Leydig Cells in Patients with Non-Obstructive Azoospermia: Do They Really Proliferate?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients, Testicular Sampling, and Tissue Processing
2.2. Immunohistochemistry
2.3. Morphometric (Stereological) Analysis
- NvLc-number of immunohistochemically positive Leydig cells on insulin-like hormone 3 (INSL 3) and testosterone (T) in a testicle unit volume (mm3 of tissue) (OA biopsies and testicle samples of patients with NOA);
- NLc-total number of immunohistochemically positive Leydig cells on insulin-like hormone 3 (INSL 3) and testosterone (T) in the whole testis (OA biopsies and testis samples of patients with NOA).
2.4. Statistical Analysis
3. Results
3.1. Hormone Values
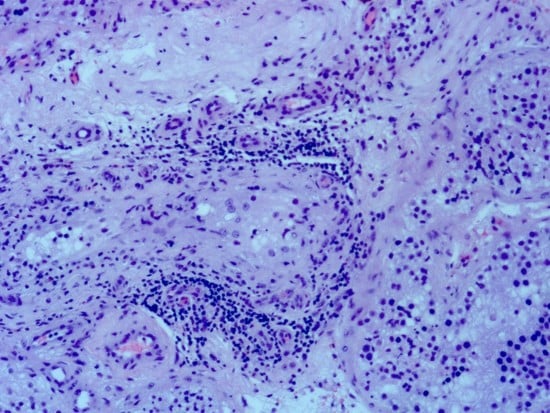
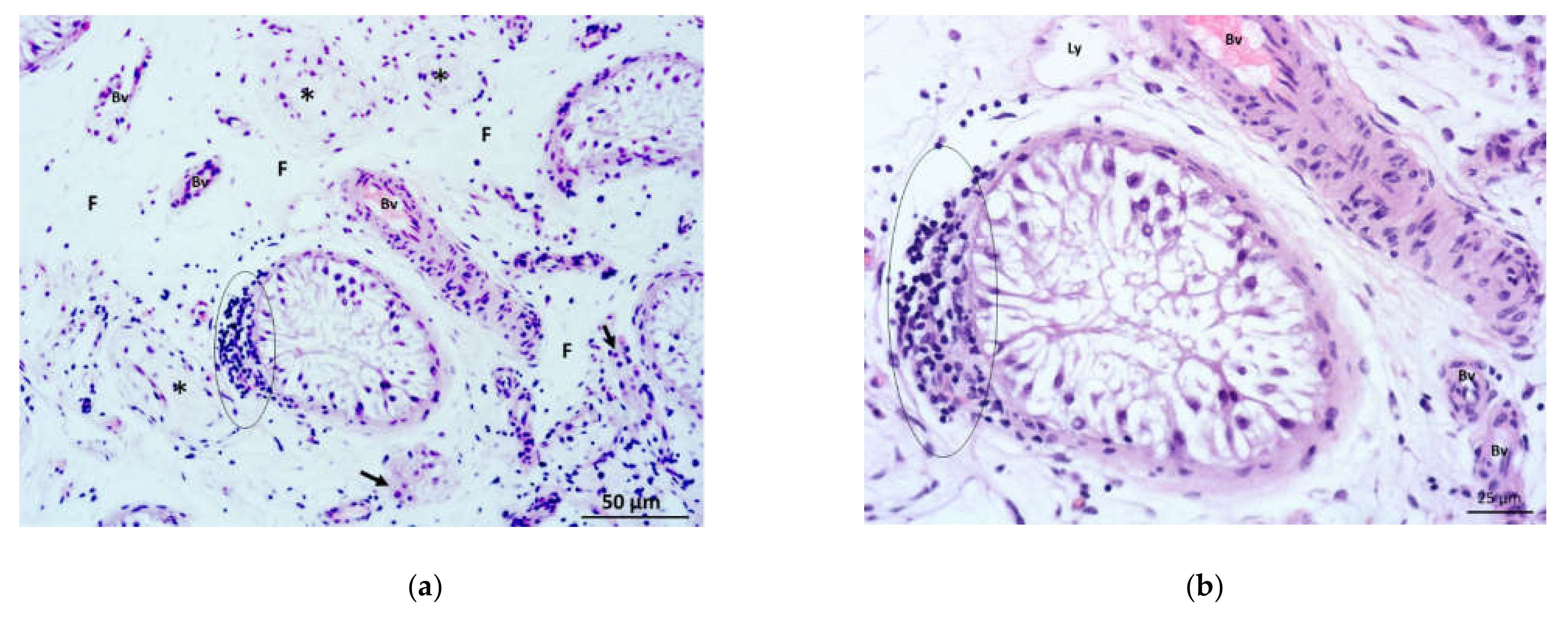
3.2. Results of the Qualitative Histological Analysis
3.2.1. Qualitative Histological Analysis of the Control Group of Biopsies
3.2.2. Qualitative Histological Analysis of Testicular Biopsies Obtained from NOA Patients
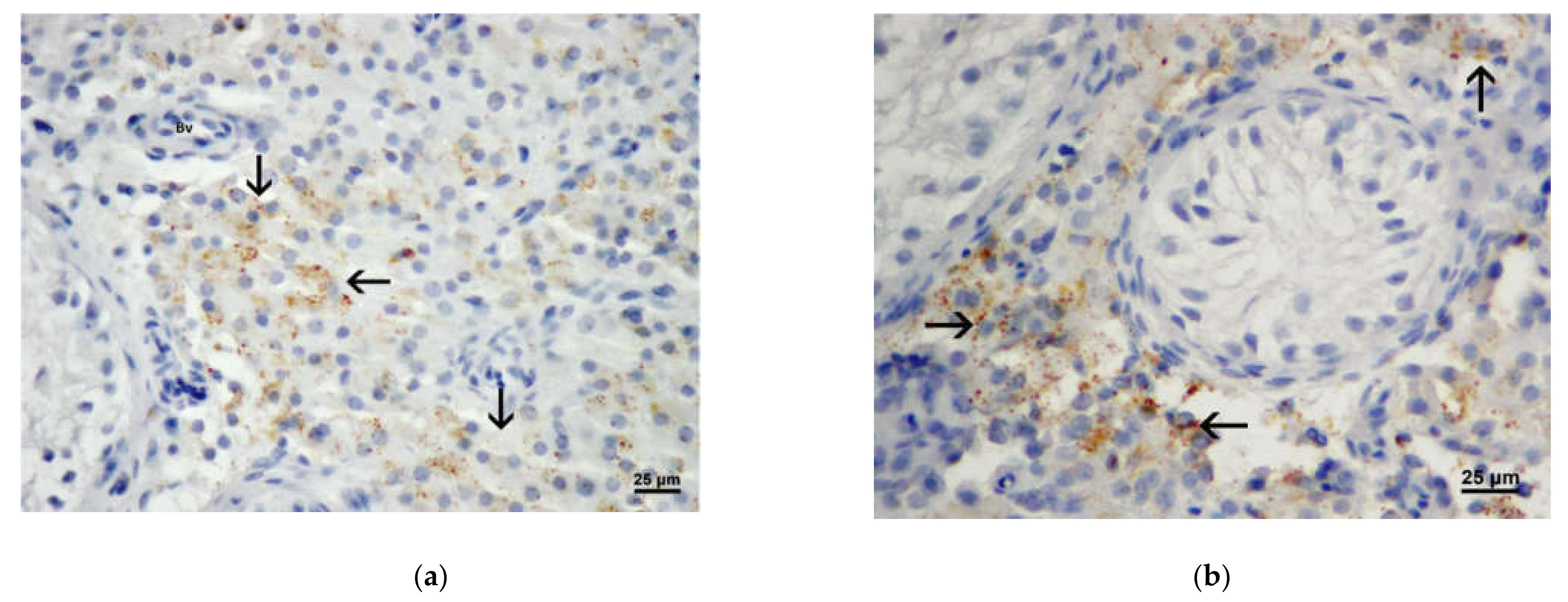
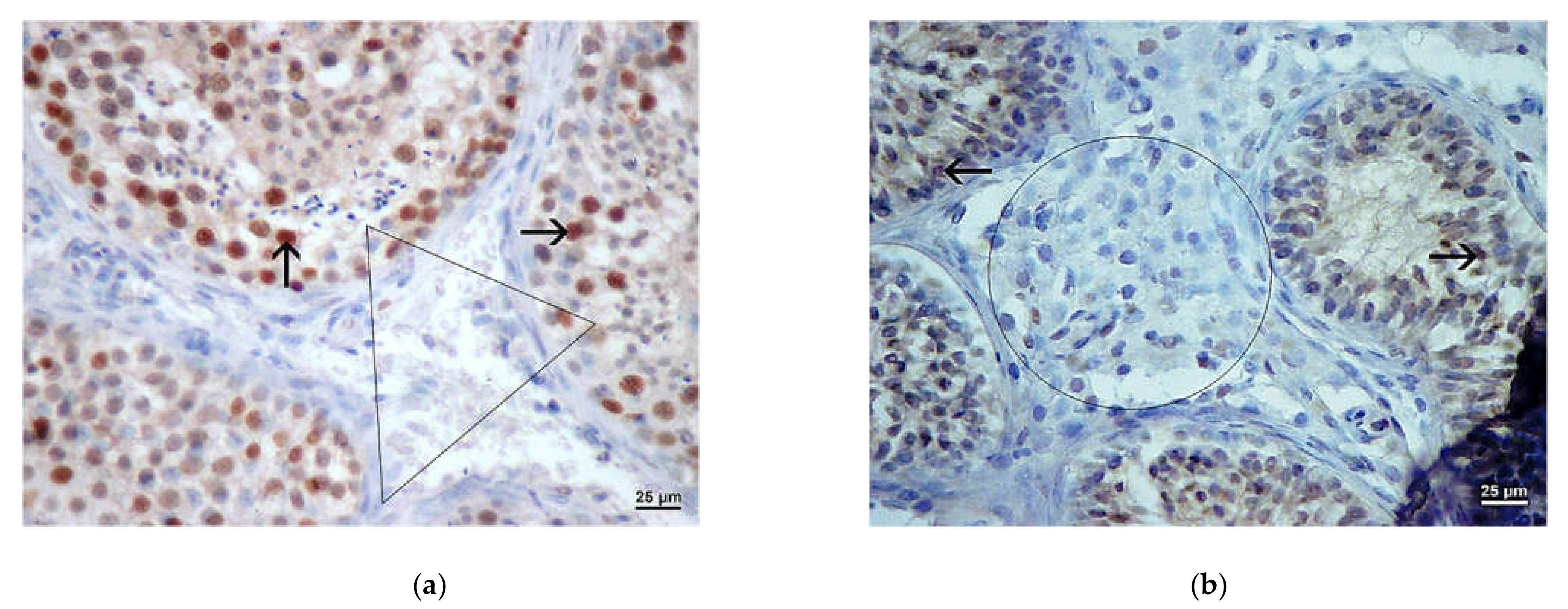
3.3. Results of Immunohistochemistry Analysis
3.4. Results of Morphometric (Stereological) Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donoso, P.; Tournaye, H.; Devroey, P. Which is the best sperm retrieval technique for non-obstructive azoospermia? A systematic review. Hum. Reprod. Update 2007, 13, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Söderström, K.O. Leydig cell hyperplasia. Arch. Androl. 1986, 17, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jezek, D.; Knuth, U.A.; Schulze, W. Successful testicular sperm extraction (TESE) in spite serum follicle stimulating hormone and azoospermia: Correlation between testicular morphology, TESE results, semen analysis and serum hormone values in 103 infertile men. Hum. Reprod. 1998, 13, 1230–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, M.; Behre, H.M.; Nieschlag, E. Serum FSH and testicular morphology in male infertility. Clin. Endocrinol. 1994, 40, 133–136. [Google Scholar] [CrossRef]
- Ježek, D.; Knežević, N.; Kalanj-Bognar, S.; Vukelić, Ž.; Krhen, I. From testicular biopsy to human embryo. Verh. Dtsch. Ges. Pathol. 2004, 88, 136–143. [Google Scholar] [CrossRef]
- Shiraishi, K.; Matsuyama, H. Gonadotoropin actions on spermatogenesis and hormonal therapies for spermatogenic disorders. Endocr. J. 2017, 64, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Main, K.M. Testicular dysgenesis syndrome: An increasingly common developmental disorder with enviromental aspects. Hum. Reprod. 2001, 16, 972–978. [Google Scholar] [CrossRef]
- Anderson, A.-M.; Jorgensen, N.; Larsen, L.F.; Rajpert-De Meyts, E.; Skakkebaek, N.E. Impaired Leydig cell function in infertile men: A study of 357 idiopathic infertile men and 318 proven fertile controls. J. Clin. Endocrinol. Metab. 2004, 89, 3161–3167. [Google Scholar] [CrossRef] [Green Version]
- Naughton, C.K.; Nadler, R.B.; Basler, J.W.; Humphrey, P.A. Leydig cell hyperplasia. Br. J. Urol. 1998, 81, 282–289. [Google Scholar] [CrossRef]
- De Kretser, D.M. Editorial: Is spermatogenic damage associated with Leydig cell dysfunction? J. Clin. Endocrinol. Metab. 2004, 89, 3158–3160. [Google Scholar] [CrossRef] [Green Version]
- Nozu, K.; Matsura, S.; Catt, K.J.; Dufau, M.L. Modulation of Leydig cell androgen biosynthesis and cytochrome P-450 levels during estrogen treatment and human chorionic gonadotropin-induced desentization. J. Biol. Chem. 1981, 256, 10012–10017. [Google Scholar] [CrossRef]
- Westerveld, G.H.; Gianotten, J.; Leschot, N.J.; van der Veen, F.; Repping, S.; Lombardi, M.P. Heterogenous nuclear ribonucleoprotein G-T (HNRNP G-T) mutations in men with impaired spermatogenesis. Mol. Hum. Reprod. 2004, 10, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Tash, J.A.; McCallum, S.; Hardy, M.P.; Knudsen, B.; Schlegel, P.N. Men with nonobstructive azoospermia have Leydig cell hypertrophy but not hyperplasia. J. Urol. 2002, 168, 1068–1070. [Google Scholar] [CrossRef]
- Holstein, A.F.; Wulfhekel, U. Die Semidünschnitt-Technik als Grundlage für eine cytologische Beurteilung der Spermatogenese des Menschen. Andrologia 1971, 3, 65–69. [Google Scholar] [CrossRef]
- Holstein, A.F.; Schulze, W.; Breucker, H. Histopathology of human testicular and epididymal tissue. In Male Infertility; Hargreave, T.B., Ed.; Springer: New York, NY, USA, 1994; pp. 105–148. [Google Scholar]
- Sabattini, E.; Bisgaard, K.; Ascani, S.; Poggi, S.; Piccioli, M.; Ceccarelli, C.; Pieri, F.; Fraternali-Orcioni, G.; Pileri, S.A. The EnVision++ system: A new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J. Clin. Pathol. 1998, 51, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Plazibat, M.; Katušić Bojanac, A.; Himerleich Perić, M.; Gamulin, O.; Rašić, M.; Radonić, V.; Škrabić, M.; Krajačić, M.; Krasić, J.; Sinčić, N.; et al. Embryo-derived teratoma in vitro biological system reveals antitumor and embryotoxic activity of valproate. FEBS J. 2020, 287, 4783–4800. [Google Scholar] [CrossRef] [Green Version]
- Bruno, S.; Darzynkiewicz, Z. Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif. 1992, 25, 31–40. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Zhao, H.; Zhang, S.; Lee, M.Y.; Lee, E.Y.; Zhang, Z. Initiation and termination of DNA replication during S phase in relation to cyclins D1, E and A, p21WAF1, Cdt1 and the p12 subunit of DNA polymerase δ revealed in individual cells by cytometry. Oncotarget 2015, 6, 11735–11750. [Google Scholar] [CrossRef]
- Noorafshan, A. Stereology as a valuable tool in the toolbox of testicular research. Ann. Anat. 2014, 196, 57–66. [Google Scholar] [CrossRef]
- Azu, O.O.; Naidu, E.C.; Naidu, J.S.; Masia, T.; Nzemande, N.F.; Chuturgoon, A.; Singh, S. Testicular histomorphologic and stereological alterations following short-term treatment with highly active antiretroviral drugs (HAART) in an experimental animal model. Andrology 2014, 2, 772–779. [Google Scholar] [CrossRef]
- Sadeghinezhad, J.; Ganji, Z.; Sadeghian Chaleshtori, S.; Bojarzadeh, H.; Aghabalazadeh Asl, M.; Khomejini, A.B.; Roominai, E.; Hosseini, M.; De Silva, M. Morphometric study of the testis in sheep embryos using unbiased design-based stereology. Anat. Histol. Embryol. 2021, 50, 1026–1033. [Google Scholar] [CrossRef]
- Kališnik, M. Temelji stereologije. Acta Stereol. 1985, 4, 1–148. [Google Scholar]
- Ježek, D.; Kozina, V.; Vukasović, A. Normal morphology of the human testis and epididymis. In Atlas on the Human Testis: Normal Morphology and Pathology; Ježek, D., Ed.; Springer: London, UK, 2013; pp. 77–98. [Google Scholar]
- Paniagua, R.; Rodriguez, M.C.; Nistal, M.; Fraile, B.; Regadera, J.; Amat, P. Changes in surface area and number of Leydig cells in relation to the 6 stages of the human seminiferous epithelium. Anat. Embryol. 1988, 178, 423–427. [Google Scholar] [CrossRef]
- Kauerhof, A.C.; Nicolas, N.; Bhushan, S.; Wahle, E.; Loveland, K.A.; Fietz, D.; Bergmann, M.; Groome, N.P.; Kliesch, S.; Schuppe, H.C.; et al. Investigation of activin A in inflammatory responses of the testis and its role in the development of testicular fibrosis. Hum. Reprod. 2019, 34, 1536–1550. [Google Scholar] [CrossRef]
- Schuppe, H.C.; Pilatz, A.; Hossain, H.; Diemer, T.; Wagenlehner, F.; Weidner, W. Urogenital Infection as a risk factor for male infertility. Dtsch. Arztebl. Int. 2017, 114, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Fijak, M.; Pilatz, A.; Hedger, M.P.; Nicolas, N.; Bhushan, S.; Michel, V.; Tung, K.S.K.; Schuppe, H.C.; Meinhardt, A. Infectious, inflammatory and ‘autoimmune’ male factor infertility: How do rodent models inform clinical practice? Hum. Reprod. Update 2018, 24, 416–441. [Google Scholar] [CrossRef] [Green Version]
- Pleuger, C.; Silva, E.J.R.; Pilatz, A.; Bhushan, S.; Meinhardt, A. Differential immune response to infection and acute inflammation along the epididymis. Front. Immunol. 2020, 27, 3125. [Google Scholar] [CrossRef]
- Wijayarathna, R.; Pasalic, A.; Nicolas, N.; Biniwale, S.; Ravinthiran, R.; Genovese, R.; Muir, J.A.; Loveland, K.L.; Meinhardt, A.; Fijak, M.; et al. Region-specific immune responses to autoimmune epididymitis in the murine reproductive tract. Cell Tissue Res. 2020, 381, 351–360. [Google Scholar] [CrossRef]
- Nicolas, N.; Michel, V.; Bhushan, S.; Wahle, E.; Hayward, S.; Ludlow, H.; de Kretser, D.M.; Loveland, K.L.; Schuppe, H.C.; Meinhardt, A.; et al. Testicular activin and follistatin levels are elevated during the course of experimental autoimmune epididymo-orchitis in mice. Sci. Rep. 2017, 7, 42391. [Google Scholar] [CrossRef]
- Willems, M.; Vloeberghs, V.; Gies, I.; De Schepper, J.; Tournaye, H.; Goossens, E.; Van Saen, D. Testicular immune cells and vasculature in Klinefelter syndrome from childhood up to adulthood. Hum. Reprod. 2020, 35, 1753–1764. [Google Scholar] [CrossRef]
- Tesi, M.; Lazzarini, G.; Magliaro, C.; Abramo, F.; Fanelli, D.; Miragliotta, V.; Rota, A. Age-related changes of seminiferous tubule morphology, interstitial fibrosis and spermatogenesis in dogs. Anim. Reprod. Sci. 2020, 219, 106534. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, B.; Qi, Y.; Zhu, L.; Cui, X.; Liu, Z. Antagonistic effects of activin A and TNF-α on the activation of L929 fibroblast cells via Smad3-independent signaling. Sci. Rep. 2020, 10, 20623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, Y.; Raman, A.; Daniel, E.; Metcalf, J.; Reif, G.; Pierucci-Alves, F.; Wallace, D.P. Overexpression of TGF-β1 induces renal fibrosis and accelerates the decline in kidney function in polycystic kidney disease. Am. J. Physiol.-Renal Physiol. 2020, 319, F1135–F1148. [Google Scholar] [CrossRef] [PubMed]
- Lottrup, G.; Nielsen, J.E.; Maroun, L.L.; Møller, L.M.; Yassin, M.; Leffers, H.; Skakkebæk, N.E.; Rajpert-De Meyts, E. Expression patterns of DLK1 and INSL3 identify stages of Leydig cell differentiation during normal development and in testicular pathologies, including testicular cancer and Klinefelter syndrome. Hum. Reprod. 2014, 29, 1637–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahyari, E.; Guo, J.; Lima, A.C.; Lewinsohn, D.P.; Stendahl, A.M.; Vigh-Conrad, K.A.; Nie, X.; Nagirnaja, L.; Rockweiler, N.B.; Carrell, D.T.; et al. Comparative single-cell analysis of biopsies clarifies pathogenic mechanisms in Klinefelter syndrome. Am. J. Hum. Genet. 2021, 108, 1924–1945. [Google Scholar] [CrossRef] [PubMed]
- Hauptman, D.; Hudolin, T.; Zimak, Z.; Kuliš, T.; Ježek, D.; Kaštelan, Ž. Conventional TESE technique: A short review and a single-centre experience in 9 years. Rad Hrvat. Akad. Znan. Umjet. Med. Znan. 2021, 547, 54–55. [Google Scholar] [CrossRef]






| Group | FSH (IU/L) Mean Range (min.–max.) | LH (IU/L) Mean Range (min.–max.) | T (nmol/L) Mean Range (min.–max.) |
|---|---|---|---|
| OA | 4.36 | 3.59 | 19.8 |
| (1.9–6.7) | (1.2–6.8) | (12.4–28.6) | |
| NOA | 29.18 | 13.24 | 17.63 |
| (2.8–41.9) | (1.7–23.7) | (7.7–39.2) |
| NV(mm−2)/Leydig Cells | ||||
|---|---|---|---|---|
| OA Left Testis | OA Right Testis | NOA Left Testis | NOA Right Testis | |
| Mean | 6243 | 6225 | 7188 | 7508 |
| SD | 3927 | 4533 | 5672 | 6602 |
| SE | 742.2 | 906.6 | 987.3 | 1167 |
| VV(mm0)/Leydig Cells | ||||
|---|---|---|---|---|
| OA Left Testis | OA Right Testis | NOA Left Testis | NOA Right Testis | |
| Mean | 0.03857 | 0.0384 | 0.08030 a | 0.08281 b |
| SD | 0.01957 | 0.01818 | 0.08278 | 0.08865 |
| SE | 0.003699 | 0.003637 | 0.01441 | 0.01567 |
| N × 106/Leydig Cells | ||||
|---|---|---|---|---|
| OA Left Testis | OA Right Testis | NOA Left Testis | NOA Right Testis | |
| Mean | 138.9 | 120.3 | 45.96 a | 34.41 b |
| SD | 81.44 | 67.18 | 38.39 | 16.66 |
| SE | 18.68 | 16.29 | 14.51 | 8.331 |
| V/mm3/Leydig Cells | ||||
|---|---|---|---|---|
| OA Left Testis | OA Right Testis | NOA Left Testis | NOA Right Testis | |
| Mean | 768.7 | 676.0 | 490.1 | 402.5 |
| SD | 490.6 | 302.9 | 349.6 | 204.1 |
| SE | 112.6 | 73.46 | 132.1 | 102.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hauptman, D.; Perić, M.H.; Marić, T.; Bojanac, A.K.; Sinčić, N.; Zimak, Z.; Kaštelan, Ž.; Ježek, D. Leydig Cells in Patients with Non-Obstructive Azoospermia: Do They Really Proliferate? Life 2021, 11, 1266. https://doi.org/10.3390/life11111266
Hauptman D, Perić MH, Marić T, Bojanac AK, Sinčić N, Zimak Z, Kaštelan Ž, Ježek D. Leydig Cells in Patients with Non-Obstructive Azoospermia: Do They Really Proliferate? Life. 2021; 11(11):1266. https://doi.org/10.3390/life11111266
Chicago/Turabian StyleHauptman, Dinko, Marta Himelreich Perić, Tihana Marić, Ana Katušić Bojanac, Nino Sinčić, Zoran Zimak, Željko Kaštelan, and Davor Ježek. 2021. "Leydig Cells in Patients with Non-Obstructive Azoospermia: Do They Really Proliferate?" Life 11, no. 11: 1266. https://doi.org/10.3390/life11111266
APA StyleHauptman, D., Perić, M. H., Marić, T., Bojanac, A. K., Sinčić, N., Zimak, Z., Kaštelan, Ž., & Ježek, D. (2021). Leydig Cells in Patients with Non-Obstructive Azoospermia: Do They Really Proliferate? Life, 11(11), 1266. https://doi.org/10.3390/life11111266