Assembly and Function of the Juxtaparanodal Kv1 Complex in Health and Disease
Abstract
1. Introduction
2. Diversity of K+ Channels at the Axon Initial Segment and Nodes of Ranvier
3. Targeting of the Kv1 Complex at The Axon Initial Segment
4. Cell Adhesion Molecules Mediate Kv1 Trapping at the Juxtaparanodes
5. Organization of the Submembrane Cytoskeleton at Paranodes and Juxtaparanodes
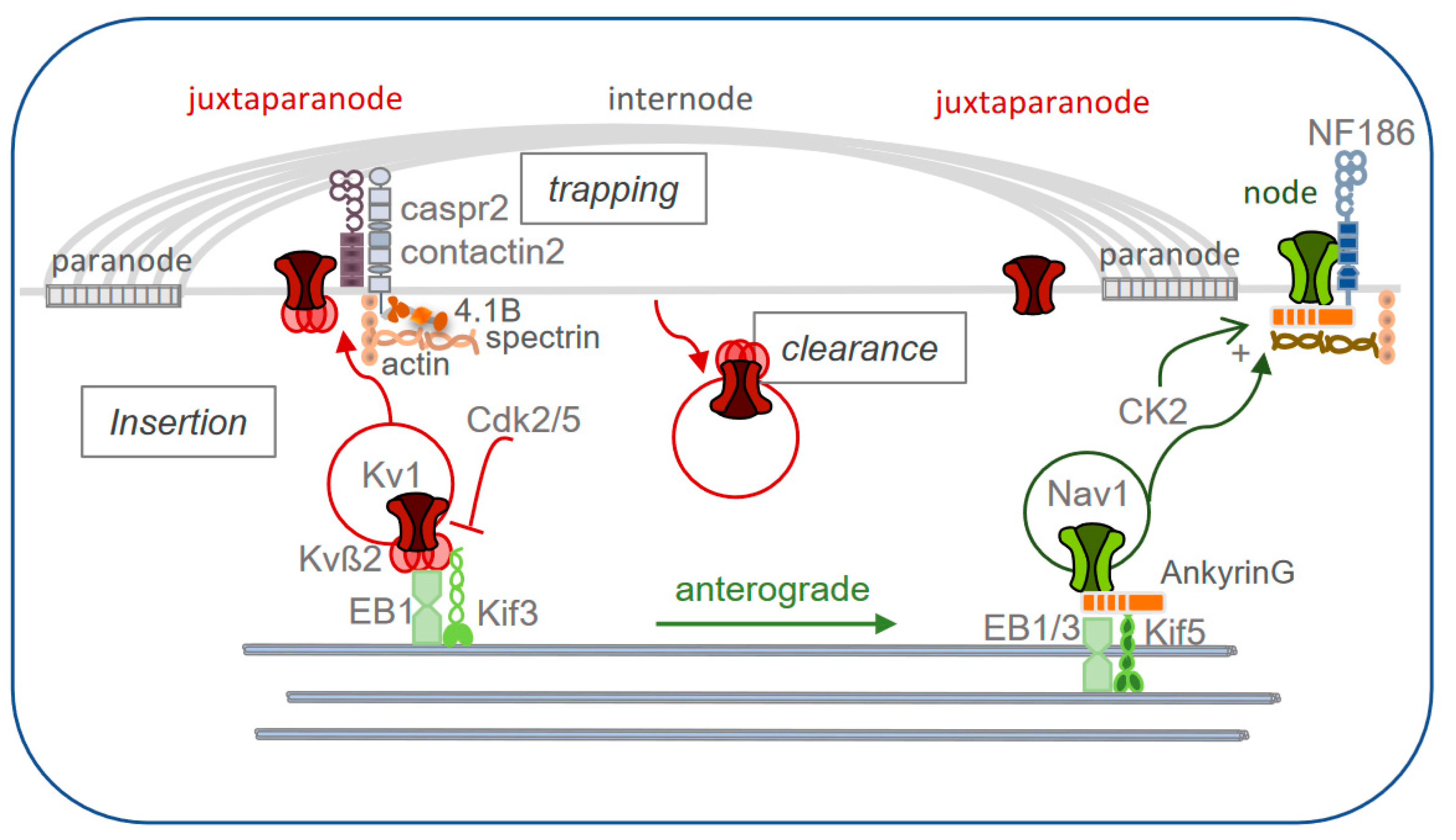
6. Trafficking and Axonal Transport of the Juxtaparanodal Components
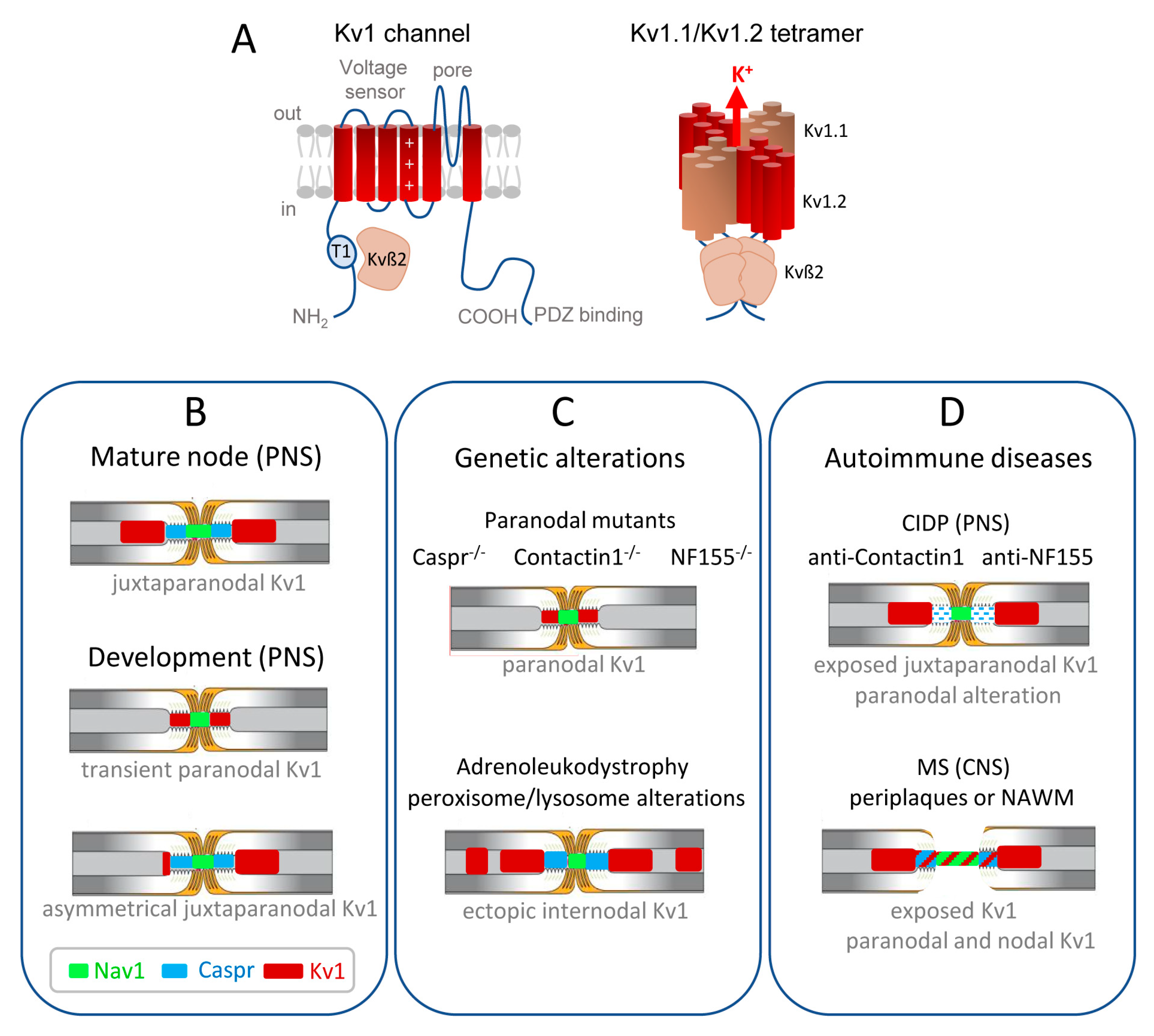
7. The Kv Channels in De- or Dys-Myelinating Neuropathy May Participate to Alterations of Axonal Conduction
8. The Kv1 Complex Components Caspr2 and LGI1 Are Target Antigens in Autoimmune Diseases Associated with Hyperexcitability
9. Conclusions and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Rasband, M.N. The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 2010, 11, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Normand, E.A.; Rasband, M.N. Subcellular patterning: Axonal domains with specialized structure and function. Dev. Cell 2015, 32, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Salzer, J.L. Switching myelination on and off. J. Cell Biol. 2008, 181, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Susuki, K.; Chang, K.J.; Zollinger, D.R.; Liu, Y.; Ogawa, Y.; Eshed-Eisenbach, Y.; Dours-Zimmermann, M.T.; Oses-Prieto, J.A.; Burlingame, A.L.; Seidenbecher, C.I.; et al. Three mechanisms assemble central nervous system nodes of ranvier. Neuron 2013, 78, 469–482. [Google Scholar] [CrossRef]
- Eshed-Eisenbach, Y.; Peles, E. The making of a node: A co-production of neurons and glia. Curr. Opin. Neurobiol. 2013, 23, 1049–1056. [Google Scholar] [CrossRef]
- Rasband, M.N.; Peles, E. The nodes of ranvier: Molecular assembly and maintenance. Cold Spring Harb. Perspect. Biol. 2015, 8, a020495. [Google Scholar] [CrossRef]
- Leterrier, C. The axon initial segment, 50years later: A nexus for neuronal organization and function. Curr. Top. Membr. 2016, 77, 185–233. [Google Scholar]
- Ghosh, A.; Sherman, D.L.; Brophy, P.J. The axonal cytoskeleton and the assembly of nodes of ranvier. Neuroscientist 2018, 24, 104–110. [Google Scholar] [CrossRef]
- Rhodes, K.J.; Strassle, B.W.; Monaghan, M.M.; Bekele-Arcuri, Z.; Matos, M.F.; Trimmer, J.S. Association and colocalization of the kvbeta1 and kvbeta2 beta-subunits with kv1 alpha-subunits in mammalian brain k+ channel complexes. J. Neurosci. 1997, 17, 8246–8258. [Google Scholar] [CrossRef]
- Devaux, J.J.; Kleopa, K.A.; Cooper, E.C.; Scherer, S.S. Kcnq2 is a nodal k+ channel. J. Neurosci. 2004, 24, 1236–1244. [Google Scholar] [CrossRef]
- Trimmer, J.S. Subcellular localization of k+ channels in mammalian brain neurons: Remarkable precision in the midst of extraordinary complexity. Neuron 2015, 85, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Inda, M.C.; DeFelipe, J.; Munoz, A. Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells. Proc. Natl. Acad. Sci. USA 2006, 103, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Kao, T.; Horvath, Z.; Lemos, J.; Sul, J.Y.; Cranstoun, S.D.; Bennett, V.; Scherer, S.S.; Cooper, E.C. A common ankyrin-g-based mechanism retains kcnq and nav channels at electrically active domains of the axon. J. Neurosci. 2006, 26, 2599–2613. [Google Scholar] [CrossRef] [PubMed]
- Devaux, J.; Alcaraz, G.; Grinspan, J.; Bennett, V.; Joho, R.; Crest, M.; Scherer, S.S. Kv3.1b is a novel component of cns nodes. J. Neurosci. 2003, 23, 4509–4518. [Google Scholar] [CrossRef] [PubMed]
- Grundemann, J.; Clark, B.A. Calcium-activated potassium channels at nodes of ranvier secure axonal spike propagation. Cell Rep. 2015, 12, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Hirono, M.; Ogawa, Y.; Misono, K.; Zollinger, D.R.; Trimmer, J.S.; Rasband, M.N.; Misonou, H. Bk channels localize to the paranodal junction and regulate action potentials in myelinated axons of cerebellar purkinje cells. J. Neurosci. 2015, 35, 7082–7094. [Google Scholar] [CrossRef]
- Brohawn, S.G.; Wang, W.W.; Handler, A.; Campbell, E.B.; Schwarz, J.R.; MacKinnon, R. The mechanosensitive ion channel traak is localized to the mammalian node of ranvier. Elife 2019, 8, e50403. [Google Scholar] [CrossRef]
- Kanda, H.; Ling, J.; Tonomura, S.; Noguchi, K.; Matalon, S.; Gu, J.G. Trek-1 and traak are principal k+ channels at the nodes of ranvier for rapid action potential conduction on mammalian myelinated afferent nerves. Neuron 2019, 104, 960–971. [Google Scholar] [CrossRef]
- Battefeld, A.; Tran, B.T.; Gavrilis, J.; Cooper, E.C.; Kole, M.H. Heteromeric kv7.2/7.3 channels differentially regulate action potential initiation and conduction in neocortical myelinated axons. J. Neurosci. 2014, 34, 3719–3732. [Google Scholar] [CrossRef]
- Schwarz, J.R.; Glassmeier, G.; Cooper, E.C.; Kao, T.C.; Nodera, H.; Tabuena, D.; Kaji, R.; Bostock, H. Kcnq channels mediate i(ks), a slow k(+) current regulating excitability in the rat node of ranvier. J. Physiol.-Lond. 2006, 573, 17–34. [Google Scholar] [CrossRef]
- Zhou, D.; Lambert, S.; Malen, P.L.; Carpenter, S.; Boland, L.M.; Bennett, V. Ankyring is required for clustering of voltage-gated na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 1998, 143, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Messing, A.; Chiu, S.Y. Determinants of excitability at transition zones in kv1.1-deficient myelinated nerves. J. Neurosci. 1999, 19, 5768–5781. [Google Scholar] [CrossRef] [PubMed]
- Tomassy, G.S.; Berger, D.R.; Chen, H.H.; Kasthuri, N.; Hayworth, K.J.; Vercelli, A.; Seung, H.S.; Lichtman, J.W.; Arlotta, P. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 2014, 344, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Micheva, K.D.; Wolman, D.; Mensh, B.D.; Pax, E.; Buchanan, J.; Smith, S.J.; Bock, D.D. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. Elife 2016, 5, e15784. [Google Scholar] [CrossRef]
- Stedehouder, J.; Couey, J.J.; Brizee, D.; Hosseini, B.; Slotman, J.A.; Dirven, C.M.F.; Shpak, G.; Houtsmuller, A.B.; Kushner, S.A. Fast-spiking parvalbumin interneurons are frequently myelinated in the cerebral cortex of mice and humans. Cereb Cortex 2017, 27, 5001–5013. [Google Scholar] [CrossRef]
- Kole, M.H.; Letzkus, J.J.; Stuart, G.J. Axon initial segment kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron 2007, 55, 633–647. [Google Scholar] [CrossRef]
- Goldberg, E.M.; Clark, B.D.; Zagha, E.; Nahmani, M.; Erisir, A.; Rudy, B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking gabaergic interneurons. Neuron 2008, 58, 387–400. [Google Scholar] [CrossRef]
- Bos, R.; Harris-Warrick, R.M.; Brocard, C.; Demianenko, L.E.; Manuel, M.; Zytnicki, D.; Korogod, S.M.; Brocard, F. Kv1.2 channels promote nonlinear spiking motoneurons for powering up locomotion. Cell Rep. 2018, 22, 3315–3327. [Google Scholar] [CrossRef]
- Rash, J.E.; Vanderpool, K.G.; Yasumura, T.; Hickman, J.; Beatty, J.T.; Nagy, J.I. Kv1 channels identified in rodent myelinated axons, linked to cx29 in innermost myelin: Support for electrically active myelin in mammalian saltatory conduction. J. Neurophysiol. 2016, 115, 1836–1859. [Google Scholar] [CrossRef]
- Larson, V.A.; Mironova, Y.; Vanderpool, K.G.; Waisman, A.; Rash, J.E.; Agarwal, A.; Bergles, D.E. Oligodendrocytes control potassium accumulation in white matter and seizure susceptibility. Elife 2018, 7, e34829. [Google Scholar] [CrossRef]
- Schirmer, L.; Mobius, W.; Zhao, C.; Cruz-Herranz, A.; Ben Haim, L.; Cordano, C.; Shiow, L.R.; Kelley, K.W.; Sadowski, B.; Timmons, G.; et al. Oligodendrocyte-encoded kir4.1 function is required for axonal integrity. Elife 2018, 7, e34829. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.L.; Hoshi, N. Modulation of kv7 channels and excitability in the brain. Cell. Mol. Life Sci. 2017, 74, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.M.; Weckhuysen, S.; Gorman, K.; King, M.D.; Lerche, H. Genetic potassium channel-associated epilepsies: Clinical review of the kv family. Eur. J. Paediatr. Neurol. 2020, 24, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Vabnick, I.; Trimmer, J.S.; Schwarz, T.L.; Levinson, S.R.; Risal, D.; Shrager, P. Dynamic potassium channel distributions during axonal development prevent aberrant firing patterns. J. Neurosci. 1999, 19, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Poliak, S.; Gollan, L.; Salomon, D.; Berglund, E.O.; Ohara, R.; Ranscht, B.; Peles, E. Localization of caspr2 in myelinated nerves depends on axon-glia interactions and the generation of barriers along the axon. J. Neurosci. 2001, 21, 7568–7575. [Google Scholar] [CrossRef]
- Hivert, B.; Pinatel, D.; Labasque, M.; Tricaud, N.; Goutebroze, L.; Faivre-Sarrailh, C. Assembly of juxtaparanodes in myelinating drg culture: Differential clustering of the kv1/caspr2 complex and scaffolding protein 4.1b. Glia 2016, 64, 840–852. [Google Scholar] [CrossRef]
- Leterrier, C.; Dargent, B. No pasaran! Role of the axon initial segment in the regulation of protein transport and the maintenance of axonal identity. Semin Cell Dev. Biol. 2014, 27, 44–51. [Google Scholar] [CrossRef]
- Hedstrom, K.L.; Ogawa, Y.; Rasband, M.N. Ankyring is required for maintenance of the axon initial segment and neuronal polarity. J. Cell Biol. 2008, 183, 635–640. [Google Scholar] [CrossRef]
- Garrido, J.J.; Giraud, P.; Carlier, E.; Fernandes, F.; Moussif, A.; Fache, M.P.; Debanne, D.; Dargent, B. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 2003, 300, 2091–2094. [Google Scholar] [CrossRef]
- Lemaillet, G.; Walker, B.; Lambert, S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J. Biol. Chem. 2003, 278, 27333–27339. [Google Scholar] [CrossRef]
- Traka, M.; Goutebroze, L.; Denisenko, N.; Bessa, M.; Nifli, A.; Havaki, S.; Iwakura, Y.; Fukamauchi, F.; Watanabe, K.; Soliven, B.; et al. Association of tag-1 with caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J. Cell Biol. 2003, 162, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Poliak, S.; Salomon, D.; Elhanany, H.; Sabanay, H.; Kiernan, B.; Pevny, L.; Stewart, C.L.; Xu, X.; Chiu, S.Y.; Shrager, P.; et al. Juxtaparanodal clustering of shaker-like k+ channels in myelinated axons depends on caspr2 and tag-1. J. Cell Biol. 2003, 162, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Van Wart, A.; Trimmer, J.S.; Matthews, G. Polarized distribution of ion channels within microdomains of the axon initial segment. J. Comp. Neurol. 2007, 500, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Horresh, I.; Trimmer, J.S.; Bredt, D.S.; Peles, E.; Rasband, M.N. Postsynaptic density-93 clusters kv1 channels at axon initial segments independently of caspr2. J. Neurosci. 2008, 28, 5731–5739. [Google Scholar] [CrossRef] [PubMed]
- Duflocq, A.; Chareyre, F.; Giovannini, M.; Couraud, F.; Davenne, M. Characterization of the axon initial segment (ais) of motor neurons and identification of a para-ais and a juxtapara-ais, organized by protein 4.1b. BMC Biol. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Oses-Prieto, J.; Kim, M.Y.; Horresh, I.; Peles, E.; Burlingame, A.L.; Trimmer, J.S.; Meijer, D.; Rasband, M.N. Adam22, a kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons. J. Neurosci. 2010, 30, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, G.; Di Scala, C. Importance of lipids for nervous system integrity: Cooperation between gangliosides and sulfatides in myelin stability. J. Neurosci. 2019, 39, 6218–6220. [Google Scholar] [CrossRef]
- Wang, F.C.; Parcej, D.N.; Dolly, J.O. Alpha subunit compositions of kv1.1-containing k+ channel subtypes fractionated from rat brain using dendrotoxins. Eur. J. Biochem 1999, 263, 230–237. [Google Scholar] [CrossRef]
- Irani, S.R.; Bien, C.G.; Lang, B. Autoimmune epilepsies. Curr. Opin. Neurol. 2010, 24, 146–153. [Google Scholar] [CrossRef]
- Lancaster, E.; Huijbers, M.G.; Bar, V.; Boronat, A.; Wong, A.; Martinez-Hernandez, E.; Wilson, C.; Jacobs, D.; Lai, M.; Walker, R.W.; et al. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann. Neurol. 2011, 69, 303–311. [Google Scholar] [CrossRef]
- Fukata, Y.; Lovero, K.L.; Iwanaga, T.; Watanabe, A.; Yokoi, N.; Tabuchi, K.; Shigemoto, R.; Nicoll, R.A.; Fukata, M. Disruption of lgi1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc. Natl. Acad. Sci. USA 2010, 107, 3799–3804. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Fukata, M.; Fukata, Y. Synaptic plasticity regulated by protein-protein interactions and posttranslational modifications. Int. Rev. Cell Mol. Biol. 2012, 297, 1–43. [Google Scholar] [PubMed]
- Hivert, B.; Marien, L.; Agbam, K.N.; Faivre-Sarrailh, C. Adam22 and adam23 modulate the targeting of the kv1 channel-associated protein lgi1 to the axon initial segment. J. Cell Sci. 2019, 132, jcs219774. [Google Scholar] [CrossRef] [PubMed]
- Seagar, M.; Russier, M.; Caillard, O.; Maulet, Y.; Fronzaroli-Molinieres, L.; De San Feliciano, M.; Boumedine-Guignon, N.; Rodriguez, L.; Zbili, M.; Usseglio, F.; et al. Lgi1 tunes intrinsic excitability by regulating the density of axonal kv1 channels. Proc. Natl. Acad. Sci. USA 2017, 114, 7719–7724. [Google Scholar] [CrossRef]
- Torii, T.; Ogawa, Y.; Liu, C.H.; Ho, T.S.; Hamdan, H.; Wang, C.C.; Oses-Prieto, J.A.; Burlingame, A.L.; Rasband, M.N. Numa1 promotes axon initial segment assembly through inhibition of endocytosis. J. Cell Biol. 2020, 219, e201907048. [Google Scholar] [CrossRef]
- Horresh, I.; Poliak, S.; Grant, S.; Bredt, D.; Rasband, M.N.; Peles, E. Multiple molecular interactions determine the clustering of caspr2 and kv1 channels in myelinated axons. J. Neurosci. 2008, 28, 14213–14222. [Google Scholar] [CrossRef]
- Pinatel, D.; Hivert, B.; Boucraut, J.; Saint-Martin, M.; Rogemond, V.; Zoupi, L.; Karagogeos, D.; Honnorat, J.; Faivre-Sarrailh, C. Inhibitory axons are targeted in hippocampal cell culture by anti-caspr2 autoantibodies associated with limbic encephalitis. Front. Cell Neurosci. 2015, 9, 265. [Google Scholar] [CrossRef]
- Gao, R.; Piguel, N.H.; Melendez-Zaidi, A.E.; Martin-de-Saavedra, M.D.; Yoon, S.; Forrest, M.P.; Myczek, K.; Zhang, G.; Russell, T.A.; Csernansky, J.G.; et al. Cntnap2 stabilizes interneuron dendritic arbors through cask. Mol. Psychiatry 2018, 23, 1832–1850. [Google Scholar] [CrossRef]
- Pinatel, D.; Hivert, B.; Saint-Martin, M.; Noraz, N.; Savvaki, M.; Karagogeos, D.; Faivre-Sarrailh, C. The kv1-associated molecules tag-1 and caspr2 are selectively targeted to the axon initial segment in hippocampal neurons. J. Cell Sci 2017, 130, 2209–2220. [Google Scholar] [CrossRef]
- Kuba, H.; Oichi, Y.; Ohmori, H. Presynaptic activity regulates na(+) channel distribution at the axon initial segment. Nature 2010, 465, 1075–1078. [Google Scholar] [CrossRef]
- Grubb, M.S.; Burrone, J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 2010, 465, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Kaphzan, H.; Buffington, S.A.; Jung, J.I.; Rasband, M.N.; Klann, E. Alterations in intrinsic membrane properties and the axon initial segment in a mouse model of angelman syndrome. J. Neurosci. 2011, 31, 17637–17648. [Google Scholar] [CrossRef] [PubMed]
- Baalman, K.L.; Cotton, R.J.; Rasband, S.N.; Rasband, M.N. Blast wave exposure impairs memory and decreases axon initial segment length. J. Neurotrauma 2013, 30, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Hinman, J.D.; Rasband, M.N.; Carmichael, S.T. Remodeling of the axon initial segment after focal cortical and white matter stroke. Stroke 2013, 44, 182–189. [Google Scholar] [CrossRef]
- Savvaki, M.; Theodorakis, K.; Zoupi, L.; Stamatakis, A.; Tivodar, S.; Kyriacou, K.; Stylianopoulou, F.; Karagogeos, D. The expression of tag-1 in glial cells is sufficient for the formation of the juxtaparanodal complex and the phenotypic rescue of tag-1 homozygous mutants in the cns. J. Neurosci. 2010, 30, 13943–13954. [Google Scholar] [CrossRef]
- Saifetiarova, J.; Liu, X.; Taylor, A.M.; Li, J.; Bhat, M.A. Axonal domain disorganization in caspr1 and caspr2 mutant myelinated axons affects neuromuscular junction integrity, leading to muscle atrophy. J. Neurosci. Res. 2017, 95, 1373–1390. [Google Scholar] [CrossRef]
- Arroyo, E.J.; Xu, Y.T.; Zhou, L.; Messing, A.; Peles, E.; Chiu, S.Y.; Scherer, S.S. Myelinating schwann cells determine the internodal localization of kv1.1, kv1.2, kvbeta2, and caspr. J. Neurocytol. 1999, 28, 333–347. [Google Scholar] [CrossRef]
- Gordon, A.; Adamsky, K.; Vainshtein, A.; Frechter, S.; Dupree, J.L.; Rosenbluth, J.; Peles, E. Caspr and caspr2 are required for both radial and longitudinal organization of myelinated axons. J. Neurosci. 2014, 34, 14820–14826. [Google Scholar] [CrossRef]
- Ivanovic, A.; Horresh, I.; Golan, N.; Spiegel, I.; Sabanay, H.; Frechter, S.; Ohno, S.; Terada, N.; Mobius, W.; Rosenbluth, J.; et al. The cytoskeletal adapter protein 4.1g organizes the internodes in peripheral myelinated nerves. J. Cell Biol. 2012, 196, 337–344. [Google Scholar] [CrossRef]
- Rasband, M.N. Clustered k+ channel complexes in axons. Neurosci. Lett. 2010, 486, 101–106. [Google Scholar] [CrossRef]
- Ozkaynak, E.; Abello, G.; Jaegle, M.; van Berge, L.; Hamer, D.; Kegel, L.; Driegen, S.; Sagane, K.; Bermingham, J.R., Jr.; Meijer, D. Adam22 is a major neuronal receptor for lgi4-mediated schwann cell signaling. J. Neurosci. 2010, 30, 3857–3864. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, J.R., Jr.; Shearin, H.; Pennington, J.; O’Moore, J.; Jaegle, M.; Driegen, S.; van Zon, A.; Darbas, A.; Ozkaynak, E.; Ryu, E.J.; et al. The claw paw mutation reveals a role for lgi4 in peripheral nerve development. Nat. Neurosci. 2006, 9, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Sagane, K.; Hayakawa, K.; Kai, J.; Hirohashi, T.; Takahashi, E.; Miyamoto, N.; Ino, M.; Oki, T.; Yamazaki, K.; Nagasu, T. Ataxia and peripheral nerve hypomyelination in adam22-deficient mice. BMC Neurosci. 2005, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Kegel, L.; Jaegle, M.; Driegen, S.; Aunin, E.; Leslie, K.; Fukata, Y.; Watanabe, M.; Fukata, M.; Meijer, D. Functional phylogenetic analysis of lgi proteins identifies an interaction motif crucial for myelination. Development 2014, 141, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Rios, J.C.; Lu, Y.; Garcia-Fresco, G.P.; Ching, W.; St Martin, M.; Li, J.; Einheber, S.; Chesler, M.; Rosenbluth, J.; et al. Axon-glia interactions and the domain organization of myelinated axons requires neurexin iv/caspr/paranodin. Neuron 2001, 30, 369–383. [Google Scholar] [CrossRef]
- Boyle, M.E.; Berglund, E.O.; Murai, K.K.; Weber, L.; Peles, E.; Ranscht, B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron 2001, 30, 385–397. [Google Scholar] [CrossRef]
- Zonta, B.; Tait, S.; Melrose, S.; Anderson, H.; Harroch, S.; Higginson, J.; Sherman, D.L.; Brophy, P.J. Glial and neuronal isoforms of neurofascin have distinct roles in the assembly of nodes of ranvier in the central nervous system. J. Cell Biol. 2008, 181, 1169–1177. [Google Scholar] [CrossRef]
- Pillai, A.M.; Thaxton, C.; Pribisko, A.L.; Cheng, J.G.; Dupree, J.L.; Bhat, M.A. Spatiotemporal ablation of myelinating glia-specific neurofascin (nfasc nf155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. J. Neurosci. Res. 2009, 87, 1773–1793. [Google Scholar] [CrossRef]
- Denisenko-Nehrbass, N.; Oguievetskaia, K.; Goutebroze, L.; Galvez, T.; Yamakawa, H.; Ohara, O.; Carnaud, M.; Girault, J.A. Protein 4.1b associates with both caspr/paranodin and caspr2 at paranodes and juxtaparanodes of myelinated fibres. Eur. J. Neurosci. 2003, 17, 411–416. [Google Scholar] [CrossRef]
- Ohara, R.; Yamakawa, H.; Nakayama, M.; Ohara, O. Type ii brain 4.1 (4.1b/kiaa0987), a member of the protein 4.1 family, is localized to neuronal paranodes. Brain Res. Mol. Brain Res. 2000, 85, 41–52. [Google Scholar] [CrossRef]
- Maurel, P.; Einheber, S.; Galinska, J.; Thaker, P.; Lam, I.; Rubin, M.B.; Scherer, S.S.; Murakami, Y.; Gutmann, D.H.; Salzer, J.L. Nectin-like proteins mediate axon schwann cell interactions along the internode and are essential for myelination. J. Cell Biol. 2007, 178, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, I.; Adamsky, K.; Eshed, Y.; Milo, R.; Sabanay, H.; Sarig-Nadir, O.; Horresh, I.; Scherer, S.S.; Rasband, M.N.; Peles, E. A central role for necl4 (syncam4) in schwann cell-axon interaction and myelination. Nat. Neurosci. 2007, 10, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Horresh, I.; Bar, V.; Kissil, J.L.; Peles, E. Organization of myelinated axons by caspr and caspr2 requires the cytoskeletal adapter protein 4.1b. J. Neurosci. 2010, 30, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Einheber, S.; Meng, X.; Rubin, M.; Lam, I.; Mohandas, N.; An, X.; Shrager, P.; Kissil, J.; Maurel, P.; Salzer, J.L. The 4.1b cytoskeletal protein regulates the domain organization and sheath thickness of myelinated axons. Glia 2013, 61, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Diaz, C.; Chareyre, F.; Garcia, M.; Devaux, J.; Carnaud, M.; Levasseur, G.; Niwa-Kawakita, M.; Harroch, S.; Girault, J.A.; Giovannini, M.; et al. Protein 4.1b contributes to the organization of peripheral myelinated axons. PLoS ONE 2011, 6, e25043. [Google Scholar] [CrossRef]
- Buttermore, E.D.; Dupree, J.L.; Cheng, J.; An, X.; Tessarollo, L.; Bhat, M.A. The cytoskeletal adaptor protein band 4.1b is required for the maintenance of paranodal axoglial septate junctions in myelinated axons. J. Neurosci. 2011, 31, 8013–8024. [Google Scholar] [CrossRef]
- Yi, C.; McCarty, J.H.; Troutman, S.A.; Eckman, M.S.; Bronson, R.T.; Kissil, J.L. Loss of the putative tumor suppressor band 4.1b/dal1 gene is dispensable for normal development and does not predispose to cancer. Mol. Cell Biol. 2005, 25, 10052–10059. [Google Scholar] [CrossRef]
- D’Este, E.; Kamin, D.; Balzarotti, F.; Hell, S.W. Ultrastructural anatomy of nodes of ranvier in the peripheral nervous system as revealed by sted microscopy. Proc. Natl. Acad. Sci. USA 2017, 114, E191–E199. [Google Scholar] [CrossRef]
- Zhang, C.; Susuki, K.; Zollinger, D.R.; Dupree, J.L.; Rasband, M.N. Membrane domain organization of myelinated axons requires betaii spectrin. J. Cell Biol. 2013, 203, 437–443. [Google Scholar] [CrossRef]
- Nie, D.Y.; Zhou, Z.H.; Ang, B.T.; Teng, F.Y.; Xu, G.; Xiang, T.; Wang, C.Y.; Zeng, L.; Takeda, Y.; Xu, T.L.; et al. Nogo-a at cns paranodes is a ligand of caspr: Possible regulation of k(+) channel localization. EMBO J. 2003, 22, 5666–5678. [Google Scholar] [CrossRef]
- Vacher, H.; Trimmer, J.S. Trafficking mechanisms underlying neuronal voltage-gated ion channel localization at the axon initial segment. Epilepsia 2012, 53 (Suppl. 9), 21–31. [Google Scholar] [CrossRef] [PubMed]
- Dumenieu, M.; Oule, M.; Kreutz, M.R.; Lopez-Rojas, J. The segregated expression of voltage-gated potassium and sodium channels in neuronal membranes: Functional implications and regulatory mechanisms. Front. Cell Neurosci. 2017, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Gu, Y. Clustering and activity tuning of kv1 channels in myelinated hippocampal axons. J. Biol. Chem. 2011, 286, 25835–25847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bekku, Y.; Dzhashiashvili, Y.; Armenti, S.; Meng, X.; Sasaki, Y.; Milbrandt, J.; Salzer, J.L. Assembly and maintenance of nodes of ranvier rely on distinct sources of proteins and targeting mechanisms. Neuron 2012, 73, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Bekku, Y.; Salzer, J.L. Independent anterograde transport and retrograde cotransport of domain components of myelinated axons. J. Cell Biol. 2020, 219, e201906071. [Google Scholar] [CrossRef]
- Barry, J.; Gu, Y.; Jukkola, P.; O’Neill, B.; Gu, H.; Mohler, P.J.; Rajamani, K.T.; Gu, C. Ankyrin-g directly binds to kinesin-1 to transport voltage-gated na+ channels into axons. Dev. Cell 2014, 28, 117–131. [Google Scholar] [CrossRef]
- Thetiot, M.; Freeman, S.A.; Roux, T.; Dubessy, A.L.; Aigrot, M.S.; Rappeneau, Q.; Lejeune, F.X.; Tailleur, J.; Sol-Foulon, N.; Lubetzki, C.; et al. An alternative mechanism of early nodal clustering and myelination onset in gabaergic neurons of the central nervous system. Glia 2020, 68, 1891–1909. [Google Scholar] [CrossRef]
- Faivre-Sarrailh, C.; Gauthier, F.; Denisenko-Nehrbass, N.; Le Bivic, A.; Rougon, G.; Girault, J.A. The glycosylphosphatidyl inositol-anchored adhesion molecule f3/contactin is required for surface transport of paranodin/contactin-associated protein (caspr). J. Cell Biol. 2000, 149, 491–502. [Google Scholar] [CrossRef]
- Leterrier, C.; Vacher, H.; Fache, M.P.; d’Ortoli, S.A.; Castets, F.; Autillo-Touati, A.; Dargent, B. End-binding proteins eb3 and eb1 link microtubules to ankyrin g in the axon initial segment. Proc. Natl. Acad. Sci. USA 2011, 108, 8826–8831. [Google Scholar] [CrossRef]
- Brechet, A.; Fache, M.P.; Brachet, A.; Ferracci, G.; Baude, A.; Irondelle, M.; Pereira, S.; Leterrier, C.; Dargent, B. Protein kinase ck2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin g. J. Cell Biol. 2008, 183, 1101–1114. [Google Scholar] [CrossRef]
- Freal, A.; Rai, D.; Tas, R.P.; Pan, X.; Katrukha, E.A.; van de Willige, D.; Stucchi, R.; Aher, A.; Yang, C.; Altelaar, A.F.M.; et al. Feedback-driven assembly of the axon initial segment. Neuron 2019, 104, 305–321e308. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Jan, Y.N.; Jan, L.Y. A conserved domain in axonal targeting of kv1 (shaker) voltage-gated potassium channels. Science 2003, 301, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zhou, W.; Puthenveedu, M.A.; Xu, M.; Jan, Y.N.; Jan, L.Y. The microtubule plus-end tracking protein eb1 is required for kv1 voltage-gated k+ channel axonal targeting. Neuron 2006, 52, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Chu, P.J.; Lewis, T.L., Jr.; Arnold, D.B. The role of kif5b in axonal localization of kv1 k(+) channels. Eur. J. Neurosci. 2007, 25, 136–146. [Google Scholar] [CrossRef]
- Vacher, H.; Yang, J.W.; Cerda, O.; Autillo-Touati, A.; Dargent, B.; Trimmer, J.S. Cdk-mediated phosphorylation of the kvbeta2 auxiliary subunit regulates kv1 channel axonal targeting. J. Cell Biol. 2011, 192, 813–824. [Google Scholar] [CrossRef]
- Bel, C.; Oguievetskaia, K.; Pitaval, C.; Goutebroze, L.; Faivre-Sarrailh, C. Axonal targeting of caspr2 in hippocampal neurons via selective somatodendritic endocytosis. J. Cell Sci. 2009, 122, 3403–3413. [Google Scholar] [CrossRef]
- Gao, R.; Pratt, C.P.; Yoon, S.; Martin-de-Saavedra, M.D.; Forrest, M.P.; Penzes, P. Cntnap2 is targeted to endosomes by the polarity protein par3. Eur. J. Neurosci. 2020, 51, 1074–1086. [Google Scholar] [CrossRef]
- Nishimura, T.; Kato, K.; Yamaguchi, T.; Fukata, Y.; Ohno, S.; Kaibuchi, K. Role of the par-3-kif3 complex in the establishment of neuronal polarity. Nat. Cell Biol. 2004, 6, 328–334. [Google Scholar] [CrossRef]
- Dupree, J.L.; Girault, J.A.; Popko, B. Axo-glial interactions regulate the localization of axonal paranodal proteins. J. Cell Biol. 1999, 147, 1145–1152. [Google Scholar] [CrossRef]
- Ishibashi, T.; Dupree, J.L.; Ikenaka, K.; Hirahara, Y.; Honke, K.; Peles, E.; Popko, B.; Suzuki, K.; Nishino, H.; Baba, H. A myelin galactolipid, sulfatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation. J. Neurosci. 2002, 22, 6507–6514. [Google Scholar] [CrossRef]
- Mathis, C.; Denisenko-Nehrbass, N.; Girault, J.A.; Borrelli, E. Essential role of oligodendrocytes in the formation and maintenance of central nervous system nodal regions. Development 2001, 128, 4881–4890. [Google Scholar] [PubMed]
- Babbs, C.F.; Shi, R. Subtle paranodal injury slows impulse conduction in a mathematical model of myelinated axons. PLoS ONE 2013, 8, e67767. [Google Scholar] [CrossRef] [PubMed]
- Arancibia-Carcamo, I.L.; Attwell, D. The node of ranvier in cns pathology. Acta Neuropathol. 2014, 128, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Querol, L.; Nogales-Gadea, G.; Rojas-Garcia, R.; Martinez-Hernandez, E.; Diaz-Manera, J.; Suarez-Calvet, X.; Navas, M.; Araque, J.; Gallardo, E.; Illa, I. Antibodies to contactin-1 in chronic inflammatory demyelinating polyneuropathy. Ann. Neurol. 2013, 73, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Labasque, M.; Hivert, B.; Nogales-Gadea, G.; Querol, L.; Illa, I.; Faivre-Sarrailh, C. Specific contactin n-glycans are implicated in neurofascin binding and autoimmune targeting in peripheral neuropathies. J. Biol. Chem. 2014, 289, 7907–7918. [Google Scholar] [CrossRef]
- Manso, C.; Querol, L.; Mekaouche, M.; Illa, I.; Devaux, J.J. Contactin-1 igg4 antibodies cause paranode dismantling and conduction defects. Brain 2016, 139, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Manso, C.; Querol, L.; Lleixa, C.; Poncelet, M.; Mekaouche, M.; Vallat, J.M.; Illa, I.; Devaux, J.J. Anti-neurofascin-155 igg4 antibodies prevent paranodal complex formation in vivo. J. Clin. Investig. 2019, 129, 2222–2236. [Google Scholar] [CrossRef]
- Vallat, J.M.; Yuki, N.; Sekiguchi, K.; Kokubun, N.; Oka, N.; Mathis, S.; Magy, L.; Sherman, D.L.; Brophy, P.J.; Devaux, J.J. Paranodal lesions in chronic inflammatory demyelinating polyneuropathy associated with anti-neurofascin 155 antibodies. Neuromuscul. Disord. 2017, 27, 290–293. [Google Scholar] [CrossRef]
- Querol, L.; Siles, A.M.; Alba-Rovira, R.; Jauregui, A.; Devaux, J.; Faivre-Sarrailh, C.; Araque, J.; Rojas-Garcia, R.; Diaz-Manera, J.; Cortes-Vicente, E.; et al. Antibodies against peripheral nerve antigens in chronic inflammatory demyelinating polyradiculoneuropathy. Sci. Rep. 2017, 7, 14411. [Google Scholar] [CrossRef]
- Lubetzki, C.; Sol-Foulon, N.; Desmazieres, A. Nodes of ranvier during development and repair in the cns. Nat. Rev. Neurol. 2020, 16, 426–439. [Google Scholar] [CrossRef]
- Coman, I.; Aigrot, M.S.; Seilhean, D.; Reynolds, R.; Girault, J.A.; Zalc, B.; Lubetzki, C. Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain 2006, 129, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Howell, O.W.; Palser, A.; Polito, A.; Melrose, S.; Zonta, B.; Scheiermann, C.; Vora, A.J.; Brophy, P.J.; Reynolds, R. Disruption of neurofascin localization reveals early changes preceding demyelination and remyelination in multiple sclerosis. Brain 2006, 129, 3173–3185. [Google Scholar] [CrossRef]
- Kastriti, M.E.; Sargiannidou, I.; Kleopa, K.A.; Karagogeos, D. Differential modulation of the juxtaparanodal complex in multiple sclerosis. Mol. Cell Neurosci. 2015, 67, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Rasband, M.N.; Peles, E.; Trimmer, J.S.; Levinson, S.R.; Lux, S.E.; Shrager, P. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing cns. J. Neurosci. 1999, 19, 7516–7528. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Karimi-Abdolrezaee, S.; Velumian, A.A.; Fehlings, M.G. Functional changes in genetically dysmyelinated spinal cord axons of shiverer mice: Role of juxtaparanodal kv1 family k+ channels. J. Neurophysiol. 2006, 95, 1683–1695. [Google Scholar] [CrossRef]
- Zoupi, L.; Markoullis, K.; Kleopa, K.A.; Karagogeos, D. Alterations of juxtaparanodal domains in two rodent models of cns demyelination. Glia 2013, 61, 1236–1249. [Google Scholar] [CrossRef] [PubMed]
- Jukkola, P.I.; Lovett-Racke, A.E.; Zamvil, S.S.; Gu, C. K+ channel alterations in the progression of experimental autoimmune encephalomyelitis. Neurobiol. Dis. 2012, 47, 280–293. [Google Scholar] [CrossRef][Green Version]
- Judge, S.I.; Bever, C.T., Jr. Potassium channel blockers in multiple sclerosis: Neuronal kv channels and effects of symptomatic treatment. Pharm. Ther. 2006, 111, 224–259. [Google Scholar] [CrossRef]
- Goodman, A.D.; Stone, R.T. Enhancing neural transmission in multiple sclerosis (4-aminopyridine therapy). Neurotherapeutics 2013, 10, 106–110. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Li, D.P.; Chen, S.R.; Pan, H.L. Aminopyridines potentiate synaptic and neuromuscular transmission by targeting the voltage-activated calcium channel beta subunit. J. Biol. Chem. 2009, 284, 36453–36461. [Google Scholar] [CrossRef]
- Hamada, M.S.; Kole, M.H. Myelin loss and axonal ion channel adaptations associated with gray matter neuronal hyperexcitability. J. Neurosci. 2015, 35, 7272–7286. [Google Scholar] [CrossRef] [PubMed]
- Kleinecke, S.; Richert, S.; de Hoz, L.; Brugger, B.; Kungl, T.; Asadollahi, E.; Quintes, S.; Blanz, J.; McGonigal, R.; Naseri, K.; et al. Peroxisomal dysfunctions cause lysosomal storage and axonal kv1 channel redistribution in peripheral neuropathy. Elife 2017, 6, e23332. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.; Dupree, J.L.; Popko, B. Myelin-associated glycoprotein and myelin galactolipids stabilize developing axo-glial interactions. J. Cell Biol. 2002, 156, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Bansal, R.; Hedstrom, K.L.; Pfeiffer, S.E.; Rasband, M.N. Does paranode formation and maintenance require partitioning of neurofascin 155 into lipid rafts? J. Neurosci. 2004, 24, 3176–3185. [Google Scholar] [CrossRef] [PubMed]
- Susuki, K.; Baba, H.; Tohyama, K.; Kanai, K.; Kuwabara, S.; Hirata, K.; Furukawa, K.; Furukawa, K.; Rasband, M.N.; Yuki, N. Gangliosides contribute to stability of paranodal junctions and ion channel clusters in myelinated nerve fibers. Glia 2007, 55, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.; Richards, N.; Schmid, A.B.; Barroso, A.; Zhu, L.; Ivulic, D.; Zhu, N.; Anwandter, P.; Bhat, M.A.; Court, F.A.; et al. Altered potassium channel distribution and composition in myelinated axons suppresses hyperexcitability following injury. Elife 2016, 5, e12661. [Google Scholar] [CrossRef]
- Zenker, J.; Poirot, O.; de Preux Charles, A.S.; Arnaud, E.; Medard, J.J.; Lacroix, C.; Kuntzer, T.; Chrast, R. Altered distribution of juxtaparanodal kv1.2 subunits mediates peripheral nerve hyperexcitability in type 2 diabetes mellitus. J. Neurosci. 2012, 32, 7493–7498. [Google Scholar] [CrossRef]
- Shibuya, K.; Misawa, S.; Arai, K.; Nakata, M.; Kanai, K.; Yoshiyama, Y.; Ito, K.; Isose, S.; Noto, Y.; Nasu, S.; et al. Markedly reduced axonal potassium channel expression in human sporadic amyotrophic lateral sclerosis: An immunohistochemical study. Exp. Neurol. 2011, 232, 149–153. [Google Scholar] [CrossRef]
- Kleopa, K.A.; Elman, L.B.; Lang, B.; Vincent, A.; Scherer, S.S. Neuromyotonia and limbic encephalitis sera target mature shaker-type k+ channels: Subunit specificity correlates with clinical manifestations. Brain 2006, 129, 1570–1584. [Google Scholar] [CrossRef]
- Irani, S.R.; Alexander, S.; Waters, P.; Kleopa, K.A.; Pettingill, P.; Zuliani, L.; Peles, E.; Buckley, C.; Lang, B.; Vincent, A. Antibodies to kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, morvan’s syndrome and acquired neuromyotonia. Brain 2010, 133, 2734–2748. [Google Scholar] [CrossRef]
- Lai, M.; Huijbers, M.G.; Lancaster, E.; Graus, F.; Bataller, L.; Balice-Gordon, R.; Cowell, J.K.; Dalmau, J. Investigation of lgi1 as the antigen in limbic encephalitis previously attributed to potassium channels: A case series. Lancet Neurol. 2010, 9, 776–785. [Google Scholar] [CrossRef]
- Van Sonderen, A.; Petit-Pedrol, M.; Dalmau, J.; Titulaer, M.J. The value of lgi1, caspr2 and voltage-gated potassium channel antibodies in encephalitis. Nat. Rev. Neurol. 2017, 13, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, T.; Fukata, Y.; Yamasaki, M.; Miyazaki, T.; Yokoi, N.; Takashima, H.; Watanabe, M.; Watanabe, O.; Fukata, M. Autoantibodies to epilepsy-related lgi1 in limbic encephalitis neutralize lgi1-adam22 interaction and reduce synaptic ampa receptors. J. Neurosci. 2013, 33, 18161–18174. [Google Scholar] [CrossRef] [PubMed]
- Petit-Pedrol, M.; Sell, J.; Planaguma, J.; Mannara, F.; Radosevic, M.; Haselmann, H.; Ceanga, M.; Sabater, L.; Spatola, M.; Soto, D.; et al. Lgi1 antibodies alter kv1.1 and ampa receptors changing synaptic excitability, plasticity and memory. Brain 2018, 141, 3144–3159. [Google Scholar] [CrossRef] [PubMed]
- Giannoccaro, M.P.; Menassa, D.A.; Jacobson, L.; Coutinho, E.; Prota, G.; Lang, B.; Leite, M.I.; Cerundolo, V.; Liguori, R.; Vincent, A. Behaviour and neuropathology in mice injected with human contactin-associated protein 2 antibodies. Brain 2019, 142, 2000–2012. [Google Scholar] [CrossRef]
- Fernandes, D.; Santos, S.D.; Coutinho, E.; Whitt, J.L.; Beltrao, N.; Rondao, T.; Leite, M.I.; Buckley, C.; Lee, H.K.; Carvalho, A.L. Disrupted ampa receptor function upon genetic- or antibody-mediated loss of autism-associated caspr2. Cereb. Cortex 2019, 29, 4919–4931. [Google Scholar] [CrossRef] [PubMed]
- Dawes, J.M.; Weir, G.A.; Middleton, S.J.; Patel, R.; Chisholm, K.I.; Pettingill, P.; Peck, L.J.; Sheridan, J.; Shakir, A.; Jacobson, L.; et al. Immune or genetic-mediated disruption of caspr2 causes pain hypersensitivity due to enhanced primary afferent excitability. Neuron 2018, 97, 806–822e810. [Google Scholar] [CrossRef]
- Saint-Martin, M.; Pieters, A.; Dechelotte, B.; Malleval, C.; Pinatel, D.; Pascual, O.; Karagogeos, D.; Honnorat, J.; Pellier-Monnin, V.; Noraz, N. Impact of anti-caspr2 autoantibodies from patients with autoimmune encephalitis on caspr2/tag-1 interaction and kv1 expression. J. Autoimmun. 2019, 103, 102284. [Google Scholar] [CrossRef]
- Bonetto, G.; Hivert, B.; Goutebroze, L.; Karagogeos, D.; Crepel, V.; Faivre-Sarrailh, C. Selective axonal expression of the kv1 channel complex in pre-myelinated gabaergic hippocampal neurons. Front. Cell Neurosci. 2019, 13, 222. [Google Scholar] [CrossRef]
- Irani, S.R.; Pettingill, P.; Kleopa, K.A.; Schiza, N.; Waters, P.; Mazia, C.; Zuliani, L.; Watanabe, O.; Lang, B.; Buckley, C.; et al. Morvan syndrome: Clinical and serological observations in 29 cases. Ann. Neurol. 2012, 72, 241–255. [Google Scholar] [CrossRef]
- Klein, C.J.; Lennon, V.A.; Aston, P.A.; McKeon, A.; Pittock, S.J. Chronic pain as a manifestation of potassium channel-complex autoimmunity. Neurology 2012, 79, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Van Sonderen, A.; Arino, H.; Petit-Pedrol, M.; Leypoldt, F.; Kortvelyessy, P.; Wandinger, K.P.; Lancaster, E.; Wirtz, P.W.; Schreurs, M.W.; Sillevis Smitt, P.A.; et al. The clinical spectrum of caspr2 antibody-associated disease. Neurology 2016, 87, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.R.; Dalmau, J.; Lancaster, E. Mechanisms of caspr2 antibodies in autoimmune encephalitis and neuromyotonia. Ann. Neurol. 2018, 83, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Raab-Graham, K.F.; Haddick, P.C.; Jan, Y.N.; Jan, L.Y. Activity- and mtor-dependent suppression of kv1.1 channel mrna translation in dendrites. Science 2006, 314, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Wu, K.; Dong, Y.; Zhou, Y.; Zhang, J.; Jiang, F.; Hu, W.P.; Li, J.D. Hyperactive akt-mtor pathway as a therapeutic target for pain hypersensitivity in cntnap2-deficient mice. Neuropharmacology 2020, 165, 107816. [Google Scholar] [CrossRef]
- Maas, D.A.; Eijsink, V.D.; Spoelder, M.; van Hulten, J.A.; De Weerd, P.; Homberg, J.R.; Valles, A.; Nait-Oumesmar, B.; Martens, G.J.M. Interneuron hypomyelination is associated with cognitive inflexibility in a rat model of schizophrenia. Nat. Commun. 2020, 11, 2329. [Google Scholar] [CrossRef]
- Benamer, N.; Vidal, M.; Angulo, M.C. The cerebral cortex is a substrate of multiple interactions between gabaergic interneurons and oligodendrocyte lineage cells. Neurosci. Lett. 2020, 715, 134615. [Google Scholar] [CrossRef]
- Freeman, S.A.; Desmazieres, A.; Simonnet, J.; Gatta, M.; Pfeiffer, F.; Aigrot, M.S.; Rappeneau, Q.; Guerreiro, S.; Michel, P.P.; Yanagawa, Y.; et al. Acceleration of conduction velocity linked to clustering of nodal components precedes myelination. Proc. Natl. Acad. Sci. USA 2015, 112, E321–E328. [Google Scholar] [CrossRef]
- Dubessy, A.L.; Mazuir, E.; Rappeneau, Q.; Ou, S.; Abi Ghanem, C.; Piquand, K.; Aigrot, M.S.; Thetiot, M.; Desmazieres, A.; Chan, E.; et al. Role of a contactin multi-molecular complex secreted by oligodendrocytes in nodal protein clustering in the cns. Glia 2019, 67, 2248–2263. [Google Scholar] [CrossRef]
- Somjen, G.G. Ion regulation in the brain: Implications for pathophysiology. Neuroscientist 2002, 8, 254–267. [Google Scholar] [CrossRef]
- Nicholas, R.; Magliozzi, R.; Campbell, G.; Mahad, D.; Reynolds, R. Temporal lobe cortical pathology and inhibitory gaba interneuron cell loss are associated with seizures in multiple sclerosis. Mult. Scler. 2016, 22, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Uribe-San-Martin, R.; Ciampi-Diaz, E.; Suarez-Hernandez, F.; Vasquez-Torres, M.; Godoy-Fernandez, J.; Carcamo-Rodriguez, C. Prevalence of epilepsy in a cohort of patients with multiple sclerosis. Seizure 2014, 23, 81–83. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinatel, D.; Faivre-Sarrailh, C. Assembly and Function of the Juxtaparanodal Kv1 Complex in Health and Disease. Life 2021, 11, 8. https://doi.org/10.3390/life11010008
Pinatel D, Faivre-Sarrailh C. Assembly and Function of the Juxtaparanodal Kv1 Complex in Health and Disease. Life. 2021; 11(1):8. https://doi.org/10.3390/life11010008
Chicago/Turabian StylePinatel, Delphine, and Catherine Faivre-Sarrailh. 2021. "Assembly and Function of the Juxtaparanodal Kv1 Complex in Health and Disease" Life 11, no. 1: 8. https://doi.org/10.3390/life11010008
APA StylePinatel, D., & Faivre-Sarrailh, C. (2021). Assembly and Function of the Juxtaparanodal Kv1 Complex in Health and Disease. Life, 11(1), 8. https://doi.org/10.3390/life11010008




