The Discordance between Network Excitability and Cognitive Performance Following Vigabatrin Treatment during Epileptogenesis
Abstract
1. Introduction
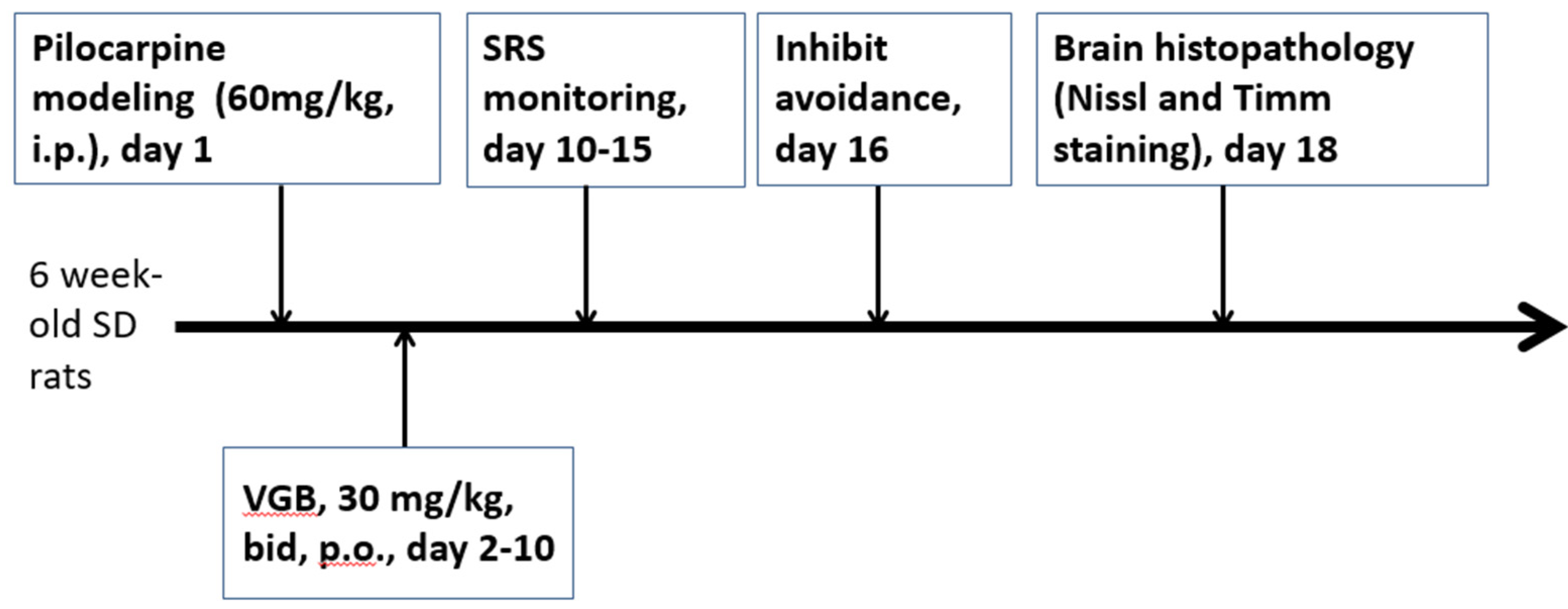
2. Materials and Methods
2.1. Animals
2.2. In Vivo Experiments
2.3. Inhibitory Avoidance Task
2.4. Histopathology
2.4.1. Cresyl Violet Staining
2.4.2. Timm’s Staining
2.5. Drugs and Solutions
2.6. Statistical Analysis
3. Results
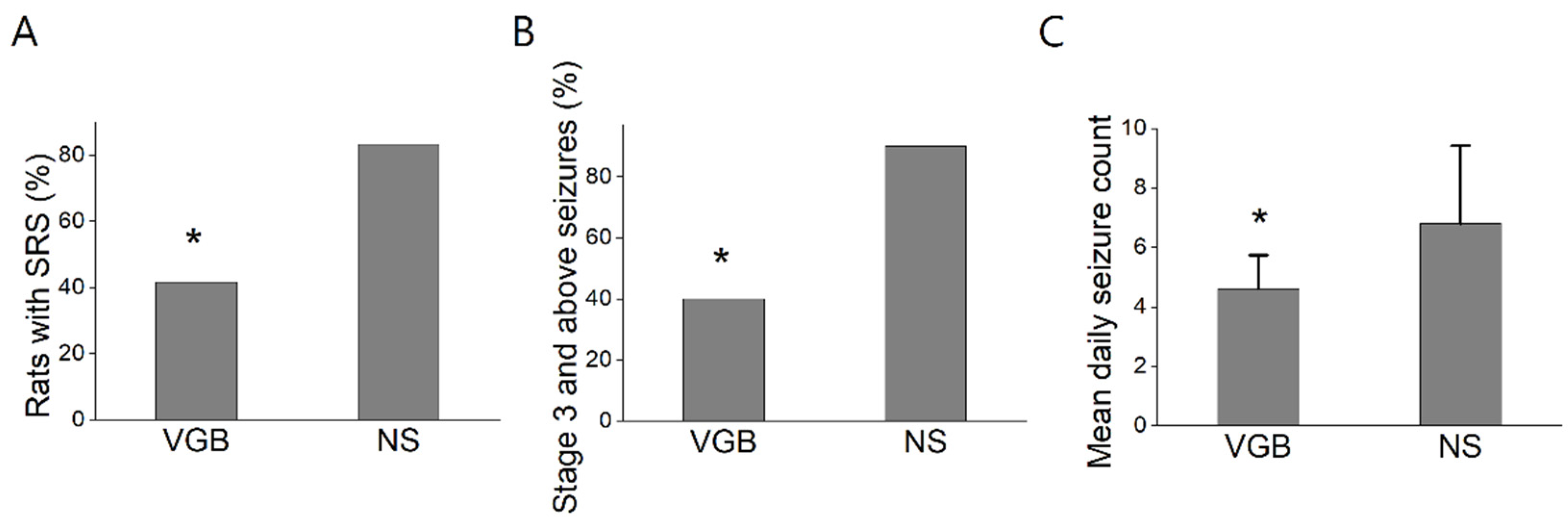
3.1. VGB-Treated Rats Had Fewer Spontaneous Recurrent Seizures
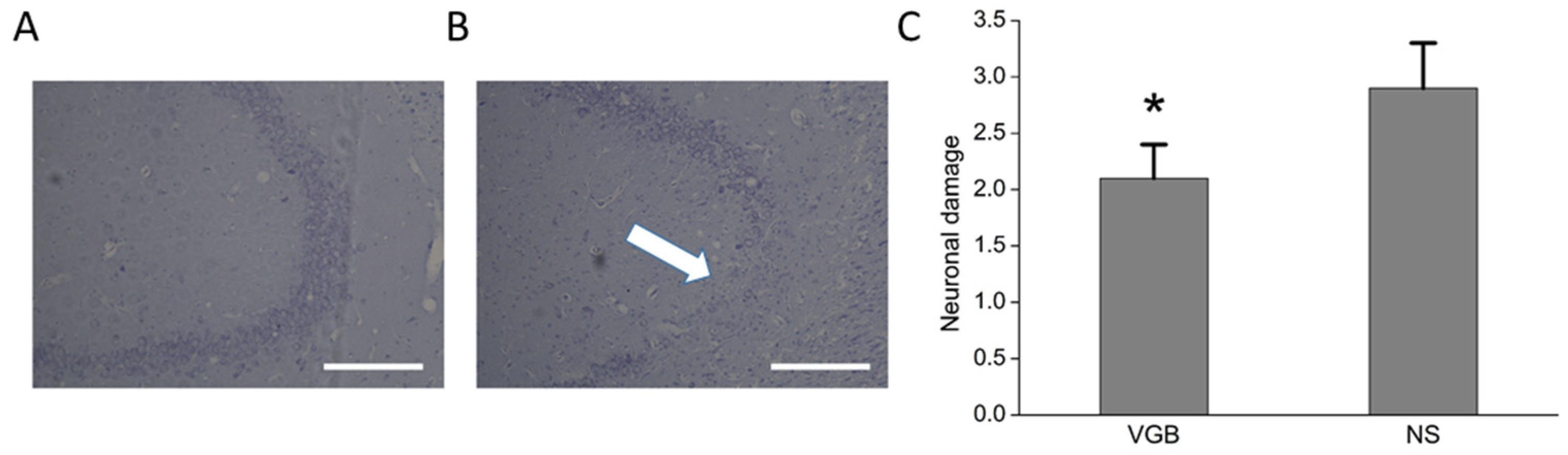
3.2. The VGB-Treated Rats Had Less Post-Status Epilepticus Chronic Hippocampal Damage
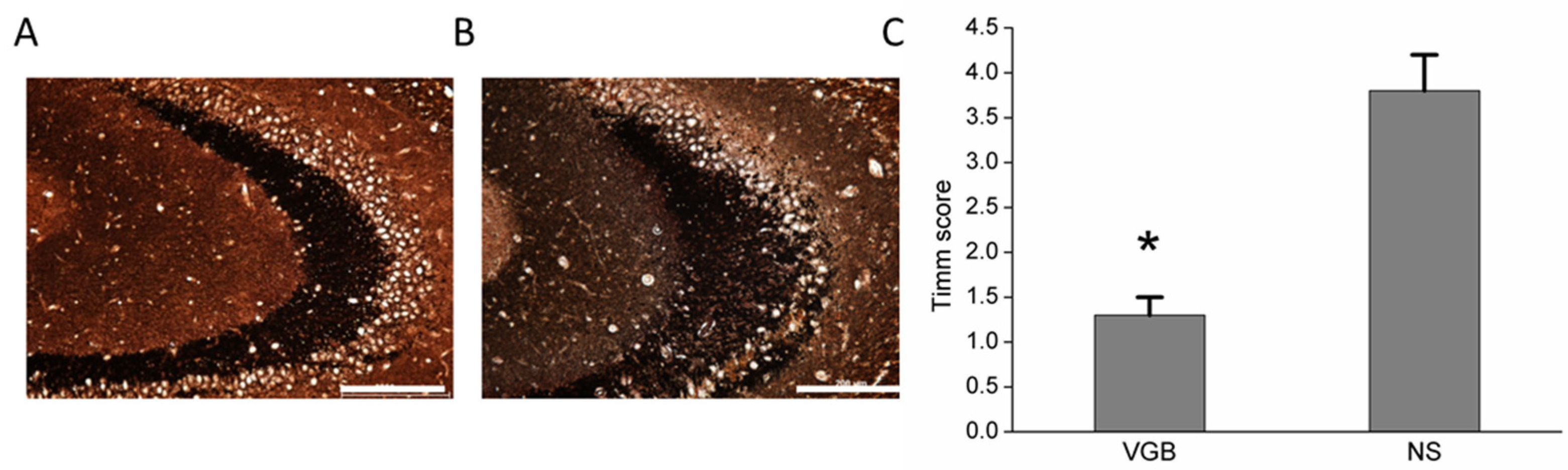
3.3. VGB-Treated Rats Had Less Aberrant Mossy Fiber Sprouting
3.4. VGB-Treated Rats Did Not Preserve Inhibitory Avoidance Test Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lux, A.L.; Edwards, S.W.; Hancock, E.; Johnson, A.L.; Kennedy, C.R.; Newton, R.W.; O’Callaghan, F.J.K.; Verity, C.M.; Osborne, J.P. The United Kingdom infantile spasms study comparing vigabatrin with prednisolone or tetracosactide at 14 days: A multicentre, randomised controlled trial. Lancet 2004, 364, 1773–1778. [Google Scholar] [CrossRef]
- Hancock, E.C.; Osborne, J.P.; Edwards, S.W. Treatment of infantile spasms. Cochrane Database Syst. Rev. 2008, 6, CD001770. [Google Scholar]
- Hemming, K.; Maguire, M.J.; Hutton, J.L.; Marson, A.G. Vigabatrin for refractory partial epilepsy. Cochrane Database Syst. Rev. 2008, 3, CD007302. [Google Scholar]
- Schechter, P.J. Vigabatrin. In Current Problems in Epilepsy 4, New Anticonvulsant Drugs; Meldrum, B.S., Porter, P.J., Eds.; John Libby: London, UK, 1986; pp. 265–275. [Google Scholar]
- Schechter, P.J.; Hanke, N.F.; Grove, J.; Huebert, N.; Sjoerdsma, A. Biochemical and clinical effects of gamma-vinyl GABA in patients with epilepsy. Neurology 1984, 34, 182–186. [Google Scholar] [CrossRef]
- Jung, M.J.; Lippert, B.; Metcalf, B.W.; Böhlen, P.; Schechter, P.J. Gamma-Vinyl GABA (4-amino-hex-5-enoic acid), a new selective irreversible inhibitor of GABA-T: Effects on brain GABA metabolism in mice. J. Neurochem. 1977, 29, 797–802. [Google Scholar] [CrossRef]
- Perry, T.L.; Kish, S.J.; Hansen, S. Gamma-Vinyl GABA: Effects of chronic administration on the metabolism of GABA and other amino compounds in rat brain. J. Neurochem. 1979, 32, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.Y.; Huang, H.Y.I.; Wu, S.N.; Huang, C.W. Depressive effectiveness of vigabatrin (γ-vinyl-GABA), an antiepileptic drug, in intermediate-conductance calcium-activated potassium channels in human glioma cells. BMC Pharmacol. Toxicol. 2021, 22, 6. [Google Scholar] [CrossRef]
- Smolders, I.; Khan, G.M.; Lindekens, H.; Prikken, S.; Marvin, C.A.; Manil, J.; Ebinger, G.; Michotte, Y. Effectiveness of vigabatrin against focally evoked pilocarpine-induced seizures and concomitant changes in extracellular hippocampal and cerebellar glutamate, gamma-aminobutyric acid and dopamine levels, a microdialysis-electrocorticography study in freely moving rats. J. Pharmacol. Exp. Ther. 1997, 283, 1239–1248. [Google Scholar]
- Świąder, M.J.; Świąder, K.; Zakrocka, I.; Krzyżanowski, M.; Wróbel, A.; Łuszczki, J.J.; Czuczwar, S.J. Long-term vigabatrin treatment modifies pentylenetetrazole-induced seizures in mice: Focused on GABA brain concentration. Pharmacol. Rep. 2020, 72, 322–330. [Google Scholar] [CrossRef]
- Frost, J.D., Jr.; Le, J.T.; Lee, C.L.; Ballester-Rosado, C.; Hrachovy, R.A.; Swann, J.W. Vigabatrin therapy implicates neocortical high frequency oscillations in an animal model of infantile spasms. Neurobiol. Dis. 2015, 82, 1–11. [Google Scholar] [CrossRef][Green Version]
- Richens, A. Potential antiepileptic drugs: Vigabatrin. In Antiepileptic Drugs, 3rd ed.; Levy, R.H., Dreifuss, F.E., Mattson, R.H., Meldrum, B.S., Penry, J.K., Eds.; Raven: New York, NY, USA, 1989; pp. 937–946. [Google Scholar]
- Montañez, S.; Kline, A.E.; Selwyn, A.P.; Suozzi, J.C.; Butler, S.E.; Hernandez, T.D. Vigabatrin directed against kindled seizures following cortical insult: Impact on epileptogenesis and somatosensory recovery. J. Neurotrauma 2001, 18, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- André, V.; Dubé, C.; François, J.; Leroy, C.; Rigoulot, M.-A.; Roch, C.; Namer, I.J.; Nehlig, A. Pathogenesis and pharmacology of epilepsy in the lithium-pilocarpine model. Epilepsia 2007, 5, 41–47. [Google Scholar] [CrossRef]
- Koene, L.M.C.; van Grondelle, S.E.; Proietti Onori, M.; Wallaard, I.; Kooijman, N.H.R.M.; van Oort, A.; Schreiber, J.; Elgersma, Y. Effects of antiepileptic drugs in a new TSC/mTOR-dependent epilepsy mouse model. Ann. Clin. Transl. Neurol. 2019, 6, 1273–1291. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, D.; Kodali, M.; Gitai, D.; Castro, O.W.; Zanirati, G.; Upadhya, R.; Attaluri, S.; Mitra, E.; Shuai, B.; Hattiangady, B.; et al. A model of chronic temporal lobe epilepsy presenting constantly rhythmic and robust spontaneous seizures, co-morbidities and hippocampal neuropathology. Aging Dis. 2019, 10, 915–936. [Google Scholar] [CrossRef] [PubMed]
- Aldenkamp, A.; Besag, F.; Gobbi, G.; Caplan, R.; Dunn, D.W.; Sillanpää, M. Psychiatric and behavioural disorders in children with epilepsy (ILAE Task Force Report): Adverse cognitive and behavioural effects of antiepileptic drugs in children. Epileptic Disord. 2016, 18, S55–S67. [Google Scholar]
- Aldenkamp, A.P.; Bodde, N. Behaviour, cognition and epilepsy. Acta Neurol. Scand. Suppl. 2005, 182, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Djuric, M.; Kravljanac, R.; Tadic, B.; Mrlješ-Popovic, N.; Appleton, R.E. Long-term outcome in children with infantile spasms treated with vigabatrin: A cohort of 180 patients. Epilepsia 2014, 55, 1918–1925. [Google Scholar] [CrossRef]
- Ljff, D.M.; Aldenkamp, A.P. Cognitive side-effects of antiepileptic drugs in children. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 111, pp. 707–718. [Google Scholar]
- Hughes, S.; Jagannath, A.; Hankins, M.W.; Foster, R.G.; Peirson, S.N. Photic regulation of clock systems. Methods Enzymol. 2015, 552, 125–143. [Google Scholar]
- Pathak, H.R.; Weissinger, F.; Terunuma, M.; Carlson, G.C.; Hsu, F.C.; Moss, S.J.; Coulter, D.A. Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J. Neurosci. 2007, 27, 14012–14022. [Google Scholar] [CrossRef]
- Hung, T.Y.; Chu, F.L.; Wu, D.C.; Wu, S.N.; Huang, C.W. The protective role of peroxisome proliferator-activated receptor-gamma in seizure and neuronal excitotoxicity. Mol. Neurobiol. 2019, 56, 5497–5506. [Google Scholar] [CrossRef]
- Lai, M.C.; Wu, S.N.; Huang, C.W. The specific effects of OD-1, a peptide activator, on voltage-gated sodium current and seizure susceptibility. Int. J. Mol. Sci. 2020, 21, 8254. [Google Scholar] [CrossRef]
- Racine, R.; Okujava, V.; Chipashvili, S. Modification of seizure activity by electrical stimulation. 3. Mechanisms. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 295–299. [Google Scholar] [CrossRef]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Aronica, E.; Iyer, A.M.; Rossetti, C.; Molteni, M.; Casalgrandi, M.; Manfredi, A.A.; et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 2010, 16, 413–419. [Google Scholar] [CrossRef]
- Chang, Y.C.; Huang, A.M.; Kuo, Y.M.; Wang, S.T.; Chang, Y.Y.; Huang, C.C. Febrile seizures impair memory and cAMP response-element binding protein activation. Ann. Neurol. 2003, 54, 706–718. [Google Scholar] [CrossRef]
- Pitkänen, A.; Schwartzkroin, P.A.; Moshe, S.L. Models of Seizures and Epilepsy; Elsevier Academic Press: Burlington, MA, USA, 2006. [Google Scholar]
- Huang, C.W.; Cheng, J.T.; Tsai, J.J.; Wu, S.N.; Huang, C.C. Diabetic hyperglycemia aggravates seizures and status epilepticus-induced hippocampal damage. Neurotox. Res. 2009, 15, 71–81. [Google Scholar] [CrossRef]
- Holmes, G.L.; Ben-Ari, Y. Seizures in the developing brain: Perhaps not so benign after all. Neuron 1998, 21, 1231–1234. [Google Scholar] [CrossRef]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.; Avoli, M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Gillham, R.A.; Blacklaw, J.; McKee, P.J.; Brodie, M.J. Effect of vigabatrin on sedation and cognitive function in patients with refractory epilepsy. J. Neurol. Neurosurg. Psychiatry 1993, 56, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Dodrill, C.B.; Arnett, J.L.; Sommerville, K.W.; Sussman, N.M. Effects of differing dosages of vigabatrin (Sabril) on cognitive abilities and quality of life in epilepsy. Epilepsia 1995, 36, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Kälviäinen, R.; Aikiä, M.; Saukkonen, A.M.; Mervaala, E.; Riekkinen, P.J., Sr. Vigabatrin vs. carbamazepine monotherapy in patients with newly diagnosed epilepsy. A randomized, controlled study. Arch. Neurol. 1995, 52, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Mederos, S.; Sánchez-Puelles, C.; Esparza, J.; Valero, M.; Ponomarenko, A.; Perea, G. GABAergic signaling to astrocytes in the prefrontal cortex sustains goal-directed behaviors. Nat. Neurosci. 2021, 24, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Vossel, K.A.; Tartaglia, M.C.; Nygaard, H.B.; Zeman, A.Z.; Miller, B.L. Epileptic activity in Alzheimer’s disease: Causes and clinical relevance. Lancet Neurol. 2017, 16, 311–322. [Google Scholar] [CrossRef]
- Pitkänen, A.; Nissinen, J.; Jolkkonen, E.; Tuunanen, J.; Halonen, T. Effects of vigabatrin treatment on status epilepticus-induced neuronal damage and mossy fiber sprouting in the rat hippocampus. Epilepsy Res. 1999, 33, 67–85. [Google Scholar] [CrossRef]
- Shin, C.; Rigsbee, L.C.; McNamara, J.O. Anti-seizure and anti-epileptogenic effect of gamma-vinyl gamma-aminobutyric acid in amygdaloid kindling. Brain Res. 1986, 398, 370–374. [Google Scholar] [CrossRef]
- Myers, C.E.; Bermudez-Hernandez, K.; Scharfman, H.E. The influence of ectopic migration of granule cells into the hilus on dentate gyrus-CA3 function. PLoS ONE 2013, 8, e68208. [Google Scholar]
- Jessberger, S.; Nakashima, K.; Clemenson, G.D., Jr.; Mejia, E.; Mathews, E.; Ure, K.; Ogawa, S.; Sinton, C.M.; Gage, F.H.; Hsieh, J. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J. Neurosci. 2007, 27, 5967–5975. [Google Scholar] [CrossRef]
- Zhu, K.; Yuan, B.; Hu, M.; Li, C.-J.; Xu, J.-H.; Feng, G.-F.; Liu, Y.; Liu, J.-X. Ablation of aberrant neurogenesis fails to attenuate cognitive deficit of chronically epileptic mice. Epilepsy Res. 2018, 142, 1–8. [Google Scholar] [CrossRef]
- Pascente, R.; Frigerio, F.; Rizzi, M.; Porcu, L.; Boido, M.; Davids, J.; Zaben, M.; Tolomeo, D.; Filibian, M.; Gray, W.; et al. Cognitive deficits and brain myo-Inositol are early biomarkers of epileptogenesis in a rat model of epilepsy. Neurobiol. Dis. 2016, 93, 146–155. [Google Scholar] [CrossRef]
- Sousa, K.; Decker, N.; Pires, T.R.; Papke, D.K.M.; Coelho, V.R.; Pflüger, P.; Pereira, P.; Picada, J.N. Neurobehavioral effects of vigabatrin and its ability to induce DNA damage in brain cells after acute treatment in rats. Psychopharmacology 2017, 234, 129–136. [Google Scholar] [CrossRef]
- Best, J.L.; Acheson, J.F. The natural history of Vigabatrin associated visual field defects in patients electing to continue their medication. Eye 2005, 19, 41–44. [Google Scholar] [CrossRef][Green Version]
- Newman, W.D.; Tocher, K.; Acheson, J.F. Vigabatrin associated visual field loss: A clinical audit to study prevalence, drug history and effects of drug withdrawal. Eye 2002, 16, 567–571. [Google Scholar] [CrossRef][Green Version]
- Braun, K.P. Preventing cognitive impairment in children with epilepsy. Curr. Opin. Neurol. 2017, 30, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Nickels, K.C.; Zaccariello, M.J.; Hamiwka, L.D.; Wirrell, E.C. Cognitive and neurodevelopmental comorbidities in paediatric epilepsy. Nat. Rev. Neurol. 2016, 12, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Citraro, R.; Scicchitano, F.; De Fazio, S.; Perrota, I.; Di Paola, E.D.; Constanti, A.; De Sarro, G. Effects of early long-term treatment with antiepileptic drugs on development of seizures and depressive-like behavior in a rat genetic absence epilepsy model. Epilepsia 2011, 52, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Tosun, D.; Siddarth, P.; Toga, A.W.; Hermann, B.; Caplan, R. Effects of childhood absence epilepsy on associations between regional cortical morphometry and aging and cognitive abilities. Hum. Brain Mapp. 2011, 32, 580–591. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, M.-C.; Huang, C.-W. The Discordance between Network Excitability and Cognitive Performance Following Vigabatrin Treatment during Epileptogenesis. Life 2021, 11, 1213. https://doi.org/10.3390/life11111213
Lai M-C, Huang C-W. The Discordance between Network Excitability and Cognitive Performance Following Vigabatrin Treatment during Epileptogenesis. Life. 2021; 11(11):1213. https://doi.org/10.3390/life11111213
Chicago/Turabian StyleLai, Ming-Chi, and Chin-Wei Huang. 2021. "The Discordance between Network Excitability and Cognitive Performance Following Vigabatrin Treatment during Epileptogenesis" Life 11, no. 11: 1213. https://doi.org/10.3390/life11111213
APA StyleLai, M.-C., & Huang, C.-W. (2021). The Discordance between Network Excitability and Cognitive Performance Following Vigabatrin Treatment during Epileptogenesis. Life, 11(11), 1213. https://doi.org/10.3390/life11111213





