Clostridioides difficile and Vancomycin-Resistant Enterococci in COVID-19 Patients with Severe Pneumonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Population and Setting
2.2. Inclusion Criteria
2.3. Detection of C. difficile (Positive Cases)
2.4. Isolation of VRE
2.5. Molecular Typing of VRE
2.6. Vancomycin Consumption Assessment
2.7. Statistical Analysis
3. Results
3.1. Patient Data Analysis—The Study and Control Groups
3.2. C. difficile Detection
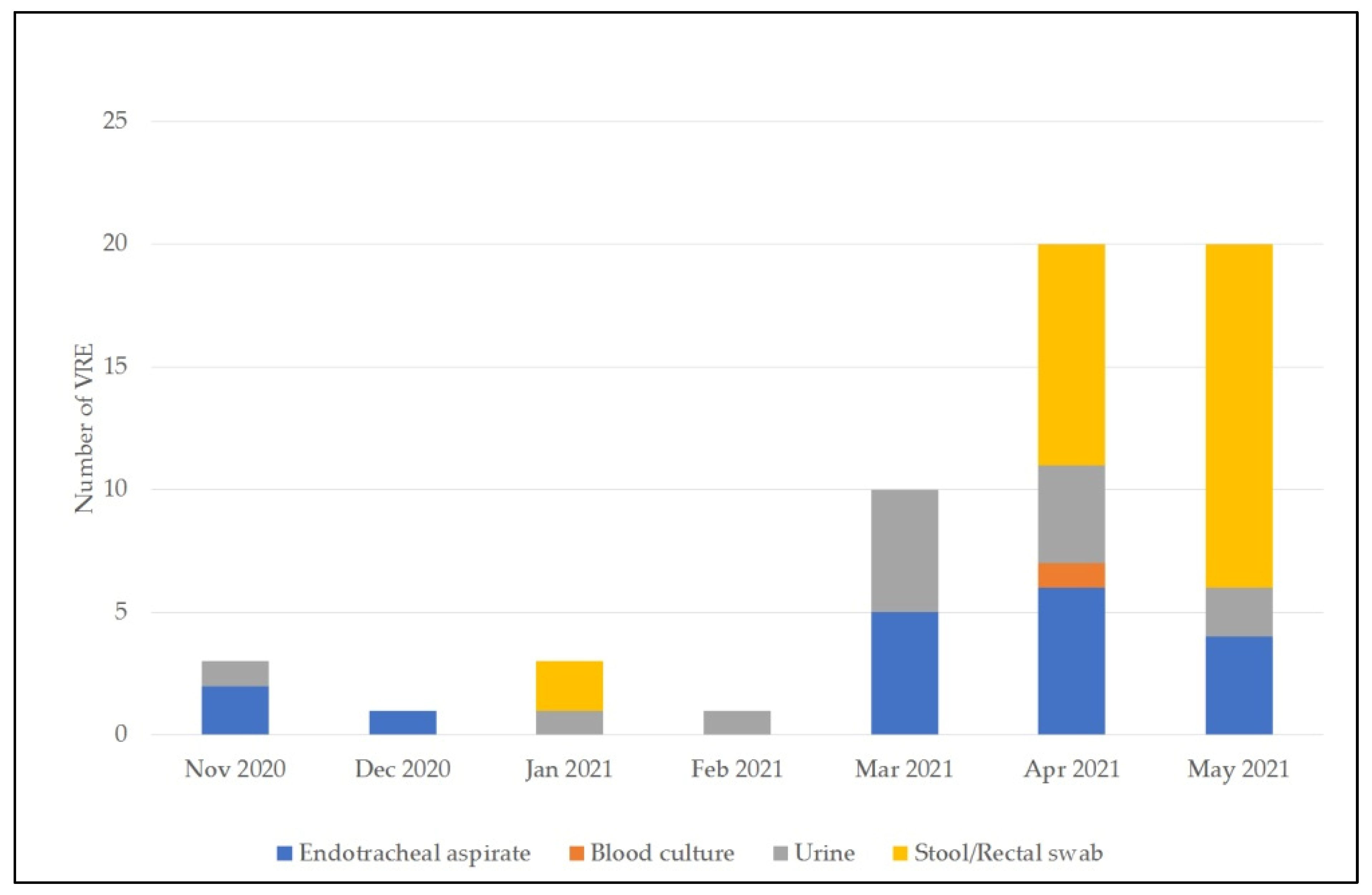
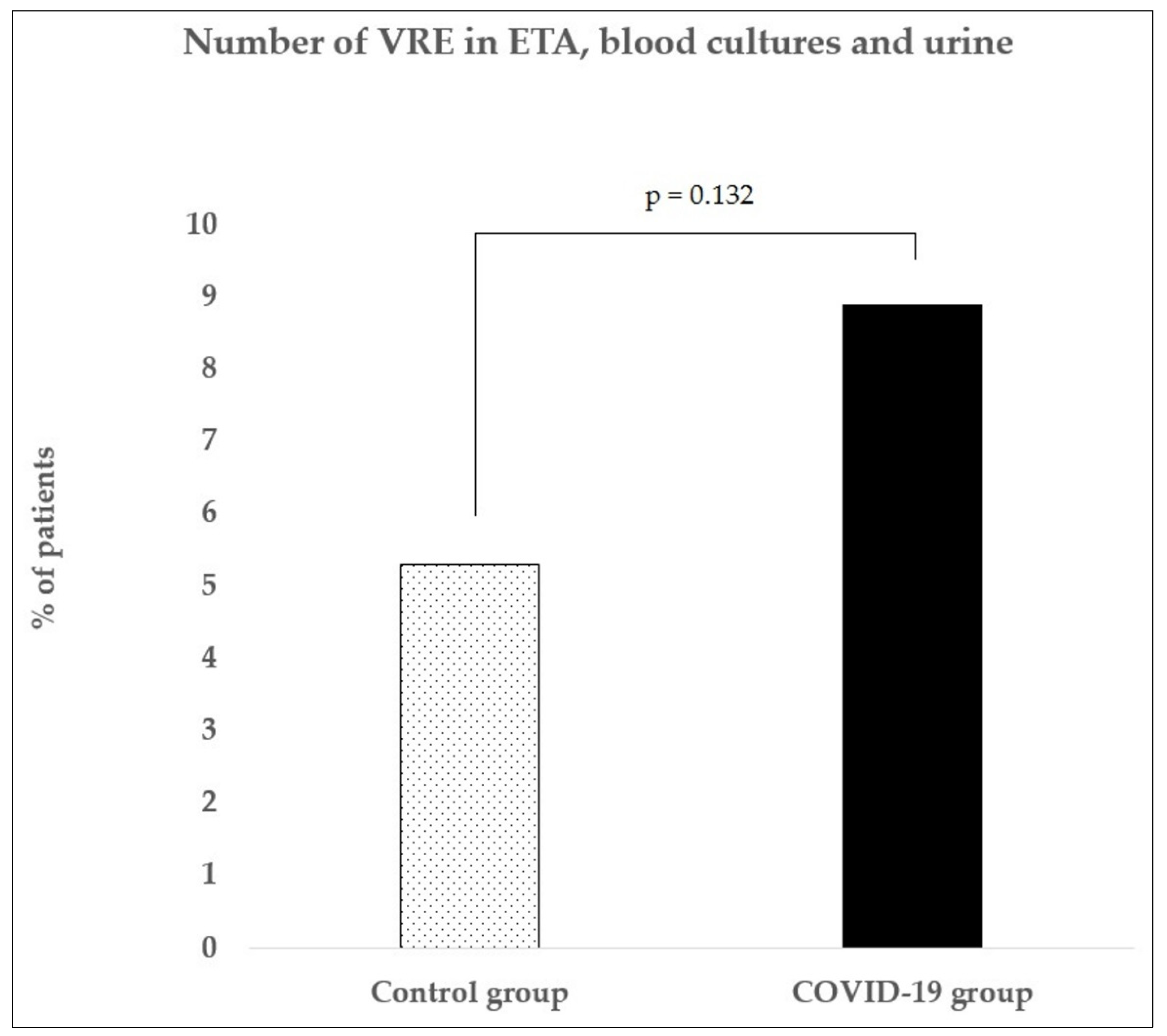
3.3. VRE Detection
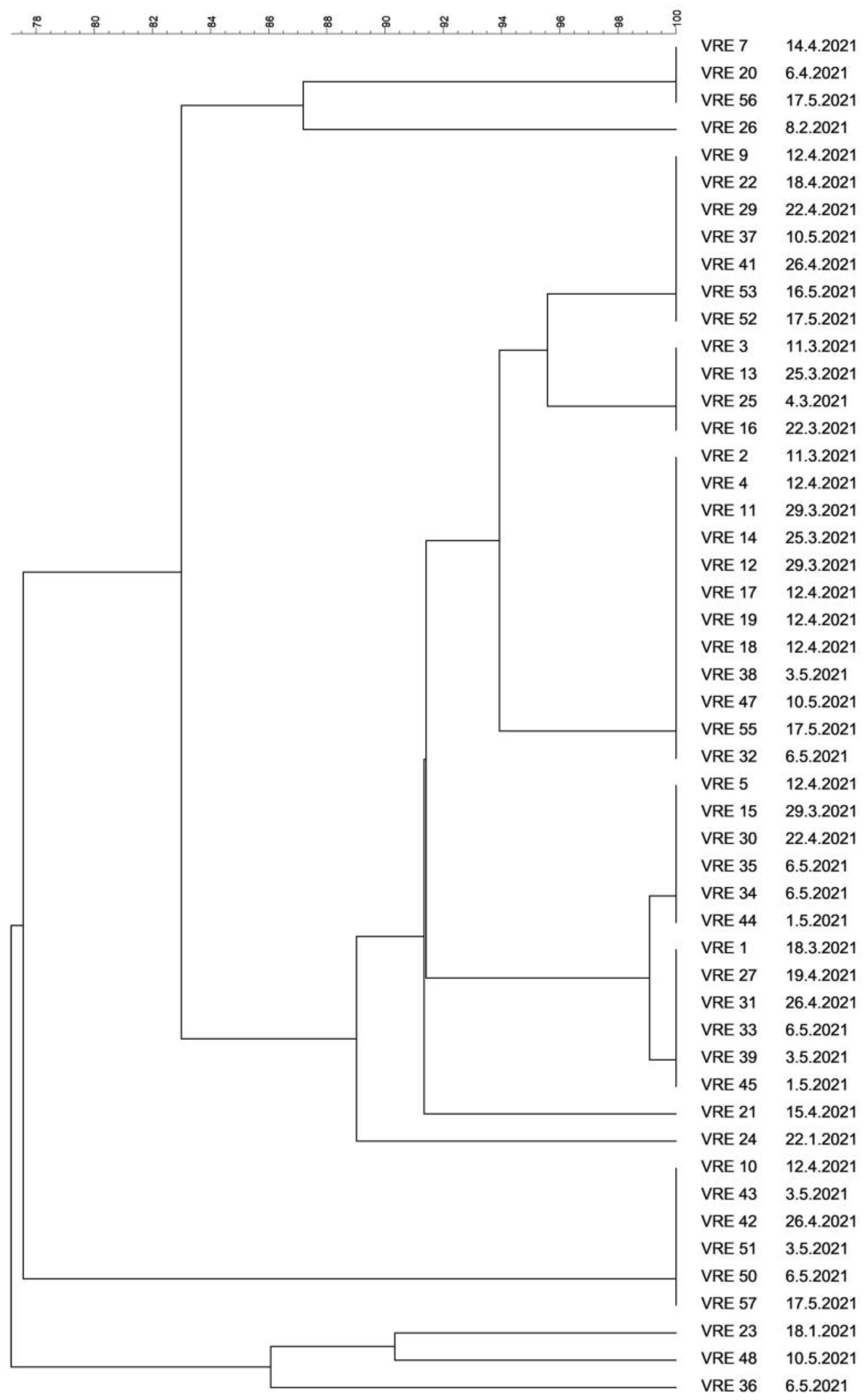
3.4. Molecular Typing of VRE
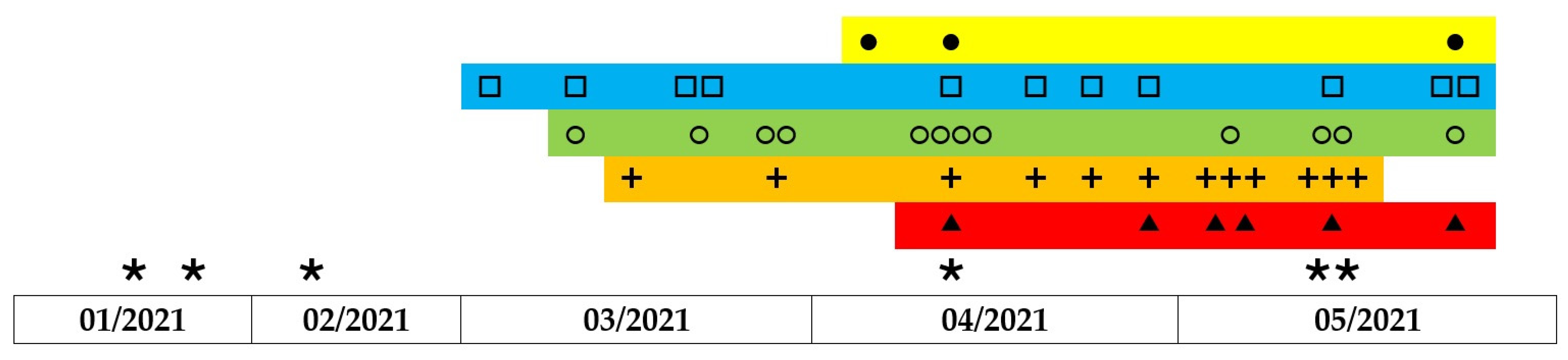
3.5. Vancomycin Consumption
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Clinical Management of COVID-19: Interim Guidance, 27 May 2020. Available online: https://apps.who.int/iris/handle/10665/332196 (accessed on 29 August 2021).
- Martín-Loeches, I.; Sanchez-Corral, A.; Diaz, E.; Granada, R.; Zaragoza, R.; Villavicencio, C.; Albaya, A.; Cerdá, E.; Catalán, R.; Luque, P.; et al. Community-Acquired Respiratory Coinfection in Critically Ill Patients with Pandemic 2009 Influenza A(H1N1). Virus. Chest. 2011, 139, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Manohar, P.; Loh, B.; Nachimuthu, R.; Hua, X.; Welburn, S.; Leptihn, S. Secondary Bacterial Infections in Patients with Viral Pneu-monia. Front. Med. 2020, 7, 420. [Google Scholar] [CrossRef]
- Brown, K.; Langford, B.; Schwartz, K.; Diong, C.; Garber, G.; Daneman, N. Antibiotic Prescribing Choices and Their Comparative C. Difficile Infection Risks: A Longitudinal Case-Cohort Study. Clin. Infect. Dis. 2021, 72, 836–844. [Google Scholar] [CrossRef] [Green Version]
- Ponce-Alonso, M.; Sáez de la Fuente, J.; Rincón-Carlavilla, A.; Moreno-Nunez, P.; Martínez-García, L.; Escudero-Sánchez, R.; Pintor, R.; García-Fernández, S.; Cobo, J. Impact of the coronavirus disease 2019 (COVID-19) pandemic on nosocomial Clostridioides difficile infection. Infect. Control. Hosp. Epidemiol. 2021, 42, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Bentivegna, E.; Alessio, G.; Spuntarelli, V.; Luciani, M.; Santino, I.; Simmaco, M.; Martelletti, P. Impact of COVID-19 prevention measures on risk of health care-associated Clostridium difficile infection. Am. J. Infect. Control 2021, 49, 640–642. [Google Scholar] [CrossRef]
- Ochoa-Hein, E.; Rajme-López, S.; Rodríguez-Aldama, J.; Huertas-Jiménez, M.; Chávez-Ríos, A.; de Paz-García, R.; Haro-Osnaya, A.; González-Colín, K.; González-González, R.; González-Lara, M.; et al. Substantial reduction of healthcare facility-onset Clostridioides difficile infection (HO-CDI) rates after conversion of a hospital for exclusive treatment of COVID-19 patients. Am. J. Infect. Control 2021, 49, 966–968. [Google Scholar] [CrossRef]
- Luo, Y.; Grinspan, L.; Fu, Y.; Adams-Sommer, V.; Willey, D.; Patel, G.; Grinspan, A. Hospital-onset Clostridioides difficile infections during the COVID-19 pandemic. Infect. Control Hosp. Epidemiol. 2020, 1–2. [Google Scholar] [CrossRef]
- Allegretti, J.; Nije, C.; McClure, E.; Redd, W.; Wong, D.; Zhou, J.; Bazarbashi, A.; McCarty, T.; Hathorn, K.; Shen, L.; et al. Prevalence and impact of Clostridioides difficile infection among hospitalized patients with coranavirus disease 201 9. JGH Open 2021, 5, 622–625. [Google Scholar] [CrossRef]
- Sandhu, A.; Tillotson, G.; Polistico, J.; Salimnia, H.; Cranis, M.; Moshos, J.; Cullen, L.; Jabbo, L.; Diebel, L.; Chopra, T. Clostridioides difficile in COVID-19 Patients, Detroit, Michigan, USA, March–April 2020. Emerg. Infect. Dis. 2020, 26, 2272–2274. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, K.; Rosołowski, M.; Kaniewska, M.; Kucha, P.; Meler, A.; Wierzba, W.; Rydzewska, G. Clostridioides difficile infection in coronavirus disease 2019: An underestimated problem? Pol. Arch. Intern. Med. 2020, 131, 121–127. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Healthcare-associated infections: Clostridium difficile. In ECDC Annual Epidemiological Report for 2016; ECDC: Stockholm, Sweden, 2018; Available online: https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-clostridium-difficile-infections-annual (accessed on 29 August 2021).
- Lessa, F.; Mu, Y.; Bamberg, W.; Beldavs, Z.; Dumyati, G.; Dunn, J.; Farley, M.; Holzbauer, S.; Meek, J.; Phipps, E.; et al. Burden of Clostridium difficile Infection in the United States. N. Engl. J. Med. 2015, 372, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Tariq, R.; Laguio-Vila, M.; Tahir, M.; Orenstein, R.; Pardi, D.; Khanna, S. Efficacy of oral vancomycin prophylaxis for prevention of Clostridioides difficile infection: A systematic review and meta-analysis. Ther. Adv. Gastroenterol. 2021, 23, 1756284821994046. [Google Scholar] [CrossRef]
- Isaac, S.; Scher, J.; Djukovic, A.; Jiménez, N.; Littman, D.; Abramson, S.; Pamer, E.; Ubeda, C. Short- and long-term effects of oral van-comycin on the human intestinal microbiota. J. Antimicrob. Chemother. 2016, 72, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Hricová, K.; Štosová, T.; Kučová, P.; Fišerová, K.; Bardoň, J.; Kolář, M. Analysis of Vancomycin-Resistant Enterococci in Hemato-Oncological Patients. Antibiotics 2020, 9, 785. [Google Scholar] [CrossRef]
- Tenover, F.; Arbeit, R.; Goering, R.; Mickelsen, P.; Murray, B.; Persing, D.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar] [CrossRef] [Green Version]
- WHO Collaborating Centre for Drug Statistics Methodology. ATC Classification Index with DDDs, 2021; WHO Collaborating Centre for Drug Statistics Methodology: Oslo, Norway, 2020. [Google Scholar]
- Yacyshyn, B. Pathophysiology of Clostridium difficile-Associated Diarrhea. Gastroenterol. Hepatol. 2016, 12, 558–560. Available online: https://pubmed.ncbi.nlm.nih.gov/27917094 (accessed on 29 August 2021).
- Zuo, T.; Zhang, F.; Lui, G.C.; Yeoh, Y.K.; Li, A.Y.; Zhan, H.; Wan, Y.; Chung, A.C.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef] [PubMed]
- Hazel, K.; Skally, M.; Glynn, E.; Foley, M.; Burns, K.; O’Toole, A.; Boland, K.; Fitzpatrick, F. The other ‘C’: Hospital-acquired Clos-tridioides difficile infection during the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control Hosp. Epidemiol. 2021, 1–2, 1–2. [Google Scholar] [CrossRef]
- Kaafarani, H.M. COVID-19: Gastrointestinal Symptoms and Complications. Available online: https://www.uptodate.com/contents/covid-19-gastrointestinal-symptoms-and-complications/print (accessed on 4 August 2021).
- Cheung, K.; Hung, I.; Chan, P.; Lung, K.; Tso, E.; Liu, R.; Ng, Y.; Chu, M.; Chung, T.; Tam, A.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples from a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef]
- Silva, F.; Brito, B.; Santos, M.; Marques, H.; Silva Júnior, R.; Carvalho, L.; Vieira, E.; Oliveira, M.; Melo, F. COVID-19 gastrointestinal manifestations: A systematic review. Rev. Da Soc. Bras. De Med. Trop. 2020, 53. [Google Scholar] [CrossRef]
- D’Amico, F.; Baumgart, D.; Danese, S.; Peyrin-Biroulet, L. Diarrhea during COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin. Gastroenterol. Hepatol. 2020, 18, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Norén, T. Clostridium Difficile and the Disease it Causes. Clostridium Difficile; Humana Press: Totowa, NJ, USA, 2010; Volume 646, pp. 9–35. [Google Scholar] [CrossRef]
- Govil, D.; Pal, D. Gastrointestinal Motility Disorders in Critically Ill. Indian J. Crit. Care Med. 2020, 24, S179–S182. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.; Longshaw, C.; Davis, G.; Bouza, E.; Barbut, F.; Barna, Z.; Delmée, M.; Fitzpatrick, F.; Ivanova, K.; Kuijper, E.; et al. Underdiagnosis of Clostridium difficile across Europe: The European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect. Dis. 2014, 14, 1208–1219. [Google Scholar] [CrossRef]
- Sieswerda, E.; de Boer, M.; Bonten, M.; Boersma, W.; Jonkers, R.; Aleva, R.; Kullberg, B.; Schouten, J.; van de Garde, E.; Verheij, T.; et al. Recommendations for antibacterial therapy in adults with COVID-19—An evidence based guideline. Clin. Microbiol. Infect. 2021, 27, 61–66. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH) COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 1 August 2021).
- Kalil, A.; Metersky, M.; Klompas, M.; Muscedere, J.; Sweeney, D.; Palmer, L.; Napolitano, L.; O’Grady, N.; Bartlett, J.; Carratalà, J.; et al. Management of Adults with Hospi-tal-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- Uvizl, R.; Adamus, M.; Cerny, V.; Dusek, L.; Jarkovsky, J.; Sramek, V.; Matejovic, M.; Stourac, P.; Kula, R.; Malaska, J.; et al. Patient survival, predictive factors and disease course of severe sepsis in Czech intensive care units: A multicentre, retrospective, observational study. Biomed. Pap. 2016, 160, 287–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herkel, T.; Uvízl, R.; Doubravská, L.; Adamus, M.; Gabrhelík, T.; Sedlakova, M.H.; Kolář, M.; Hanulík, V.; Pudová, V.; Langová, K.; et al. Epidemiology of hospital-acquired pneumonia: Results of a Central European multicenter, prospective, observational study compared with data from the European region. Biomed. Pap. J. Fac. Med. Dent. Palacky Univ. Olomouc 2016, 160, 448–455. Available online: http://www.medvik.cz/link/bmc16028823 (accessed on 29 August 2021). [CrossRef]
- Rawson, T.; Moore, L.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Langford, B.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.; Soucy, J.; Daneman, N. Bacterial co-infection and sec-ondary infection in patients with COVID-19: A living rapid review and meta-analysis: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W. Co-infections in people with COVID-19: A systematic review and meta-analysis: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalised patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Vaughn, V.; Gandhi, T.; Petty, L.; Patel, P.; Prescott, H.; Malani, A.; Ratz, D.; McLaughlin, E.; Chopra, V.; Flanders, S. Empiric Antibacterial Therapy and Community-onset Bacterial Coinfection in Patients Hospitalized with Coronavirus Disease 2019 (COVID-19): A Multi-hospital Cohort Study. Clin. Infect. Dis. 2021, 72, e533–e541. [Google Scholar] [CrossRef]
- Youngs, J.; Wyncoll, D.; Hopkins, P.; Arnold, A.; Ball, J.; Bicanic, T. Improving antibiotic stewardship in COVID-19: Bacterial co-infection is less common than with influenza. J. Infect. 2020, 81, e55–e57. [Google Scholar] [CrossRef]
- Clancy, C.; Nguyen, M. Coronavirus Disease 2019, Superinfections, and Antimicrobial Development: What Can We Expect? Clin. Infect. Dis. 2020, 71, 2736–2743. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Garcia-Telles, N.; Aggarwal, G.; Lavie, C.; Lippi, G.; Henry, B. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis 2020, 7, 91–96. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; et al. Clinical Characteristics of COVID-19 Patients with Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retro-spective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 769–777. [Google Scholar] [CrossRef] [Green Version]
- Borba, M.; Val, F.; Sampaio, V.; Alexandre, M.; Melo, G.; Brito, M.; Mourão, M.; Brito-Sousa, J.; Baía-da-Silva, D.; Guerra, M.; et al. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Jama Netw. Open 2020, 3, e208857. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Xu, X.; Yin, H.; Hu, Q.; Xiong, T.; Tang, Y.; Yang, A.; Yu, B.; Huang, Z. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: A retrospective study. BMC Infect. Dis. 2020, 20, 311. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, H.P.; Hildebrandt, T.; Poulsen, A.; Uslu, B.; Knudsen, H.H.; Roed, J.; Poulsen, T.D.; Nielsen, H.B. Initial experiences from patients with COVID-19 on ventilatory support in Denmark. Dan. Med. J. 2020, 67, A04200232. [Google Scholar] [PubMed]
- Alhazzani, W.; Møller, M.; Arabi, Y.; Loeb, M.; Gong, M.; Fan, E.; Oczkowski, S.; Levy, M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19): Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 46, 854–887. [Google Scholar] [CrossRef] [Green Version]
- Kluge, S.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Marx, G.; Karagiannidis, C. German recommendations for critically ill patients with COVID 19. Med. Klin.—Intensivmed. Und Notfallmedizin. 2020, 115, 111–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strategies to Prevent Clostridioides difficile Infection in Acute Care Facilities. Available online: https://www.cdc.gov/hai/prevent/cdi-prevention-strategies.html (accessed on 29 August 2021).
- Johnson, S.; Brown, S.; Priest, D. Effectiveness of Oral Vancomycin for Prevention of Healthcare Facility–Onset Clostridioides difficile Infection in Targeted Patients during Systemic Antibiotic Exposure. Clin. Infect. Dis. 2020, 71, 1133–1139. [Google Scholar] [CrossRef]
- Ganetsky, A.; Han, J.; Hughes, M.; Babushok, D.; Frey, N.; Gill, S.; Hexner, E.; Loren, A.; Luger, S.; Mangan, J.; et al. Oral Vancomycin Prophylaxis Is Highly Effective in Preventing Clostridium difficile Infection in Allogeneic Hematopoietic Cell Transplant Recipients. Clin. Infect. Dis. 2019, 68, 2003–2009. [Google Scholar] [CrossRef]
- Babar, S.; El Kurdi, B.; El Iskandarani, M.; Haddad, I.; Imam, Z.; Alomari, M.; Myers, J.; Moorman, J. Oral vancomycin prophylaxis for the prevention of Clostridium difficile infection: A systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2020, 41, 1302–1309. [Google Scholar] [CrossRef]
- Van Hise, N.; Bryant, A.; Hennessey, E.; Crannage, A.; Khoury, J.; Manian, F. Efficacy of Oral Vancomycin in Preventing Recurrent Clostridium difficile Infection in Patients Treated With Systemic Antimicrobial Agents: Table 1. Clin. Infect. Dis. 2016, 63, 651–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carignan, A.; Poulin, S.; Martin, P.; Labbé, A.; Valiquette, L.; Al-Bachari, H.; Montpetit, L.; Pépin, J. Efficacy of Secondary Prophylaxis with Vancomycin for Preventing Recurrent Clostridium difficile Infections. Am. J. Gastroenterol. 2016, 111, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Splinter, L.; Kerstenetzky, L.; Jorgenson, M.; Descourouez, J.; Leverson, G.; Saddler, C.; Smith, J.; Safdar, N.; Redfield, R. Vancomycin Prophylaxis for Prevention of Clostridium difficile Infection Recurrence in Renal Transplant Patients. Ann. Pharma-Cotherapy 2017, 52, 113–119. [Google Scholar] [CrossRef]
- Papic, N.; Maric, L.; Vince, A. Efficacy of oral vancomycin in primary prevention of Clostridium Difficile infection in elderly patients treated with systemic antibiotic therapy. Infect. Dis. 2018, 50, 483–486. [Google Scholar] [CrossRef]
- Morrisette, T.; Van Matre, A.; Miller, M.; Mueller, S.; Bajrovic, V.; Abidi, M.; Benamu, E.; Kaiser, J.; Barber, G.; Chase, S.; et al. Oral Vancomycin Prophylaxis as Secondary Prevention Against Clostridioides difficile Infection in the Hemato-poietic Stem Cell Transplantation and Hematologic Malignancy Population. Biol. Blood Marrow Transplant. 2019, 25, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.; Schiller, D.; Fulman, M.; Rastogi, R. Long-Term Efficacy of Oral Vancomycin Prophylaxis for the Prevention of Clos-tridium difficile Recurrence. J. Pharm. Pract. 2020, 33, 633–639. [Google Scholar] [CrossRef]
- McDonald, L.; Gerding, D.; Johnson, S.; Bakken, J.; Carroll, K.; Coffin, S.; Dubberke, E.; Garey, K.; Gould, C.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.; Pepin, J.; Frost, E.; Carrier, J.; Sirard, S.; Fortier, L.; Valiquette, L. Faecal pharmacokinetics of orally administered vancomycin in patients with suspected Clostridium difficile infection. BMC Infect. Dis. 2010, 10, 363. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S. Editorial Commentary: Potential Risks and Rewards with Prophylaxis for Clostridium difficile Infection. Clin. Infect. Dis. 2016, 63, 654–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abujamel, T.; Cadnum, J.; Jury, L.; Sunkesula, V.; Kundrapu, S.; Jump, R.; Stintzi, A.; Donskey, C.; Paredes-Sabja, D. Defining the Vul-nerable Period for Re-Establishment of Clostridium difficile Colonization after Treatment of C. difficile Infection with Oral Vancomycin or Metronidazole. PLoS ONE 2013, 8, e76269. [Google Scholar] [CrossRef]
- Lewis, B.; Buffie, C.; Carter, R.; Leiner, I.; Toussaint, N.; Miller, L.; Gobourne, A.; Ling, L.; Pamer, E. Loss of Microbiota-Mediated Colonization Resistance to Clostridium difficile Infection with Oral Vancomycin Compared with Metronidazole. J. Infect. Dis. 2015, 212, 1656–1665. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S. Treatment of Asymptomatic Clostridium difficile Carriers (Fecal Excretors) with Vancomycin or Metronidazole. Ann. Intern. Med. 1992, 117, 297–302. [Google Scholar] [CrossRef]
- Kampmeier, S.; Tönnies, H.; Correa-Martinez, C.; Mellmann, A.; Schwierzeck, V. A nosocomial cluster of vancomycin resistant enterococci among COVID-19 patients in an intensive care unit. Antimicrob. Resist. Infect. Control. 2020, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Correa-Martinez, C.L.; Stollenwerk, V.B.; Kossow, A.; Schaumburg, F.; Mellmann, A.; Kampmeier, S. Risk Factors for Long-Term Vancomycin-Resistant Enterococci Persistence—A Prospective Longitudinal Study. Microorganisms 2019, 7, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Control Group 2018–2019 (n = 189) | COVID-19 Group 2020–2021 (n = 372) | p-Value |
|---|---|---|---|
| Gender—males (%) | 136 (72) | 241 (64.2) | 0.087 |
| Age (median, range, IQR) | 65, 18–97, 20 | 67, 22–90, 16 | 0.753 |
| Admission up to 48 h after admission to hospital (%) | 82 (43.4) | 186 (50) | 0.138 |
| Transfer from another hospital or department | 107 (56.6) | 186 (50) | 0.138 |
| No. of patients on mechanical ventilation only | 180 (95.2) | 78 (21.0) | <0.0001 |
| No. of patients on HFOT only | 3 (1.6) | 112 (30.1) | <0.0001 |
| No. of patient on both mechanical ventilation and HFOT | 6 (3.2) | 182 (48.9) | <0.0001 |
| No. of patients on ECMO | 0 (0.0) | 24 (6.5) | 0.004 |
| ICU mortality | 74 (39.2) | 158 (42.5) | 0.450 |
| Month | Control Group 2018–2019 | COVID-19 Group 2020–2021 | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of Admitted Patients | No. (%) of CD-Tested Patients | No. (%) of CD-Positive Cases among Admitted Patients | No. (%) of CD Toxin-Positive Cases among Admitted Patients | No. of Admitted Patients | No. (%) of CD-Tested Patients | No. (%) of CD-Positive Cases among Admitted Patients | No. (%) of CD Toxin-Positive Cases among Admitted Patients | |
| November | 23 | 2 (8.7) | 0 | 0 | 55 | 8 (14.5) | 3 (5.5) | 2 (3.6) |
| December | 28 | 2 (7.14) | 0 | 0 | 56 | 7 (12.5) | 5 (8.9) | 3 (5.4) |
| January | 30 | 4 (13.3) | 1 (3.3) | 1 (3.3) | 64 | 20 (31.2) | 15 (23.4) | 9 (14.1) |
| February | 35 | 5 (14.3) | 1 (2.9) | 1 (2.9) | 40 | 25 (62.5) | 16 (40) | 10 (25) |
| March | 28 | 3 (10.7) | 1 (3.6) | 0 | 84 | 71 (84.5) | 40 (47.6) | 13 (15.5) |
| April | 22 | 2 (9.1) | 2 (9.1) | 1 (4.5) | 52 | 44 (84.6) | 16 (30.8) | 7 (13.5) |
| May | 23 | 2 (8.7) | 0 | 0 | 21 | 17 (81) | 7 (33.3) | 2 (9.5) |
| Total | 189 | 20 (10.6) | 5 (2.6) | 3 (1.6) | 372 | 192 (51.6) | 102 (27.4) | 46 (12.4) |
| Month | Control Group 2018–2019 | COVID-19 Group 2020–2021 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Admitted Patients | No. of VRE in ETA | No. of VRE in Blood Cultures | No. of VRE in Urine | Absolute no. (%) of VRE-Positive Patients | No. of Admitted Patients | No. of VRE in ETA | No. of VRE in Blood Cultures | No. of VRE in Urine | Absolute no. (%) of VRE-Positive Patients | |
| November | 23 | 2 | 0 | 1 | 3 (13.1) | 55 | 2 | 0 | 1 | 3 (5.5) |
| December | 28 | 0 | 0 | 0 | 0 | 56 | 1 | 0 | 0 | 1 (1.8) |
| January | 30 | 1 | 1 | 0 | 2 (6.7) | 64 | 0 | 0 | 1 | 1 (1.6) |
| February | 35 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 1 | 1 (2.5) |
| March | 28 | 3 | 1 | 0 | 4 (14.3) | 84 | 5 | 0 | 5 | 10 (11.9) |
| April | 22 | 0 | 0 | 0 | 0 | 52 | 6 | 1 | 4 | 11 (21.2) |
| May | 23 | 0 | 1 | 0 | 1 (4.3) | 21 | 4 | 0 | 2 | 6 (28.6) |
| Total | 189 | 6 | 2 | 1 | 10 (5.3) | 372 | 18 | 1 | 14 | 33 (8.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanová, K.; Doubravská, L.; Vágnerová, I.; Hricová, K.; Pudová, V.; Röderová, M.; Papajk, J.; Uvízl, R.; Langová, K.; Kolář, M. Clostridioides difficile and Vancomycin-Resistant Enterococci in COVID-19 Patients with Severe Pneumonia. Life 2021, 11, 1127. https://doi.org/10.3390/life11111127
Bogdanová K, Doubravská L, Vágnerová I, Hricová K, Pudová V, Röderová M, Papajk J, Uvízl R, Langová K, Kolář M. Clostridioides difficile and Vancomycin-Resistant Enterococci in COVID-19 Patients with Severe Pneumonia. Life. 2021; 11(11):1127. https://doi.org/10.3390/life11111127
Chicago/Turabian StyleBogdanová, Kateřina, Lenka Doubravská, Iva Vágnerová, Kristýna Hricová, Vendula Pudová, Magdaléna Röderová, Jan Papajk, Radovan Uvízl, Kateřina Langová, and Milan Kolář. 2021. "Clostridioides difficile and Vancomycin-Resistant Enterococci in COVID-19 Patients with Severe Pneumonia" Life 11, no. 11: 1127. https://doi.org/10.3390/life11111127
APA StyleBogdanová, K., Doubravská, L., Vágnerová, I., Hricová, K., Pudová, V., Röderová, M., Papajk, J., Uvízl, R., Langová, K., & Kolář, M. (2021). Clostridioides difficile and Vancomycin-Resistant Enterococci in COVID-19 Patients with Severe Pneumonia. Life, 11(11), 1127. https://doi.org/10.3390/life11111127







