Organic Matter in Cometary Environments
Abstract
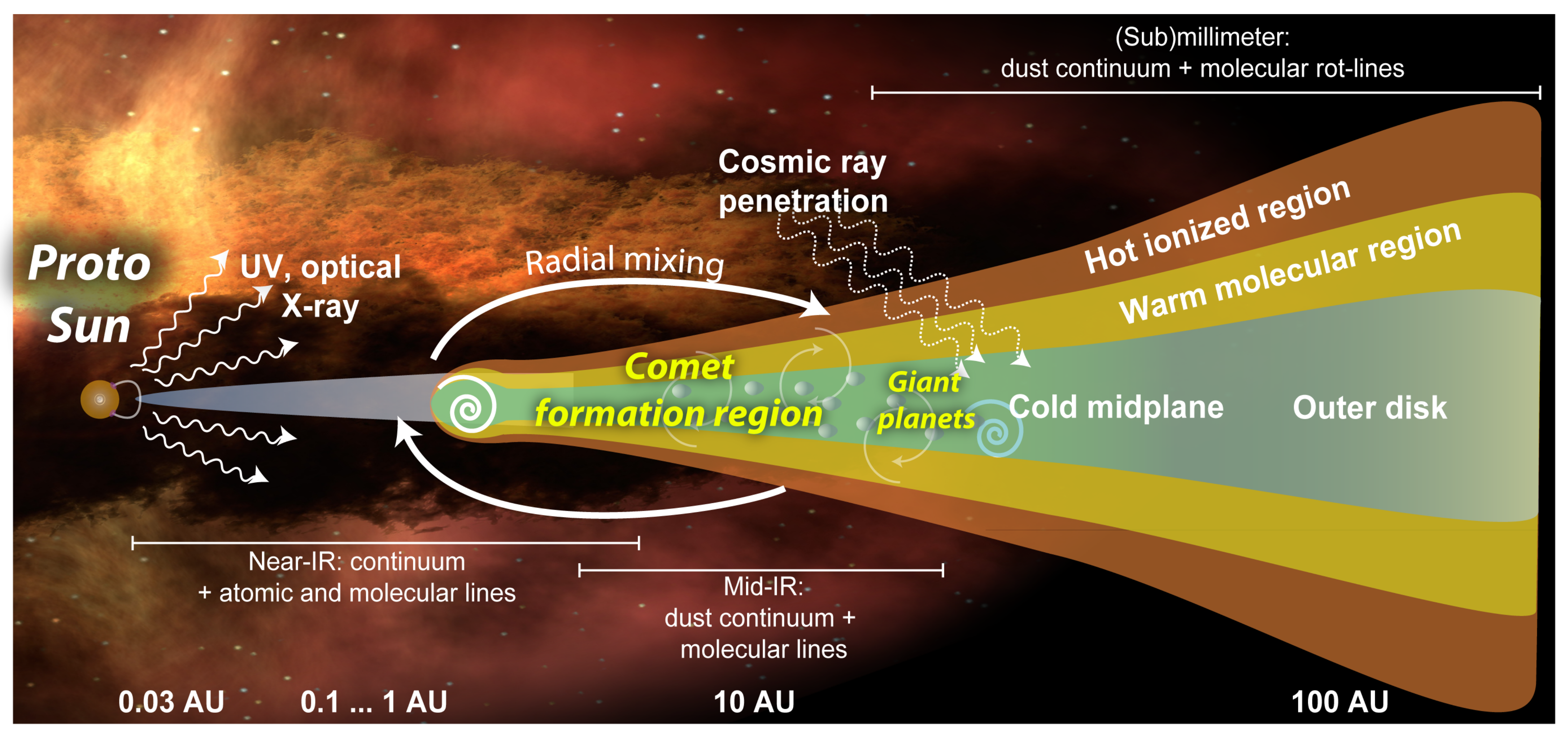
1. Introduction
2. Tools for Studying Cometary Organic Matter
2.1. Remote Sensing
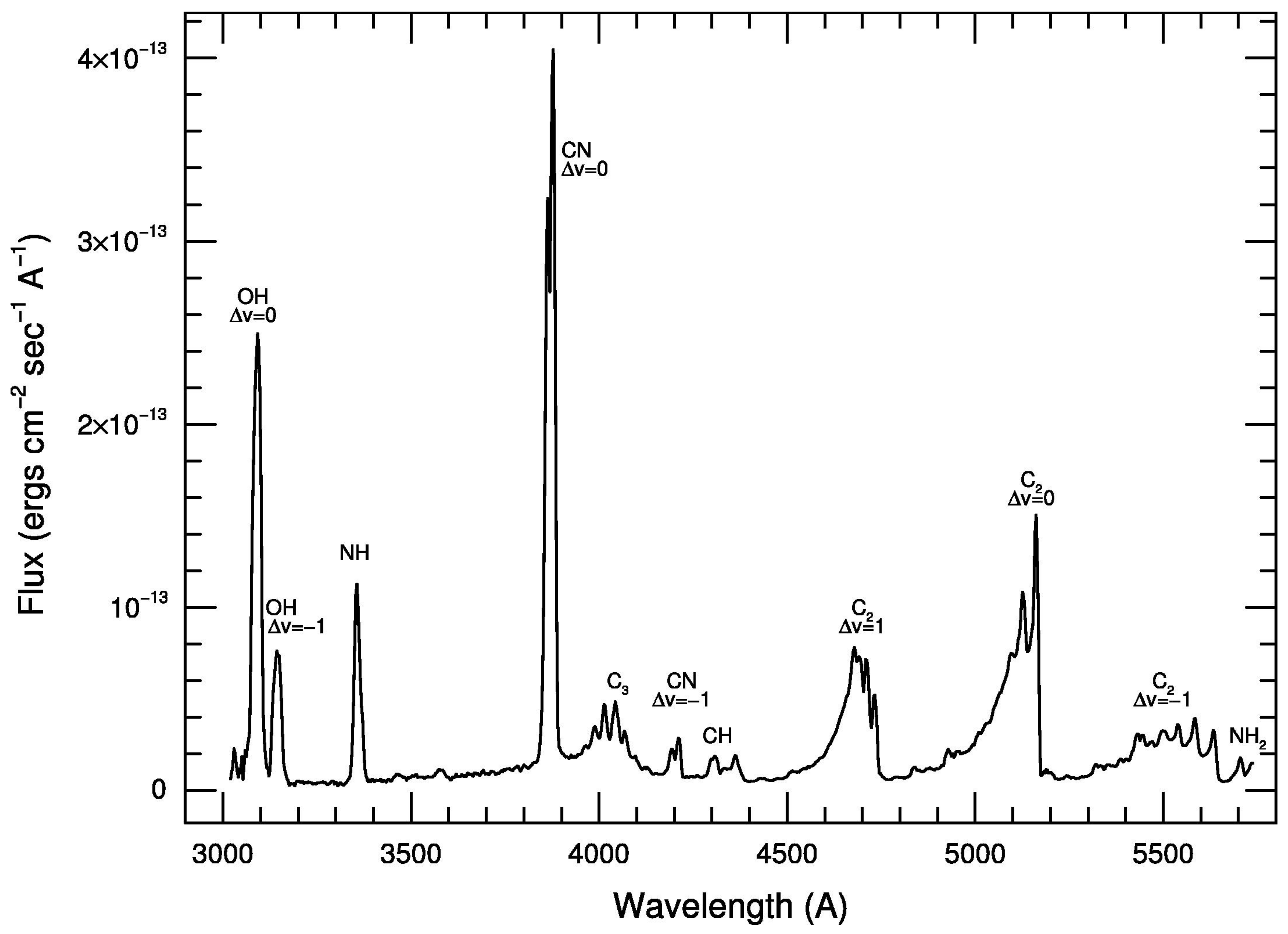
2.1.1. Optical
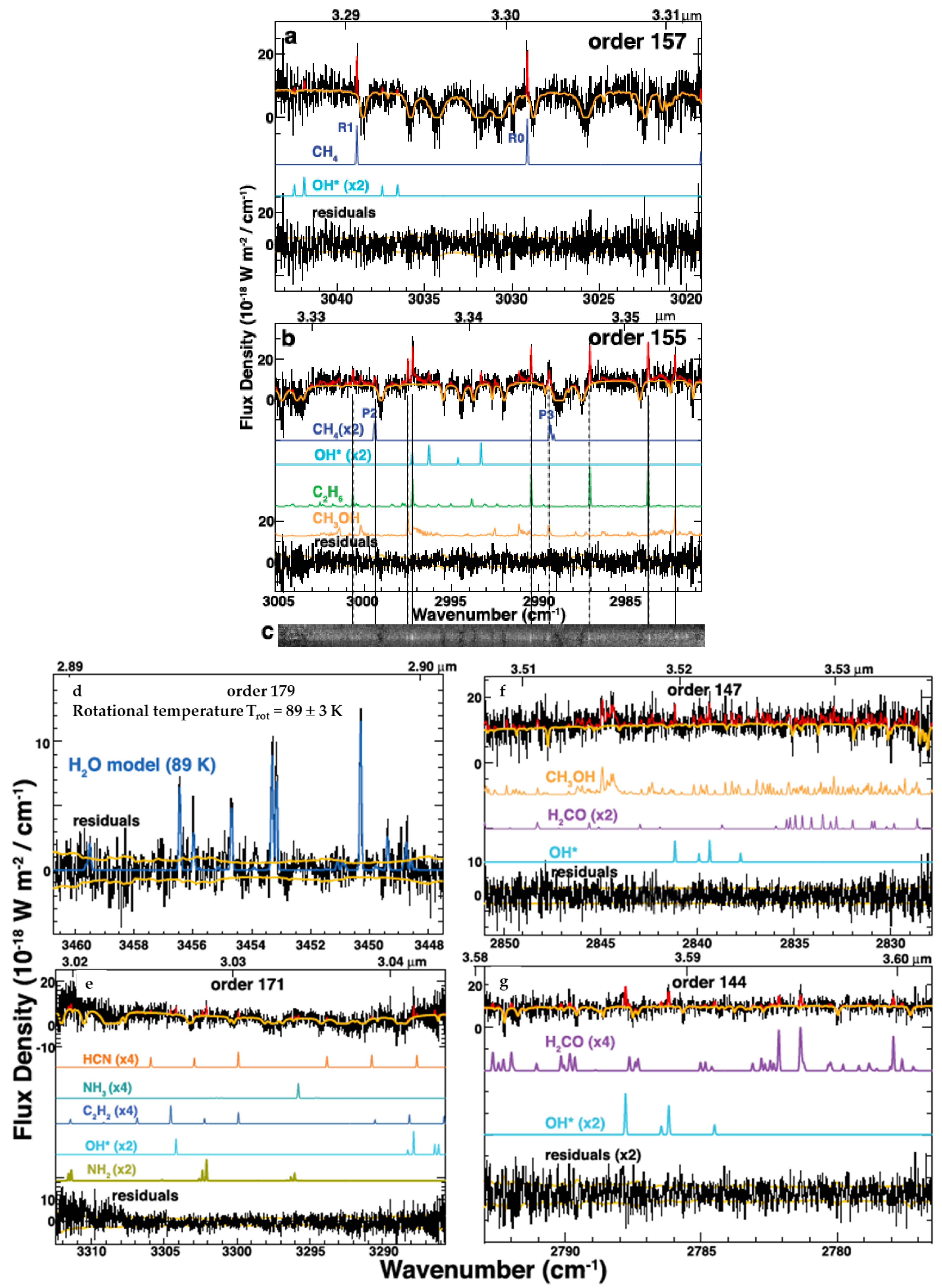
2.1.2. Near Infrared (NIR)
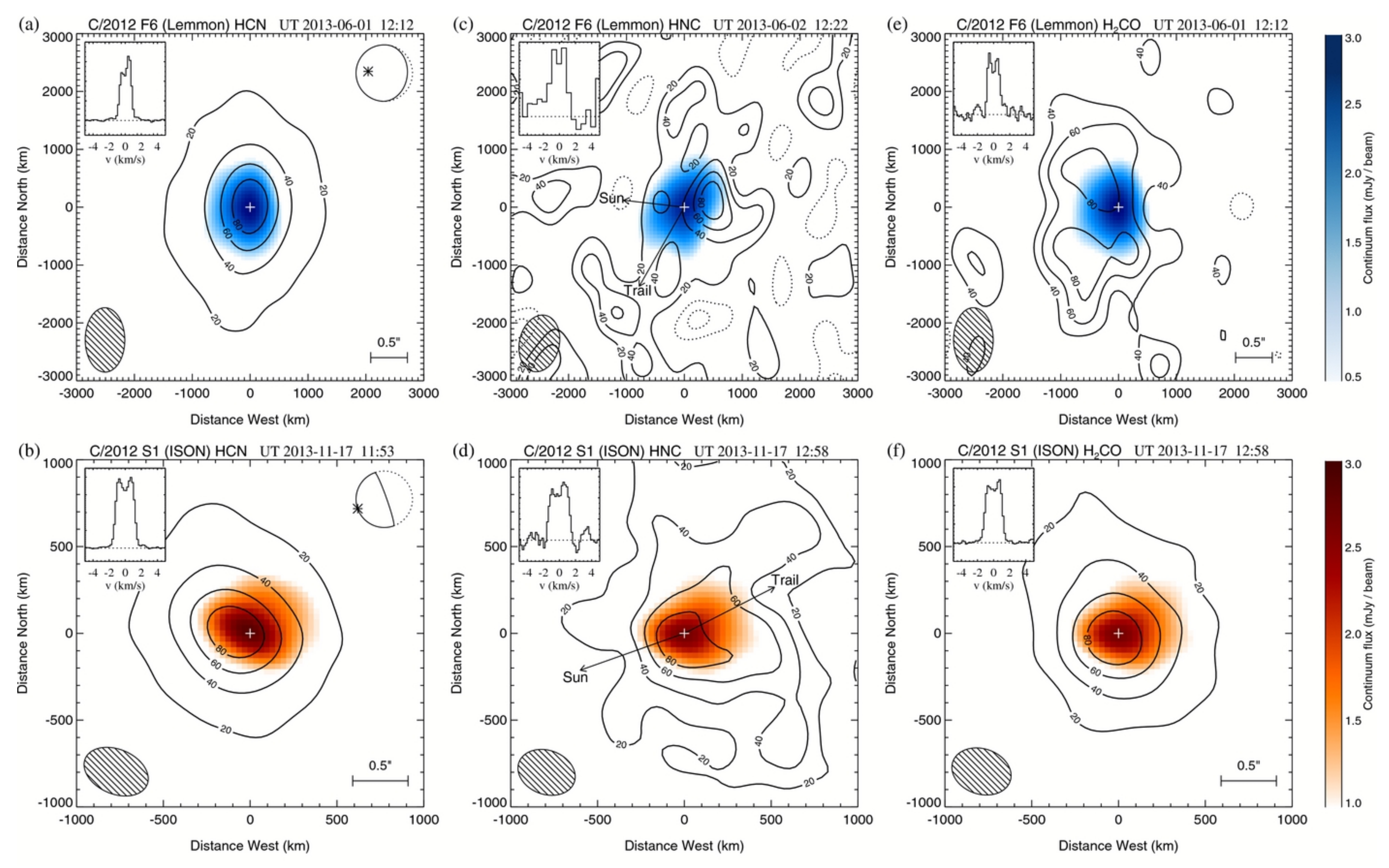
2.1.3. Mm/Sub-mm
2.2. Spacecraft Missions
2.2.1. The Halley Armada
2.2.2. Stardust
2.2.3. Rosetta
3. Results for Specific Species
3.1. HCN and HNC
3.2. HCO
3.3. CHOH
3.4. CH
3.5. CH
3.6. CH
3.7. Other Organic Molecules
3.8. Complex Hydrocarbons, PAHs, and CHON Particles
3.9. Organic Tracers
3.9.1. CN
3.9.2. C
3.9.3. C
3.9.4. CH
4. Implications
5. Future Directions for the Study of Cometary Organic Matter
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Comet | Abundance (X/HO in %) | ||||||
|---|---|---|---|---|---|---|---|
| HCN | HNC | CH | CH | CH | HCO | CHOH | |
| 2P/Encke [110] | 0.12 | 0.11 | 0.04 | 0.27 | 0.87 | ||
| 21P/Giacobini-Zinner [111,114] | 0.15 | 1.1 | 0.23 | 2.3 | |||
| 45P/Honda-Mrkos-Pajdušáková [52,113] | 0.05–0.15 | 0.08 | 0.79–1.0 | 0.52–0.81 | 0.14–0.36 | 3.6–4.5 | |
| 46P/Wirtanen [154] | 0.09 | ||||||
| 67P/Churymov-Gerasimenko [108,127,137,153] | 0.14 | 0.34–0.47 | 0.29 | 0.32 | 0.21 | ||
| 252P/LINEAR [155] | 0.23 | 0.95 | 4.9 | ||||
| C/2012 F6 (Lemmon) [156] | 0.06 | 0.003 | 0.1 | 0.97 | |||
| C/2012 K1 (PanSTARRS) [50,157] | 0.14 | 0.46 | 0.87 | 1.7–2.7 | |||
| C/2012 S1 (ISON) [75,156,158] | 0.11–0.26 | 0.05 | 0.21 | 0.33 | 0.29 | 0.23–1.1 | 0.49–1.4 |
| C/2013 R1 (Lovejoy) [159] | 0.2 | ||||||
| C/2013 V5 (Oukaimeden) [107] | 0.08 | 0.07 | 0.28 | 0.2 | 0.13 | 0.95 | |
| C/2014 Q2 (Lovejoy) [159,160] | 0.09 | 0.2 | 2.2 | ||||
| C/2017 E4 (Lovejoy) [161] | 0.17 | 0.14 | 0.34 | 0.39 | 0.36 | 1.62 | |
References
- Altwegg, K.; Balsiger, H.; Berthelier, J.J.; Bieler, A.; Calmonte, U.; De Keyser, J.; Fiethe, B.; Fuselier, S.A.; Gasc, S.; Gombosi, T.I.; et al. D2O and HDS in the coma of 67P/Churyumov-Gerasimenko. Philos. Trans. R. Soc. Lond. Ser. A 2017, 375, 20160253. [Google Scholar] [CrossRef]
- Eistrup, C.; Walsh, C.; van Dishoeck, E.F. Cometary compositions compared with protoplanetary disk midplane chemical evolution. An emerging chemical evolution taxonomy for comets. Astron. Astrophys. 2019, 629, A84. [Google Scholar] [CrossRef]
- Whipple, F.L. A comet model. I. The acceleration of Comet Encke. Astorphys. J. 1950, 111, 375–394. [Google Scholar] [CrossRef]
- Fulle, M.; Blum, J.; Green, S.F.; Gundlach, B.; Herique, A.; Moreno, F.; Mottola, S.; Rotundi, A.; Snodgrass, C. The refractory-to-ice mass ratio in comets. Mon. Not. R. Astron. Soc. 2019, 482, 3326–3340. [Google Scholar] [CrossRef]
- Mumma, M.J.; Charnley, S.B. The Chemical Composition of Comets—Emerging Taxonomies and Natal Heritage. Annu. Rev. Astonomy Astrophys. 2011, 49, 471–524. [Google Scholar] [CrossRef]
- Cochran, A.L.; Levasseur-Regourd, A.C.; Cordiner, M.; Hadamcik, E.; Lasue, J.; Gicquel, A.; Schleicher, D.G.; Charnley, S.B.; Mumma, M.J.; Paganini, L.; et al. The Composition of Comets. Space Sci. Rev. 2015, 197, 9–46. [Google Scholar] [CrossRef]
- Bieler, A.; Altwegg, K.; Balsiger, H.; Bar-Nun, A.; Berthelier, J.J.; Bochsler, P.; Briois, C.; Calmonte, U.; Combi, M.; de Keyser, J.; et al. Abundant molecular oxygen in the coma of comet 67P/Churyumov-Gerasimenko. Nature 2015, 526, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Dello Russo, N.; Kawakita, H.; Vervack, R.J.; Weaver, H.A. Emerging trends and a comet taxonomy based on the volatile chemistry measured in thirty comets with high-resolution infrared spectroscopy between 1997 and 2013. Icarus 2016, 278, 301–332. [Google Scholar] [CrossRef]
- Helbert, J.; Rauer, H.; Boice, D.C.; Huebner, W.F. The chemistry of C2 and C3 in the coma of Comet C/1995 O1 (Hale-Bopp) at heliocentric distances rh≥ 2.9 AU. Astron. Astrophys. 2005, 442, 1107–1120. [Google Scholar] [CrossRef]
- Biver, N.; Bockelée-Morvan, D.; Moreno, R.; Crovisier, J.; Colom, P.; Lis, D.C.; Sandqvist, A.; Boissier, J.; Despois, D.; Milam, S.N. Ethyl alcohol and sugar in comet C/2014 Q2 (Lovejoy). Sci. Adv. 2015, 1, 1500863. [Google Scholar] [CrossRef]
- Rivilla, V.M.; Drozdovskaya, M.N.; Altwegg, K.; Caselli, P.; Beltrán, M.T.; Fontani, F.; van der Tak, F.F.S.; Cesaroni, R.; Vasyunin, A.; Rubin, M.; et al. ALMA and ROSINA detections of phosphorus-bearing molecules: The interstellar thread between star-forming regions and comets. Mon. Not. R. Astron. Soc. 2020, 492, 1180–1198. [Google Scholar] [CrossRef]
- Altwegg, K.; Balsiger, H.; Bar-Nun, A.; Berthelier, J.J.; Bieler, A.; Bochsler, P.; Briois, C.; Calmonte, U.; Combi, M.R.; Cottin, H.; et al. Prebiotic chemicals–amino acid and phosphorus–in the coma of comet 67P/Churyumov-Gerasimenko. Sci. Adv. 2016, 2, e1600285. [Google Scholar] [CrossRef] [PubMed]
- Combes, M.; Crovisier, J.; Encrenaz, T.; Moroz, V.I.; Bibring, J.P. The 2.5–12 micron spectrum of Comet Halley from the IKS-VEGA Experiment. Icarus 1988, 76, 404–436. [Google Scholar] [CrossRef]
- Moreels, G.; Clairemidi, J.; Hermine, P.; Brechignac, P.; Rousselot, P. Detection of a polycyclic aromatic molecule in comet P/Halley. Astron. Astrophys. 1994, 282, 643–656. [Google Scholar]
- Lisse, C.M.; VanCleve, J.; Adams, A.C.; A’Hearn, M.F.; Fernández, Y.R.; Farnham, T.L.; Armus, L.; Grillmair, C.J.; Ingalls, J.; Belton, M.J.S.; et al. Spitzer Spectral Observations of the Deep Impact Ejecta. Science 2006, 313, 635–640. [Google Scholar] [CrossRef]
- Sandford, S.A.; Aléon, J.; Alexand er, C.M.O.D.; Araki, T.; Bajt, S.; Baratta, G.A.; Borg, J.; Bradley, J.P.; Brownlee, D.E.; Brucato, J.R.; et al. Organics Captured from Comet 81P/Wild 2 by the Stardust Spacecraft. Science 2006, 314, 1720. [Google Scholar] [CrossRef]
- Clairemidi, J.; Bréchignac, P.; Moreels, G.; Pautet, D. Tentative identification of pyrene as a polycyclic aromatic molecule in UV spectra of comet P/Halley: An emission from 368 to 384 nm. Planet. Space Sci. 2004, 52, 761–772. [Google Scholar] [CrossRef]
- Clairemidi, J.; Moreels, G.; Mousis, O.; Bréchignac, P. Identification of anthracene in Comet 1P/Halley. Astron. Astrophys. 2008, 492, 245–250. [Google Scholar] [CrossRef]
- Ootsubo, T.; Kawakita, H.; Shinnaka, Y.; Watanabe, J.I.; Honda, M. Unidentified infrared emission features in mid-infrared spectrum of comet 21P/Giacobini-Zinner. Icarus 2020, 338, 113450. [Google Scholar] [CrossRef]
- Altwegg, K.; Balsiger, H.; Berthelier, J.J.; Bieler, A.; Calmonte, U.; Fuselier, S.A.; Goesmann, F.; Gasc, S.; Gombosi, T.I.; Le Roy, L.; et al. Organics in comet 67P—A first comparative analysis of mass spectra from ROSINA-DFMS, COSAC and Ptolemy. Mon. Not. R. Astron. Soc. 2017, 469, S130–S141. [Google Scholar] [CrossRef]
- Clark, B.C.; Mason, L.W.; Kissel, J. Systematics of the CHON and Other Light Element Particle Populations in Comet p/Halley. Astron. Astrophys. 1987, 187, 779. [Google Scholar]
- Combi, M.R.; Fink, U. A Critical Study of Molecular Photodissociation and CHON Grain Sources for Cometary C 2. Astrophys. J. 1997, 484, 879. [Google Scholar] [CrossRef]
- McKay, A.J.; Chanover, N.J.; DiSanti, M.A.; Morgenthaler, J.P.; Cochran, A.L.; Harris, W.M.; Russo, N.D. Rotational variation of daughter species production rates in Comet 103P/Hartley: Implications for the progeny of daughter species and the degree of chemical heterogeneity. Icarus 2014, 231, 193–205. [Google Scholar] [CrossRef]
- Milam, S.N.; Remijan, A.J.; Womack, M.; Abrell, L.; Ziurys, L.M.; Wyckoff, S.; Apponi, A.J.; Friedel, D.N.; Snyder, L.E.; Veal, J.M.; et al. Formaldehyde in Comets C/1995 O1 (Hale-Bopp), C/2002 T7 (LINEAR), and C/2001 Q4 (NEAT): Investigating the Cometary Origin of H2CO. Astorphysical J. 2006, 649, 1169–1177. [Google Scholar] [CrossRef]
- Donati, G.B. Schreiben des Herrn Prof. Donati an den Herausgeber. Astron. Nachrichten 1864, 62, 375. [Google Scholar] [CrossRef]
- Huggins, W. Further Observations on the Spectra of Some of the Stars and Nebulae, with an Attempt to Determine Therefrom Whether These Bodies are Moving towards or from the Earth, Also Observations on the Spectra of the Sun and of Comet II., 1868. Philos. Trans. R. Soc. Lond. Ser. I 1868, 158, 529–564. [Google Scholar]
- Draper, H. Note on photographs of the spectrum of comet b 1881. Obs 1881, 4, 252–253. [Google Scholar]
- Farnham, T.L.; Schleicher, D.G.; A’Hearn, M.F. The HB Narrowband Comet Filters: Standard Stars and Calibrations. Icarus 2000, 147, 180–204. [Google Scholar] [CrossRef]
- Cochran, A.L.; Barker, E.S.; Gray, C.L. Thirty years of cometary spectroscopy from McDonald Observatory. Icarus 2012, 218, 144–168. [Google Scholar] [CrossRef]
- A’Hearn, M.F.; Millis, R.L.; Schleicher, D.G.; Osip, D.J.; Birch, P.V. The ensemble properties of comets: Results from narrowband photometry of 85 comets, 1976–1992. Icarus 1995, 118, 223–270. [Google Scholar] [CrossRef]
- Fink, U. A taxonomic survey of comet composition 1985–2004 using CCD spectroscopy. Icarus 2009, 201, 311–334. [Google Scholar] [CrossRef]
- Schleicher, D.; Bair, A. Chemical and physical properties of comets in the Lowell database: Results from 35 years of narrow-band photometry. In Asteroids, Comets, Meteors 2014; Muinonen, K., Penttilä, A., Granvik, M., Virkki, A., Fedorets, G., Wilkman, O., Kohout, T., Eds.; Asteroids, Comets, Meteors Meeting: Helsinki, Finland, 2014; p. 475. [Google Scholar]
- Weiler, M. The chemistry of C3 and C2 in cometary comae. I. Current models revisited. Astron. Astrophys. 2012, 538, A149. [Google Scholar] [CrossRef]
- Wyckoff, S.; Lindholm, E.; Wehinger, P.A.; Peterson, B.A.; Zucconi, J.M.; Festou, M.C. The C-12/C-13 abundance ratio in Comet Halley. Astrophys. J. 1989, 339, 488–500. [Google Scholar] [CrossRef]
- Manfroid, J.; Jehin, E.; Hutsemékers, D.; Cochran, A.; Zucconi, J.M.; Arpigny, C.; Schulz, R.; Stüwe, J.A.; Ilyin, I. The CN isotopic ratios in comets. Astron. Astrophys. 2009, 503, 613–624. [Google Scholar] [CrossRef]
- Wyckoff, S.; Kleine, M.; Peterson, B.A.; Wehinger, P.A.; Ziurys, L.M. Carbon Isotope Abundances in Comets. Astrophys. J. 2000, 535, 991–999. [Google Scholar] [CrossRef]
- Rousselot, P.; Pirali, O.; Jehin, E.; Vervloet, M.; Hutsemékers, D.; Manfroid, J.; Cordier, D.; Martin-Drumel, M.A.; Gruet, S.; Arpigny, C.; et al. Toward a Unique Nitrogen Isotopic Ratio in Cometary Ices. Astrophys. J. Lett. 2014, 780, L17. [Google Scholar] [CrossRef]
- Shinnaka, Y.; Kawakita, H.; Kobayashi, H.; Nagashima, M.; Boice, D.C. 14NH2/15NH2 Ratio in Comet C/2012 S1 (ISON) Observed during its Outburst in 2013 November. Astrophys. J. Lett. 2014, 782, L16. [Google Scholar] [CrossRef]
- Shinnaka, Y.; Kawakita, H.; Jehin, E.; Decock, A.; Hutsemékers, D.; Manfroid, J.; Arai, A. Nitrogen isotopic ratios of NH2 in comets: Implication for 15N-fractionation in cometary ammonia. Mon. Not. R. Astron. Soc. 2016, 462, S195–S209. [Google Scholar] [CrossRef]
- Mumma, M.J.; Weaver, H.A.; Larson, H.P.; Williams, M.; Davis, D.S. Detection of water vapor in Halley’s comet. Science 1986, 232, 1523–1528. [Google Scholar] [CrossRef]
- Dello Russo, N.; DiSanti, M.A.; Mumma, M.J.; Magee-Sauer, K.; Rettig, T.W. Carbonyl Sulfide in Comets C/1996 B2 (Hyakutake) and C/1995 O1 (Hale-Bopp): Evidence for an Extended Source in Hale-Bopp. Icarus 1998, 135, 377–388. [Google Scholar] [CrossRef]
- Mumma, M.J.; Dello Russo, N.; DiSanti, M.A.; Magee-Sauer, K.; Novak, R.E.; Brittain, S.; Rettig, T.; McLean, I.S.; Reuter, D.C.; Xu, L.H. Organic Composition of C/1999 S4 (LINEAR): A Comet Formed Near Jupiter? Science 2001, 292, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.E. Detection of OH at 18-CENTIMETER Wavelength in Comet Kohoutek (1973f). Astrophys. J. 1974, 189, L137–L139. [Google Scholar] [CrossRef]
- Huebner, W.F.; Snyder, L.E.; Buhl, D. HCN Radio Emission from Comet Kohoutek (1973f). Icarus 1974, 23, 580–584. [Google Scholar] [CrossRef]
- Biver, N.; Bockelée-Morvan, D.; Crovisier, J.; Davies, J.K.; Matthews, H.E.; Wink, J.E.; Rauer, H.; Colom, P.; Dent, W.R.F.; Despois, D.; et al. Spectroscopic Monitoring of Comet C/1996 B2 (Hyakutake) with the JCMT and IRAM Radio Telescopes. Astron. J. 1999, 118, 1850. [Google Scholar] [CrossRef]
- Biver, N.; Bockelée-Morvan, D.; Colom, P.; Crovisier, J.; Henry, F.; Lellouch, E.; Winnberg, A.; Johansson, L.; Gunnarsson, M.; Rickman, H.; et al. The 1995–2002 Long-Term Monitoring of Comet C/1995 O1 (Hale-Bopp) at Radio Wavelength. Earth Moon Planets 2002, 90, 5–14. [Google Scholar] [CrossRef]
- Biver, N.; Bockelée-Morvan, D.; Crovisier, J.; Lis, D.C.; Moreno, R.; Colom, P.; Henry, F.; Herpin, F.; Paubert, G.; Womack, M. Radio wavelength molecular observations of comets C/1999 T1 (McNaught-Hartley), C/2001 A2 (LINEAR), C/2000 WM1 (LINEAR) and 153P/Ikeya-Zhang. Astron. Astrophys. 2006, 449, 1255–1270. [Google Scholar] [CrossRef]
- Cordiner, M.A.; Remijan, A.J.; Boissier, J.; Milam, S.N.; Mumma, M.J.; Charnley, S.B.; Paganini, L.; Villanueva, G.; Bockelée-Morvan, D.; Kuan, Y.J.; et al. Mapping the Release of Volatiles in the Inner Comae of Comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON) Using the Atacama Large Millimeter/Submillimeter Array. Astrophys. J. 2014, 792, L2. [Google Scholar] [CrossRef]
- Cordiner, M.A.; Boissier, J.; Charnley, S.B.; Remijan, A.J.; Mumma, M.J.; Villanueva, G.L.; Lis, D.C.; Milam, S.N.; Paganini, L.; Crovisier, J.; et al. ALMA Mapping of Rapid Gas and Dust Variations in Comet C/2012 S1 (ISON):New Insights into the Origin of Cometary HNC. Astrophys. J. 2017, 838, 147. [Google Scholar] [CrossRef]
- Cordiner, M.A.; Biver, N.; Crovisier, J.; Bockelée-Morvan, D.; Mumma, M.J.; Charnley, S.B.; Villanueva, G.L.; Paganini, L.; Lis, D.C.; Milam, S.N. Thermal Physics of the Inner Coma: ALMA Studies of the Methanol Distribution and Excitation in Comet C/2012 K1 (PanSTARRS). Astrophys. J. 2017, 837, 177. [Google Scholar] [CrossRef]
- Krankowsky, D.; Lammerzahl, P.; Herrwerth, I.; Woweries, J.; Eberhardt, P.; Dolder, U.; Herrmann, U.; Schulte, W.; Berthelier, J.J.; Illiano, J.M. In situ gas and ion measurements at comet Halley. Nature 1986, 321, 326–329. [Google Scholar] [CrossRef]
- DiSanti, M.A.; Bonev, B.P.; Dello Russo, N.; Vervack, R.J., Jr.; Gibb, E.L.; Roth, N.X.; McKay, A.J.; Kawakita, H.; Feaga, L.M.; Weaver, H.A. Hypervolatiles in a Jupiter-family Comet: Observations of 45P/Honda-Mrkos-Pajdušáková Using iSHELL at the NASA-IRTF. Astron. J. 2017, 154, 246. [Google Scholar] [CrossRef]
- Flynn, G.J.; Bleuet, P.; Borg, J.; Bradley, J.P.; Brenker, F.E.; Brennan, S.; Bridges, J.; Brownlee, D.E.; Bullock, E.S.; Burghammer, M.; et al. Elemental Compositions of Comet 81P/Wild 2 Samples Collected by Stardust. Science 2006, 314, 1731. [Google Scholar] [CrossRef]
- Ogliore, R.C.; Nagashima, K.; Huss, G.R.; Westphal, A.J.; Gainsforth, Z.; Butterworth, A.L. Oxygen isotopic composition of coarse- and fine-grained material from comet 81P/Wild 2. Geochim. Cosmochim. Acta 2015, 166, 74–91. [Google Scholar] [CrossRef]
- Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. Cometary glycine detected in samples returned by Stardust. Meteorit. Planet. Sci. 2009, 44, 1323–1330. [Google Scholar] [CrossRef]
- Flynn, G.J.; Keller, L.P.; Feser, M.; Wirick, S.; Jacobsen, C. The origin of organic matter in the solar system: Evidence from the interplanetary dust particles. Geochim. Cosmochim. Acta 2003, 67, 4791–4806. [Google Scholar] [CrossRef]
- Starkey, N.A.; Franchi, I.A.; Lee, M.R. Isotopic diversity in interplanetary dust particles and preservation of extreme 16O-depletion. Geochim. Cosmochim. Acta 2014, 142, 115–131. [Google Scholar] [CrossRef][Green Version]
- Busemann, H.; Nguyen, A.N.; Cody, G.D.; Hoppe, P.; Kilcoyne, A.L.D.; Stroud, R.M.; Zega, T.J.; Nittler, L.R. Ultra-primitive interplanetary dust particles from the comet 26P/Grigg-Skjellerup dust stream collection. Earth Planet. Sci. Lett. 2009, 288, 44–57. [Google Scholar] [CrossRef]
- Boehnhardt, H.; Bibring, J.P.; Apathy, I.; Auster, H.U.; Ercoli Finzi, A.; Goesmann, F.; Klingelhöfer, G.; Knapmeyer, M.; Kofman, W.; Krüger, H.; et al. The Philae lander mission and science overview. Philos. Trans. R. Soc. Lond. Ser. A 2017, 375, 20160248. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, M.C.; Capaccioni, F.; Ciarniello, M.; Filacchione, G.; Formisano, M.; Mottola, S.; Raponi, A.; Tosi, F.; Bockelée-Morvan, D.; Erard, S.; et al. The diurnal cycle of water ice on comet 67P/Churyumov-Gerasimenko. Nature 2015, 525, 500–503. [Google Scholar] [CrossRef]
- Filacchione, G.; de Sanctis, M.C.; Capaccioni, F.; Raponi, A.; Tosi, F.; Ciarniello, M.; Cerroni, P.; Piccioni, G.; Capria, M.T.; Palomba, E.; et al. Exposed water ice on the nucleus of comet 67P/Churyumov-Gerasimenko. Nature 2016, 529, 368–372. [Google Scholar] [CrossRef]
- Filacchione, G.; Raponi, A.; Capaccioni, F.; Ciarniello, M.; Tosi, F.; Capria, M.T.; De Sanctis, M.C.; Migliorini, A.; Piccioni, G.; Cerroni, P.; et al. Seasonal exposure of carbon dioxide ice on the nucleus of comet 67P/Churyumov-Gerasimenko. Science 2016, 354, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Quirico, E.; Moroz, L.V.; Schmitt, B.; Arnold, G.; Faure, M.; Beck, P.; Bonal, L.; Ciarniello, M.; Capaccioni, F.; Filacchione, G.; et al. Refractory and semi-volatile organics at the surface of comet 67P/Churyumov-Gerasimenko: Insights from the VIRTIS/Rosetta imaging spectrometer. Icarus 2016, 272, 32–47. [Google Scholar] [CrossRef]
- Raponi, A.; Ciarniello, M.; Capaccioni, F.; Mennella, V.; Filacchione, G.; Vinogradoff, V.; Poch, O.; Beck, P.; Quirico, E.; De Sanctis, M.C.; et al. Infrared detection of aliphatic organics on a cometary nucleus. Nat. Astron. 2020, 4, 500–505. [Google Scholar] [CrossRef]
- Altwegg, K.; Balsiger, H.; Fuselier, S.A. Cometary Chemistry and the Origin of Icy Solar System Bodies: The View after Rosetta. Annu. Rev. Astron. Astrophys. 2019, 57, 113–155. [Google Scholar] [CrossRef]
- Bockelée-Morvan, D.; Calmonte, U.; Charnley, S.; Duprat, J.; Engrand, C.; Gicquel, A.; Hässig, M.; Jehin, E.; Kawakita, H.; Marty, B.; et al. Cometary Isotopic Measurements. Space Sci. Rev. 2015, 197, 47–83. [Google Scholar] [CrossRef]
- Bockelée-Morvan, D.; Biver, N. The composition of cometary ices. Philos. Trans. R. Soc. A 2017, 375, 20160252. [Google Scholar] [CrossRef]
- Filacchione, G.; Groussin, O.; Herny, C.; Kappel, D.; Mottola, S.; Oklay, N.; Pommerol, A.; Wright, I.; Yoldi, Z.; Ciarniello, M.; et al. Comet 67P/CG Nucleus Composition and Comparison to Other Comets. Space Sci. Rev. 2019, 215, 19. [Google Scholar] [CrossRef]
- Oro, J.; Mills, T.; Lazcano, A. Comets and the formation of biochemical compounds on the primitive Earth A review. Orig. Life Evol. Biosph. 1991, 21, 267. [Google Scholar] [CrossRef]
- Despois, D.; Crovisier, J.; Bockelée-Morvan, D.; Gerard, E.; Schraml, J. Observations of hydrogen cyanide in comet halley. Astron. Astrophys. 1986, 160, L11. [Google Scholar]
- Schloerb, F.P.; Kinzel, W.M.; Swade, D.A.; Irvine, W.M. Observations of HCN in comet P/Halley. Astron. Astrophys. 1987, 187, 475. [Google Scholar]
- Altwegg, K.; Balsiger, H.; Hänni, N.; Rubin, M.; Schuhmann, M.; Schroeder, I.; Sémon, T.; Wampfler, S.; Berthelier, J.J.; Briois, C.; et al. Evidence of ammonium salts in comet 67P as explanation for the nitrogen depletion in cometary comae. Nat. Astron. 2020, 4, 533–540. [Google Scholar] [CrossRef]
- Poch, O.; Istiqomah, I.; Quirico, E.; Beck, P.; Schmitt, B.; Theulé, P.; Faure, A.; Hily-Blant, P.; Bonal, L.; Raponi, A.; et al. Ammonium salts are a reservoir of nitrogen on a cometary nucleus and possibly on some asteroids. Science 2020, 367, aaw7462. [Google Scholar] [CrossRef] [PubMed]
- Dello Russo, N.; Vervack, R.J., Jr.; Weaver, H.A.; Kawakita, H.; Kobayashi, H.; Biver, N.; Bockelée-Morvan, D.; Crovisier, J. The Parent Volatile Composition of 6p/d’Arrest and a Chemical Comparison of Jupiter-Family Comets Measured at Infrared Wavelengths. Astrophys. J. 2009, 703, 187–197. [Google Scholar] [CrossRef]
- Dello Russo, N.; Vervack, R.J.; Kawakita, H.; Cochran, A.; McKay, A.J.; Harris, W.M.; Weaver, H.A.; Lisse, C.M.; DiSanti, M.A.; Kobayashi, H.; et al. The compositional evolution of C/2012 S1 (ISON) from ground-based high-resolution infrared spectroscopy as part of a worldwide observing campaign. Icarus 2016, 266, 152–172. [Google Scholar] [CrossRef]
- Irvine, W.M.; Bockelée-Morvan, D.; Lis, D.C.; Matthews, H.E.; Biver, N.; Crovisier, J.; Davies, J.K.; Dent, W.R.F.; Gautier, D.; Godfrey, P.D.; et al. Spectroscopic evidence for interstellar ices in comet Hyakutake. Nature 1996, 383, 418. [Google Scholar] [CrossRef] [PubMed]
- Biver, N.; Bockelée-Morvan, D.; Colom, P.; Crovisier, J.; Germain, B.; Lellouch, E.; Davies, J.K.; Dent, W.R.F.; Moreno, R.; Paubert, G.; et al. Long-term Evolution of the Outgassing of Comet Hale-Bopp From Radio Observations. Earth Moon Planets 1997, 78, 5–11. [Google Scholar] [CrossRef]
- Rodgers, S.D.; Charnley, S.B. HNC and HCN in Comets. Astrophys. J. Lett. 1998, 501, L227. [Google Scholar] [CrossRef]
- Rodgers, S.D.; Charnley, S.B. On the origin of HNC in Comet Lee. Mon. Not. R. Astron. Soc. 2001, 323, 84. [Google Scholar]
- Lis, D.C.; Bockelée-Morvan, D.; Boissier, J.; Crovisier, J.; Biver, N.; Charnley, S.B. Hydrogen Isocyanide in Comet 73P/Schwassmann-Wachmann (Fragment B). Astrophys. J. 2008, 675, 931. [Google Scholar] [CrossRef]
- Bockelée-Morvan, D.; Colom, P.; Crovisier, J.; Despois, D.; Paubert, G. Microwave detection of hydrogen sulphide and methanol in comet Austin (1989c1). Nature 1991, 350, 318. [Google Scholar]
- Colom, P.; Crovisier, J.; Bockelée-Morvan, D.; Despois, D.; Paubert, G. Formaldehyde in comets. I - Microwave observations of P/Brorsen-Metcalf (1989 X), Austin (1990 V) and Levy (1990 XX). Astron. Astrophys. 1992, 264, 270–281. [Google Scholar]
- Moroz, V.I.; Combes, M.; Bibring, J.P.; Coron, N.; Crovisier, J.; Encrenaz, T.; Crifo, J.F.; Sanko, N.; Grigoryev, A.V.; Bockelée-Morvan, D.; et al. Detection of Parent Molecules in Comet p/ Halley from the IKS VEGA Experiment. Astron. Astrophys. 1987, 187, 513. [Google Scholar]
- Danks, A.C.; Encrenaz, T.; Bouchet, P.; Le Bertre, T.; Chalabaev, A. The spectrum of comet P/Halley from 3.0 to 4.0 microns. Astron. Astrophys. 1987, 184, 329. [Google Scholar]
- Snyder, L.E.; Palmer, P.; de Pater, I. Radio Detection of Formaldehyde Emission from Comet Halley. Astron. J. 1989, 97, 246. [Google Scholar] [CrossRef]
- Meier, R.; Eberhardt, P.; Krankowsky, D.; Hodges, R.R. The extended formaldehyde source in comet P/Halley. Astron. Astrophys. 1993, 277, 677. [Google Scholar]
- Bockelée-Morvan, D.; Lis, D.C.; Wink, J.E.; Despois, D.; Crovisier, J.; Bachiller, R.; Benford, D.J.; Biver, N.; Colom, P.; Davies, J.K.; et al. New molecules found in comet C/1995 O1 (Hale-Bopp). Investigating the link between cometary and interstellar material. Astron. Astrophys. 2000, 353, 1101. [Google Scholar]
- DiSanti, M.A.; Bonev, B.P.; Magee-Sauer, K.; Dello Russo, N.; Mumma, M.J.; Reuter, D.C.; Villanueva, G.L. Detection of Formaldehyde Emission in Comet C/2002 T7 (LINEAR) at Infrared Wavelengths: Line-by-Line Validation of Modeled Fluorescent Intensities. Astrophys. J. 2006, 650, 470–483. [Google Scholar] [CrossRef]
- Cottin, H.; Fray, N. Distributed Sources in Comets. Space Sci. Rev. 2008, 183, 179. [Google Scholar] [CrossRef]
- Capaccioni, F.; Coradini, A.; Filacchione, G.; Erard, S.; Arnold, G.; Drossart, P.; De Sanctis, M.C.; Bockelée-Morvan, D.; Capria, M.T.; Tosi, F.; et al. The organic-rich surface of comet 67P/Churyumov-Gerasimenko as seen by VIRTIS/Rosetta. Science 2015, 6220, aaa0628. [Google Scholar] [CrossRef]
- Fray, N.; Bénilan, Y.; Biver, N.; Bockelée-Morvan, D.; Cottin, H.; Crovisier, J.; Gazeau, M.C. Heliocentric evolution of the degradation of polyoxymethylene: Application to the origin of the formaldehyde (H 2CO) extended source in Comet C/1995 O1 (Hale-Bopp). Icarus 2006, 184, 239. [Google Scholar] [CrossRef]
- Garrod, R.T.; Widicus Weaver, S.L.; Herbst, E. Complex Chemistry in Star-forming Regions: An Expanded Gas-Grain Warm-up Chemical Model. Astrophys. J. 2008, 682, 283–302. [Google Scholar] [CrossRef]
- Öberg, K.I.; Garrod, R.T.; van Dishoeck, E.F.; Linnartz, H. Formation rates of complex organics in UV irradiated CH3OH-rich ices. I. Experiments. Astron. Astrophys. 2009, 504, 891–913. [Google Scholar] [CrossRef]
- Herbst, E.; van Dishoeck, E.F. Complex Organic Interstellar Molecules. Annu. Rev. Astron. Astrophys. 2009, 47, 427–480. [Google Scholar] [CrossRef]
- Bockelée-Morvan, D.; Crovisier, J.; Colom, P.; Despois, D. The rotational lines of methanol in comets Austin 1990 V and Levy 1990 XX. Astron. Astrophys. 1994, 287, 647. [Google Scholar]
- Dello Russo, N.; Vervack, R.J., Jr.; Lisse, C.M.; Weaver, H.A.; Kawakita, H.; Kobayashi, H.; Cochran, A.L.; Harris, W.M.; McKay, A.J.; Biver, N.; et al. The Volatile Composition and Activity of Comet 103P/Hartley 2 During the EPOXI Closest Approach. Astrophys. J. Lett. 2011, 734, L8. [Google Scholar] [CrossRef]
- Mumma, M.J.; Bonev, B.P.; Villanueva, G.L.; Paganini, L.; DiSanti, M.A.; Gibb, E.L.; Keane, J.V.; Meech, K.J.; Blake, G.A.; Ellis, R.S.; et al. Temporal and Spatial Aspects of Gas Release During the 2010 Apparition of Comet 103P/Hartley 2. Astrophys. J. 2011, 734, L7. [Google Scholar] [CrossRef]
- Bonev, B.P.; Villanueva, G.L.; Paganini, L.; DiSanti, M.A.; Gibb, E.L.; Keane, J.V.; Meech, K.J.; Mumma, M.J. Evidence for two modes of water release in Comet 103P/Hartley 2: Distributions of column density, rotational temperature, and ortho-para ratio. Icarus 2013, 222, 740–751. [Google Scholar] [CrossRef]
- Kawakita, H.; Kobayashi, H.; Dello Russo, N.; Vervack, R.J.; Hashimoto, M.; Weaver, H.A.; Lisse, C.M.; Cochran, A.L.; Harris, W.M.; Bockelée-Morvan, D.; et al. Parent volatiles in Comet 103P/Hartley 2 observed by Keck II with NIRSPEC during the 2010 apparition. Icarus 2013, 222, 723–733. [Google Scholar] [CrossRef]
- Villanueva, G.L.; Mumma, M.J.; DiSanti, M.A.; Bonev, B.P.; Gibb, E.L.; Magee-Sauer, K.; Blake, G.A.; Salyk, C. The molecular composition of Comet C/2007 W1 (Boattini): Evidence of a peculiar outgassing and a rich chemistry. Icarus 2011, 216, 227–240. [Google Scholar] [CrossRef]
- Mumma, M.J.; DiSanti, M.A.; dello Russo, N.; Fomenkova, M.; Magee-Sauer, K.; Kaminski, C.D.; Xie, D.X. Detection of Abundant Ethane and Methane, Along with Carbon Monoxide and Water, in Comet C/1996 B2 Hyakutake: Evidence for Interstellar Origin. Science 1996, 272, 1310–1314. [Google Scholar] [CrossRef]
- Tielens, A.G.G.M. Grain Surface Chemistry. In Chemistry and Spectroscopy of Interstellar Molecules; Bohme, D.K., Ed.; University of Tokyo Press: Tokyo, Japan, 1992; p. 237. [Google Scholar]
- Kobayashi, H.; Hidaka, H.; Lamberts, T.; Hama, T.; Kawakita, H.; Kästner, J.; Watanabe, N. Hydrogenation and Deuteration of C2H2 and C2H4 on Cold Grains: A Clue to the Formation Mechanism of C2H6 with Astronomical Interest. Astrophys. J. 2017, 837, 155. [Google Scholar] [CrossRef]
- Gerakines, P.A.; Schutte, W.A.; Ehrenfreund, P. Ultraviolet processing of interstellar ice analogs. I. Pure ices. Astron. Astrophys. 1996, 312, 289–305. [Google Scholar]
- Bennett, C.J.; Jamieson, C.S.; Osamura, Y.; Kaiser, R.I. Laboratory Studies on the Irradiation of Methane in Interstellar, Cometary, and Solar System Ices. Astrophys. J. 2006, 653, 792–811. [Google Scholar] [CrossRef]
- De Barros, A.L.F.; da Silveira, E.F.; Fulvio, D.; Rothard, H.; Boduch, P. Ion Irradiation of Ethane and Water Mixture Ice at 15 K: Implications for the Solar System and the ISM. Astrophys. J. 2016, 824, 81. [Google Scholar] [CrossRef]
- DiSanti, M.A.; Bonev, B.P.; Gibb, E.L.; Roth, N.X.; Dello Russo, N.; Vervack, R.J., Jr. Comet C/2013 V5 (Oukaimeden): Evidence for Depleted Organic Volatiles and Compositional Heterogeneity as Revealed through Infrared Spectroscopy. Astron. J. 2018, 156, 258. [Google Scholar] [CrossRef]
- Bockelée-Morvan, D.; Crovisier, J.; Erard, S.; Capaccioni, F.; Leyrat, C.; Filacchione, G.; Drossart, P.; Encrenaz, T.; Biver, N.; de Sanctis, M.C.; et al. Evolution of CO2, CH4, and OCS abundances relative to H2O in the coma of comet 67P around perihelion from Rosetta/VIRTIS-H observations. Mon. Not. R. Astron. Soc. 2016, 462, S170–S183. [Google Scholar] [CrossRef]
- Le Roy, L.; Altwegg, K.; Balsiger, H.; Berthelier, J.J.; Bieler, A.; Briois, C.; Calmonte, U.; Combi, M.R.; De Keyser, J.; Dhooghe, F. Inventory of the volatiles on comet 67P/Churyumov-Gerasimenko from Rosetta/ROSINA. Astron. Astrophys. 2015, 583, A1. [Google Scholar] [CrossRef]
- Roth, N.X.; Gibb, E.L.; Bonev, B.P.; DiSanti, M.A.; Dello Russo, N.; Vervack, R.J., Jr.; McKay, A.J.; Kawakita, H. A Tale of “Two” Comets: The Primary Volatile Composition of Comet 2P/Encke Across Apparitions and Implications for Cometary Science. Astron. J. 2018, 156, 251. [Google Scholar] [CrossRef]
- Faggi, S.; Mumma, M.J.; Villanueva, G.L.; Paganini, L.; Lippi, M. Quantifying the Evolution of Molecular Production Rates of Comet 21P/Giacobini—Zinner with iSHELL/NASA-IRTF. Astron. J. 2019, 158, 254. [Google Scholar] [CrossRef]
- McKay, A.; DiSanti, M.; Bonev, B.; Dello Russo, N.; Vervack, R.J.; Gibb, E.; Roth, N.; Saki, M.; Kawakita, H. Hypervolatiles in Jupiter Family Comet 46P/Wirtanen Observed with IRTF iSHELL. In Proceedings of the EPSC-DPS Joint Meeting 2019, Geneva, Switzerland, 15–20 September 2019; Volume 2019, p. EPSC–DPS2019–1061. [Google Scholar]
- Dello Russo, N.; Kawakita, H.; Bonev, B.P.; Vervack, R.J.; Gibb, E.L.; Shinnaka, Y.; Roth, N.X.; DiSanti, M.A.; McKay, A.J. Post-perihelion volatile production and release from Jupiter-family comet 45P/Honda-Mrkos-Pajdušáková. Icarus 2020, 335, 113411. [Google Scholar] [CrossRef]
- Roth, N.X.; Gibb, E.L.; Bonev, B.P.; DiSanti, M.A.; Dello Russo, N.; McKay, A.J.; Vervack, R.J., Jr.; Kawakita, H.; Saki, M.; Biver, N.; et al. Probing the Evolutionary History of Comets: An Investigation of the Hypervolatiles CO, CH4, and C2H6 in the Jupiter-family Comet 21P/Giacobini—Zinner. Astron. J. 2020, 159, 42. [Google Scholar] [CrossRef]
- Qasim, D.; Fedoseev, G.; Chuang, K.J.; He, J.; Ioppolo, S.; van Dishoeck, E.F.; Linnartz, H. An experimental study of the surface formation of methane in interstellar molecular clouds. Nat. Astron. 2020, 4, 781–785. [Google Scholar] [CrossRef]
- Martínez, L.; Santoro, G.; Merino, P.; Accolla, M.; Lauwaet, K.; Sobrado, J.; Sabbah, H.; Pelaez, R.J.; Herrero, V.J.; Tanarro, I.; et al. Prevalence of non-aromatic carbonaceous molecules in the inner regions of circumstellar envelopes. Nat. Astron. 2020, 4, 97–105. [Google Scholar] [CrossRef]
- Frenklach, M.; Feigelson, E.D. Formation of Polycyclic Aromatic Hydrocarbons in Circumstellar Envelopes. Astrophys. J. 1989, 341, 372. [Google Scholar] [CrossRef]
- Cernicharo, J. The Polymerization of Acetylene, Hydrogen Cyanide, and Carbon Chains in the Neutral Layers of Carbon-rich Proto-planetary Nebulae. Astrophys. J. 2004, 608, L41–L44. [Google Scholar] [CrossRef]
- Biver, N.; Bockelée-Morvan, D.; Debout, V.; Crovisier, J.; Boissier, J.; Lis, D.C.; Dello Russo, N.; Moreno, R.; Colom, P.; Paubert, G.; et al. Complex organic molecules in comets C/2012 F6 (Lemmon) and C/2013 R1 (Lovejoy): Detection of ethylene glycol and formamide. Astron. Astrophys. 2014, 566, L5. [Google Scholar]
- Crovisier, J.; Bockelée-Morvan, D.; Biver, N.; Colom, P.; Despois, D.; Lis, D.C. Ethylene glycol in comet C/1995 O1 (Hale-Bopp). Astron. Astrophys. 2004, 418, L35. [Google Scholar] [CrossRef]
- Goesmann, F.; Rosenbauer, H.; Bredehöft, J.H.; Cabane, M.; Ehrenfreund, P.; Gautier, T.; Giri, C.; Krüger, H.; Le Roy, L.; MacDermott, A.R.J.; et al. Organic compounds on comet 67P/Churyumov-Gerasimenko revealed by COSAC mass spectrometry. Science 2015, 349, 2–689. [Google Scholar] [CrossRef]
- Hadraoui, K.; Cottin, H.; Ivanovski, S.L.; Zapf, P.; Altwegg, K.; Benilan, Y.; Biver, N.; Della Corte, V.; Fray, N.; Lasue, J.; et al. Distributed glycine in comet 67P/Churyumov-Gerasimenko. Astron. Astrophys. 2019, 630, A32. [Google Scholar] [CrossRef]
- Wright, I.P.; Sheridan, S.; Barber, S.J.; Morgan, G.H.; Andrews, D.J.; Morse, A.D. CHO-bearing organic compounds at the surface of 67P/Churyumov-Gerasimenko revealed by Ptolemy. Science 2015, 349, aab0673. [Google Scholar] [CrossRef]
- Fray, N.; Bardyn, A.; Cottin, H.; Altwegg, K.; Baklouti, D.; Briois, C.; Colangeli, L.; Engrand, C.; Fischer, H.; Glasmachers, A.; et al. High-molecular-weight organic matter in the particles of comet 67P/Churyumov-Gerasimenko. Nature 2016, 538, 72–74. [Google Scholar] [CrossRef] [PubMed]
- A’Hearn, M.F.; Belton, M.J.S.; Delamere, W.A.; Kissel, J.; Klaasen, K.P.; McFadden, L.A.; Meech, K.J.; Melosh, H.J.; Schultz, P.H.; Sunshine, J.M.; et al. Deep Impact: Excavating Comet Tempel 1. Science 2005, 310, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Clemett, S.J.; Sandford, S.A.; Nakamura-Messenger, K.; Hörz, F.; McKay, D.S. Complex aromatic hydrocarbons in Stardust samples collected from comet 81P/Wild 2. Meteorit. Planet. Sci. 2010, 45, 701–722. [Google Scholar] [CrossRef]
- Schuhmann, M.; Altwegg, K.; Balsiger, H.; Berthelier, J.J.; De Keyser, J.; Fiethe, B.; Fuselier, S.A.; Gasc, S.; Gombosi, T.I.; Hänni, N.; et al. Aliphatic and aromatic hydrocarbons in comet 67P/Churyumov-Gerasimenko seen by ROSINA. Astron. Astrophys. 2019, 630, A31. [Google Scholar] [CrossRef]
- Gibb, E.L.; Mumma, M.J.; Dello Russo, N.; DiSanti, M.A.; Magee-Sauer, K. Methane in Oort cloud comets. Icarus 2003, 165, 391–406. [Google Scholar] [CrossRef]
- Swings, P. Complex structure of cometary bands tentatively ascribed to the contour of the solar spectrum. Lick Obs. Bull. 1941, 19, 131–136. [Google Scholar]
- Vaughan, C.M.; Pierce, D.M.; Cochran, A.L. Jet Morphology and Coma Analysis of Comet 103P/Hartley 2. Astron. J. 2017, 154, 219. [Google Scholar] [CrossRef]
- Heays, A.N.; Visser, R.; Gredel, R.; Ubachs, W.; Lewis, B.R.; Gibson, S.T.; van Dishoeck, E.F. Isotope selective photodissociation of N2 by the interstellar radiation field and cosmic rays. Astron. Astrophys. 2014, 562, A61. [Google Scholar] [CrossRef]
- Visser, R.; Bruderer, S.; Cazzoletti, P.; Facchini, S.; Heays, A.N.; van Dishoeck, E.F. Nitrogen isotope fractionation in protoplanetary disks. Astron. Astrophys. 2018, 615, A75. [Google Scholar] [CrossRef]
- Hily-Blant, P.; Magalhaes de Souza, V.; Kastner, J.; Forveille, T. Multiple nitrogen reservoirs in a protoplanetary disk at the epoch of comet and giant planet formation. Astron. Astrophys. 2019, 632, L12. [Google Scholar] [CrossRef]
- Yang, B.; Hutsemékers, D.; Shinnaka, Y.; Opitom, C.; Manfroid, J.; Jehin, E.; Meech, K.J.; Hainaut, O.R.; Keane, J.V.; Gillon, M. Isotopic ratios in outbursting comet C/2015 ER61. Astron. Astrophys. 2018, 609, L4. [Google Scholar] [CrossRef]
- Douglas, A.E. Laboratory Studies of the λ 4050 Group of Cometary Spectra. Astrophys. J. 1951, 114, 466. [Google Scholar] [CrossRef]
- Dufay, J. CH Bands in Comet Spectra. Astrophys. J. 1940, 91, 91. [Google Scholar] [CrossRef]
- Rubin, M.; Bekaert, D.V.; Broadley, M.W.; Drozdovskaya, M.N.; Wampfler, S.F. Volatile Species in Comet 67P/Churyumov-Gerasimenko: Investigating the Link from the ISM to the Terrestrial Planets. ACS Earth Space Chem. 2019, 3, 1792–1811. [Google Scholar] [CrossRef]
- Snodgrass, C.; Jones, G.H. The European Space Agency’s Comet Interceptor lies in wait. Nat. Commun. 2019, 10, 5418. [Google Scholar] [CrossRef] [PubMed]
- Bockelée-Morvan, D.; Filacchione, G.; Altwegg, K.; Bianchi, E.; Bizzarro, M.; Blum, J.; Bonal, L.; Capaccioni, F.; Codella, C.; Choukroun, M.; et al. AMBITION—Comet Nucleus Cryogenic Sample Return (White paper for ESA’s Voyage 2050 programme). arXiv 2019, arXiv:1907.11081. [Google Scholar]
- Kelley, M.S.P.; Woodward, C.E.; Bodewits, D.; Farnham, T.L.; Gudipati, M.S.; Harker, D.E.; Hines, D.C.; Knight, M.M.; Kolokolova, L.; Li, A.; et al. Cometary Science with the James Webb Space Telescope. Publ. Astron. Soc. Pac. 2016, 2016. 128, 018009. [Google Scholar] [CrossRef][Green Version]
- Opitom, C.; Fitzsimmons, A.; Jehin, E.; Moulane, Y.; Hainaut, O.; Meech, K.J.; Yang, B.; Snodgrass, C.; Micheli, M.; Keane, J.V.; et al. 2I/Borisov: A C2 depleted interstellar comet. arXiv 2019, arXiv:1910.09078. [Google Scholar] [CrossRef]
- Kareta, T.; Andrews, J.; Noonan, J.W.; Harris, W.M.; Smith, N.; O’Brien, P.; Sharkey, B.N.L.; Reddy, V.; Springmann, A.; Lejoly, C.; et al. Carbon Chain Depletion of 2I/Borisov. Astrophys. J. 2020, 889, L38. [Google Scholar] [CrossRef]
- McKay, A.J.; Cochran, A.L.; Dello Russo, N.; DiSanti, M.A. Detection of a Water Tracer in Interstellar Comet 2I/Borisov. Astrophys. J. 2020, 889, L10. [Google Scholar] [CrossRef]
- Cordiner, M.A.; Milam, S.N.; Biver, N.; Bockelée-Morvan, D.; Roth, N.X.; Bergin, E.A.; Jehin, E.; Remijan, A.J.; Charnley, S.B.; Mumma, M.J.; et al. Unusually high CO abundance of the first active interstellar comet. Nat. Astron. 2020, 4, 861–866. [Google Scholar] [CrossRef]
- Frattin, E.; Cremonese, G.; Simioni, E.; Bertini, I.; Lazzarin, M.; Ott, T.; Drolshagen, E.; La Forgia, F.; Sierks, H.; Barbieri, C.; et al. Post-perihelion photometry of dust grains in the coma of 67P Churyumov-Gerasimenko. Mon. Not. R. Astron. Soc. 2017, 469, S195–S203. [Google Scholar] [CrossRef]
- Filacchione, G.; Capaccioni, F.; Ciarniello, M.; Raponi, A.; Rinaldi, G.; De Sanctis, M.C.; Bockelèe-Morvan, D.; Erard, S.; Arnold, G.; Mennella, V.; et al. An orbital water-ice cycle on comet 67P from colour changes. Nature 2020, 578, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.L.; McKay, A.J. Strong CO+ and {{∖rm{N}}}_{2}{+} Emission in Comet C/2016 R2 (Pan-STARRS). Astrophys. J. 2018, 854, L10. [Google Scholar] [CrossRef]
- Wierzchos, K.; Womack, M. C/2016 R2 (PANSTARRS): A Comet Rich in CO and Depleted in HCN. Astron. J. 2018, 156, 34. [Google Scholar] [CrossRef]
- Biver, N.; Bockelée-Morvan, D.; Paubert, G.; Moreno, R.; Crovisier, J.; Boissier, J.; Bertrand, E.; Boussier, H.; Kugel, F.; McKay, A.; et al. The extraordinary composition of the blue comet C/2016 R2 (PanSTARRS). Astron. Astrophys. 2018, 619, A127. [Google Scholar] [CrossRef]
- Opitom, C.; Hutsemékers, D.; Jehin, E.; Rousselot, P.; Pozuelos, F.J.; Manfroid, J.; Moulane, Y.; Gillon, M.; Benkhaldoun, Z. High resolution optical spectroscopy of the N2-rich comet C/2016 R2 (PanSTARRS). Astron. Astrophys. 2019, 624, A64. [Google Scholar] [CrossRef]
- McKay, A.J.; DiSanti, M.A.; Kelley, M.S.P.; Knight, M.M.; Womack, M.; Wierzchos, K.; Harrington Pinto, O.; Bonev, B.; Villanueva, G.L.; Dello Russo, N.; et al. The Peculiar Volatile Composition of CO-dominated Comet C/2016 R2 (PanSTARRS). Astron. J. 2019, 158, 128. [Google Scholar] [CrossRef]
- Calmonte, U.; Altwegg, K.; Balsiger, H.; Berthelier, J.J.; Bieler, A.; Cessateur, G.; Dhooghe, F.; van Dishoeck, E.F.; Fiethe, B.; Fuselier, S.A.; et al. Sulphur-bearing species in the coma of comet 67P/Churyumov-Gerasimenko. Mon. Not. R. Astron. Soc. 2016, 462, S253–S273. [Google Scholar] [CrossRef]
- Schuhmann, M.; Altwegg, K.; Balsiger, H.; Berthelier, J.J.; De Keyser, J.; Fuselier, S.A.; Gasc, S.; Gombosi, T.I.; Hänni, N.; Rubin, M.; et al. CHO-bearing molecules in Comet 67P/Churyumov-Gerasimenko. ACS Earth Space Chem. 2019, 3, 1854–1861. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.B.; Tseng, W.L.; Sun, J.X.; Liao, Y.; Ip, W.H.; Zheng, X.W.; Wang, N.; Lu, D.R.; Chen, L.; et al. Observations of the Hydrogen Cyanide in Comet 46P/Wirtanen at a 3.4 mm Wavelength. Astron. J. 2020, 159, 240. [Google Scholar] [CrossRef]
- Paganini, L.; Camarca, M.N.; Mumma, M.J.; Faggi, S.; Lippi, M.; Villanueva, G.L. Observations of Jupiter Family Comet 252P/LINEAR During a Close Approach to Earth Reveal Large Abundances of Methanol and Ethane. Astron. J. 2019, 158, 98. [Google Scholar] [CrossRef]
- Bøgelund, E.G.; Hogerheijde, M.R. Exploring the volatile composition of comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON) with ALMA. Astron. Astrophys. 2017, 604, A131. [Google Scholar] [CrossRef]
- Roth, N.X.; Gibb, E.L.; Bonev, B.P.; DiSanti, M.A.; Mumma, M.J.; Villanueva, G.L.; Paganini, L. The Composition of Comet C/2012 K1 (PanSTARRS) and the Distribution of Primary Volatile Abundances among Comets. Astron. J. 2017, 153, 168. [Google Scholar] [CrossRef]
- DiSanti, M.A.; Bonev, B.P.; Gibb, E.L.; Paganini, L.; Villanueva, G.L.; Mumma, M.J.; Keane, J.V.; Blake, G.A.; Dello Russo, N.; Meech, K.J.; et al. En Route to Destruction: The Evolution in Composition of Ices in Comet D/2012 S1 (ISON) between 1.2 and 0.34 AU from the Sun as Revealed at Infrared Wavelengths. Astrophys. J. 2016, 820, 34. [Google Scholar] [CrossRef]
- Wirström, E.S.; Lerner, M.S.; Källström, P.; Levinsson, A.; Olivefors, A.; Tegehall, E. HCN observations of comets C/2013 R1 (Lovejoy) and C/2014 Q2 (Lovejoy). Astron. Astrophys. 2016, 588, A72. [Google Scholar] [CrossRef]
- De Val-Borro, M.; Milam, S.N.; Cordiner, M.A.; Charnley, S.B.; Villanueva, G.L.; Kuan, Y.J. CO-activity in comet C/2016 R2 (PANSTARRS). Astron. Telegr. 2018, 11254. [Google Scholar]
- Faggi, S.; Villanueva, G.L.; Mumma, M.J.; Paganini, L. The Volatile Composition of Comet C/2017 E4 (Lovejoy) before its Disruption, as Revealed by High-resolution Infrared Spectroscopy with iSHELL at the NASA/IRTF. Astron. J. 2018, 156, 68. [Google Scholar] [CrossRef]
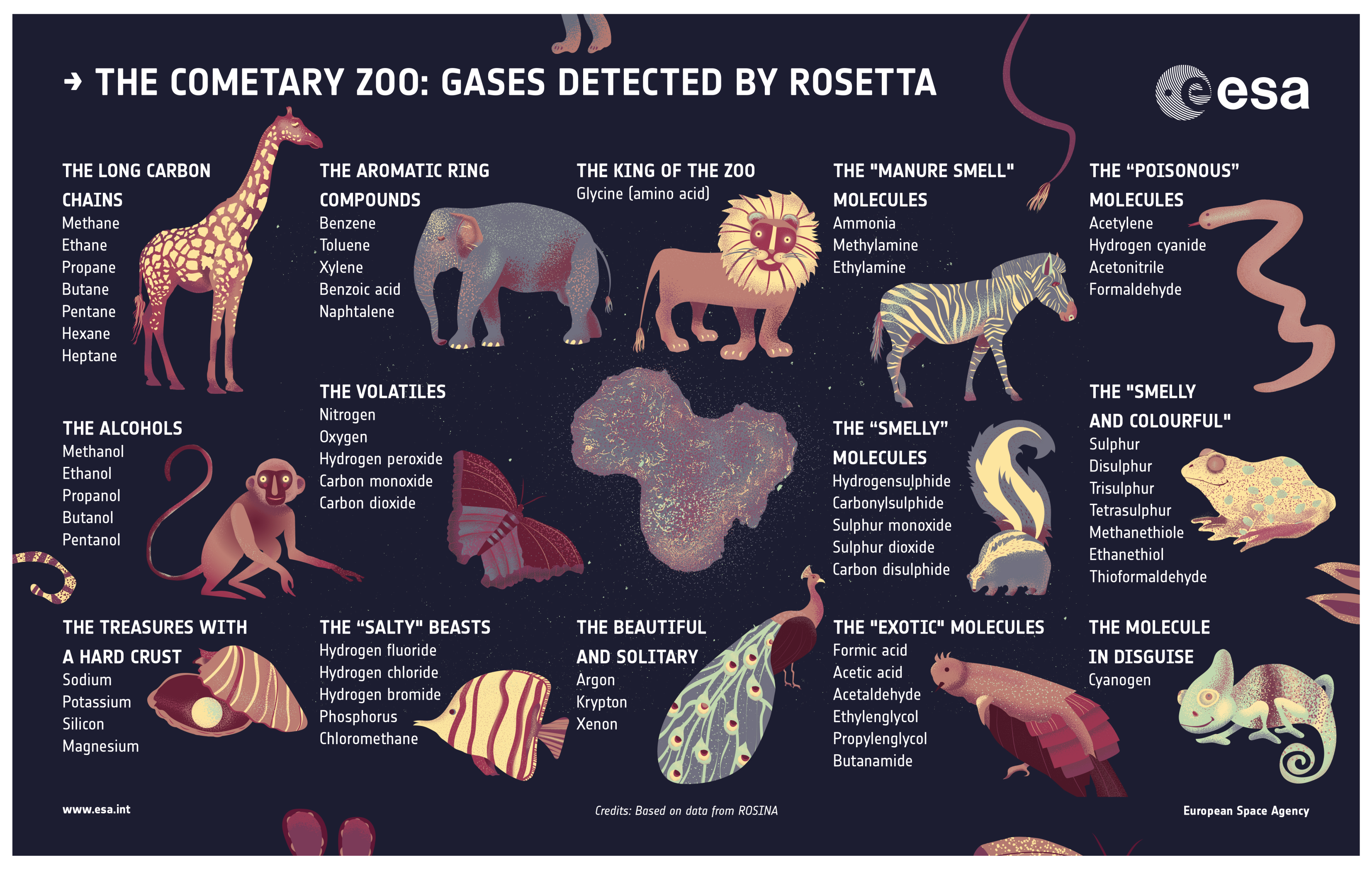
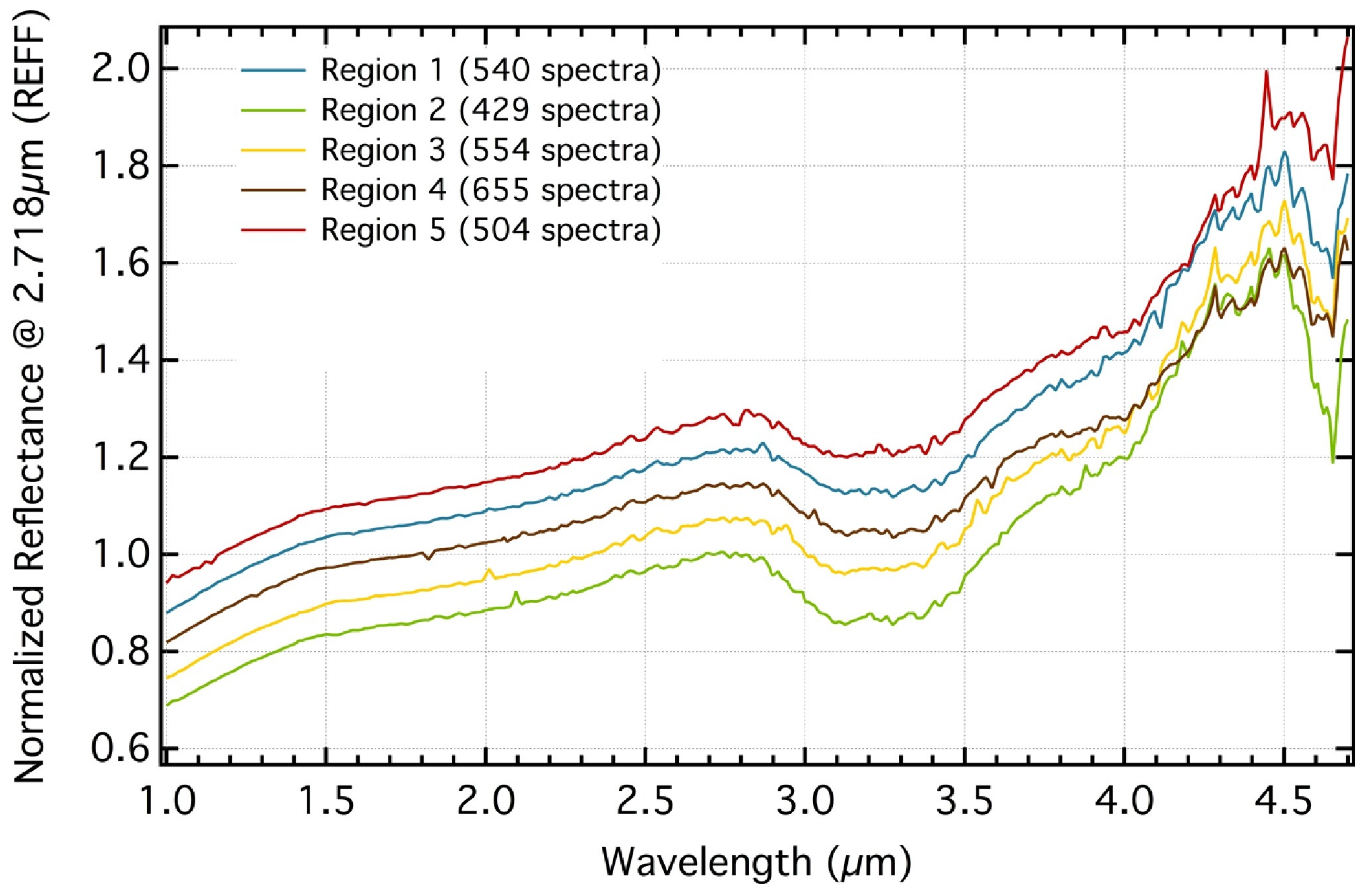
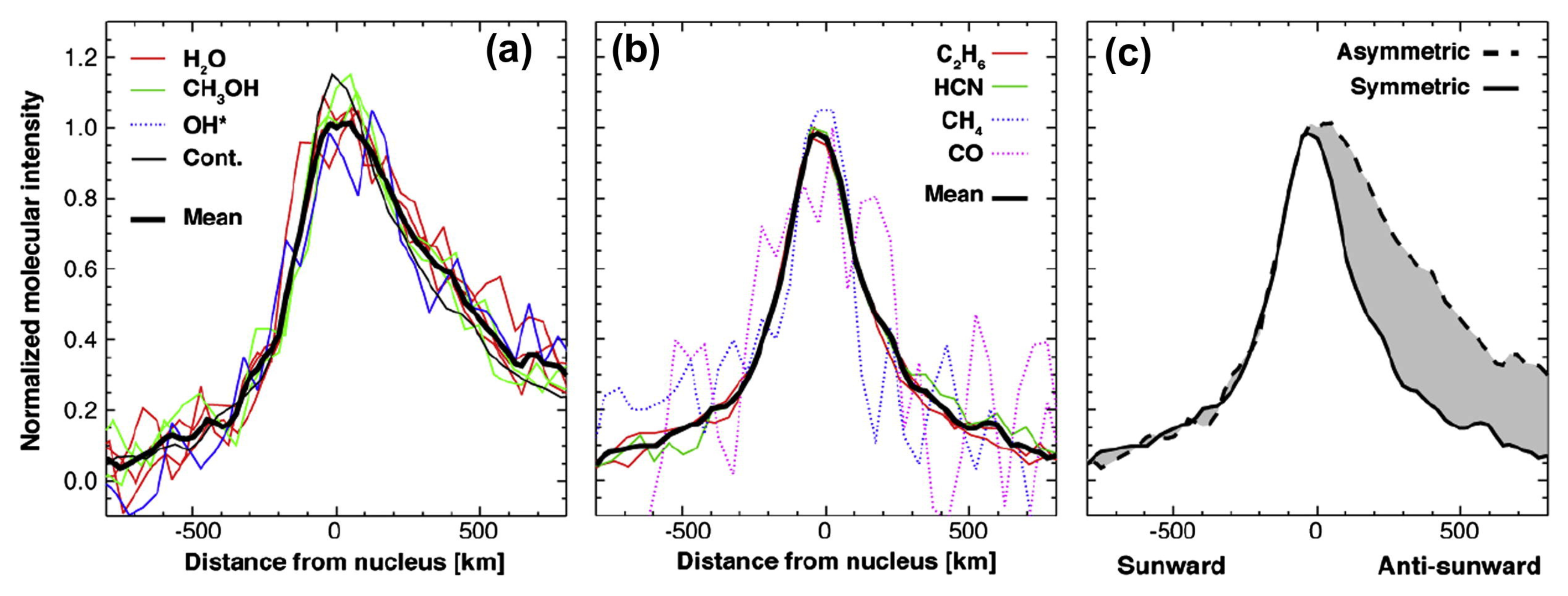
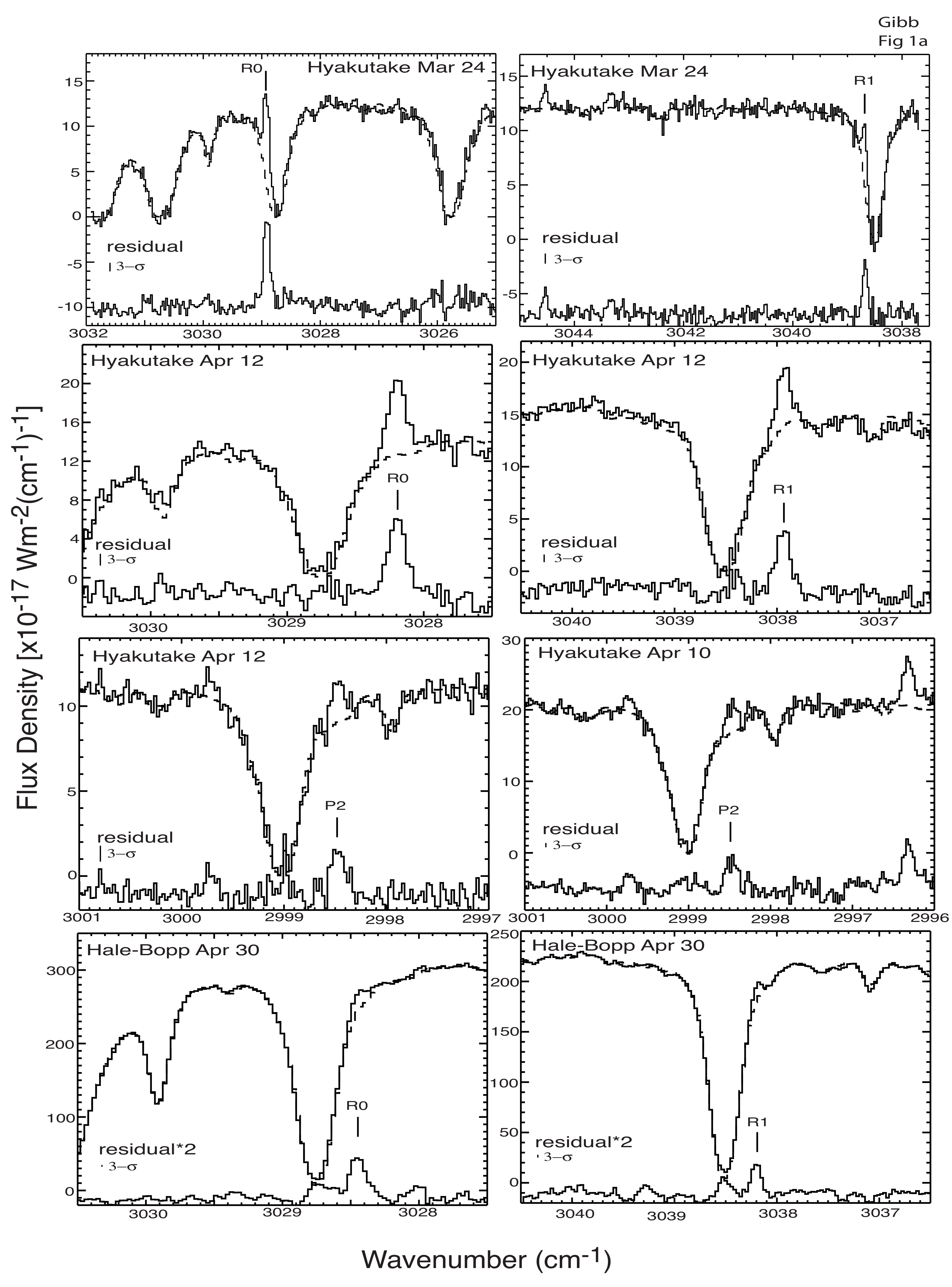
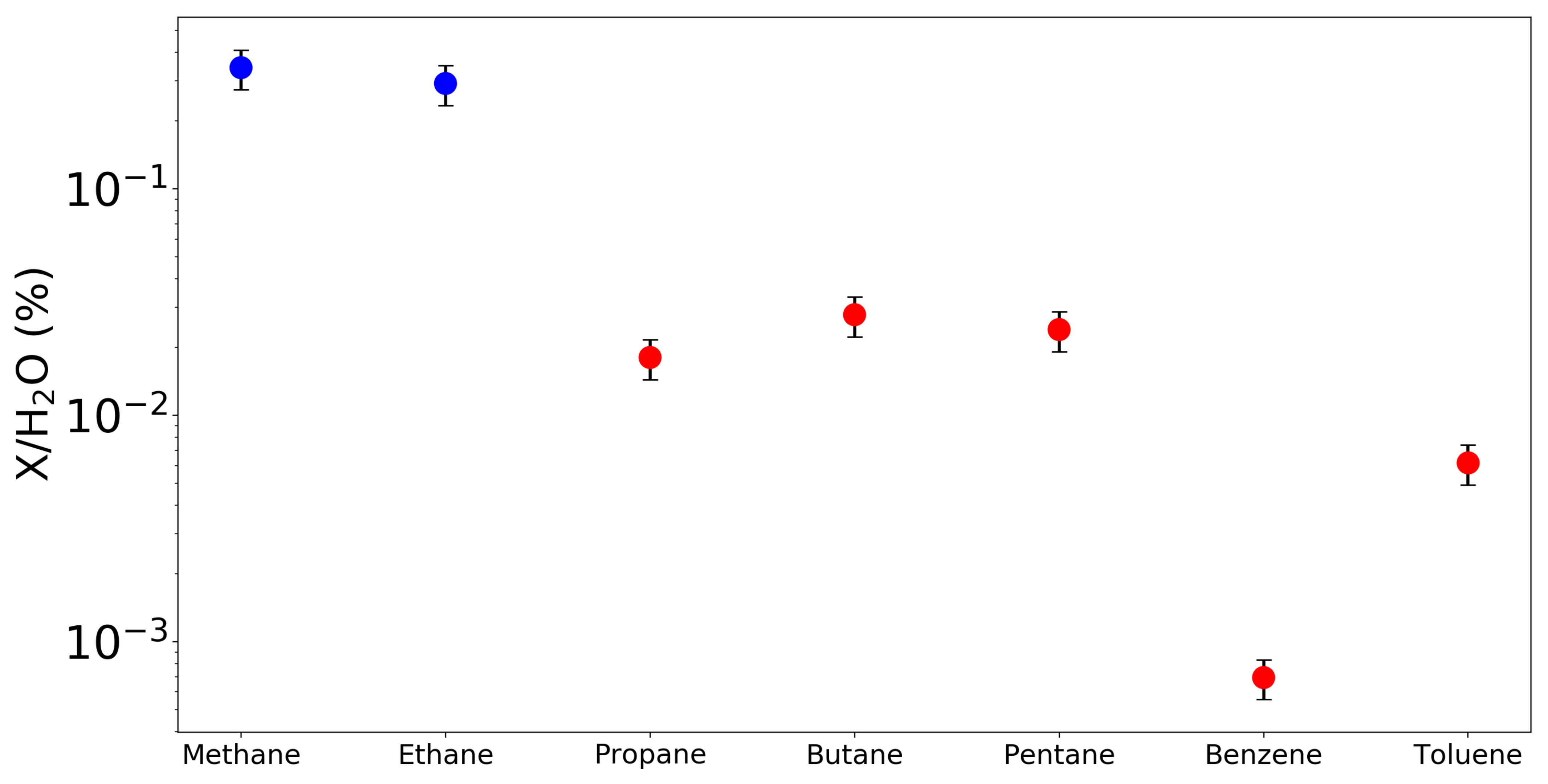
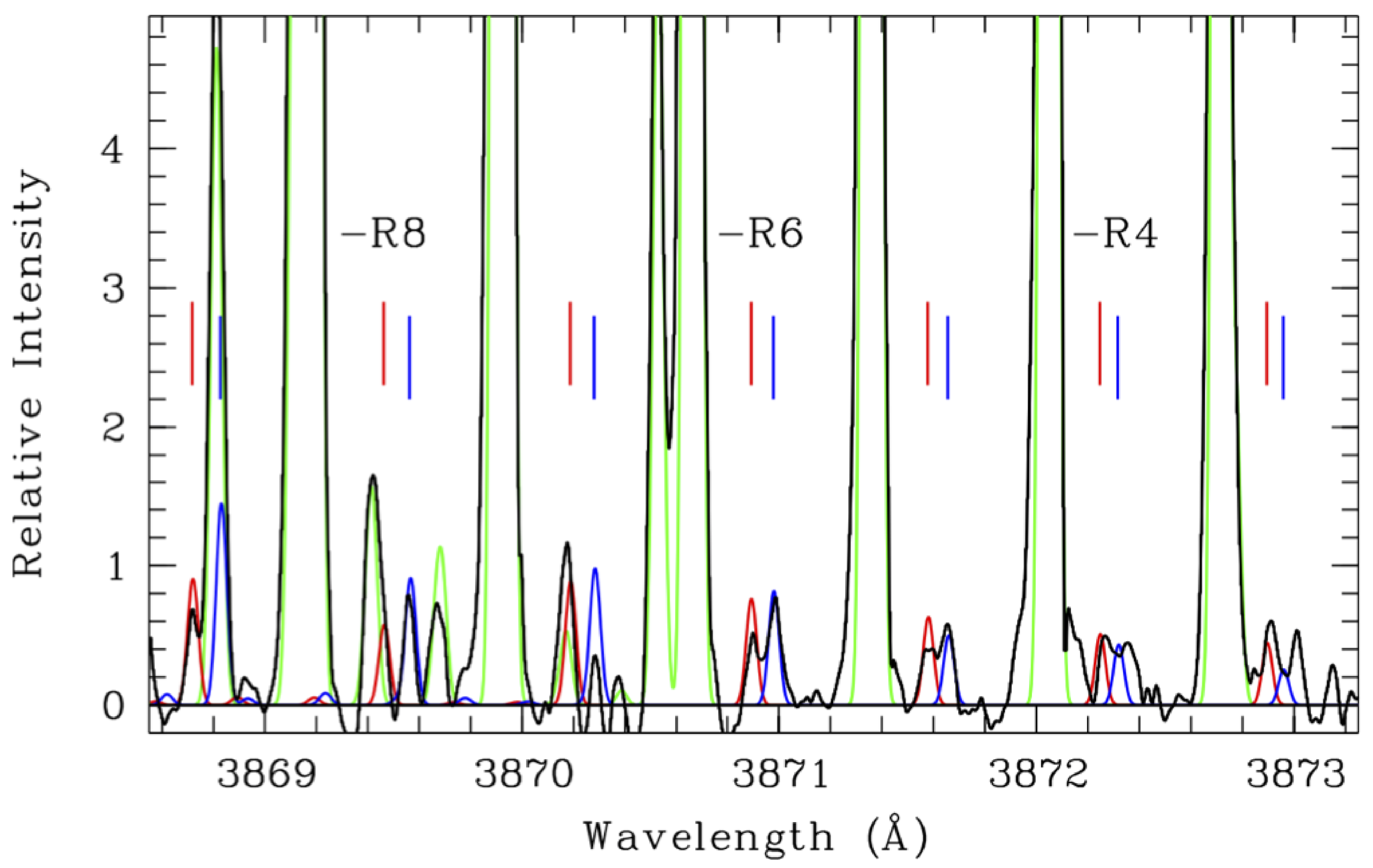
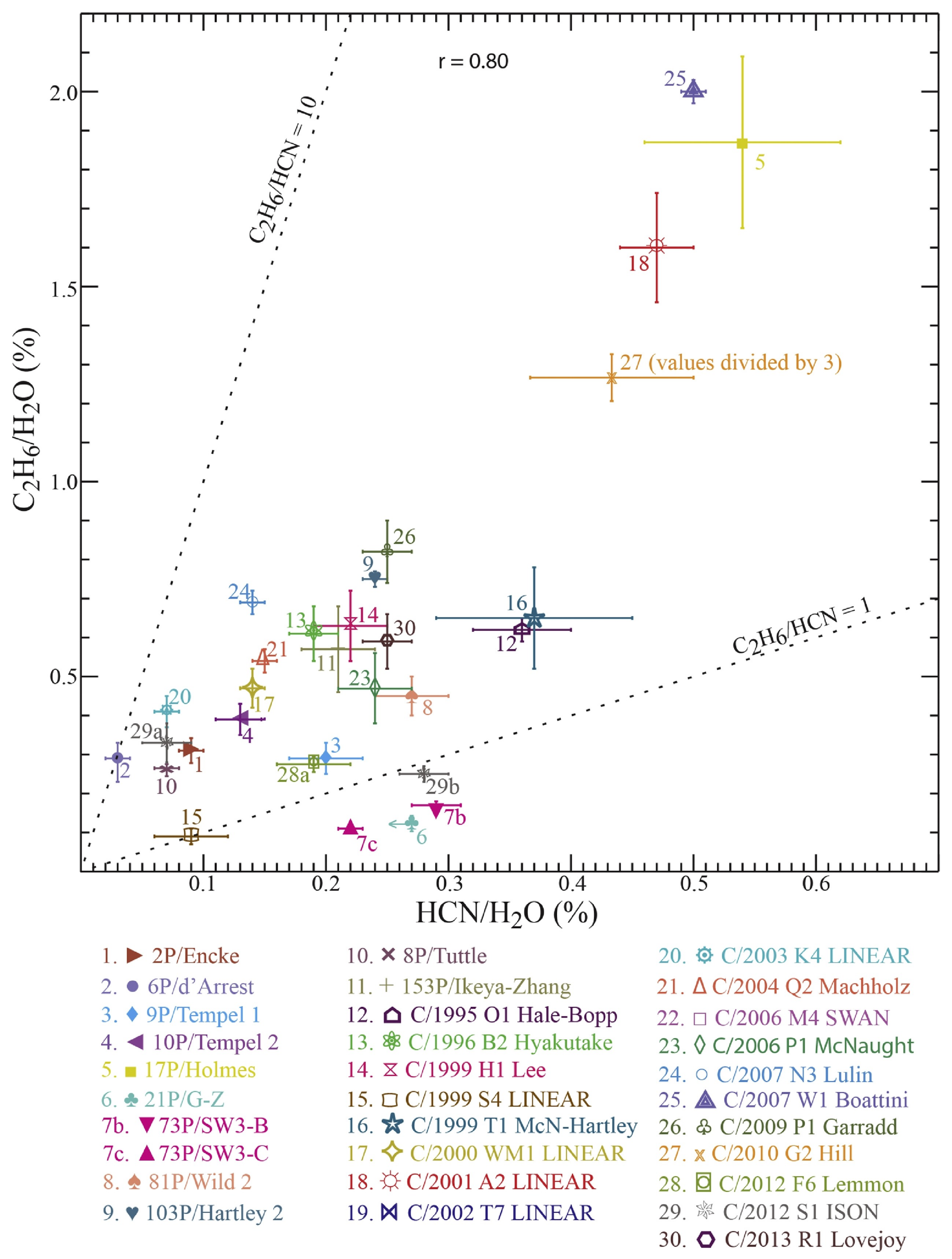
| Species | Chemical Formula | Typical Abundance (X/HO in %) | Number of Comets |
|---|---|---|---|
| Hydrogen Cyanide | HCN | 0.1–0.2 a | 57 |
| Hydrogen Isocyanide | HNC | 0.01 | 21 |
| Isocyanic Acid | HNCO | 0.02 | 16 |
| Acetonitrile | CHCN | 0.02 | 22 |
| Cyanoacetylene | HCN | 0.02 | 11 |
| Acetylene | CH | 0.13 | 30 |
| Methane | CH | 0.72 | 32 |
| Ethane | CH | 0.52 | 35 |
| Formaldehyde | HCO | 0.30 | 31 |
| Methanol | CHOH | 2.05 | 35 |
| Formic Acid | HCOOH | 0.09 | 10 |
| Methyl Formate | HCOOCH | 0.003–0.08 | 3 |
| Acetaldehyde | CHCHO | 0.05–0.08 | 4 |
| Ethylene Glycol | (CHOH) | 0.01–0.35 | 5 |
| Ethanol | CHOH | 0.04–0.12 | 2 |
| Glycolaldehyde | CHOHCHO | 0.02 | 2 |
| Formamide | NHCHO | 0.004–0.021 | 5 |
| Thioformaldehyde | HCS | 0.003–0.09 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKay, A.J.; Roth, N.X. Organic Matter in Cometary Environments. Life 2021, 11, 37. https://doi.org/10.3390/life11010037
McKay AJ, Roth NX. Organic Matter in Cometary Environments. Life. 2021; 11(1):37. https://doi.org/10.3390/life11010037
Chicago/Turabian StyleMcKay, Adam J., and Nathan X. Roth. 2021. "Organic Matter in Cometary Environments" Life 11, no. 1: 37. https://doi.org/10.3390/life11010037
APA StyleMcKay, A. J., & Roth, N. X. (2021). Organic Matter in Cometary Environments. Life, 11(1), 37. https://doi.org/10.3390/life11010037





