Considering Predictive Factors in the Diagnosis of Clinically Significant Prostate Cancer in Patients with PI-RADS 3 Lesions
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Study Cohort
3.2. Biopsy Results
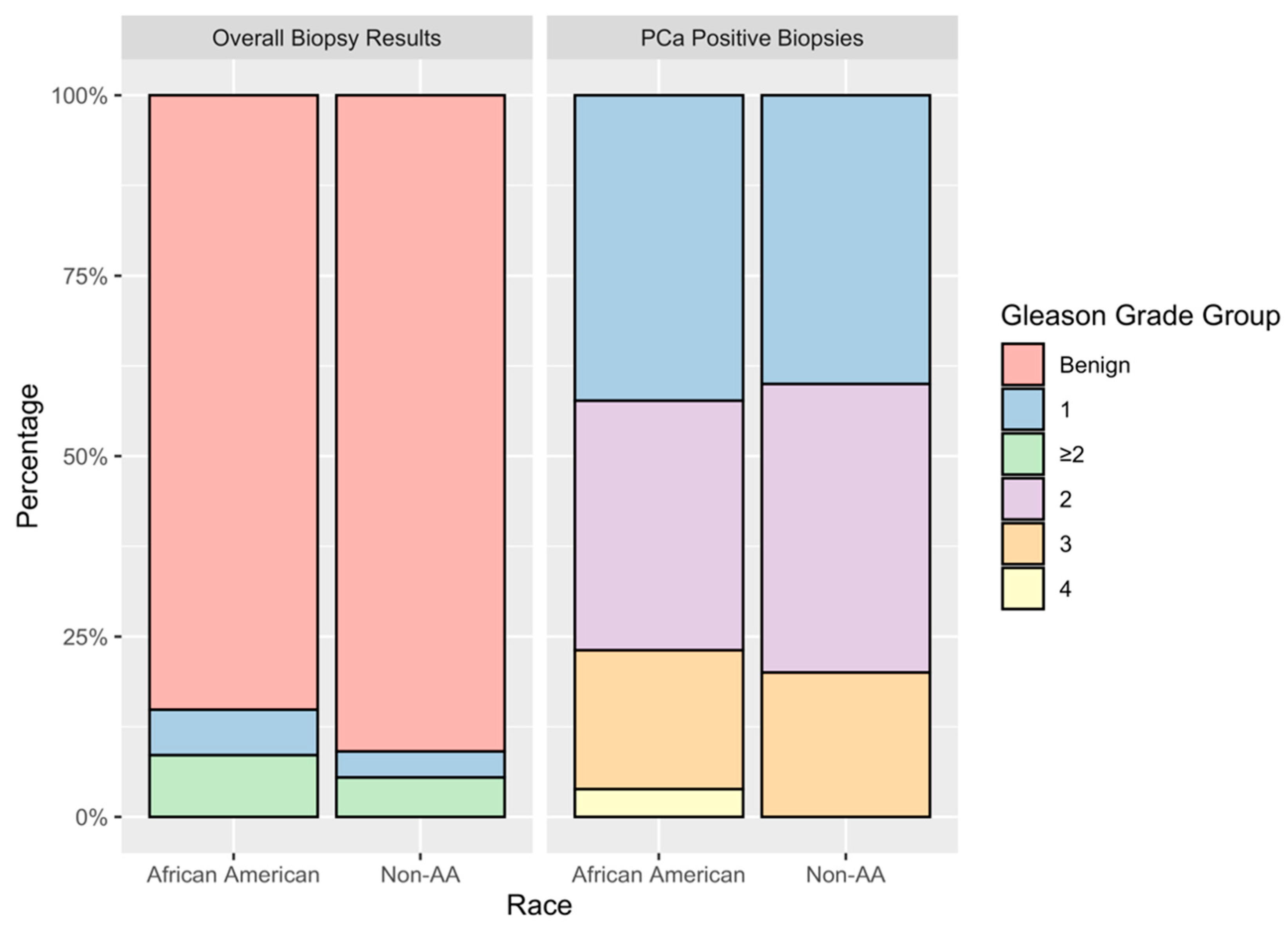
3.3. Racial Compassions of Disease
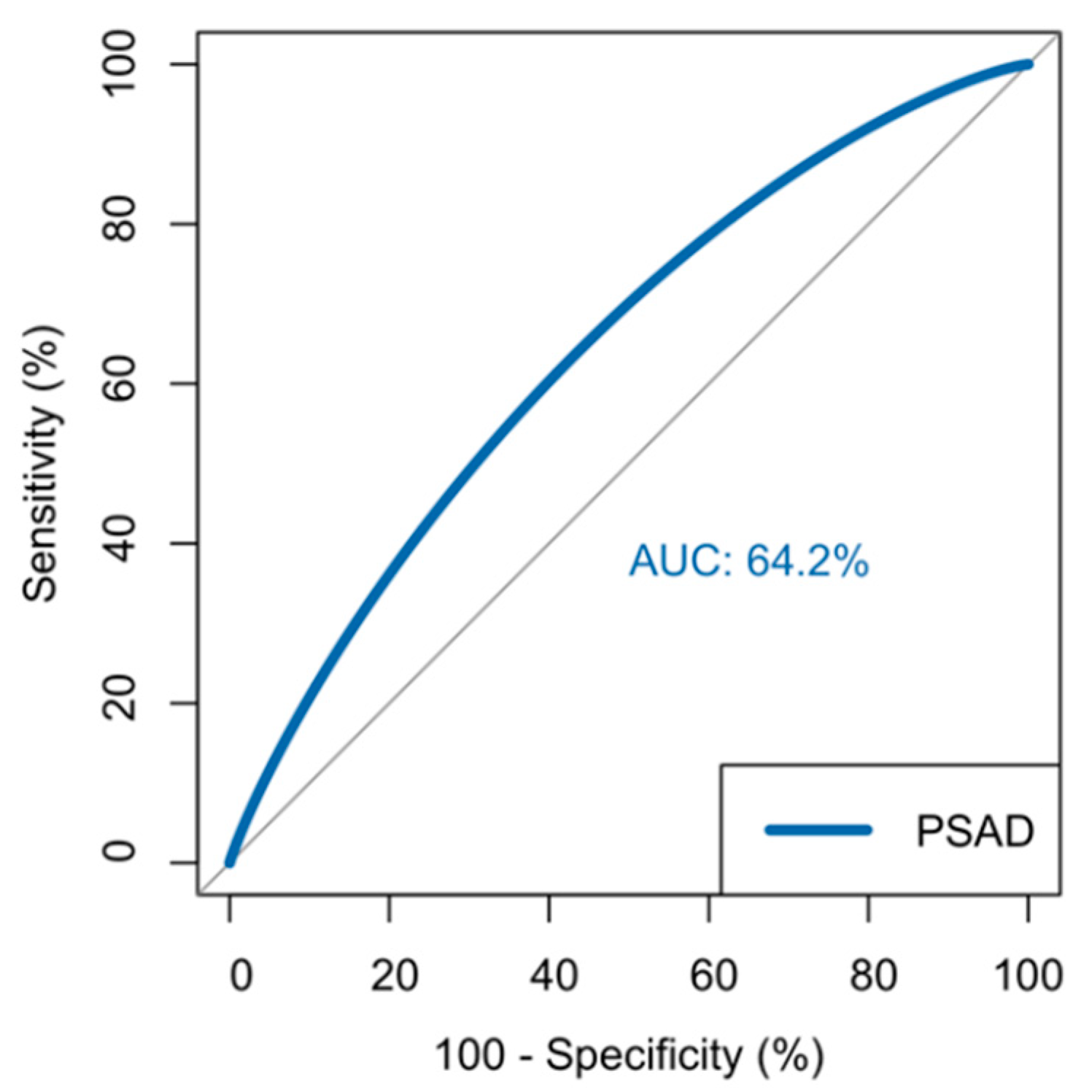
3.4. Predicting Clinically Significant Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stabile, A.; Giganti, F.; Rosenkrantz, A.; Taneja, S.; Villeirs, G.; Gill, I.S.; Allen, C.; Emberton, M.; Moore, C.M.; Kasivisvanathan, V. Multiparametric MRI for prostate cancer diagnosis: Current status and future directions. Nat. Rev. Urol. 2019, 17, 41–61. [Google Scholar] [CrossRef]
- Sedláčková, H.; Dolejšová, O.; Hora, M.; Ferda, J.; Hes, O.; Topolčan, O.; Fuchsová, R.; Kučera, R. Prostate Cancer Diagnostic Algorithm as a “Road Map” from the First Stratification of the Patient to the Final Treatment Decision. Life 2021, 11, 324. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Bosaily, A.E.-S.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Bloom, J.B.; Hale, G.R.; Gold, S.A.; Rayn, K.N.; Smith, C.; Mehralivand, S.; Czarniecki, M.; Valera, V.; Wood, B.J.; Merino, M.J.; et al. Predicting Gleason Group progression for men on prostate cancer active surveillance: Role of a negative confirmatory magnetic resonance imaging-ultrasound fusion biopsy. J. Urol. 2019, 201, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Bs, M.D.G.; Brown, A.M.; Shih, J.H.; Summers, R.M.; Marko, J.; Law, Y.M.; Sankineni, S.; George, A.K.; Merino, M.J.; Pinto, P.A.; et al. Accuracy and agreement of PIRADSv2 for prostate cancer mpMRI: A multireader study. J. Magn. Reson. Imaging 2017, 45, 579–585. [Google Scholar] [CrossRef]
- Mazzone, E.; Stabile, A.; Pellegrino, F.; Basile, G.; Cignoli, D.; Cirulli, G.O.; Sorce, G.; Barletta, F.; Scuderi, S.; Bravi, C.A.; et al. Positive Predictive Value of Prostate Imaging Reporting and Data System Version 2 for the Detection of Clinically significant prostate cancer: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2020, 4, 697–713. [Google Scholar] [CrossRef]
- Richenberg, J.; Løgager, V.; Panebianco, V.; Rouviere, O.; Villeirs, G.; Schoots, I.G. The primacy of multiparametric MRI in men with suspected prostate cancer. Eur. Radiol. 2019, 29, 6940–6952. [Google Scholar] [CrossRef] [Green Version]
- Maggi, M.; Panebianco, V.; Mosca, A.; Salciccia, S.; Gentilucci, A.; Di Pierro, G.; Busetto, G.M.; Barchetti, G.; Campa, R.; Sperduti, I.; et al. Prostate Imaging Reporting and Data System 3 Category Cases at Multiparametric Magnetic Resonance for Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2020, 6, 463–478. [Google Scholar] [CrossRef]
- Rivas, J.G.; Giganti, F.; Álvarez-Maestro, M.; Freire, M.J.; Kasivisvanathan, V.; Martinez-Piñeiro, L.; Emberton, M. Prostate Indeterminate Lesions on Magnetic Resonance Imaging-Biopsy Versus Surveillance: A Literature Review. Eur. Urol. Focus 2019, 5, 799–806. [Google Scholar] [CrossRef] [Green Version]
- Padhani, A.R.; Weinreb, J.; Rosenkrantz, A.B.; Villeirs, G.; Turkbey, B.; Barentsz, J. Prostate Imaging-Reporting and Data System Steering Committee: PI-RADS v2 Status Update and Future Directions. Eur. Urol. 2019, 75, 385–396. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.S.L.; De Visschere, P.; Van Praet, C.; Villeirs, G. How does PI-RADS v2.1 impact patient classification? A head-to-head comparison between PI-RADS v2.0 and v2.1. Acta Radiol. 2021, 62, 839–847. [Google Scholar] [CrossRef]
- Scialpi, M.; Martorana, E.; Aisa, M.C.; Rondoni, V.; D’Andrea, A.; Bianchi, G. Score 3 prostate lesions: A gray zone for PI-RADS v2. Turk. J. Urol. 2017, 43, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Liddell, H.; Jyoti, R.; Haxhimolla, H.Z. mp-MRI Prostate Characterised PIRADS 3 Lesions are Associated with a Low Risk of Clinically significant prostate cancer—A Retrospective Review of 92 Biopsied PIRADS 3 Lesions. Curr. Urol. 2015, 8, 96–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonn, G.A.; Chang, E.; Natarajan, S.; Margolis, D.J.; Macairan, M.; Lieu, P.; Huang, J.; Dorey, F.J.; Reiter, R.E.; Marks, L.S. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur. Urol. 2014, 65, 809–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermie, I.; Van Besien, J.; De Visschere, P.; Lumen, N.; Decaestecker, K. Which clinical and radiological characteristics can predict clinically significant prostate cancer in PI-RADS 3 lesions? A retrospective study in a high-volume academic center. Eur. J. Radiol. 2019, 114, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Schlenker, B.; Apfelbeck, M.; Armbruster, M.; Chaloupka, M.; Stief, C.; Clevert, D.-A. Comparison of PIRADS 3 lesions with histopathological findings after MRI-fusion targeted biopsy of the prostate in a real world-setting. Clin. Hemorheol. Microcirc. 2019, 71, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Jue, J.S.; Barboza, M.P.; Prakash, N.S.; Venkatramani, V.; Sinha, V.R.; Pavan, N.; Nahar, B.; Kanabur, P.; Ahdoot, M.; Dong, Y.; et al. Re-examining Prostate-specific Antigen (PSA) Density: Defining the Optimal PSA Range and Patients for Using PSA Density to Predict Prostate Cancer Using Extended Template Biopsy. Urology 2017, 105, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Koller, C.; Greenberg, J.; Shelton, T.; Hughes, W.; Sanekommu, G.; Silberstein, J.; Krane, L. Prostate Cancer Lesions by Zone and Race: Does Multiparametric MRI Demonstrate Racial Difference in Prostate Cancer Lesions for African American Men? Curr. Oncol. 2021, 28, 212. [Google Scholar] [CrossRef]
- Washino, S.; Okochi, T.; Saito, K.; Konishi, T.; Hirai, M.; Kobayashi, Y.; Miyagawa, T. Combination of prostate imaging reporting and data system (PI-RADS) score and prostate-specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naïve patients. BJU Int. 2017, 119, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Görtz, M.; Radtke, J.P.; Hatiboglu, G.; Schütz, V.; Tosev, G.; Güttlein, M.; Leichsenring, J.; Stenzinger, A.; Bonekamp, D.; Schlemmer, H.-P.; et al. The Value of Prostate-specific Antigen Density for Prostate Imaging-Reporting and Data System 3 Lesions on Multiparametric Magnetic Resonance Imaging: A Strategy to Avoid Unnecessary Prostate Biopsies. Eur. Urol. Focus 2021, 7, 325–331. [Google Scholar] [CrossRef]
- Stamey, T.A.; Freiha, F.S.; McNeal, J.E.; Redwine, E.A.; Whittemore, A.S.; Schmid, H.-P. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 1993, 71, 933–938. [Google Scholar] [CrossRef]
- Rico, L.; Blas, L.; Vitagliano, G.; Contreras, P.; Pita, H.R.; Ameri, C. PI-RADS 3 lesions: Does the association of the lesion volume with the prostate-specific antigen density matter in the diagnosis of clinically significant prostate cancer? Urol. Oncol. Semin. Orig. Investig. 2021, 39, 431.e9–431.e13. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Siegel, R.L.; Sauer, A.G.; Miller, K.D.; Fedewa, S.A.; Alcaraz, K.I.; Jemal, A. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J. Clin. 2016, 66, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.D.; Doshi, C.P.; Koehne, E.L.; Hart, S.; Van Kuiken, M.; Quek, M.L.; Flanigan, R.C.; Gupta, G.N. African American Men have Increased Risk of Prostate Cancer Detection Despite Similar Rates of Anterior Prostatic Lesions and PI-RADS Grade on Multiparametric Magnetic Resonance Imaging. Urology 2021. [Google Scholar] [CrossRef] [PubMed]
- Walton, E.; Yaguchi, G.; Keeley, J.; Deebajah, M.; Menon, M.; Peabody, J.; Dabaja, A.; Alanee, S. Effect of Lesion Location on Prostate Cancer Detection Rate with Magnetic Resonance Imaging Targeted Biopsy in African Americans. J. Urol. 2019, 201, 503–509. [Google Scholar] [CrossRef]
- Greenberg, J.W.; Leinwand, G.; Feibus, A.H.; Haney, N.M.; Krane, L.S.; Thomas, R.; Sartor, O.; Silberstein, J.L. Prospective Observational Study of a Racially Diverse Group of Men on Active Surveillance for Prostate Cancer. Urology 2021, 148, 203–210. [Google Scholar] [CrossRef]
- Gaziev, G.; Wadhwa, K.; Barrett, T.; Koo, B.C.; Gallagher, F.A.; Serrao, E.; Frey, J.; Seidenader, J.; Carmona, L.; Warren, A.; et al. Defining the learning curve for multiparametric magnetic resonance imaging (MRI) of the prostate using MRI-transrectal ultrasonography (TRUS) fusionguided trans-perineal prostate biopsies as a validation tool. BJU Int. 2016, 117, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Shelton, T.M.; Greenberg, J.W.; Silberstein, J.L.; Krane, L.S. Hematologic parameters are not predictors of upgrading or treatment in a racially diverse prospective study of men with prostate cancer on active surveillance. Aging Male Off. J. Int. Soc. Study Aging Male 2020, 23, 1400–1408. [Google Scholar] [CrossRef]
- Coughlin, S.S.; Vernon, M.; Klaassen, Z.; Tingen, M.S.; Cortes, J.E. Knowledge of prostate cancer among African American men: A systematic review. Prostate 2021, 81, 202–213. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total PI-RADS 3 Lesions |
|---|---|
| Median Age (IQR) | 68.2 (63.9–70.8) years |
| Median PSA (IQR) | 6.26 (4.66–9.12) ng/mL |
| Median PSA Density (IQR) | 0.13 (0.08–0.22) ng/mL2 |
| Median MRI Prostate Volume (IQR) | 53.6 (38.2–75.2) mL |
| Median BMI (IQR) | 28.9 (25.7–32.4) |
| n, On Active Surveillance (%) | 94 (41%) |
| Race (%) | –––––– |
| n, African-American | 99 (75%) |
| n, Caucasian | 31 (23%) |
| n, Other | 2 (1.5%) |
| Location (%) | –––––– |
| n, Peripheral Zone | 158 (69%) |
| n, Transitional Zone | 31 (13%) |
| n, Anterior Lesion | 41 (18%) |
| Number of PIRADS 3 Lesions | Rates of GGG ≥1 Cancer | p-Value |
|---|---|---|
| 1 (n = 78) | 18% | ––– |
| 2 (n = 98) | 12.2% | ––– |
| 3 (n = 42) | 11.9% | ––– |
| 4 (n = 12) | 0% | 0.15 |
| Variable | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Age | 1.032 | 0.942–1.130 | 0.493 |
| Race | 1.562 | 0.415–5.88 | 0.641 |
| BMI | 0.970 | 0.888–1.060 | 0.495 |
| PSA | 0.835 * | 0.687–1.016 | 0.031 * |
| PSA Density | 5.39 * | 1.531–18.980 | 0.009 ** |
| On Active Surveillance | 1.027 | 0.369–2.858 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natale, C.; Koller, C.R.; Greenberg, J.W.; Pincus, J.; Krane, L.S. Considering Predictive Factors in the Diagnosis of Clinically Significant Prostate Cancer in Patients with PI-RADS 3 Lesions. Life 2021, 11, 1432. https://doi.org/10.3390/life11121432
Natale C, Koller CR, Greenberg JW, Pincus J, Krane LS. Considering Predictive Factors in the Diagnosis of Clinically Significant Prostate Cancer in Patients with PI-RADS 3 Lesions. Life. 2021; 11(12):1432. https://doi.org/10.3390/life11121432
Chicago/Turabian StyleNatale, Caleb, Christopher R. Koller, Jacob W. Greenberg, Joshua Pincus, and Louis S. Krane. 2021. "Considering Predictive Factors in the Diagnosis of Clinically Significant Prostate Cancer in Patients with PI-RADS 3 Lesions" Life 11, no. 12: 1432. https://doi.org/10.3390/life11121432
APA StyleNatale, C., Koller, C. R., Greenberg, J. W., Pincus, J., & Krane, L. S. (2021). Considering Predictive Factors in the Diagnosis of Clinically Significant Prostate Cancer in Patients with PI-RADS 3 Lesions. Life, 11(12), 1432. https://doi.org/10.3390/life11121432







