Effectiveness of Telemonitoring for Respiratory and Systemic Symptoms of Asthma and COPD: A Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Literature Resources
2.3. Selection Procedure and Data Extraction
3. Results
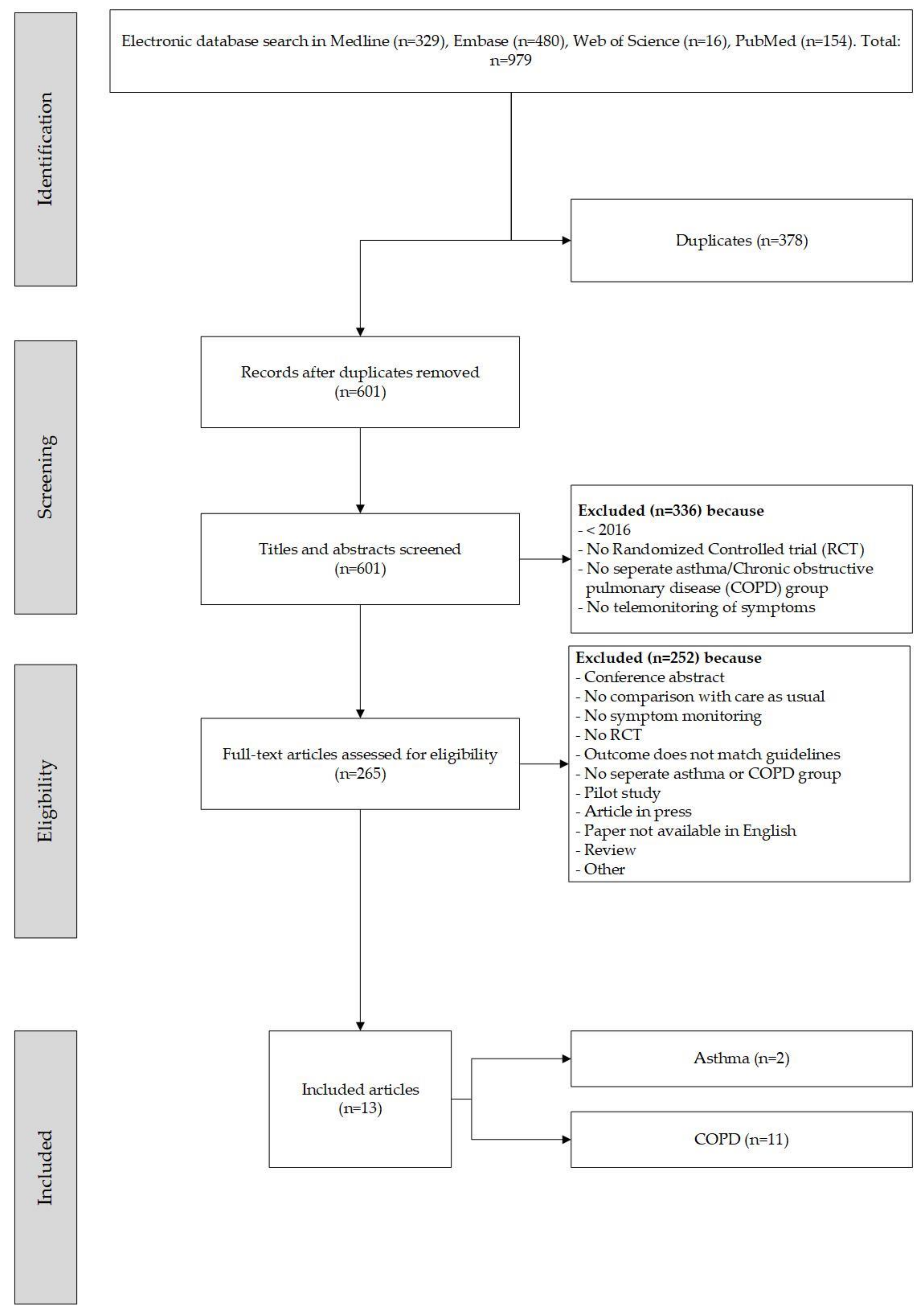
3.1. Selection of Papers
3.2. Patient Characteristics
| Platform for Patients | Monitoring Device(s) | Phone Monitoring | Structured Educational Component | |
|---|---|---|---|---|
| ASTHMA STUDIES * | ||||
| Kim, M.-Y. et al. (2016). | Smartphone application with short message service (SMS) feedback | Smartphone application (snuCare), peak flow meter, symptom questionnaire in app | If the values require intervention | Automated personalized feedback and treatment support in the app based on an action plan |
| Nemanic, T. et al. (2019). | Web-based application or SMS | Peak expiratory flow device, online questionnaires | None | Patients were educated in guided self-management and on how to use an action plan |
| COPD STUDIES * | ||||
| Bernocchi, P. et al. (2018). | Phone calls by healthcare provider to collect information on disease status and symptoms | Pulse oximeter, portable electrocardiogram, pedometer | Weekly phone call to monitor disease status and symptoms | Project started with educational intervention |
| Ho, T.-W. et al. (2016). | Web-based electronic diary | Pulse oximeter, thermometer, BP meter. Electronic symptoms, vital signs and weight diary | If the values require intervention | Education after alert if the alert was considered innocent |
| Kessler, R. et al. (2018). | Telephone-based questionnaire and telephone/web platform | None | Weekly phone-based questionnaires to monitor symptoms | Self-management/coaching program “living well with COPD” |
| North, M. et al. (2020). | Online self-management app platform | MyCOPD app | Monthly phone calls to collect adverse events and CAT scores | Online self-management support app with “how to use the app” videos, and online education |
| Vasilopoulou, M. et al. (2017). | Tablet for exercises and secure web platform to collect data | Spirometry, heart rate meter, saturation 13 m, pedometer, tablet for questionnaires | Weekly phone call for dietary, psychological, and self-management advice | Training prior to the intervention on how to use devices |
| Ritchie, C. et al. (2016). | Interactive Discharge Assistant via phone calls | None | Phone calls with interactive voice response system to monitor symptoms and to provide customized patient education | Training prior to using devices and self-management intervention |
| Lilholt, P. H. et al. (2017). | Tablet that is connected to the devices | Telekit system with BP monitor, pulse oximeter, tablet, weight scale | None | None |
| Mínguez Clemente, P. et al. (2020). | Multiparametric recording unit that uploaded data to an online web platform | Pulse oximeter, portable electrocardiogram, BP gage, temperature and respiratory rate | If the values require intervention | None |
| Soriano, J. B. et al. (2018). | Electronic case report form | Pulse oximeter, BP gage, spirometer, respiratory rate and oxygen therapy compliance monitor | Training before the intervention on how to use devices | |
| Stamenova, V. et al. (2020). | Cloud DX platform and connected Health Kit with tablet | Pulse wave wrist cuff monitor, oximeter, weighting scale, thermometer, tablet for questionnaires | Weekly feedback phone calls by the Respiratory Therapist | None |
| Walker, P. P. et al. (2018). | CHROMED (Clinical trials for Elderly Patients with Multiple Disease) monitoring platform | Within breath respiratory mechanical impedance | Monitoring of rescue medication, symptoms and QoL by phone | None |
3.3. Intervention Characteristics
3.4. Outcome Measurements and Effects of the Intervention
3.4.1. Parameters Used
3.4.2. Effects of the Intervention
3.5. Integration of the Telemonitoring Programs in the Healthcare Organization
3.5.1. Feasibility and Safety
3.5.2. Acceptability and Adherence
3.6. Education and Self-Management
4. Discussion
4.1. Main Results
4.2. Comparison with Current Literature
4.2.1. Telemonitoring and Patient Education
4.2.2. Accetability, Feasibility and Adherence
4.3. Strengths and Limitations
4.4. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
Appendix A. Search Terms
Appendix B. Study Characteristics and Intervention Effects
| Authors | Population | Selection | Aim | Telemonitoring | Comparison | Effect | Study Design | Follow-Up | Additional Support | Acceptability and Feasibility |
|---|---|---|---|---|---|---|---|---|---|---|
| ASTHMA STUDIES | ||||||||||
| Kim, M.-Y. et al. (2016). | I (n = 22), 18% male, mean age 49 (19–72), C (n = 22), 36% male, mean age 51 (34–67) years. | Asthma patients >19 years | Explore feasibility and effectiveness of the snuCare in adult patients with asthma. | snuCare app with peak flow meter, daily symptom scores. App provided feedback based on action plan. | 1:1 CAU. | Enhanced medication adherence Intervention group: p = 0.017, control group p = 0.571. FEV1, asthma control and QoL did not improve. | Random controlled trial. | 8 weeks | 3 visits. Researchers received automatic warnings through the system if measurements were worrisome. | Application was deemed feasible to use and was effective. |
| Nemanic, T. et al. (2019). | I (N = 51), 47% male, mean age 45 (39–61) years. C (n = 49), 49% male, mean age 53 (40–60) years. | Confirmed asthma diagnosis, ≥1 exacerbation last year or symptoms more than twice a week and activity limitation | To test the influence of telemonitoring on asthma control, exacerbation rate and severity. | Via SMS or webportal: ACT, pulmonary function tests, eNO, questionnaire data on knowledge, compliance, symptoms, exacerbations. | 1:1 CAU. | No difference between control and intervention group in asthma control change for patients with moderate asthma symptoms. For severe patients with asthma (i.e., patients with two or more exacerbations prior to study inclusion), a significant increase in ACT was detected. | Single center prospective randomized controlled trial. | 12 months | Study nurse received automatic warning if values are worrisome. | Home monitoring in asthma is feasible and effective for patients with more severe symptoms. |
| COPD STUDIES | ||||||||||
| Bernocchi, P. et al. (2018). | I: 88% male, 71 ± 9 years, C: 75% male, 70 ± 10 years. | Patients with COPD (GOLD B, C, D) and CHF undergoing hospital rehabilitation with ≥6 months life expectancy | Feasibility and effectiveness of intervention on exercise tolerance and time to event hospitalization, death, dyspnea, physical activity, disability and QoL. | Weekly phone call to assess symptoms and disease status. Pulse oximeter, portable ECG, mini ergo meter, pedometer, diary. | 1:1 CAU. | After 4 months change in Δ6MWT: I 60, C −15 (p = 0.004) in favor of I. Time to hospitalization/death: I 113 days, C 105 days (p = 0.0484). I improved more than C in disease status: ΔMRC, ΔPASE, ΔBarthel, ΔMLHFQ and ΔCAT. | Randomized controlled trial. | 4 months | Intervention group received weekly monitoring calls from nurse tutor within addition educational and motivational input. | Intervention is feasible and safe. No major side effects observed. |
| Ho, T.-W. et al. (2016). | I (n = 53), C (n = 53). 76% male, mean age 80 ± 9 years. | COPD (FEV1/FVC < 70%) patients discharged after exacerbation, current or former smokers | Reduce the frequency of readmission. | Pulse oximeter, thermometer, blood pressure meter, online symptom diary. | 1:1 CAU. | Number of all cause exacerbation episodes/patient improved. Hospital admissions I: 0.23, C: 0.68 (p = 0.002). Emergency room visits I: 0.36, C: 1.29 (p = 0.006). | Randomized controlled trial. | 6 months | Healthcare providers receive notification if values are concerning according to algorithm. Patients in both groups had access to medical counseling via phoneline. | |
| Kessler, R. et al. (2018). | I (n = 172), 69% male, mean age 67 ± 9 years. C (n = 173), 69% male, mean age 67 ± 9 years. | Patients with COPD (post bronchodilator FEV1/FVC < 70% and FEV1 < 50%), ≥ 10 pack-years, ≥ 1 severe exacerbation in the past year, ≥ 6 months life expectancy | Reduce hospitalization, reduce length of stay and improve patients coping behaviors. | Weekly/daily symptom monitoring, spirometry, pulse oximeter, heart rate. Patients on long-term oxygen therapy were monitored with NOWOX. | 1:1 CAU. | Intervention: 23% fewer all cause hospitalization days (p = 0.047), BODE index (Body mass index, Obstruction, Dyspnea, Exercise capacity). After 12 months 0.8 points lower in intervention group (p = 0.010), less mortality in Intervention group (1.9% vs. 14.2%, p < 0.001). | International Randomized controlled clinical trial. | 12 months | Study performed in France, Germany, Italy and Spain; 3 monthly hospital visits and regular phone call by hospital staff. | Safety was assessed and intervention was safe. |
| Lilholt, P. H. et al. (2017). | I (n = 578), 48% male, mean age 70 ± 9 years. C (n = 647), 44% male, mean age 70 ± 10 years. | Patients with COPD with CAT ≥ 10 or MRC ≥3 or mMRC ≥ 2 or ≥ 2 exacerbations in the past 12 months | To assess the effect of telehealthcare on HR-QoL. | Telekit system with tablet, blood pressure monitor, pulse oximeter, health precision scale. | 1:1 CAU. | No HR-QoL improvement in either intervention or control group. | Pragmatic cluster randomized trial. | 12 months | High attrition rate (52% lost to follow-up). Patients were contacted if measurements were not performed or is values were worrisome. | Telehealthcare was deemed feasible. |
| Mínguez Clemente, P. et al. (2020). | I (n = 49), 77% male, mean age 68 ± 8 years. C (n = 52), 60% male, mean age 70 ± 8 years. | patients with COPD admitted for exacerbation. Clinical stabilization in 4 days ** | Effectiveness of telemonitoring on time until first exacerbation post discharge. | Temperature, Blood pressure, respiratory rate, Oxygen saturation, heart rate, ECG. | Traditional follow-up based on daily visits. | No HR-QoL improvement in either intervention or control group. However, intervention group had the same results with less home visits compared to control group. | Randomized controlled trial. | 6 months | Patients received telephone calls from the physician to evaluate clinical situation and actions. If values were worrisome, the physician received an SMS warning. | |
| North, M. et al. (2020). | I (n = 20), 65% male, mean age 65 ± 6 years. C (n = 21), 52% male, mean age 68 ± 7 years. | patients with COPD using an inhaler, current or ex-smoker | Evaluation of safety and effectiveness of myCOPD. | CAT evaluation every 4 weeks. | 1:1 CAU. | The treatment effect on the CAT score was 4.49 (95% CI: −8.41, −0.58) points lower in the myCOPD arm. Patients’ inhaler technique improved in the digital intervention arm (101 improving to 20 critical errors) compared to usual care (100 to 72 critical errors). Exacerbations tended to be less frequent in the digital arm compared to usual care; 18 vs. 34 events. Hospital readmissions risk was numerically lower in the digital intervention arm: OR for readmission 0.383 (95% CI: 0.074, 1.987; n = 35). | Parallel arm feasibility randomized controlled trial with blinded outcome assessment. | 90 days | All patients were contacted by phone at 30, 60 and 90 days to record symptom data. About 50% of the eligible patients declined to participate. Authors did not report p-values. | The use of digital platforms in patients with COPD is feasible. |
| Ritchie, C. et al. (2016). | patients with COPD: I (n = 65), 42% male, mean age 64 ± 11 years. C (n = 67), 69% male, mean age 63 ± 11 years. | Hospitalized COPD/CHF patients who are expected to be discharged, ≥ 6 months life expectancy. Patients with impaired cognition could participate of a caregiver could serve as proxy | Evaluation of the eCoach effectiveness on rehospitalization, mortality and measure of community tenure. | eCoach: automated calls from the interactive discharge assistant for risk assessment. | 1:1 CAU. | In the COPD subgroup the intervention was related to fewer hospital days after 30 days compared to control (0.5 vs. 1.6, p = 0.03). | Pragmatic randomized trial. | 90 days | Healthcare providers had access to dashboard to review the data. | |
| Soriano, J. B. et al. (2018). | I (n = 115), C (n = 114). Total: 80% male, mean age 71 ± 8 years. | patients with COPD 50–90 years, with FEV1 <50%, treated with chronic home oxygen therapy, ≥2 exacerbations past year, currently clinically stable | Estimate effectiveness of intervention on exacerbations leading to emergency department visits/hospital admissions with telehealth. | Modem, pulse oximeter, blood pressure gage, spirometer, respiratory rate and oxygen therapy compliance monitor. | 1:1 CAU. | No effect on exacerbations or hospitalizations. | Multicenter, nonblind, randomized controlled trial. | 12 months | No additional support. Monitoring Center for healthcare provider with traffic light indicator. | Patients and doctors were satisfied with the program. |
| Stamenova, V. et al. (2020). | Self-monitoring (n = 41), 56% male, mean age 72 ± 7 years. Remote monitoring (n = 41), 56% male, mean age 72 ± 10 years. CAU (n = 40), 52% male, 73 ± 9 years. | COPD diagnosis | Evaluate effectiveness of technology enables self-management program with remote monitoring and CAU. | Bluetooth enabled device kit for oxygen saturation, blood pressure, temperature, weight, symptoms. | 2 intervention groups, 1 CAU group. | No difference in self-efficacy, disease knowledge or disease severity between the groups. No changes compared to baseline in symptoms or activity scores in any of the groups. No differences in healthcare utilization, emergency room visits, hospital admissions or healthcare visits. | 3 arm randomized controlled trial. | 6 months | Weekly evaluation and education phone calls. All patients could email or call the clinic with non-urgent questions. Clinical project specialist received automatic warning if values are worrisome. | |
| Vasilopoulou, M. et al. (2017). | C (n = 50), 74%, mean age 64 ± 8 years. Tele-rehabilitation (n = 50), 88% male, mean age 67 ± 10 years. Hospital-based rehabilitation (n = 50), 76% male, 67 ± 7 years. | patients with COPD, with FEV1 <80%, optimal medical treatment without regular use of OCS, ≥1 exacerbation past year | Evaluate the effectiveness of regular home monitoring of vital signs combined with teleconsultation sessions on acute exacerbations, hospitalizations and emergency department (ED) visits. | Tablet and heart rate monitor, pulse oximeter, symptoms, pedometer, spirometry, oximetry, HR-QoL, CAT, Hospital Anxiety and Depression Scale (HADS), mMRC. | 3 groups: (1) CAU, (2) 2-month outpatient rehabilitation and home maintenance tele-rehabilitation and (3) 2-month outpatient rehabilitation and hospital maintenance rehabilitation. | Home-based tele-rehabilitation and hospital-based pulmonary rehabilitation remained independent predictors of a lower risk for (1) acute exacerbation: IRR 0.52, 95% CI 0.39–0.69, and IRR 0.64, 95% CI 0.47–0.85), and (2) hospitalization: IRR 0.19, 95% CI 0.10–0.36, and IRR 0.38, 95% CI 0.21–0.68). Only home-based maintenance tele-rehabilitation was an independent predictor of reduced ED visits (IRR 0.12, 95% CI 0.07–0.19). | Prospective randomized controlled trial. | 12 months | Access call center, psychological support, weekly diary and self-management advice. | |
| Walker, P. P. et al. (2018). | I (n = 154), 66% male, mean age 71. C (n = 158), 66% male, mean age 71 (65–76) years. | COPD GOLD II, III, IV, ≥ 60 years, ≥ 1 exacerbation in the past year, ≥ 10 pack-years, ≥ 1 non-pulmonary chronic condition | Evaluate efficacy of home monitoring of lung mechanisms by forced oscillation technique and cardiac parameters on time to hospitalization, HR-QoL and reduce hospital costs. | Within breath respiratory mechanical impedance. CHF patients also measured blood pressure, oxygen saturation, heart rate and body temperature. | 1:1 CAU. | No effects were found. | Multi center randomized clinical trial. | 9 months | Every 3 months evaluation phone calls. The study nurse received automatic warning if values are worrisome. | The approach is feasible. |
References
- Asthma. Available online: https://www.who.int/news-room/facts-in-pictures/detail/asthma (accessed on 2 September 2021).
- Chronic Obstructive Pulmonary Disease (COPD). Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 6 September 2021).
- Forum of International Respiratory Societies The Global Impact of Respiratory Disease. Available online: https://theunion.org/technical-publications/the-global-impact-of-respiratory-disease. (accessed on 6 September 2021).
- Kavanagh, J.; Jackson, D.J.; Kent, B.D. Over- and under-diagnosis in asthma. Breathe 2019, 15, e20–e27. [Google Scholar] [CrossRef] [PubMed]
- Langsetmo, L.; Platt, R.W.; Ernst, P.; Bourbeau, J. Underreporting Exacerbation of Chronic Obstructive Pulmonary Disease in a Longitudinal Cohort. Am. J. Respir. Crit. Care Med. 2008, 177, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.A.R.; Donaldson, G.C.; Bhowmik, A.; Jeffries, D.J.; Wedzicha, J.A. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2000, 161, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.M.A.; Donaldson, G.C.; Hurst, J.R.; Seemungal, T.A.R.; Wedzicha, J.A. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2004, 169, 1298–1303. [Google Scholar] [CrossRef]
- Marchetti, N.; Criner, G.J.; Albert, R.K. Preventing Acute Exacerbations and Hospital Admissions in COPD. Chest 2013, 143, 1444–1454. [Google Scholar] [CrossRef]
- Doosty, F.; Maleki, M.R.; Yarmohammadian, M.H. An investigation on workload indicator of staffing need: A scoping review. J. Educ. Health Promot. 2019, 8, 22. [Google Scholar] [CrossRef]
- Barbosa, M.T.; Sousa, C.S.; Morais-Almeida, M.; Simões, M.J.; Mendes, P. Telemedicine in COPD: An Overview by Topics. COPD: J. Chronic Obstr. Pulm. Dis. 2020, 17, 601–617. [Google Scholar] [CrossRef]
- Doshi, H.; Hsia, B.; Shahani, J.; Mowrey, W.; Jariwala, S.P. Impact of Technology-Based Interventions on Patient-Reported Outcomes in Asthma: A Systematic Review. J. Allergy Clin. Immunol. Pract. 2021, 9, 2336–2341. [Google Scholar] [CrossRef]
- Ding, H.; Jayasena, R.; Chen, S.H.; Maiorana, A.; Dowling, A.; Layland, J.; Good, N.; Karunanithi, M.; Edwards, I. The Effects of Telemonitoring on Patient Compliance With Self-Management Recommendations and Outcomes of the Innovative Telemonitoring Enhanced Care Program for Chronic Heart Failure: Randomized Controlled Trial. J. Med. Internet Res. 2020, 22, e17559. [Google Scholar] [CrossRef]
- Aikens, J.E.; Rosland, A.-M.; Piette, J.D. Improvements in illness self-management and psychological distress associated with telemonitoring support for adults with diabetes. Prim. Care Diabetes 2015, 9, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Mäkelä, M.J.; Backer, V.; Hedegaard, M.; Larsson, K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir. Med. 2013, 107, 1481–1490. [Google Scholar] [CrossRef] [Green Version]
- Lavorini, F.; Magnan, A.; Dubus, J.C.; Voshaar, T.; Corbetta, L.; Broeders, M.; Dekhuijzen, R.; Sanchis, J.; Viejo, J.L.; Barnes, P.; et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir. Med. 2008, 102, 593–604. [Google Scholar] [CrossRef] [Green Version]
- Tashkin, D.P.; Celli, B.; Senn, S.; Burkhart, D.; Kesten, S.; Menjoge, S.; Decramer, M. A 4-Year Trial of Tiotropium in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2009, 58, 848–849. [Google Scholar] [CrossRef] [Green Version]
- Lorig, K.R.; Holman, H.R. Self-management education: History, definition, outcomes, and mechanisms. Ann. Behav. Med. 2003, 26, 1–7. [Google Scholar] [CrossRef]
- Warsi, A.; Wang, P.S.; LaValley, M.P.; Avorn, J.; Solomon, D.H. Self-management education programs in chronic disease: A systematic review and methodological critique of the literature. Arch. Intern. Med. 2004, 164, 1641–1649. [Google Scholar] [CrossRef]
- 2021 GINA Main Report—Global Initiative for Asthma—GINA. Available online: https://ginasthma.org/gina-reports/ (accessed on 10 September 2021).
- 2021 GOLD Reports—Global Initiative for Chronic Obstructive Lung Disease—GOLD. Available online: https://goldcopd.org/2021-gold-reports/ (accessed on 10 September 2021).
- Bonnevie, T.; Smondack, P.; Elkins, M.; Gouel, B.; Medrinal, C.; Combret, Y.; Muir, J. Cuvelier, A.; Prieur, G.; Gravier, F. Advanced telehealth technology improves home-based exercise therapy for people with stable chronic obstructive pulmonary disease: A systematic review. J. Physiother. 2021, 67, 27–40. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, S.H. Effectiveness of tele-monitoring by patient severity and intervention type in chronic obstructive pulmonary disease patients: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2019, 92, 1–15. [Google Scholar] [CrossRef]
- Alghamdi, S.M.; Alqahtani, J.S.; Aldhahir, A.M.; Alrajeh, A.M.; Aldabayan, Y.S. Effectiveness of telehealth-based interventions with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Am. J. Respir. Crit. Care Med. 2020, 201, A4308. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L632377796&from=export (accessed on 1 September 2021).
- Jang, S.; Kim, Y.; Cho, W.K. A Systematic Review and Meta-Analysis of Telemonitoring Interventions on Severe COPD Exacerbations. Int. J. Environ. Res. Public Health 2021, 18, 6757. [Google Scholar] [CrossRef]
- Kruse, C.; Pesek, B.; Anderson, M.; Brennan, K.; Comfort, H. Telemonitoring to Manage Chronic Obstructive Pulmonary Disease: Systematic Literature Review. JMIR Med. Inform. 2019, 7, e11496. [Google Scholar] [CrossRef]
- Paré, G.; Moqadem, K.; Pineau, G.; St-Hilaire, C. Clinical effects of home telemonitoring in the context of diabetes, asthma, heart failure and hypertension: A systematic review. J. Med. Internet Res. 2010, 12, e21. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L359408353&from=export (accessed on 1 September 2021). [CrossRef] [PubMed] [Green Version]
- Almojaibel, A. Delivering pulmonary rehabilitation for patients with chronic obstructive pulmonary disease at home using telehealth: A review of the literature. Saudi J. Med. Med. Sci. 2016, 4, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci. 2009, 4, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gemert-Pijnen, L.v.; Kelders, S.; Kip, H.; Sanderman, R. (Eds.) eHealth Research, Theory and Development. A Multidisciplinary Approach, 1st ed.; Routledge, Taylor & Francis Group: Abingdon, UK, 2018. [Google Scholar]
- Eurostat—Internet Activities 2019. Available online: https://appsso.eurostat.ec.europa.eu/nui/show.do?query=BOOKMARK_DS-053730_QID_2A8E1206_UID_-3F171EB0&layout=TIME,C,X,0;GEO,L,Y,0;INDIC_IS,L,Z,0;UNIT,L,Z,1;IND_TYPE,L,Z,2;INDICATORS,C,Z,3;&zSelection=DS-053730INDICATORS,OBS_FLAG;DS-053730UNIT,PC_IND;DS-053 (accessed on 19 November 2020).
- North, M.; Bourne, S.; Green, B.; Chauhan, A.J.; Brown, T.; Winter, J.; Jones, T.; Neville, D.; Blythin, A.; Watson, A.; et al. A randomised controlled feasibility trial of E-health application supported care vs. usual care after exacerbation of COPD: The RESCUE trial. NPJ Digit. Med. 2020, 3, 145. [Google Scholar] [CrossRef]
- Ritchie, C.S.; Houston, T.K.; Richman, J.S.; Sobko, H.J.; Berner, E.S.; Taylor, B.B.; Salanitro, A.H.; Locher, J.L. The E-Coach technology-assisted care transition system: A pragmatic randomized trial. Transl. Behav. Med. 2016, 6, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Vasilopoulou, M.; Papaioannou, A.I.; Kaltsakas, G.; Louvaris, Z.; Chynkiamis, N.; Spetsioti, S.; Kortianou, E.; Genimata, S.A.; Palamidas, A.; Kostikas, K.; et al. Home-based maintenance tele-rehabilitation reduces the risk for acute exacerbations of COPD, hospitalisations and emergency department visits. Eur. Respir. J. 2017, 49, 1602129. [Google Scholar] [CrossRef]
- Mínguez Clemente, P.; Pascual-Carrasco, M.; Mata Hernández, C.; Malo de Molina, R.; Arvelo, L.A.; Cadavid, B.; López, F.; Sánchez-Madariaga, R.; Sam, A.; Trisan Alonso, A.; et al. Follow-up with Telemedicine in Early Discharge for COPD Exacerbations: Randomized Clinical Trial (TELEMEDCOPD-Trial). COPD: J. Chronic Obstr. Pulm. Dis. 2020, 18, 62–69. [Google Scholar] [CrossRef]
- Bernocchi, P.; Vitacca, M.; La Rovere, M.T.; Volterrani, M.; Galli, T.; Baratti, D.; Paneroni, M.; Campolongo, G.; Sposato, B.; Scalvini, S. Home-based telerehabilitation in older patients with chronic obstructive pulmonary disease and heart failure: A randomised controlled trial. Age Ageing 2018, 47, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Kessler, R.; Casan-Clara, P.; Koehler, D.; Tognella, S.; Luis Viejo, J.; Dal Negro, R.W.; Díaz-Lobato, S.; Reissig, K.; Rodríguez González-Moro, J.M.; Devouassoux, G.; et al. CoMET: A multicomponent home-based disease-management programme versus routine care in severe COPD. Eur. Respir. J. 2018, 51, 1701612. [Google Scholar] [CrossRef] [Green Version]
- Soriano, J.B.; García-Río, F.; Vázquez-Espinosa, E.; Ignacio Conforto, J.; Hernando-Sanz, A.; López-Yepes, L.; Galera-Martínez, R.; Peces-Barba, G.; Gotera-Rivera, C.M.; Pérez-Warnisher, M.T.; et al. A multicentre, randomized controlled trial of telehealth for the management of COPD. Respir. Med. 2018, 144, 74–81. [Google Scholar] [CrossRef]
- Walker, P.P.; Pompilio, P.P.; Zanaboni, P.; Bergmo, T.S.; Prikk, K.; Malinovschi, A.; Montserrat, J.M.; Middlemass, J.; Šonc, S.; Munaro, G.; et al. Telemonitoring in chronic obstructive pulmonary disease (CHROMED) A randomized clinical trial. Am. J. Respir. Crit. Care Med. 2018, 198, 620–628. [Google Scholar] [CrossRef]
- Ho, T.-W.; Huang, C.-T.; Chiu, H.-C.; Ruan, S.-Y.; Tsai, Y.-J.; Yu, C.-J.; Lai, F. Effectiveness of telemonitoring in patients with chronic obstructive pulmonary disease in Taiwan—A randomized Controlled Trial. Sci. Rep. 2016, 6, 23797. [Google Scholar] [CrossRef] [Green Version]
- Nemanic, T.; Sarc, I.; Skrgat, S.; Flezar, M.; Cukjati, I.; Malovrh, M.M. Telemonitoring in asthma control: A randomized controlled trial. J. Asthma 2019, 56, 782–790. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Lee, S.-Y.; Jo, E.-J.; Lee, S.-E.; Kang, M.-G.; Song, W.-J.; Kim, S.-H.; Cho, S.-H.; Min, K.-U.; Ahn, K.-H.; et al. Feasibility of a smartphone application based action plan and monitoring in asthma. Asia Pac. Allergy 2016, 6, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Vasilopoulou, M.; Papaioannou, A.I.; Kaltsakas, G.; Gennimata, S.A.; Palamidas, A.F.; Feridou, C.; Chynkiamis, N.; Vasilogiannakopoulou, T.; Spetsioti, S.; Louvaris, Z.; et al. Evidence of benefit from home tele-rehabilitation on chronic dyspnea and quality of life in patients with COPD. Eur. Respir. J. 2015, 46, PA3721. [Google Scholar] [CrossRef]
- Lilholt, P.H.; Udsen, F.W.; Ehlers, L.; Hejlesen, O.K. Telehealthcare for patients suffering from chronic obstructive pulmonary disease: Effects on health-related quality of life: Results from the Danish ‘TeleCare North’ cluster-randomised trial. BMJ Open 2017, 7, e014587. [Google Scholar] [CrossRef]
- Stamenova, V.; Liang, K.; Yang, R.; Engel, K.; van Lieshout, F.; Lalingo, E.; Cheung, A.; Erwood, A.; Radina, M.; Greenwald, A.; et al. Technology-enabled self-management of chronic obstructive pulmonary disease with or without asynchronous remote monitoring: Randomized controlled trial. J. Med. Internet Res. 2020, 22, e18598. [Google Scholar] [CrossRef]
- North, M.; Bourne, S.; Green, B.; Chauhan, A.; Brown, T.; Winter, J.; Johnson, M.; Culliford, D.; Wilkinson, T. A randomised controlled feasibility trial of an e-health platform supported care vs. usual care after exacerbation of COPD. (Rescue COPD). Thorax 2018, 73, A231. [Google Scholar] [CrossRef]
- Bernocchi, P.; Scalvini, S.; Galli, T.; Paneroni, M.; Baratti, D.; Turla, O.; La Rovere, M.T.; Volterrani, M.; Vitacca, M. A multidisciplinary telehealth program in patients with combined chronic obstructive pulmonary disease and chronic heart failure: Study protocol for a randomized controlled trial. Trials 2016, 17, 17–462. [Google Scholar] [CrossRef] [Green Version]
- Price, D.; David-Wang, A.; Cho, S.-H.; Ho, J.C.-M.; Jeong, J.W.; Liam, C.-K.; Lin, J.; Muttalif, A.R.; Perng, D.-W.; Tan, T.-L.; et al. Asthma in Asia: Physician perspectives on control, inhaler use and patient communications. J. Asthma 2016, 53, 761–769. [Google Scholar] [CrossRef]
- Hanlon, P.; Daines, L.; Campbell, C.; McKinstry, B.; Weller, D.; Pinnock, H. Telehealth interventions to support self-management of long-term conditions: A systematic metareview of diabetes, heart failure, asthma, chronic obstructive pulmonary disease, and cancer. J. Med. Internet Res. 2017, 19, e172. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.E. What is value in health care? N. Engl. J. Med. 2010, 363, 2477–2481. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.R.; Diamond, E.J. Emerging role of remote patient monitoring in pulmonary care: Telemedicine to smart phone. Chest 2021, 159, 477–478. [Google Scholar] [CrossRef] [PubMed]
| Asthma | COPD | ||||||
|---|---|---|---|---|---|---|---|
| Primary | Reference | Secondary | Reference | Primary | Reference | Secondary | Reference |
| Feasibility | [40,41] | Medication adherence | [41] | Exercise tolerance | [35] | Physical activity | [33] |
| Asthma Control | [40] | Asthma health status | [40] | Time to hospitalization or Hospitalization duration | [32,36,38,39] | HR-QoL | [33,38] |
| Events * | [40] | HR-QoL | [45] | Healthcare usage | [43] | ||
| Exacerbation frequency | [33] | QALYs | [38] | ||||
| COPD health status | [31] | BODE index | [36] | ||||
| COPD health status | [31] | Smoking cessation | [43] | ||||
| Events * | [36] | ||||||
| Dyspnea | [35] | ||||||
| Slower lung function decline | [33] | ||||||
| Time to event | [32,34,36,38,39,43] | ||||||
| COPD Health status | [43] | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metting, E.; Dassen, L.; Aardoom, J.; Versluis, A.; Chavannes, N. Effectiveness of Telemonitoring for Respiratory and Systemic Symptoms of Asthma and COPD: A Narrative Review. Life 2021, 11, 1215. https://doi.org/10.3390/life11111215
Metting E, Dassen L, Aardoom J, Versluis A, Chavannes N. Effectiveness of Telemonitoring for Respiratory and Systemic Symptoms of Asthma and COPD: A Narrative Review. Life. 2021; 11(11):1215. https://doi.org/10.3390/life11111215
Chicago/Turabian StyleMetting, Esther, Lizayra Dassen, Jiska Aardoom, Anke Versluis, and Niels Chavannes. 2021. "Effectiveness of Telemonitoring for Respiratory and Systemic Symptoms of Asthma and COPD: A Narrative Review" Life 11, no. 11: 1215. https://doi.org/10.3390/life11111215
APA StyleMetting, E., Dassen, L., Aardoom, J., Versluis, A., & Chavannes, N. (2021). Effectiveness of Telemonitoring for Respiratory and Systemic Symptoms of Asthma and COPD: A Narrative Review. Life, 11(11), 1215. https://doi.org/10.3390/life11111215






