Effect of Quercetin on Injury to Indomethacin-Treated Human Embryonic Kidney 293 Cells
Abstract
1. Introduction
2. Results
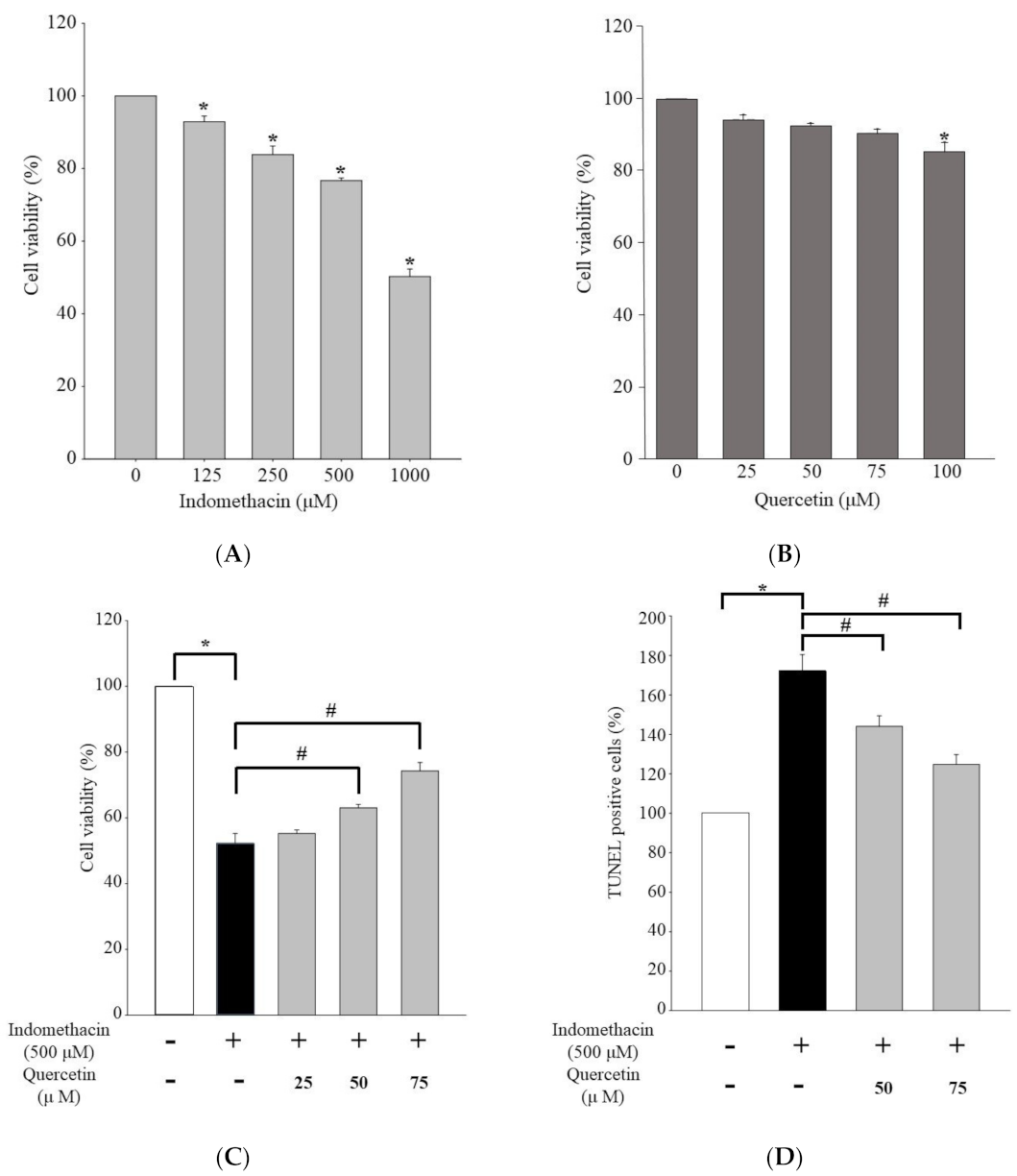
2.1. Effect of Quercetin on Viability of Human Embryonic Kidney 293 Cells Treated with Indomethacin
2.2. Effect of Quercetin on Caspase-Dependent Apoptosis in HEK293 Cells Treated with Indomethacin
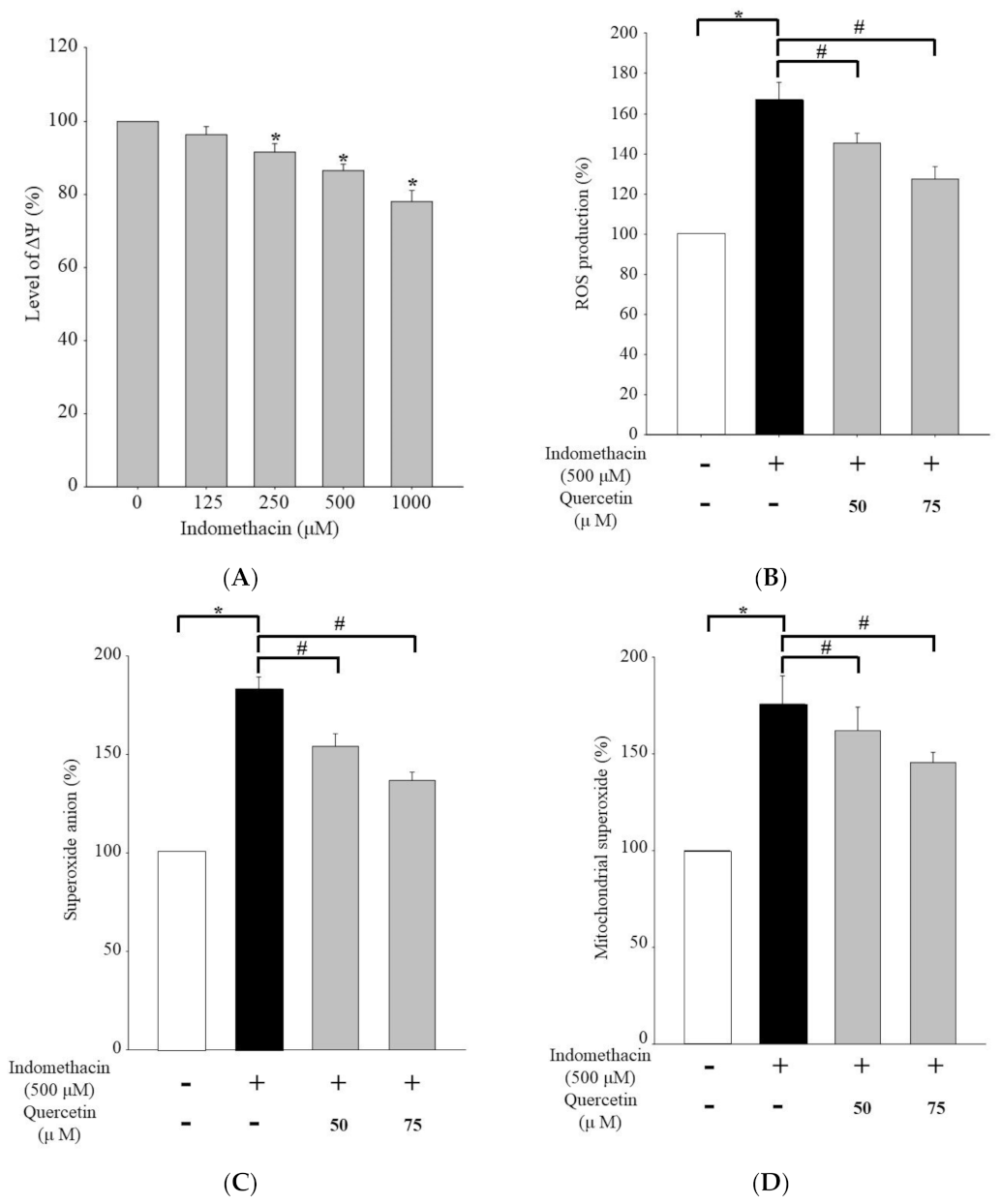
2.3. Effects of Quercetin on Superoxide Anion, Mitochondrial Superoxide, and Reactive Oxygen Species Production in HEK293 Cells Treated with Indomethacin
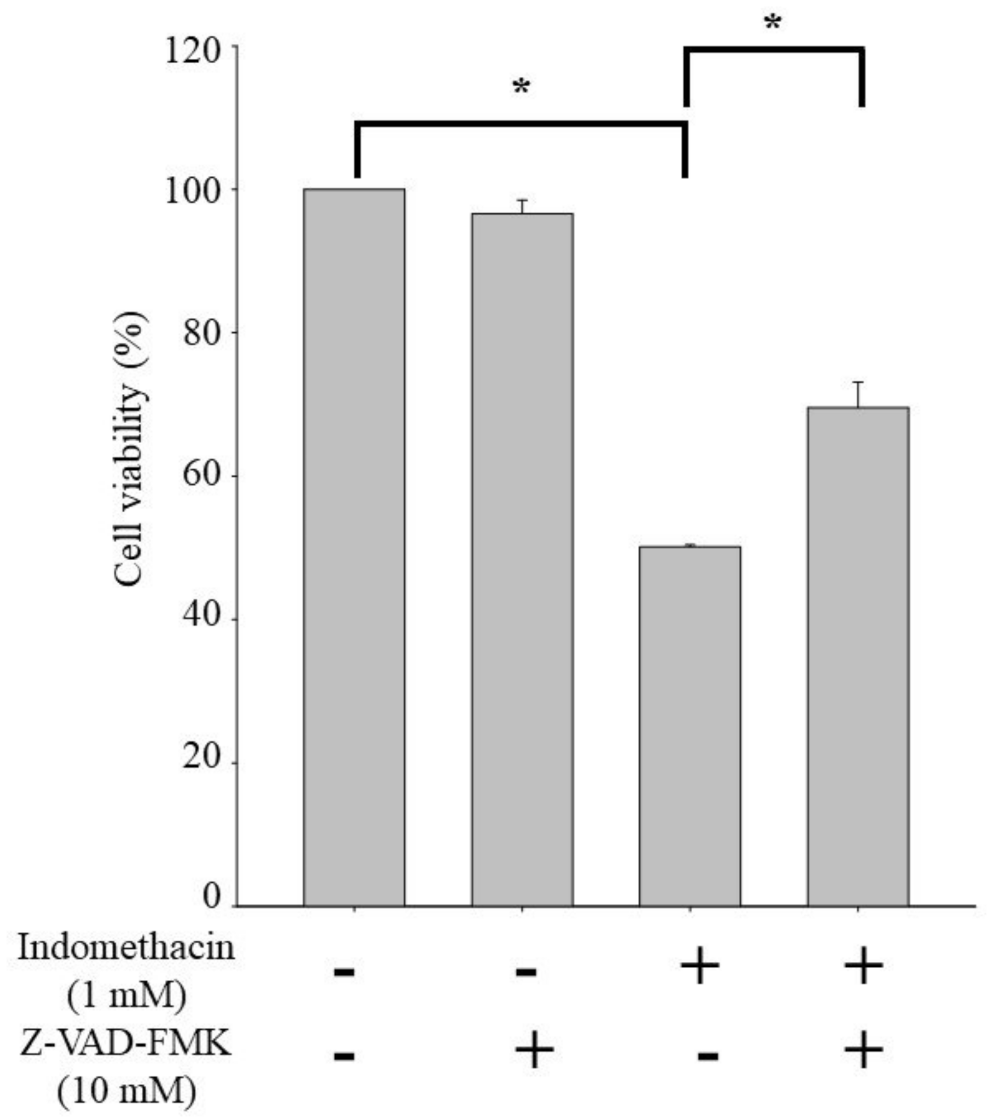
2.4. Effects of Pan-Caspase Inhibitor Z-VAD-FMK on Apoptosis in HEK293 Cells Treated with Indomethacin
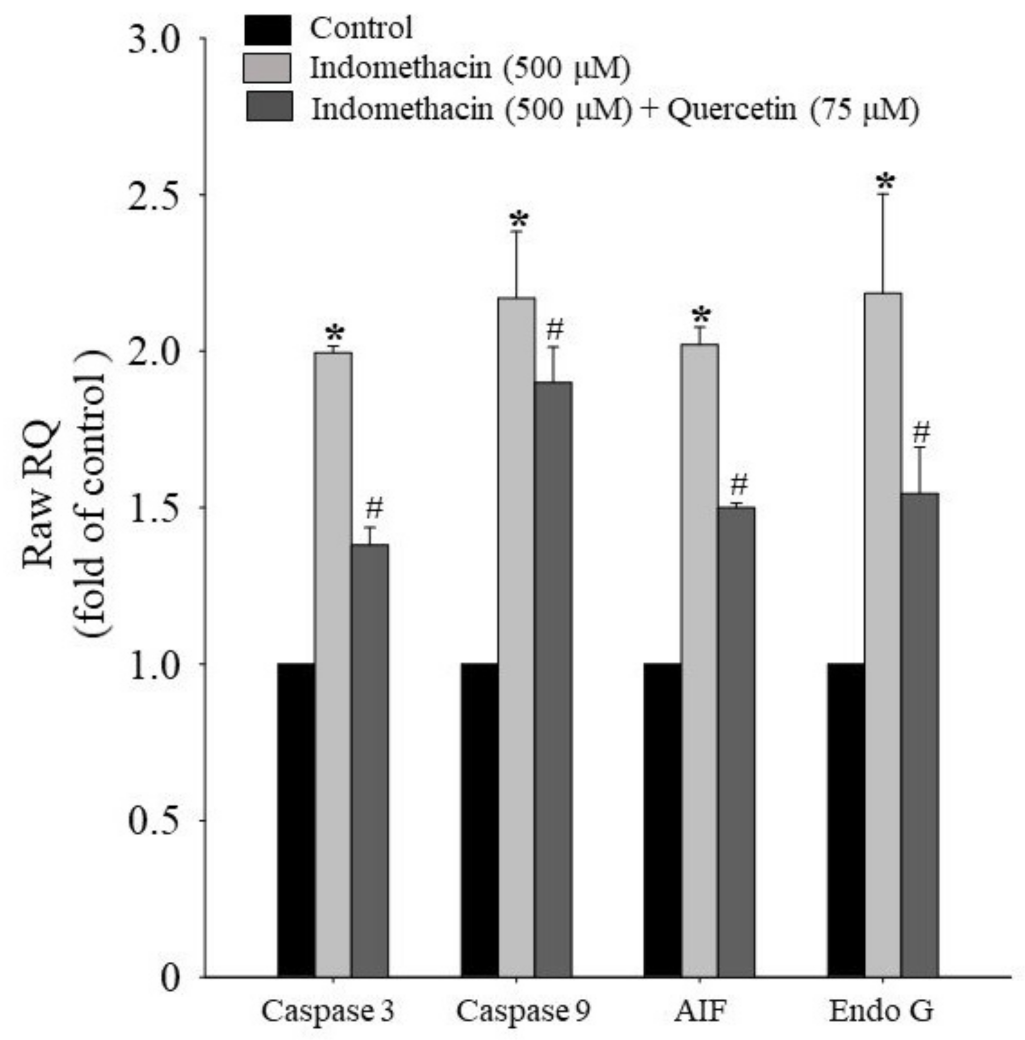
2.5. Effects of Quercetin on Apoptotic mRNA Expression in HEK293 Cells Treated with Indomethacin
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. TUNEL Assay for Apoptosis Analysis
4.5. Determination of Caspase-3 and -9 Activity
4.6. Determination of ROS and Mitochondrial Superoxide Production through Flow Cytometry
4.7. Detection of ΔΨm
4.8. Apoptotic mRNA Level Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| Apaf-1 | Apoptotic protease activating factor-1 |
| AIF | Apoptosis-Inducing Factor |
| Fas | Fas ligand |
| CD95 | Receptor CD95 |
| DR4 | Death receptor 4 |
| DR5 | Death receptor 5 |
| TNFR | TNF-receptor |
| EndoG | Endonuclease G |
| EDTA | Ethylenediaminetetraacetic acid |
References
- Orczyk, G.P.; Behrman, H.R. Ovulation blockade by aspirin or indomethacin—In vivo evidence for a role of prostaglandin in gonadotrophin secretion. Prostaglandins 1972, 1, 3–20. [Google Scholar] [CrossRef]
- Inamdar, S.; Han, D.; Passi, M.; Sejpal, D.V.; Trindade, A.J. Rectal indomethacin is protective against post-ERCP pancreatitis in high-risk patients but not average-risk patients: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Melilli, B.; Piazza, C.; Zurria, M.; Drago, F. Ocular Pharmacokinetics Profile of Different Indomethacin Topical Formulations. J. Ocul. Pharmacol. Ther. 2011, 27, 571–576. [Google Scholar] [CrossRef]
- Elmunzer, B.J.; Hernandez, I.; Gellad, W.F. The Skyrocketing Cost of Rectal Indomethacin. JAMA Intern. Med. 2020, 180, 631. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Ochi, H.; Kitakoji, H. Effects of tender point acupuncture on delayed onset muscle soreness (DOMS)—A pragmatic trial. Chin. Med. 2008, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Kawakita, K. Effect of Indomethacin on the Development of Eccentric Exercise-Induced Localized Sensitive Region in the Fascia of the Rabbit. Jpn. J. Physiol. 2002, 52, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Helleberg, L. Clinical Pharmacokinetics of Indomethacin. Clin. Pharmacokinet. 1981, 6, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Lago, P.; Bettiol, T.; Salvadori, S.; Pitassi, I.; Vianello, A.; Chiandetti, L.; Saia, O.S. Safety and efficacy of ibuprofen versus indomethacin in preterm infants treated for patent ductus arteriosus: A randomised controlled trial. Eur. J. Pediatr. 2002, 161, 202–207. [Google Scholar] [CrossRef]
- Hilton, J.E.; Summers, M. The effect of polyvinylpyrrolidones on intestinal ulceration caused by indomethacin. Int. J. Pharm. 1986, 32, 13–19. [Google Scholar] [CrossRef]
- Langenbach, R.; Morham, S.G.; Tiano, H.F.; Loftin, C.D.; Ghanayem, B.I.; Chulada, P.C.; Mahler, J.F.; Lee, C.A.; Goulding, E.H.; Kluckman, K.D.; et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 1995, 83, 483–492. [Google Scholar] [CrossRef]
- Pickard, J.; Tamura, A.; Stewart, M.; McGeorge, A.; Fitch, W. Prostacyclin, indomethacin and the cerebral circulation. Brain Res. 1980, 197, 425–431. [Google Scholar] [CrossRef]
- Gary, N.E.; Dodelson, R.; Eisinger, R.P. Indomethacin-associated acute renal failure. Am. J. Med. 1980, 69, 135–136. [Google Scholar] [CrossRef]
- Walker, R.J.; Fawcett, J.P.; Flannery, E.M.; Gerrard, D.F. Indomethacin potentiates exercise-induced reduction in renal hemodynamics in athletes. Med. Sci. Sports Exerc. 1994, 26, 1302–1306. [Google Scholar] [CrossRef]
- Warner, D.C.; Schnepf, G.; Barrett, M.S.; Dian, D.; Swigonski, N.L. Prevalence, attitudes, and behaviors related to the use of nonsteroidal anti-inflammatory drugs (NSAIDs) in student athletes. J. Adolesc. Health 2002, 30, 150–153. [Google Scholar] [CrossRef]
- Ciocca, M. Medication and Supplement Use by Athletes. Clin. Sports Med. 2005, 24, 719–738. [Google Scholar] [CrossRef]
- Davis, B.R. Non-Steroidal Anti-Inflammatory Drug Use in Collegiate Athletes. Master’s Thesis, Portland State University, Portland, OR, USA, 2015. [Google Scholar]
- O’Connor, S.; McCaffrey, N.; Whyte, E.; Moran, K.; Lacey, P. Nonsteroidal anti-inflammatory drug use, knowledge, and behaviors around their use and misuse in Irish collegiate student-athletes. Physician Sportsmed. 2019, 47, 318–322. [Google Scholar] [CrossRef]
- Gorski, T.; Cadore, E.L.; Pinto, S.S.; da Silva, E.M.; Correa, C.S.; Beltrami, F.G.; Kruel, L.F.M. Use of NSAIDs in triathletes: Prevalence, level of awareness and reasons for use. Br. J. Sports Med. 2011, 45, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Warden, S.J. Prophylactic Use of NSAIDs by Athletes: A Risk/Benefit Assessment. Physician Sportsmed. 2010, 38, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.E.; Moore, J.H. Diagnosis and Treatment of Acute Exertional Rhabdomyolysis. J. Orthop. Sports Phys. Ther. 2003, 33, 104–108. [Google Scholar] [CrossRef]
- Patel, D.R.; Gyamfi, R.; Torres, A. Exertional Rhabdomyolysis and Acute Kidney Injury. Physician Sportsmed. 2009, 37, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.L.; Maxwell, L.E.; Koina, M.E.; Falk, M.C.; Willenborg, D.; Dahlstrom, J.E. Renal Glomeruli and Tubular Injury Following Indomethacin, Ibuprofen, and Gentamicin Exposure in a Neonatal Rat Model. Pediatr. Res. 2007, 62, 307–312. [Google Scholar] [CrossRef]
- Nelson, D.A.; Marks, E.S.; Deuster, P.A.; O’Connor, F.G.; Kurina, L.M. Association of Nonsteroidal Anti-inflammatory Drug Prescriptions With Kidney Disease Among Active Young and Middle-aged Adults. JAMA Netw. Open 2019, 2, e187896. [Google Scholar] [CrossRef] [PubMed]
- Christopher, S.; Tadlock, B.A.; Veroneau, B.J.; Harnish, C.; Perera, N.K.P.; Knab, A.M.; Vallabhajosula, S.; Bullock, G.S. Epidemiological profile of pain and non-steroid anti-inflammatory drug use in collegiate athletes in the United States. BMC Musculoskelet. Disord. 2020, 21, 561. [Google Scholar] [CrossRef]
- Zhang, X.; Donnan, P.T.; Bell, S.; Guthrie, B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: Systematic review and meta-analysis. BMC Nephrol. 2017, 18, 256. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Chen, C.; Yang, J.-S.; Lu, C.-C.; Chiu, Y.-J.; Chen, H.-C.; Chung, M.-I.; Wu, Y.-T.; Chen, F.-A. Effect of Quercetin on Dexamethasone-Induced C2C12 Skeletal Muscle Cell Injury. Molecules 2020, 25, 3267. [Google Scholar] [CrossRef]
- Dallak, M.; Dawood, A.F.; Haidara, M.A.; Kader, D.H.A.; Eid, R.A.; Kamar, S.; Eldeen, A.M.S.; Al-Ani, B. Suppression of glomerular damage and apoptosis and biomarkers of acute kidney injury induced by acetaminophen toxicity using a combination of resveratrol and quercetin. Drug Chem. Toxicol. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Kenan Kinaci, M.; Erkasap, N.; Kucuk, A.; Koken, T.; Tosun, M. Effects of quercetin on apoptosis, NF-κB and NOS gene expression in renal ischemia/reperfusion injury. Exp. Ther. Med. 2012, 3, 249–254. [Google Scholar] [CrossRef]
- Yagmurca, M.; Yasar, Z.; Bas, O. Effects of quercetin on kidney injury induced by doxorubicin. Bratisl Lek Listy 2015, 116, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Zyurt, H.; Çevik, Ö.; Özgen, Z.; Özden, A.S.; Çadırcı, S.; Elmas, M.A.; Ercan, F.; Gören, M.Z.; Şener, G. Quercetin protects radiation-induced DNA damage and apoptosis in kidney and bladder tissues of rats. Free Radic. Res. 2014, 48, 1247–1255. [Google Scholar]
- Inal, M.; Altinişik, M.; Bilgin, M.D. The effect of quercetin on renal ischemia and reperfusion injury in the rat. Cell Biochem. Funct. 2002, 20, 291–296. [Google Scholar] [CrossRef]
- Leonard, C.E.; Freeman, C.P.; Newcomb, C.W.; Reese, P.P.; Herlim, M.; Bilker, W.B.; Hennessy, S.; Strom, B.L. Proton pump inhibitors and traditional nonsteroidal anti-inflammatory drugs and the risk of acute interstitial nephritis and acute kidney injury. Pharmacoepidemiol. Drug Saf. 2012, 21, 1155–1172. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.D.; Brater, D.C.; Tierney, W.M.; Hui, S.L.; McDonald, C.J. Ibuprofen-associated Renal Impairment in a Large General Internal Medicine Practice. Am. J. Med Sci. 1990, 299, 222–229. [Google Scholar] [CrossRef]
- Patel, K.; Diamantidis, C.; Zhan, M.; Hsu, V.D.; Walker, L.D.; Gardner, J.; Weir, M.R.; Fink, J.C. Influence of Creatinine versus Glomerular Filtration Rate on Non-Steroidal Anti-Inflammatory Drug Prescriptions in Chronic Kidney Disease. Am. J. Nephrol. 2012, 36, 19–26. [Google Scholar] [CrossRef]
- Silverstein, F.E.; Faich, G.; Goldstein, J.L.; Simon, L.S.; Pincus, T.; Whelton, A.; Makuch, R.; Eisen, G.; Agrawal, N.M.; Stenson, W.F.; et al. Gastrointestinal toxicity with celecoxib vs. nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000, 284, 1247–1255. [Google Scholar] [CrossRef]
- Catella-Lawson, F.; McAdam, B.; Morrison, B.W.; Kapoor, S.; Kujubu, D.; Antes, L.; Lasseter, K.C.; Quan, H.; Gertz, B.J.; Fitzgerald, G.A. Effects of specific inhibition of cyclooxygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J. Pharmacol. Exp. Ther. 1999, 289, 735–741. [Google Scholar]
- Whelton, A.; Schulman, G.; Wallemark, C.; Drower, E.J.; Isakson, P.C.; Verburg, K.M.; Geis, G.S. Effects of Celecoxib and Naproxen on Renal Function in the Elderly. Arch. Intern. Med. 2000, 160, 1465–1470. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 138. [Google Scholar]
- Dreischulte, T.; Donnan, P.; Grant, A.; Hapca, A.; McCowan, C.; Guthrie, B. Safer Prescribing—A Trial of Education, Informatics, and Financial Incentives. N. Engl. J. Med. 2016, 374, 1053–1064. [Google Scholar] [CrossRef]
- Guthrie, B.; Kavanagh, K.; Robertson, C.; Barnett, K.; Treweek, S.; Petrie, D.; Ritchie, L.; Bennie, M. Data feedback and behavioural change intervention to improve primary care prescribing safety (EFIPPS): Multicentre, three arm, cluster randomised controlled trial. BMJ 2016, 354, i4079. [Google Scholar] [CrossRef]
- Dreischulte, T.; Morales, D.R.; Bell, S.; Guthrie, B. Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin–angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int. 2015, 88, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Bouck, Z.; Mecredy, G.C.; Ivers, N.M.; Barua, M.; Martin, D.; Austin, P.C.; Tepper, J.; Bhatia, R.S. Frequency and Associations of Prescription Nonsteroidal Anti-inflammatory Drug Use Among Patients With a Musculoskeletal Disorder and Hypertension, Heart Failure, or Chronic Kidney Disease. JAMA Intern. Med. 2018, 178, 1516–1525. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States; US Department of Health and Human Services: Atlanta, GA, USA, 2019.
- Plantinga, L.; Grubbs, V.; Sarkar, U.; Hsu, C.-Y.; Hedgeman, E.; Robinson, B.; Saran, R.; Geiss, L.; Burrows, N.R.; Eberhardt, M.; et al. Nonsteroidal Anti-Inflammatory Drug Use Among Persons With Chronic Kidney Disease in the United States. Ann. Fam. Med. 2011, 9, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Abbas, E.E.; AlBawab, I.M.; Safarini, S.K.; Alhassan, R.A.; Khatim, M.S. The Effect of Short Term Use of Indomethacin, a Non-Steroidal Anti-inflammatory Drug, on Peritoneal Dialysis Patients. Saudi J. Kidney Dis. Transplant. 1996, 7, 10–14. [Google Scholar]
- McCarthy, J.T.; Torres, V.E.; Romero, J.C.; Wochos, D.N.; Velosa, J.A. Acute intrinsic renal failure induced by indomethacin: Role of prostaglandin synthetase inhibition. Mayo Clin. Proc. 1982, 57, 289–296. [Google Scholar] [PubMed]
- Dixit, M.; Doan, T.; Kirschner, R.; Dixit, N. Significant Acute Kidney Injury Due to Non-steroidal Anti-inflammatory Drugs: Inpatient Setting. Pharmaceuticals 2010, 3, 1279–1285. [Google Scholar] [CrossRef]
- Ravnskov, U. Glomerular, tubular and interstitial nephritis associated with non-steroidal anti-inflammatory drugs. Evidence of a common mechanism. Br. J. Clin. Pharmacol. 1999, 47, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Perazella, M.A. Drug-induced acute interstitial nephritis: Pathology, pathogenesis, and treatment. Iran. J. Kidney Dis. 2015, 9, 3–13. [Google Scholar]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res. 2011, 10, 655–683. [Google Scholar] [PubMed]
- Patrignani, P.; Tacconelli, S.; Sciulli, M.G.; Capone, M.L. New insights into COX-2 biology and inhibition. Brain Res. Rev. 2005, 48, 352–359. [Google Scholar] [CrossRef]
- Bertolini, A.; Ottani, A.; Sandrini, M. Dual acting anti-inflammatory drugs: A reappraisal. Pharmacol. Res. 2001, 44, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.S.; Cuzzocrea, S.; Collino, M.; Chaterjee, P.K.; Mazzon, E.; Britti, D.; Yaqoob, M.M.; Thiemermann, C. The role of cycloxygenase-2 in the rodent kidney following ischaemia/reperfusion injury in vivo. Eur. J. Pharmacol. 2007, 562, 148–154. [Google Scholar] [CrossRef]
- Harirforoosh, S.; Jamali, F. Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin. Drug Saf. 2009, 8, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.-C.; Yang, C.-R.; Cheng, C.-L.; Li, J.-R.; Raung, S.-L.; Hung, Y.-Y.; Chen, C.-J. Indomethacin causes renal epithelial cell injury involving Mcl-1 down-regulation. Biochem. Biophys. Res. Commun. 2009, 380, 531–536. [Google Scholar] [CrossRef]
- Ou, Y.-C.; Yang, C.-R.; Cheng, C.-L.; Raung, S.-L.; Hung, Y.-Y.; Chen, C.-J. Indomethacin induces apoptosis in 786-O renal cell carcinoma cells by activating mitogen-activated protein kinases and AKT. Eur. J. Pharmacol. 2007, 563, 49–60. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Li, Z.; Jo, J.; Jia, J.-M.; Lo, S.-C.; Whitcomb, D.; Jiao, S.; Cho, K.; Sheng, M. Caspase-3 Activation via Mitochondria Is Required for Long-Term Depression and AMPA Receptor Internalization. Cell 2010, 141, 859–871. [Google Scholar] [CrossRef]
- Hao, C.-M.; Kömhoff, M.; Guan, Y.; Redha, R.; Breyer, M.D. Selective targeting of cyclooxygenase-2 reveals its role in renal medullary interstitial cell survival. Am. J. Physiol. Content 1999, 277, F352–F359. [Google Scholar] [CrossRef]
- Danon, A.; Leibson, V.; Assouline, G. Effects of aspirin, indomethacin, flufenamic acid and paracetamol on prostaglandin output from rat stomach and renal papilla in-vitro and ex-vivo. J. Pharm. Pharmacol. 1983, 35, 576–579. [Google Scholar] [CrossRef]
- Schmidt, J.; Klingler, F.-M.; Proschak, E.; Steinhilber, D.; Schubert-Zsilavecz, M.; Merk, D. NSAIDs Ibuprofen, Indometacin and Diclofenac do not interact with Farnesoid X Receptor. Sci. Rep. 2015, 5, 14782. [Google Scholar] [CrossRef]
- Kavsan, V.M.; Iershov, A.V.; Balynska, O.V. Immortalized cells and one oncogene in malignant transformation: Old insights on new explanation. BMC Cell Biol. 2011, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.; Morse, S.; Ararat, M.; Graham, F.L. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002, 16, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Boone, M.; Meuris, L.; Lemmens, I.; Van Roy, N.; Soete, A.; Reumers, J.; Moisse, M.; Plaisance, S.; Drmanac, R.; et al. Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nat. Commun. 2014, 5, 4767. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Estaquier, J.; Vallette, F.; Vayssiere, J.L.; Mignotte, B. The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 2012, 942, 157–183. [Google Scholar]
- Lodi, A.; Tiziani, S.; Khanim, F.L.; Drayson, M.; Günther, U.L.; Bunce, C.M.; Viant, M.R. Hypoxia Triggers Major Metabolic Changes in AML Cells without Altering Indomethacin-Induced TCA Cycle Deregulation. ACS Chem. Biol. 2011, 6, 169–175. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Pastene, E.; Vergara, C.; Zapata, M.; Sandoval, C.; Gotteland, M. Stimulation of cytosolic and mitochondrial calcium mobilization by indomethacin in Caco-2 cells: Modulation by the polyphenols quercetin, resveratrol and rutin. Biochim. Biophys. Acta BBA Gen. Subj. 2012, 1820, 2052–2061. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Gotoh, T.; Tomisato, W.; Mima, S.; Hoshino, T.; Hwang, H.-J.; Takenaka, H.; Tsuchiya, T.; Mori, M.; Mizushima, T. Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death Differ. 2004, 11, 1009–1016. [Google Scholar] [CrossRef]
- Qi, X.; Cai, Y.; Gong, L.; Liu, L.; Chen, F.; Xiao, Y.; Wu, X.; Li, Y.; Xue, X.; Ren, J. Role of mitochondrial permeability transition in human renal tubular epithelial cell death induced by aristolochic acid. Toxicol. Appl. Pharmacol. 2007, 222, 105–110. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef]
- Lu, H.; Wu, L.; Liu, L.; Ruan, Q.; Zhang, X.; Hong, W.; Wu, S.; Jin, G.; Bai, Y. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem. Pharmacol. 2018, 154, 203–212. [Google Scholar] [CrossRef]
- Zhang, M.; Swarts, S.G.; Yin, L.; Liu, C.; Tian, Y.; Cao, Y.; Swarts, M.; Yang, S.; Zhang, S.B.; Zhang, K.; et al. Antioxidant Properties of Quercetin. Adv. Exp. Med. Biol. 2011, 701, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Manvelian, G.; Daniels, S.; Altman, R. A Phase I Study Evaluating the Pharmacokinetic Profile of a Novel, Proprietary, Nano-formulated, Lower-Dose Oral Indomethacin. Postgrad. Med. 2012, 124, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Pountos, I.; Georgouli, T.; Calori, G.M.; Giannoudis, P.V. Do Nonsteroidal Anti-Inflammatory Drugs Affect Bone Healing? A Critical Analysis. Sci. World J. 2012, 2012, 606404. [Google Scholar] [CrossRef] [PubMed]
- Shu-Ping, L.I.; Guo-Fu, H.U. Mechanism and Function of Angiogenin in Apoptosis Regulation. Chin. J. Biochem. Mol. Biol. 2015, 31, 1258–1260. [Google Scholar] [CrossRef]
- Martinvalet, D. ROS signaling during granzyme B-mediated apoptosis. Mol. Cell. Oncol. 2015, 2, e992639. [Google Scholar] [CrossRef]
- Tian, B.; Yang, Q.; Mao, Z. Phosphorylation of ATM by Cdk5 mediates DNA damage signalling and regulates neuronal death. Nat. Cell Biol. 2009, 11, 211–218. [Google Scholar] [CrossRef][Green Version]
- Kozlov, S.V.; Graham, M.E.; Jakob, B.; Tobias, F.; Kijas, A.W.; Tanuji, M.; Chen, P.; Robinson, P.J.; Taucher-Scholz, G.; Suzuki, K.; et al. Autophosphorylation and ATM activation: Additional sites add to the complexity. J. Biol. Chem. 2011, 286, 9107–9119. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, J.; Tan, J.; Wang, M.; Yang, J.; Zhang, Z.-M.; Li, C.; Basnakian, A.G.; Tang, H.-W.; Perrimon, N.; et al. Endonuclease G promotes autophagy by suppressing mTOR signaling and activating the DNA damage response. Nat. Commun. 2021, 12, 476. [Google Scholar] [CrossRef]
- Yan, C.; Xin-Ming, Q.; Li-Kun, G.; Lin-Lin, L.; Fang-Ping, C.; Ying, X.; Xiong-Fei, W.; Xiang-Hong, L.; Jin, R. Tetrandrine-induced apoptosis in rat primary hepatocytes is initiated from mitochondria: Caspases and Endonuclease G (Endo G) pathway. Toxicology 2006, 218, 1–12. [Google Scholar] [CrossRef]
- Wiehe, R.S.; Gole, B.; Chatre, L.; Walther, P.; Calzia, E.; Ricchetti, M.; Wiesmüller, L. Endonuclease G promotes mitochondrial genome cleavage and replication. Oncotarget 2018, 9, 18309–18326. [Google Scholar] [CrossRef]
- Rodríguez-Vargas, J.M.; Magaña, M.J.R.; Ruiz, M.R.; Majuelos-Melguizo, J.; Peralta-Leal, A.; Rodriguez, J.M.; Muñoz-Gámez, J.A.; De Almodóvar, M.R.; Siles, E.; Rivas, A.L.; et al. ROS-induced DNA damage and PARP-1 are required for optimal induction of starvation-induced autophagy. Cell Res. 2012, 22, 1181–1198. [Google Scholar] [CrossRef] [PubMed]
- Sevrioukova, I.F. Apoptosis-Inducing Factor: Structure, Function, and Redox Regulation. Antioxid. Redox Sig. 2011, 14, 2545–2579. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.S.; Lee, H.Y.; Kim, J.; Advani, S.M.; Peng, H.-L.; Banfield, E.; Hawk, E.T.; Chang, S.; Wood, L. Use of non-steroidal anti-inflammatory drugs in US adults: Changes over time and by demographic. Open Heart 2017, 4, e000550. [Google Scholar] [CrossRef]
- Zhou, X.; Zuo, S.; Xin, W. miR-27b overexpression improves mitochondrial function in a Sirt1-dependent manner. J. Physiol. Biochem. 2015, 71, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Boonyong, C.; Vardhanabhuti, N.; Jianmongkol, S. Natural polyphenols prevent indomethacin-induced and diclofenac-induced Caco-2 cell death by reducing endoplasmic reticulum stress regardless of their direct reactive oxygen species scavenging capacity. J. Pharm. Pharmacol. 2020, 72, 583–591. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Souza, M.T.D.S.; Duarte, A.B.S.; de Sousa, D.P. Mechanistic Aspects and Therapeutic Potential of Quercetin against COVID-19-Associated Acute Kidney Injury. Molecules 2020, 25, 5772. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.Z.; Wang, C.; Deng, C.; Zhong, X.; Yan, Y.; Luo, Y.; Lan, H.Y.; He, T.; Wang, L. Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-κB signaling maintained macrophage inflammation. Phytother. Res. 2020, 34, 139–152. [Google Scholar] [CrossRef]
- Alshanwani, A.R.; Shaheen, S.; Faddah, L.M.; Alhusaini, A.M.; Ali, H.M.; Hasan, I.; Hagar, H.; Ahmed, R.; Alharbi, F.M.B.; Al Harthii, A. Manipulation of Quercetin and Melatonin in the Down-Regulation of HIF-1α, HSP-70 and VEGF Pathways in Rat’s Kidneys Induced by Hypoxic Stress. Dose Response 2020, 18, 1559325820949797. [Google Scholar] [CrossRef]
- Wang, C.; Pan, Y.; Zhang, Q.-Y.; Wang, F.-M.; Kong, L.-D. Quercetin and Allopurinol Ameliorate Kidney Injury in STZ-Treated Rats with Regulation of Renal NLRP3 Inflammasome Activation and Lipid Accumulation. PLoS ONE 2012, 7, e38285. [Google Scholar] [CrossRef]
- Ha, H.A.; Chiang, J.H.; Tsai, F.J.; Bau, D.T.; Juan, Y.N.; Lo, Y.H.; Hour, M.J.; Yang, J.S. Novel quinazolinone MJ33 induces AKT/mTORmediated autophagyassociated apoptosis in 5FUresistant colorectal cancer cells. Oncol. Rep. 2021, 45, 680–692. [Google Scholar] [CrossRef]
- Lee, C.-F.; Yang, J.-S.; Tsai, F.-J.; Chiang, N.-N.; Lu, C.-C.; Huang, Y.-S.; Chen, C.; Chen, F.-A. Kaempferol induces ATM/p53-mediated death receptor and mitochondrial apoptosis in human umbilical vein endothelial cells. Int. J. Oncol. 2016, 48, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chen, K.B.; Tsai, C.H.; Tsai, F.J.; Huang, C.Y.; Tang, C.H.; Yang, J.S.; Hsu, Y.M.; Peng, S.F.; Chung, J.G. Casticin inhibits human prostate cancer DU 145 cell migration and invasion via Ras/Akt/NF-kappaB signaling pathways. J. Food. Biochem. 2019, 43, e12902. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-F.; Chiang, N.-N.; Lu, Y.-H.; Huang, Y.-S.; Yang, J.-S.; Tsai, S.-C.; Lu, C.-C.; Chen, F.-A. Benzyl isothiocyanate (BITC) triggers mitochondria-mediated apoptotic machinery in human cisplatin-resistant oral cancer CAR cells. BioMedicine 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-M.; Hsu, Y.-M.; Ying, M.-C.; Tsai, F.-J.; Tsai, C.-H.; Chung, J.-G.; Yang, J.-S.; Tang, C.-H.; Cheng, L.-Y.; Su, P.-H.; et al. High-density lipoprotein ameliorates palmitic acid-induced lipotoxicity and oxidative dysfunction in H9c2 cardiomyoblast cells via ROS suppression. Nutr. Metab. 2019, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-R.; Lin, C.; Lu, C.-C.; Kuo, S.-C.; Tsao, J.-W.; Juan, Y.-N.; Chiu, H.-Y.; Lee, F.-Y.; Yang, J.-S.; Tsai, F.-J. YC-1 induces G0/G1phase arrest and mitochondria-dependent apoptosis in cisplatin-resistant human oral cancer CAR cells. BioMedicine 2017, 7, 12. [Google Scholar] [CrossRef]
- Chung, J.-G.; Yang, J.-S.; Huang, L.-J.; Lee, F.-Y.; Teng, C.-M.; Tsai, S.-C.; Lin, K.-L.; Wang, S.-F.; Kuo, S.-C. Proteomic approach to studying the cytotoxicity of YC-1 on U937 leukemia cells and antileukemia activity in orthotopic model of leukemia mice. Proteomics 2007, 7, 3305–3317. [Google Scholar] [CrossRef]
- Ho, Y.-T.; Lu, C.-C.; Yang, J.-S.; Chiang, J.-H.; Li, T.-C.; Ip, S.-W.; Hsia, T.-C.; Liao, C.-L.; Lin, J.-G.; Wood, W.G.; et al. Berberine induced apoptosis via promoting the expression of caspase-8, -9 and -3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Antican. Res. 2009, 29, 4063–4070. [Google Scholar]

| Primers | Sequences |
|---|---|
| homo caspase-3 | 5′-CAGTGGAGGCCGACTTCTTG-3′ 3′-TGGCACAAAGCGACTGGAT-5′ |
| homo caspase-9 | 5′-TGTCCTACTCTACTTTCCCAGGTTTT-3′ 3′-GTGAGCCCACTGCTCAAAGAT-5′ |
| homo AIF | 5′-GGGAGGACTACGGCAAAGGT-3′ 3′-CTTCCTTGCTATTGGCATTCG-5′ |
| homo Endo G | 5′-GTACCAGGTCATCGGCAAGAA-3′ 3′-CGTAGGTGCGGAGCTCAATT-5′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Yang, J.-S.; Lu, C.-C.; Wu, Y.-T.; Chen, F.-A. Effect of Quercetin on Injury to Indomethacin-Treated Human Embryonic Kidney 293 Cells. Life 2021, 11, 1134. https://doi.org/10.3390/life11111134
Chen C, Yang J-S, Lu C-C, Wu Y-T, Chen F-A. Effect of Quercetin on Injury to Indomethacin-Treated Human Embryonic Kidney 293 Cells. Life. 2021; 11(11):1134. https://doi.org/10.3390/life11111134
Chicago/Turabian StyleChen, Chun, Jai-Sing Yang, Chi-Cheng Lu, Yu-Tse Wu, and Fu-An Chen. 2021. "Effect of Quercetin on Injury to Indomethacin-Treated Human Embryonic Kidney 293 Cells" Life 11, no. 11: 1134. https://doi.org/10.3390/life11111134
APA StyleChen, C., Yang, J.-S., Lu, C.-C., Wu, Y.-T., & Chen, F.-A. (2021). Effect of Quercetin on Injury to Indomethacin-Treated Human Embryonic Kidney 293 Cells. Life, 11(11), 1134. https://doi.org/10.3390/life11111134







