Current Overview on the Use of Mesenchymal Stem Cells for Perianal Fistula Treatment in Patients with Crohn’s Disease
Abstract
:1. Introduction
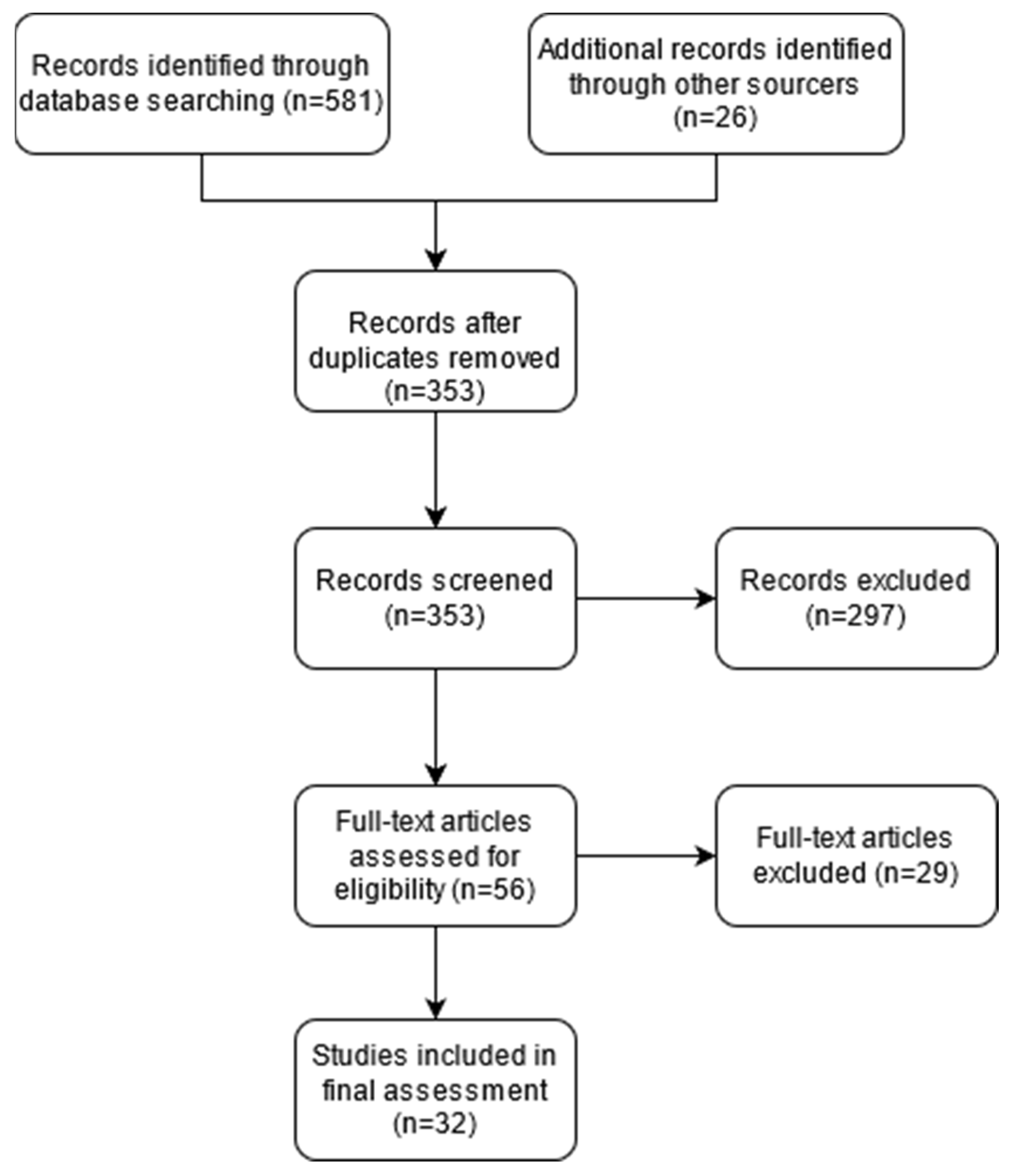
2. Methods
2.1. Searching Strategy
2.2. Study Selection and Risk of Bias
2.3. Outcome Assessment
2.4. Ethical Considerations
3. Mesenchymal Stem Cells Mechanism in Crohn’s Disease Perianal Fistula
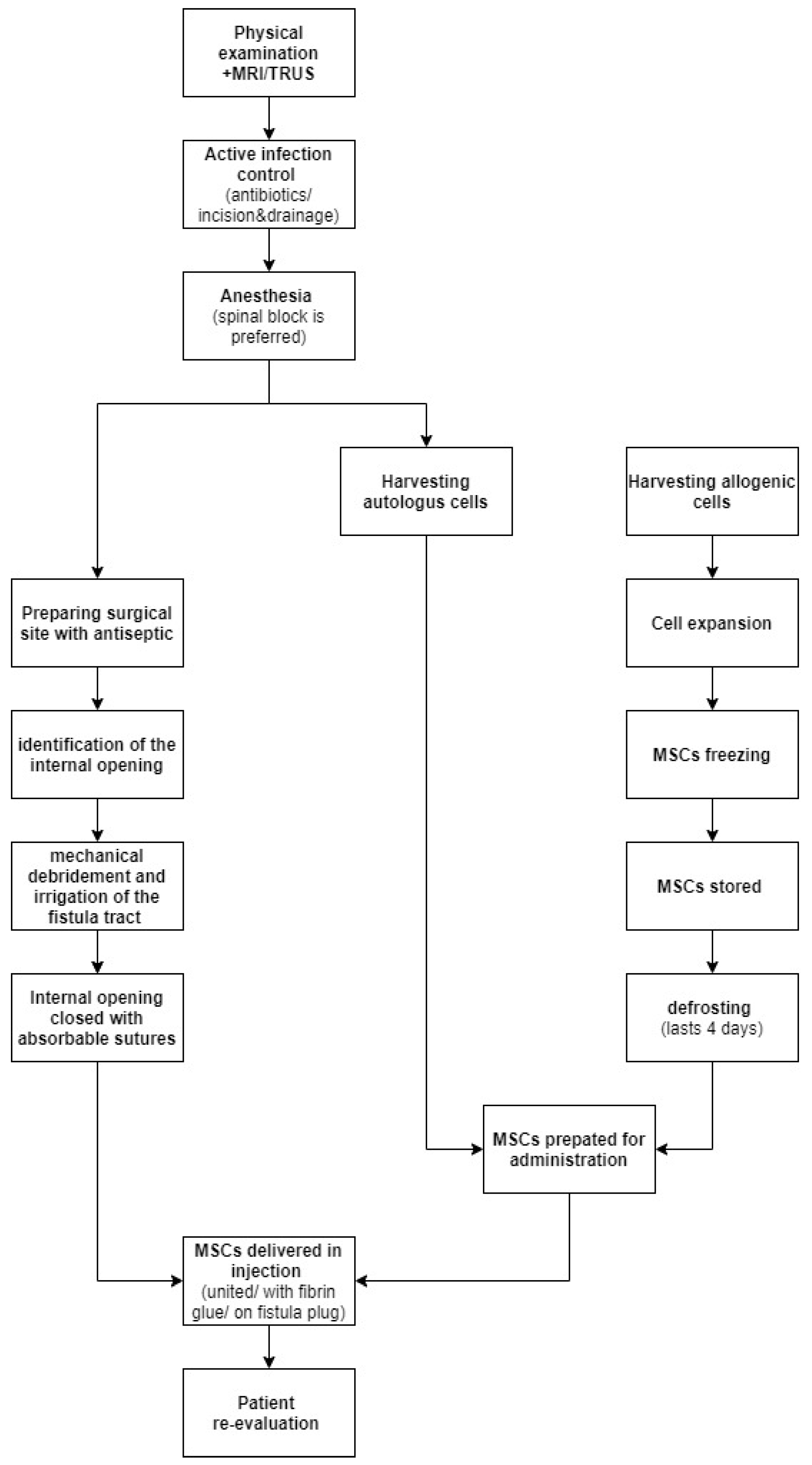
4. Mesenchymal Stem Cells Application in Perianal Crohn’s Disease
5. Analysis of Recent Clinical Studies Outcomes
5.1. ADMIRE-CD
5.2. 2009. Garcia-Olmo
5.3. 2013. Lee, Park and Cho Study and 2015 Cho, Park, Yoon Study
5.4. 2013. De la Portilla et al. Study
5.5. 2017. Dietz et al.
5.6. 2015. Molendijk et al.
5.7. 2020. Barnhoorn et al.
5.8. 2020. Laureti et al.
5.9. 2020. Zhou et al.
5.10. Drawbacks
5.11. Secondary Outcomes
6. Limitations of the Paper
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar]
- Pogacnik, J.S.; Salgado, G. Perianal Crohn’s Disease. Clin. Colon Rectal Surg. 2019, 32, 377–385. [Google Scholar] [CrossRef]
- Multidisciplinary Team (MDT) Approach to Diagnosis & Management of Perianal Crohn’s Disease | Crohn’s & Colitis Foundation. Available online: https://www.crohnscolitisfoundation.org/clinical-pearls/mdt-perianal-crohns-disease (accessed on 30 June 2021).
- Panes, J.; Reinisch, W.; Rupniewska, E.; Khan, S.; Forns, J.; Khalid, J.M.; Bojic, D.; Patel, H. Burden and outcomes for complex perianal fistulas in Crohn’s disease: Systematic review. World J. Gastroenterol. 2018, 24, 4821–4834. [Google Scholar] [CrossRef]
- Herreros, M.D.; Garcia-Olmo, D.; Guadalajara, H.; Georgiev-Hristov, T.; Brandariz, L.; Garcia-Arranz, M. Stem cell therapy: A compassionate use program in perianal fistula. Stem Cells Int. 2019, 2019, 6132340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372. [Google Scholar] [CrossRef]
- Tozer, P.J.; Lung, P.; Lobo, A.J.; Sebastian, S.; Brown, S.R.; Hart, A.L.; Fearnhead, N.; Adegbola, S.O.; Heywood, N.; Hind, D.; et al. Review article: Pathogenesis of Crohn’s perianal fistula—Understanding factors impacting on success and failure of treatment strategies. Aliment. Pharmacol. Ther. 2018, 48, 260–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardi, L.; Dos Santos, C.H.M.; Pinheiro, V.A.Z.; Oliveira, R.J.; Antoniolli-Silva, A.C.M.B. Transplantation of adipose-derived mesenchymal stem cells in refractory crohn’s disease: Systematic review. Arq. Bras. Cir. Dig. 2019, 32, e1465. [Google Scholar] [CrossRef]
- Verstockt, B.; Ferrante, M.; Vermeire, S.; Van Assche, G. New treatment options for inflammatory bowel diseases. J. Gastroenterol. 2018, 53, 585–590. [Google Scholar] [CrossRef] [Green Version]
- Hoogduijn, M.J.; Lombardo, E. Mesenchymal Stromal Cells Anno 2019: Dawn of the Therapeutic Era? Concise Review. Stem Cells Transl. Med. 2019, 8, 1126–1134. [Google Scholar] [CrossRef] [Green Version]
- Carvello, M.; Lightner, A.; Yamamoto, T.; Kotze, P.G.; Spinelli, A. Mesenchymal Stem Cells for Perianal Crohn’s Disease. Cells 2019, 8, 764. [Google Scholar] [CrossRef] [Green Version]
- Georgiev-Hristov, T.; Guadalajara, H.; Herreros, M.D.; Lightner, A.L.; Dozois, E.J.; García-Arranz, M.; García-Olmo, D. A Step-By-Step Surgical Protocol for the Treatment of Perianal Fistula with Adipose-Derived Mesenchymal Stem Cells. J. Gastrointest. Surg. 2018, 22, 2003–2012. [Google Scholar] [CrossRef]
- Banasiewicz, T.; Eder, P.; Rydzewska, G.; Reguła, J.; Dobrowolska, A.; Durlik, M.; Wallner, G. Position of the expert group on the current practice and prospects for the treatment of complex perirectal fistulas in the course of Crohn’s disease. Polish J. Surg. 2019, 91, 1–9. [Google Scholar] [CrossRef]
- Herreros, M.D.; Garcia-Arranz, M.; Guadalajara, H.; De-La-Quintana, P.; Garcia-Olmo, D. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: A phase III randomized clinical trial (FATT 1: Fistula advanced therapy trial 1) and long-term evaluation. Dis. Colon Rectum 2012, 55, 762–772. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Klersy, C.; Leffler, D.A.; Rogers, R.; Bennett, D.; Corazza, G.R. Systematic review with meta-analysis: Safety and efficacy of local injections of mesenchymal stem cells in perianal fistulas. JGH Open 2019, 3, 249–260. [Google Scholar] [CrossRef]
- Cao, Y.; Su, Q.; Zhang, B.; Shen, F.; Li, S. Efficacy of stem cells therapy for Crohn’s fistula: A meta-analysis and systematic review. Stem Cell Res. Ther. 2021, 12, 32. [Google Scholar] [CrossRef]
- Avivar-Valderas, A.; Martín-Martín, C.; Ramírez, C.; Del Río, B.; Menta, R.; Mancheño-Corvo, P.; Ortiz-Virumbrales, M.; Herrero-Méndez, Á.; Panés, J.; García-Olmo, D.; et al. Dissecting allo-sensitization after local administration of human allogeneic adipose mesenchymal stem cells in perianal fistulas of Crohn’s disease patients. Front. Immunol. 2019, 10, 1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molendijk, I.; Bonsing, B.A.; Roelofs, H.; Peeters, K.C.M.J.; Wasser, M.N.J.M.; Dijkstra, G.; Van Der Woude, C.J.; Duijvestein, M.; Veenendaal, R.A.; Zwaginga, J.J.; et al. Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cells Promote Healing of Refractory Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology 2015, 149, 918–927.e6. [Google Scholar] [CrossRef] [Green Version]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Long-term Efficacy and Safety of Stem Cell Therapy (Cx601) for Complex Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology 2018, 154, 1334–1342.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lightner, A.L.; Ashburn, J.H.; Brar, M.S.; Carvello, M.; Chandrasinghe, P.; van Overstraeten, A.d.B.; Fleshner, P.R.; Gallo, G.; Kotze, P.G.; Holubar, S.D.; et al. Fistulizing Crohn’s disease. Curr. Probl. Surg. 2020, 57, 100808. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Herreros, D.; Pascual, I.; Pascual, J.A.; Del-Valle, E.; Zorrilla, J.; De-La-Quintana, P.; Garcia-Arranz, M.; Pascual, M. Expanded Adipose-Derived Stem Cells for the Treatment of Complex Perianal Fistula. Dis. Colon Rectum 2009, 52, 79–86. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, K.J.; Cho, Y.B.; Yoon, S.N.; Song, K.H.; Kim, D.S.; Jung, S.H.; Kim, M.; Yoo, H.W.; Kim, I.; et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for crohn’s fistula. Stem Cells 2013, 31, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.B.; Park, K.J.; Yoon, S.N.; Song, K.H.; Kim, D.S.; Jung, S.H.; Kim, M.; Jeong, H.Y.; Yu, C.S. Long-Term Results of Adipose-Derived Stem Cell Therapy for the Treatment of Crohn’s Fistula. Stem Cells Transl. Med. 2015, 4, 532–537. [Google Scholar] [CrossRef]
- De La Portilla, F.; Alba, F.; García-Olmo, D.; Herrerías, J.M.; González, F.X.; Galindo, A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: Results from a multicenter phase I/IIa clinical trial. Int. J. Colorectal Dis. 2013, 28, 313–323. [Google Scholar] [CrossRef]
- Dietz, A.B.; Dozois, E.J.; Fletcher, J.G.; Butler, G.W.; Radel, D.; Lightner, A.L.; Dave, M.; Friton, J.; Nair, A.; Camilleri, E.T.; et al. Autologous Mesenchymal Stem Cells, Applied in a Bioabsorbable Matrix, for Treatment of Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology 2017, 153, 59–62.e2. [Google Scholar] [CrossRef]
- Barnhoorn, M.C.; Wasser, M.N.J.M.; Roelofs, H.; Maljaars, P.W.J.; Molendijk, I.; Bonsing, B.A.; Oosten, L.E.M.; Dijkstra, G.; Van Der Woude, C.J.; Roelen, D.L.; et al. Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn’s disease perianal fistulas. J. Crohn’s Colitis 2020, 14, 64–70. [Google Scholar] [CrossRef]
- Laureti, S.; Gionchetti, P.; Cappelli, A.; Vittori, L.; Contedini, F.; Rizzello, F.; Golfieri, R.; Campieri, M.; Poggioli, G. Refractory Complex Crohn’s Perianal Fistulas: A Role for Autologous Microfragmented Adipose Tissue Injection. Inflamm. Bowel Dis. 2020, 26, 321–330. [Google Scholar] [CrossRef]
- Zhou, C.; Li, M.; Zhang, Y.; Ni, M.; Wang, Y.; Xu, D.; Shi, Y.; Zhang, B.; Chen, Y.; Huang, Y.; et al. Autologous adipose-derived stem cells for the treatment of Crohn’s fistula-in-ano: An open-label, controlled trial. Stem Cell Res. Ther. 2020, 11, 124. [Google Scholar] [CrossRef] [Green Version]
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; de Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, eaba6884. [Google Scholar] [CrossRef]
- Borycka-Kiciak, K.; Pietrzak, A.; Kielar, M.; Tarnowski, W. Mesenchymal stem cells for the treatment of complex perianal fistulas in patients with Crohn disease. Polish J. Surg. 2019, 91, 764. [Google Scholar] [CrossRef]
- Lopez, N.; Ramamoorthy, S.; Sandborn, W.J. Recent advances in the management of perianal fistulizing Crohn’s disease: Lessons for the clinic. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 563–577. [Google Scholar] [CrossRef]
- Gallo, G.; Tiesi, V.; Fulginiti, S.; De Paola, G.; Vescio, G.; Sammarco, G. Mesenchymal Stromal Cell Therapy in the Management of Perianal Fistulas in Crohn’s Disease: An Up-To-Date Review. Medicina 2020, 56, 563. [Google Scholar] [CrossRef]
- Lightner, A.L.; Wang, Z.; Zubair, A.C.; Dozois, E.J. A systematic review and meta-analysis of mesenchymal stem cell injections for the treatment of perianal Crohn’s disease: Progress made and future directions. Dis. Colon Rectum 2018, 61, 629–640. [Google Scholar] [CrossRef]
| Group | Number of Injected MSCs | Healing Rate (%) | ||
|---|---|---|---|---|
| Week 6 | Week 12 | Week 24 | ||
| 1 | 1 × 107 | 60 | 40 | 80 |
| 2 | 3 × 107 | 80 | 80 | 80 |
| 3 | 9 × 107 | 20 | 20 | 20 |
| placebo | 0.9% NaCl/5% human albumin solution consisting no cells. | 16.7 | 33 | 33 |
| Authors | Year | Patients | Cell Type | Aplication/Intervention | Time-Point | Healing Rate (%) | Follow-Up | Recurrence Rate % |
|---|---|---|---|---|---|---|---|---|
| ADMIRE-CD study [20,21] | 2016, 2018 | 212 | Adipose allogenic (Cx601) | Local application of 120 million Cx601 cells vs. placebo (control group) | 24 weeks | 50% vs. 34% in control group | 1 year | 25% vs. 44.1% in control group |
| Garcia-Olmo et al. [23] | 2009 | 14 | Adipose autologous | Local application of 2 × 106 stem cells+ fibrin glue | 8 weeks | 71% | 52 weeks | 17.6% |
| Lee et al. [24,25] | 2013 2015 | 33 | Adipose autologous | Local application of 3 × 107 or 6 × 107 stem cells | 8 weeks | 81.8% | 1 year 2 year | 11.5% 16.7% |
| De la Portilla et al. [26] | 2013 | 24 | Adipose allogenic | Local application of 2 × 107 (+4 × 107) stem cells | 24 weeks | Reduction in the number of draining fistula—69% of patients Full fistula closure—30% of patients | 6 months | 20.8% |
| Dietz et al. [27] | 2017 | 12 | Adipose autologous | Local application of 2 × 107 of stem cells on a biological plug | 12 weeks 6 months | Complete healing—75% 83% of patients presented with fistula healing | - | N/A |
| Molendijk et al. + Barnhoorn et al. [19,28] | 2015 2020 | 21 | Bone marrow allogenic | Local application of 1-; 3-; 9 × 107 of stem cells | 24 weeks | 1 × 107 cells—80% 3 × 107 cells—80% 9 × 107 cells—20% | 3.5 years | 1 × 107 cells—all patients managed to have full fistula closure 3 × 107 cells—0% 9 × 107 cells—0% |
| Laureti et al. [29] | 2020 | 15 | Adipose autologous | Local application of 20 cc of microfragmented adipose tissu stem cells | 24 weeks | 66.7% | 24 weeks | 0% |
| Zhou et al. [30] | 2020 | 22 | Adipose autologous | Local application of 5 × 106 stem cells vs. incision-thread-drawing procedure (control group) | 1 year | 63.6% vs. 54.5% (in control group) | - | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk, M.; Czerwińska, K.; Włodarczyk, J.; Fichna, J.; Dziki, A.; Dziki, Ł. Current Overview on the Use of Mesenchymal Stem Cells for Perianal Fistula Treatment in Patients with Crohn’s Disease. Life 2021, 11, 1133. https://doi.org/10.3390/life11111133
Włodarczyk M, Czerwińska K, Włodarczyk J, Fichna J, Dziki A, Dziki Ł. Current Overview on the Use of Mesenchymal Stem Cells for Perianal Fistula Treatment in Patients with Crohn’s Disease. Life. 2021; 11(11):1133. https://doi.org/10.3390/life11111133
Chicago/Turabian StyleWłodarczyk, Marcin, Katarzyna Czerwińska, Jakub Włodarczyk, Jakub Fichna, Adam Dziki, and Łukasz Dziki. 2021. "Current Overview on the Use of Mesenchymal Stem Cells for Perianal Fistula Treatment in Patients with Crohn’s Disease" Life 11, no. 11: 1133. https://doi.org/10.3390/life11111133
APA StyleWłodarczyk, M., Czerwińska, K., Włodarczyk, J., Fichna, J., Dziki, A., & Dziki, Ł. (2021). Current Overview on the Use of Mesenchymal Stem Cells for Perianal Fistula Treatment in Patients with Crohn’s Disease. Life, 11(11), 1133. https://doi.org/10.3390/life11111133






