.997 | 0.940 | 1.058 | 0.280 | 1.020 | 0.984 | 1.057 |
| T stage | 0.021 | 2.910 | 1.175 | 7.210 | 0.439 | 1.239 | 0.720 | 2.131 |
| N stage | 0.599 | 1.189 | 0.625 | 2.261 | 0.007 | 1.982 | 1.205 | 3.263 |
| M stage | 0.347 | 1.752 | 0.544 | 5.647 | 0.227 | 1.710 | 0.717 | 4.082 |
| Carcinoma in situ | 0.208 | 1.974 | 0.685 | 5.685 | 0.674 | 1.190 | 0.529 | 2.679 |
| Positive surgical margins | 0.333 | 2.795 | 0.349 | 22.405 | 0.463 | 1.445 | 0.541 | 3.861 |
| Tumor recurrence | 0.624 | 0.046 | 0 | 10,387.347 | 0.443 | 0.455 | 0.061 | 3.400 |
| PD-L1-Positive Group | PD-L1-Negative Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical Factors | p-Value | Hazard Ratio (HR) | 95.0% CI for HR | p-Value | Hazard Ratio (HR) | 95.0% CI for HR | ||
| Lower | Upper | Lower | Upper | |||||
| Gender | 0.750 | 0.671 | 0.058 | 7.812 | 0.402 | 1.538 | 0.562 | 4.213 |
| Age (years) | 0.481 | 0.973 | 0.903 | 1.049 | 0.193 | 1.029 | 0.986 | 1.074 |
| T stage | 0.027 | 3.018 | 1.132 | 8.046 | 0.659 | 0.870 | 0.469 | 1.613 |
| N stage | 0.441 | 1.375 | 0.612 | 3.091 | 0.008 | 2.601 | 1.287 | 5.260 |
| M stage | 0.547 | 1.527 | 0.385 | 6.052 | 0.098 | 2.625 | 0.837 | 8.234 |
| Tumor recurrence | 0.991 | 0 | 0 | 0 | 0.819 | 0.766 | 0.078 | 7.508 |
| Positive surgical margins | 0.898 | 1.174 | 0.100 | 13.745 | 0.977 | 0.981 | 0.269 | 3.572 |
| Carcinoma in situ | 0.767 | 1.346 | 0.189 | 9.589 | 0.881 | 1.076 | 0.413 | 2.807 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nechifor-Boilă, I.A.; Loghin, A.; Nechifor-Boilă, A.; Decaussin-Petrucci, M.; Voidăzan, S.; Chibelean, B.C.; Martha, O.; Borda, A. PD-L1 Expression in Muscle Invasive Urothelial Carcinomas as Assessed via Immunohistochemistry: Correlations with Specific Clinical and Pathological Features, with Emphasis on Prognosis after Radical Cystectomy. Life 2021, 11, 404. https://doi.org/10.3390/life11050404
Nechifor-Boilă IA, Loghin A, Nechifor-Boilă A, Decaussin-Petrucci M, Voidăzan S, Chibelean BC, Martha O, Borda A. PD-L1 Expression in Muscle Invasive Urothelial Carcinomas as Assessed via Immunohistochemistry: Correlations with Specific Clinical and Pathological Features, with Emphasis on Prognosis after Radical Cystectomy. Life. 2021; 11(5):404. https://doi.org/10.3390/life11050404
Chicago/Turabian StyleNechifor-Boilă, Ioan Alin, Andrada Loghin, Adela Nechifor-Boilă, Myriam Decaussin-Petrucci, Septimiu Voidăzan, Bogdan Călin Chibelean, Orsolya Martha, and Angela Borda. 2021. "PD-L1 Expression in Muscle Invasive Urothelial Carcinomas as Assessed via Immunohistochemistry: Correlations with Specific Clinical and Pathological Features, with Emphasis on Prognosis after Radical Cystectomy" Life 11, no. 5: 404. https://doi.org/10.3390/life11050404
APA StyleNechifor-Boilă, I. A., Loghin, A., Nechifor-Boilă, A., Decaussin-Petrucci, M., Voidăzan, S., Chibelean, B. C., Martha, O., & Borda, A. (2021). PD-L1 Expression in Muscle Invasive Urothelial Carcinomas as Assessed via Immunohistochemistry: Correlations with Specific Clinical and Pathological Features, with Emphasis on Prognosis after Radical Cystectomy. Life, 11(5), 404. https://doi.org/10.3390/life11050404






