Development of a Dual ELISA for the Detection of CD2v-Unexpressed Lower-Virulence Mutational ASFV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Swine Serum and Secondary Antibody
2.2. Eukaryotic Expression and Purification of CD2v and p30 Proteins
2.3. Western Blotting Analysis
2.4. Establishment of Enzyme-Linked Immunosorbent Assay (ELISA) and ELISA Kit
2.5. Statistical Analysis
3. Results
3.1. Expression of ASFV CD2v and p30 and Immunogenic Identification
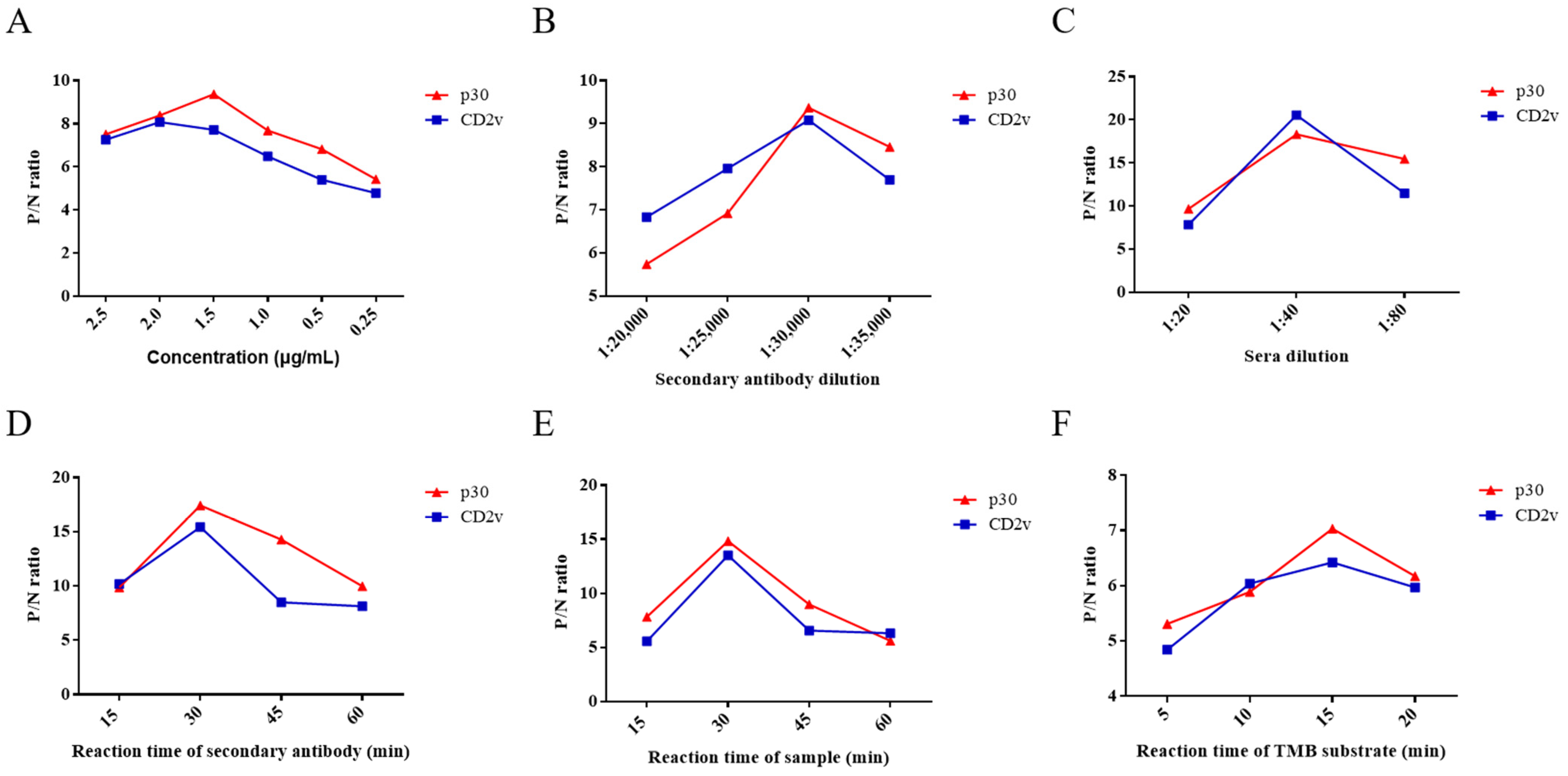
3.2. Optimization of Experimental Conditions for ELISA
3.3. Determination of the Cut-off Value of ELISA
3.4. Detection Sensitivity and Specificity of ELISA
3.5. Dual ELISA Kit Detection of ASFV- or ASFV-ΔCD2v-Infected Sera
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alejo, A.; Matamoros, T.; Guerra, M.; Andrés, G. A Proteomic Atlas of the African Swine Fever Virus Particle. J. Virol. 2018, 92, 01293-18. [Google Scholar] [CrossRef] [Green Version]
- Borca, M.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Espinoza, N.; Velazquez-Salinas, L.; Gay, C.; Gladue, D. ASFV-G-∆I177L as an Effective Oral Nasal Vaccine against the Eurasia Strain of Africa Swine Fever. Viruses 2021, 13, 765. [Google Scholar] [CrossRef]
- Ai, Q.; Lin, X.; Xie, H.; Li, B.; Liao, M.; Fan, H. Proteome Analysis in PAM Cells Reveals That African Swine Fever Virus Can Regulate the Level of Intracellular Polyamines to Facilitate Its Own Replication through ARG1. Viruses 2021, 13, 1236. [Google Scholar] [CrossRef]
- Donald, P.; King, A.; Scott, M.; Reid, B.; Geoffrey, H.; Hutchings, B.; Sylvia, S.; Grierson, A.; Philip, J.; Wilkinson, B.; et al. Development of a TaqMan PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods 2003, 107, 53–61. [Google Scholar]
- Awosanya, E.J.; Olugasa, B.O.; Gimba, F.I.; Sabri, M.Y.; Ogundipe, G.A. Detection of African swine fever virus in pigs in Southwest Nigeria. Veter-World 2021, 14, 1840–1845. [Google Scholar] [CrossRef]
- Nan, W.; Dongming, Z.; Jialing, W.; Yangling, Z.; Ming, W.; Yan, G.; Fang, L.; Zhigao, B.; Zihe, R.; Xiangxi, W. Architecture of African swine fever virus and implications for viral assembly. Science 2019, 366, 640–644. [Google Scholar]
- Revilla, Y.; Pérez-Núñez, D.; Richt, J.A. African Swine Fever Virus Biology and Vaccine Approaches. Adv. Appl. Microbiol. 2018, 100, 41–74. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, R.; Zhang, X.; Li, F.; Wang, J.; Zhang, J.; Liu, X.; Wang, L.; Zhang, J.; Wu, X.; et al. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg. Microbes Infect. 2019, 8, 438–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallardo, C.; Soler, A.; Rodze, I.; Nieto, R.; Cano-Gomez, C.; Fernandez-Pinero, J.; Arias, M. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound. Emerg. Dis. 2019, 66, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Boinas, F.S.; Hutchings, G.H.; Dixon, L.K.; Wilkinson, P.J. Characterization of pathogenic and non-pathogenic African swine fever virus isolates from Ornithodoros erraticus inhabiting pig premises in Portugal. J. Gen. Virol. 2004, 85, 2177–2187. [Google Scholar] [CrossRef]
- Sun, E.; Zhang, Z.; Wang, Z.; He, X.; Zhang, X.; Wang, L.; Wang, W.; Huang, L.; Xi, F.; Huangfu, H.; et al. Emergence and prevalence of naturally occurring lower virulent African swine fever viruses in domestic pigs in China in 2020. Sci. China Life Sci. 2021, 64, 752–765. [Google Scholar] [CrossRef]
- Giménez-Lirola, L.G.; Mur, L.; Rivera, B.; Mogler, M.; Sun, Y.; Lizano, S.; Goodell, C.; Harris, D.L.H.; Rowland, R.R.R.; Gallardo, C.; et al. Detection of African Swine Fever Virus Antibodies in Serum and Oral Fluid Specimens Using a Recombinant Protein 30 (p30) Dual Matrix Indirect ELISA. PLoS ONE 2016, 11, e0161230. [Google Scholar] [CrossRef] [Green Version]
- Damle, R.G.; Patil, A.A.; Bhide, V.S.; Pawar, S.D.; Sapkal, G.N.; Bondre, V.P. Development of a novel rapid mi-cro-neutralization ELISA for the detection of neutralizing antibodies against Chandipura virus. J. Virol. Methods 2017, 240, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rui, P.D.; Young, M.P.; Jong, Y.C.; Boo, S.K.; Guhung, J. Detection of antibodies against DNA polymerase of hepatitis B virus in HBsAg-positive sera using ELISA. Korean J. Intern. Med. 1998, 13, 95–98. [Google Scholar]
- Zhang, Q.; Zhang, H.; Gao, J.; Huang, K.; Yang, Y.; Hui, X.; He, X.; Li, C.; Gong, W.; Zhang, Y.; et al. A serological survey of SARS-CoV-2 in cat in Wuhan. Emerg. Microbes Infect. 2020, 9, 2013–2019. [Google Scholar] [CrossRef]
- Goatley, L.C.; Dixon, L.K. Processing and Localization of the African Swine Fever Virus CD2v Transmembrane Protein. J. Virol. 2011, 85, 3294–3305. [Google Scholar] [CrossRef] [Green Version]
- Pan, Q.; Wang, J.; Hussain, A.; Gao, Y.; Cui, H.; Li, K.; Qi, X.; Liu, C.; Zhang, Y.; Zhang, S.; et al. An optimized secretory expression system and immunogenicity evaluation for glycosylated gp90 of avian reticuloendotheliosis virus. Veter-Res. 2020, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Lowe, A.D.; Rodríguez, Y.Y.; Murgia, M.V.; Dodd, K.A.; Rowland, R.R.; Jia, W. Antigenic Regions of African Swine Fever Virus Phosphoprotein P30. Transbound. Emerg. Dis. 2020. [Google Scholar] [CrossRef]
- Kazakova, A.S.; Imatdinov, A.; Dubrovskaya, O.; Sidlik, M.V.; Balyshev, V.M.; Красочко, П.А.; Sereda, D. Recombinant Protein p30 for Serological Diagnosis of African Swine Fever by Immunoblotting Assay. Transbound. Emerg. Dis. 2016, 64, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Filgueira, D.M.; González-Camacho, F.; Gallardo, C.; Resino-Talaván, P.; Blanco, E.; Gómez-Casado, E.; Alonso, C.; Escribano, J.M. Optimization and Validation of Recombinant Serological Tests for African Swine Fever Diagnosis Based on Detection of the p30 Protein Produced in Trichoplusia ni Larvae. J. Clin. Microbiol. 2006, 44, 3114–3121. [Google Scholar] [CrossRef] [Green Version]
- Teklue, T.; Wang, T.; Luo, Y.; Hu, R.; Sun, Y.; Qiu, H.-J. Generation and Evaluation of an African Swine Fever Virus Mutant with Deletion of the CD2v and UK Genes. Vaccines 2020, 8, 763. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, P.L.; Lacasta, A.; López, E.; Bosch, L.; Collado, J.; Pina-Pedrero, S.; Correa-Fiz, F.; Accensi, F.; Navas, M.J.; Vidal, E.; et al. BA71ΔCD2: A New Recombinant Live Attenuated African Swine Fever Virus with Cross-Protective Capabilities. J. Virol. 2017, 91, 01058-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Lv, C.; Fan, J.; Zhao, Y.; Jiang, L.; Sun, X.; Zhang, Q.; Jin, M. Development of a chemiluminescence immunoassay to accurately detect African swine fever virus antibodies in serum. J. Virol. Methods 2021, 298, 114269. [Google Scholar] [CrossRef]
- Breard, E.; Garnier, A.; Despres, P.; Boisseau, S.B.; Comtet, L.; Viarouge, C.; Bakkali-Kassimi, L.; Pourquier, P.; Hudelet, P.; Vitour, D.; et al. Development of a Double-Antigen Microsphere Immunoassay for Simultaneous Group and Serotype Detection of Bluetongue Virus Antibodies. Transbound. Emerg. Dis. 2016, 64, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.; Sinclair, J.; Entrican, G.; Herring, J.; Malloy, C.; Nettleton, P. A monoclonal antibody capture ELISA to detect antibody to border disease virus in sheep serum. Veter-Microbiol. 1991, 28, 327–333. [Google Scholar] [CrossRef]
- Alroqi, F.; Masuadi, E.; Alabdan, L.; Nogoud, M.; Aljedaie, M.; Abu-Jaffal, A.S.; Barhoumi, T.; Almasoud, A.; Alharbi, N.K.; Alsaedi, A.; et al. Seroprevalence of SARS-CoV-2 among high-risk healthcare workers in a MERS-CoV endemic area. J. Infect. Public Health 2021, 14, 1268–1273. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, C.; Zhao, Y.; Jiang, L.; Zhao, L.; Wu, C.; Hui, X.; Hu, X.; Shao, Z.; Xia, X.; Sun, X.; et al. Development of a Dual ELISA for the Detection of CD2v-Unexpressed Lower-Virulence Mutational ASFV. Life 2021, 11, 1214. https://doi.org/10.3390/life11111214
Lv C, Zhao Y, Jiang L, Zhao L, Wu C, Hui X, Hu X, Shao Z, Xia X, Sun X, et al. Development of a Dual ELISA for the Detection of CD2v-Unexpressed Lower-Virulence Mutational ASFV. Life. 2021; 11(11):1214. https://doi.org/10.3390/life11111214
Chicago/Turabian StyleLv, Changjie, Ya Zhao, Lili Jiang, Li Zhao, Chao Wu, Xianfeng Hui, Xiaotong Hu, Ziqi Shao, Xiaohan Xia, Xiaomei Sun, and et al. 2021. "Development of a Dual ELISA for the Detection of CD2v-Unexpressed Lower-Virulence Mutational ASFV" Life 11, no. 11: 1214. https://doi.org/10.3390/life11111214
APA StyleLv, C., Zhao, Y., Jiang, L., Zhao, L., Wu, C., Hui, X., Hu, X., Shao, Z., Xia, X., Sun, X., Zhang, Q., & Jin, M. (2021). Development of a Dual ELISA for the Detection of CD2v-Unexpressed Lower-Virulence Mutational ASFV. Life, 11(11), 1214. https://doi.org/10.3390/life11111214




