Shear Stress and RBC-NOS Serine1177 Phosphorylation in Humans: A Dose Response
Abstract
1. Introduction
2. Material and Methods
2.1. Subjects and Sampling
2.2. Experimental Overview
2.3. Exposure of Red Blood Cells to Shear Stress
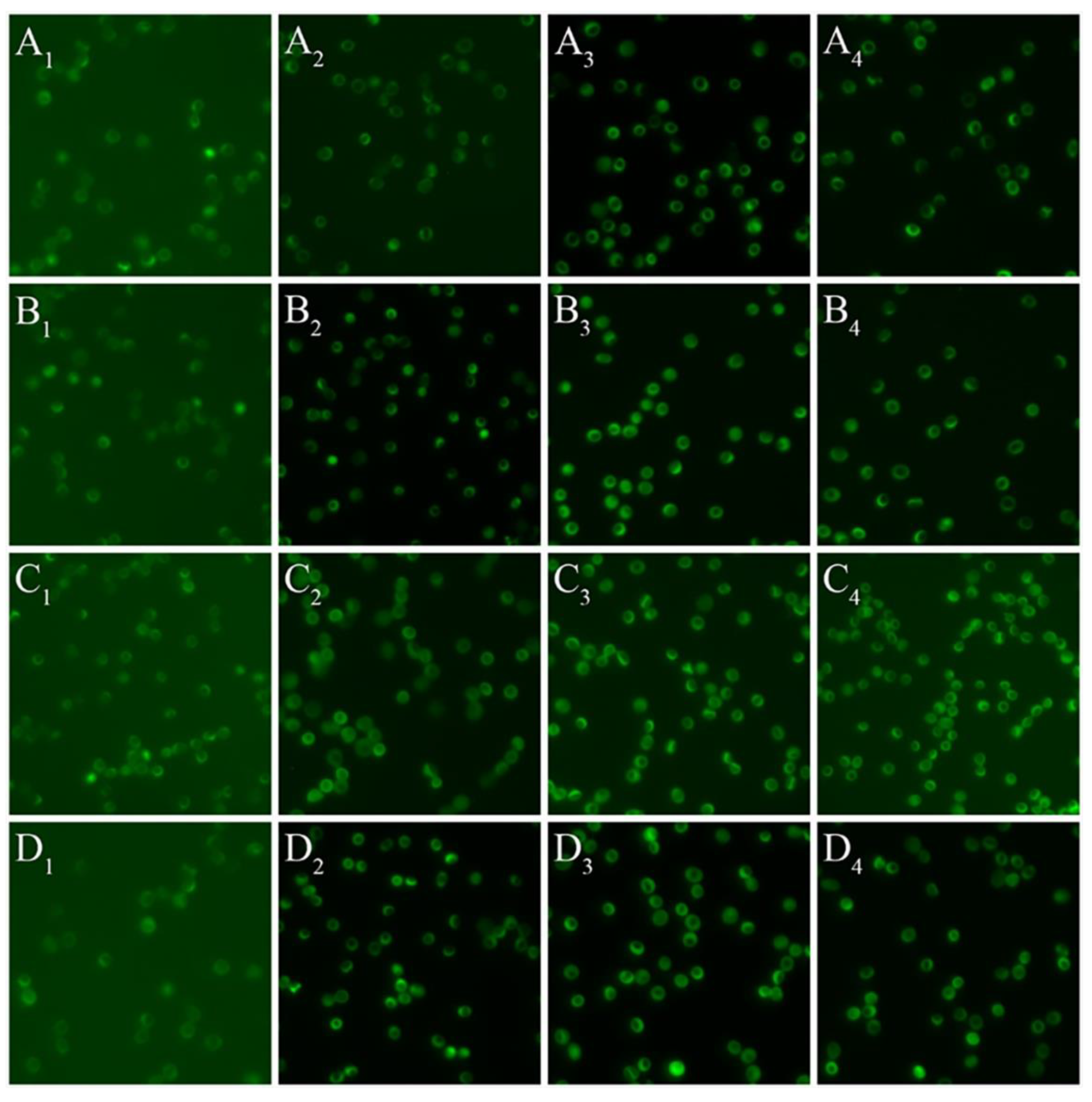
2.4. Red Blood Cell Nitric Oxide Synthase Serine1177 Phosphorylation
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Whole Blood Viscosity
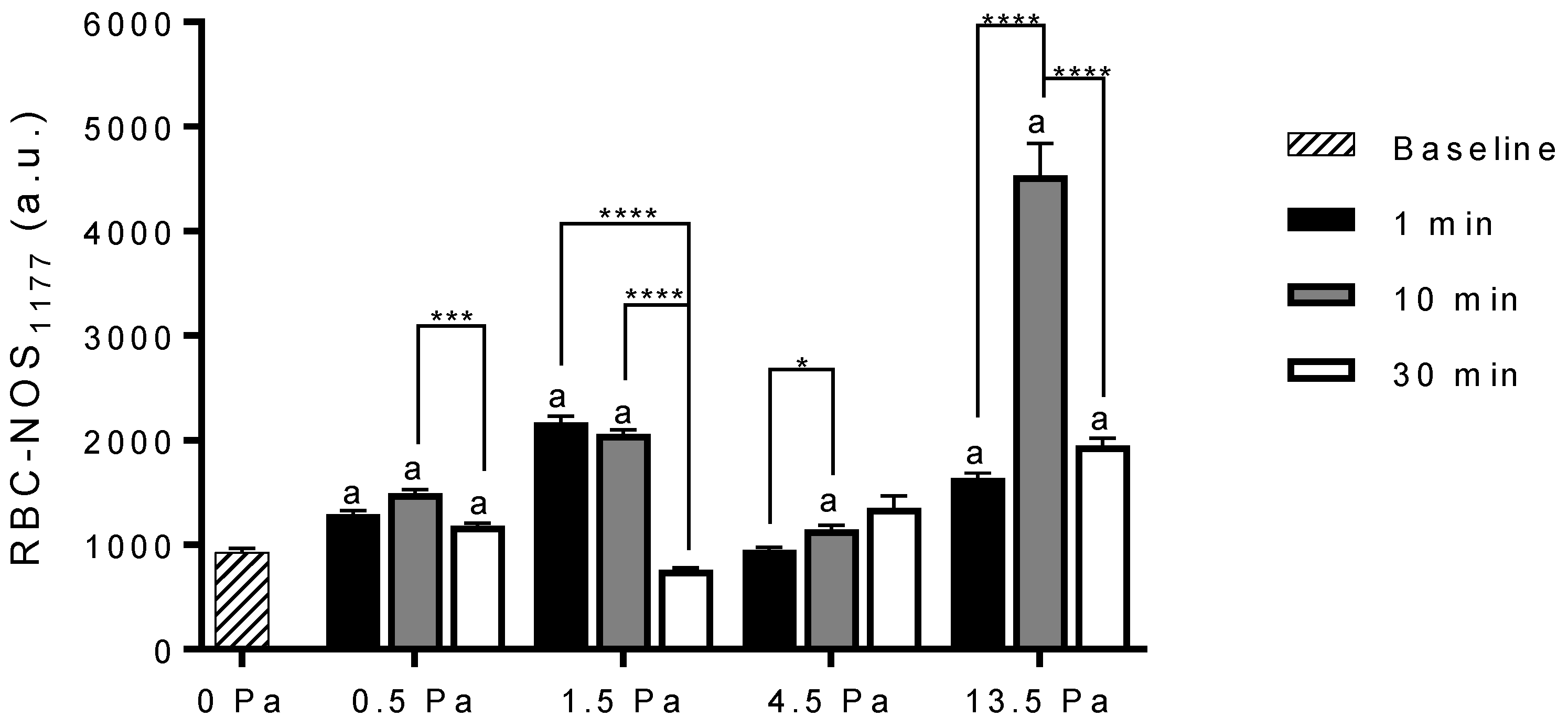
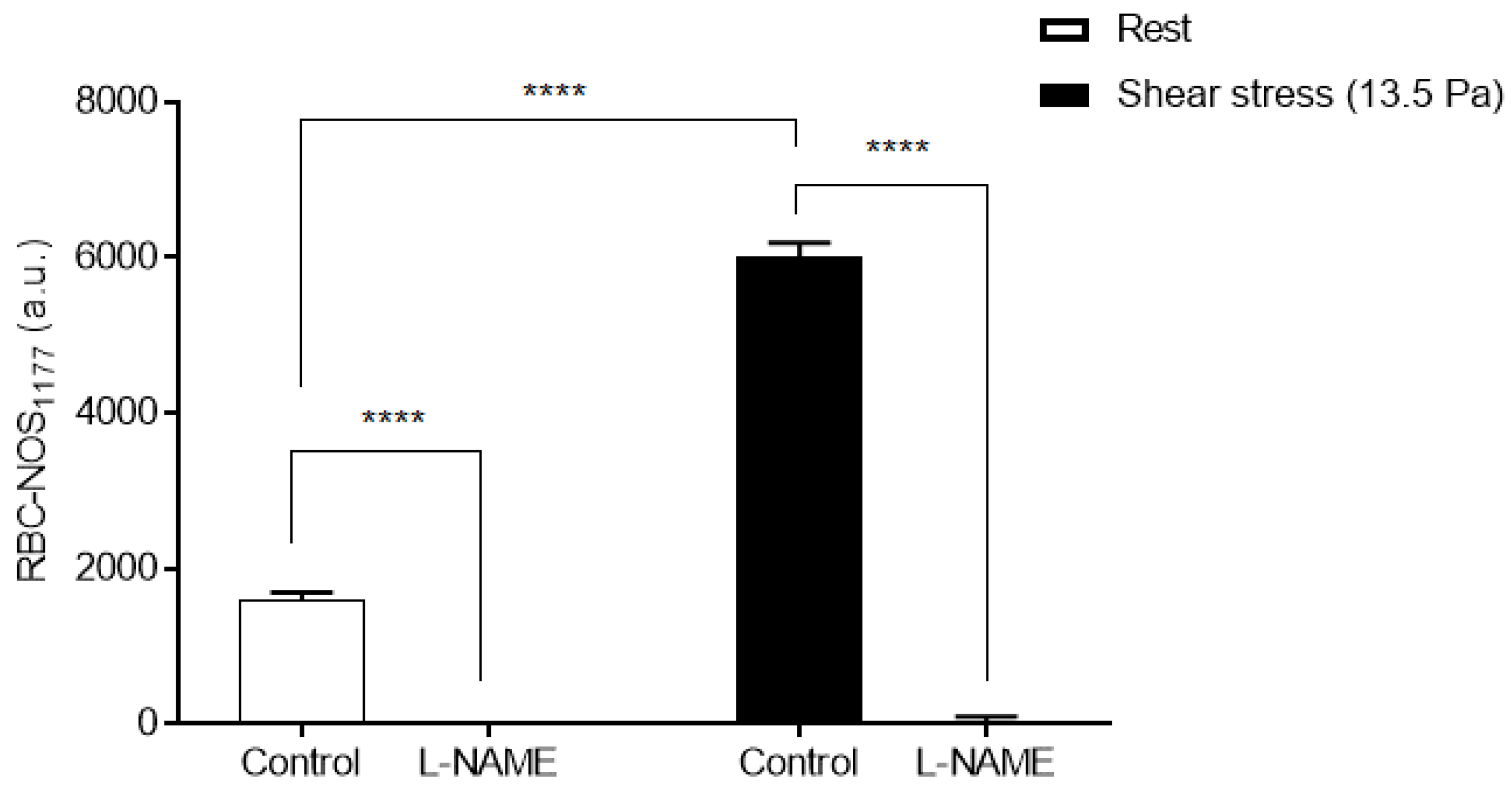
3.2. Red Blood Cell Nitric Oxide Synthase Serine1177 Phosphorylation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gow, A.J.; Ischiropoulos, H. Nitric oxide chemistry and cellular signaling. J. Cell Physiol. 2001, 187, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Owusu, B.Y.; Stapley, R.; Patel, R.P. Nitric oxide formation versus scavenging: The red blood cell balancing act. J. Physiol. 2012, 590, 4993–5000. [Google Scholar] [CrossRef] [PubMed]
- Eligini, S.; Porro, B.; Lualdi, A.; Squellerio, I.; Veglia, F.; Chiorino, E.; Crisci, M.; Garlaschè, A.; Giovannardi, M.; Werba, J.P.; et al. Nitric oxide synthetic pathway in red blood cells is impaired in coronary artery disease. PLoS ONE 2013, 8, e66945. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Kampoli, A.M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Schulz, R.; Rassaf, T.; Lauer, T.; Dejam, A.; Jax, T.; Kumara, I.; Gharini, P.; Kabanova, S.; Ozüyaman, B.; et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood 2006, 107, 2943–2951. [Google Scholar] [CrossRef]
- Grau, M.; Pauly, S.; Ali, J.; Walpurgis, K.; Thevis, M.; Bloch, W.; Suhr, F. RBC-NOS-dependent S-nitrosylation of cytoskeletal proteins improves RBC deformability. PLoS ONE 2013, 8, e56759. [Google Scholar] [CrossRef]
- Walder, J.A.; Chatterjee, R.; Steck, T.L.; Low, P.S.; Musso, G.F.; Kaiser, E.T.; Rogers, P.H.; Arnone, A. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J. Biol. Chem. 1984, 259, 10238–10246. [Google Scholar] [CrossRef]
- Jia, L.; Bonaventura, C.; Bonaventura, J.; Stamler, J.S. S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature 1996, 380, 221–226. [Google Scholar] [CrossRef]
- Stamler, J.S.; Jia, L.; Eu, J.P.; McMahon, T.J.; Demchenko, I.T.; Bonaventura, J.; Gernert, K.; Piantadosi, C.A. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 1997, 276, 2034–2037. [Google Scholar] [CrossRef]
- Pawloski, J.R.; Hess, D.T.; Stamler, J.S. Export by red blood cells of nitric oxide bioactivity. Nature 2001, 409, 622–626. [Google Scholar] [CrossRef]
- Wennmalm, A.; Benthin, G.; Petersson, A.S. Dependence of the metabolism of nitric oxide (NO) in healthy human whole blood on the oxygenation of its red cell haemoglobin. Br. J. Pharmacol. 1992, 106, 507–508. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Fukuto, J.M.; Griscavage, J.M.; Rogers, N.E.; Byrns, R.E. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: Comparison with enzymatically formed nitric oxide from L-arginine. Proc. Natl. Acad. Sci. USA 1993, 90, 8103–8107. [Google Scholar] [CrossRef] [PubMed]
- Eich, R.F.; Li, T.; Lemon, D.D.; Doherty, D.H.; Curry, S.R.; Aitken, J.F.; Mathews, A.J.; Johnson, K.A.; Smith, R.D.; Phillips, G.N., Jr.; et al. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry 1996, 35, 6976–6983. [Google Scholar] [CrossRef]
- Vitturi, D.A.; Teng, X.; Toledo, J.C.; Matalon, S.; Lancaster, J.R., Jr.; Patel, R.P. Regulation of nitrite transport in red blood cells by hemoglobin oxygen fractional saturation. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1398–H1407. [Google Scholar] [CrossRef]
- Grau, M.; Mozar, A.; Charlot, K.; Lamarre, Y.; Weyel, L.; Suhr, F.; Collins, B.; Jumet, S.; Hardy-Dessources, M.D.; Romana, M.; et al. High red blood cell nitric oxide synthase activation is not associated with improved vascular function and red blood cell deformability in sickle cell anaemia. Br. J. Haematol. 2015, 168, 728–736. [Google Scholar] [CrossRef]
- Nader, E.; Grau, M.; Fort, R.; Collins, B.; Cannas, G.; Gauthier, A.; Walpurgis, K.; Martin, C.; Bloch, W.; Poutrel, S.; et al. Hydroxyurea therapy modulates sickle cell anemia red blood cell physiology: Impact on RBC deformability, oxidative stress, nitrite levels and nitric oxide synthase signalling pathway. Nitric Oxide 2018, 81, 28–35. [Google Scholar] [CrossRef]
- Naumann, H.N.; Diggs, L.W.; Barreras, L.; Williams, B.J. Plasma hemoglobin and hemoglobin fractions in sickle cell crisis. Am. J. Clin. Pathol. 1971, 56, 137–147. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, E.A. Nitric oxide and the vascular endothelium. Handb. Exp. Pharmacol. 2006, 176 Pt 1, 213–254. [Google Scholar]
- Ongini, E.; Impagnatiello, F.; Bonazzi, A.; Guzzetta, M.; Govoni, M.; Monopoli, A.; Del Soldato, P.; Ignarro, L.J. Nitric oxide (NO)-releasing statin derivatives, a class of drugs showing enhanced antiproliferative and antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2004, 101, 8497–8502. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Mehta, J.L. Evidence for the presence of L-arginine-nitric oxide pathway in human red blood cells: Relevance in the effects of red blood cells on platelet function. J. Cardiovasc. Pharmacol. 1998, 32, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Ulker, P.; Gunduz, F.; Meiselman, H.J.; Baskurt, O.K. Nitric oxide generated by red blood cells following exposure to shear stress dilates isolated small mesenteric arteries under hypoxic conditions. Clin. Hemorheol. Microcirc. 2013, 54, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Bohmer, A.; Beckmann, B.; Sandmann, J.; Tsikas, D. Doubts concerning functional endothelial nitric oxide synthase in human erythrocytes. Blood 2012, 119, 1322–1323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ulker, P.; Yaras, N.; Yalcin, O.; Celik-Ozenci, C.; Johnson, P.C.; Meiselman, H.J.; Baskurt, O.K. Shear stress activation of nitric oxide synthase and increased nitric oxide levels in human red blood cells. Nitric Oxide 2011, 24, 184–191. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Rodriguez-Mateos, A.; Sansone, R.; Kuhnle, G.G.; Thasian-Sivarajah, S.; Krenz, T.; Horn, P.; Krisp, C.; Wolters, D.; Heiss, C.; et al. Human red blood cells at work: Identification and visualization of erythrocytic eNOS activity in health and disease. Blood 2012, 120, 4229–4237. [Google Scholar] [CrossRef]
- Boo, Y.C.; Sorescu, G.; Boyd, N.; Shiojima, I.; Walsh, K.; Du, J.; Jo, H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: Role of protein kinase A. J. Biol. Chem. 2002, 277, 3388–3396. [Google Scholar] [CrossRef]
- Michel, T.; Feron, O. Nitric oxide synthases: Which, where, how, and why? J. Clin. Investig. 1997, 100, 2146–2152. [Google Scholar] [CrossRef]
- Garcin, E.D.; Bruns, C.M.; Lloyd, S.J.; Hosfield, D.J.; Tiso, M.; Gachhui, R.; Stuehr, D.J.; Tainer, J.A.; Getzoff, E.D. Structural basis for isozyme-specific regulation of electron transfer in nitric-oxide synthase. J. Biol. Chem. 2004, 279, 37918–37927. [Google Scholar] [CrossRef]
- Burgering, B.M.; Coffer, P.J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 1995, 376, 599–602. [Google Scholar] [CrossRef]
- Suhr, F.; Brenig, J.; Muller, R.; Behrens, H.; Bloch, W.; Grau, M. Moderate exercise promotes human RBC-NOS activity, NO production and deformability through Akt kinase pathway. PLoS ONE 2012, 7, e45982. [Google Scholar] [CrossRef] [PubMed]
- Stimpel, M.; Neyses, L.; Locher, R.; Groth, H.; Vetter, W. Human red blood cells--an ideal model system for the action of calcium agonists and antagonists. J. Hypertens. Suppl. 1984, 2, S577–S580. [Google Scholar] [PubMed]
- Lin, M.S.; Huang, C.S.; Leen, D.Y. Lack of effects of calcium antagonists on red blood cell deformability in hypertension. Int. J. Clin. Pharmacol. Ther. Toxicol. 1988, 26, 585–587. [Google Scholar] [PubMed]
- Koutsiaris, A.G.; Tachmitzi, S.V.; Batis, N. Wall shear stress quantification in the human conjunctival pre-capillary arterioles in vivo. Microvasc. Res. 2013, 85, 34–39. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Stefanadis, C. Vascular wall shear stress: Basic principles and methods. Hellenic. J. Cardiol. 2005, 46, 9–15. [Google Scholar]
- Horobin, J.T.; Simmonds, M.J.; Nandakumar, D.; Gregory, S.D.; Tansley, G.; Pauls, J.P.; Girnghuber, A.; Balletti, N.; Fraser, J.F. Speed Modulation of the HeartWare HVAD to Assess In Vitro Hemocompatibility of Pulsatile and Continuous Flow Regimes in a Rotary Blood Pump. Artif. Organs 2018, 42, 879–890. [Google Scholar] [CrossRef]
- Grau, M.; Cremer, J.M.; Schmeichel, S.; Kunkel, M.; Bloch, W. Comparisons of Blood Parameters, Red Blood Cell Deformability and Circulating Nitric Oxide Between Males and Females Considering Hormonal Contraception: A Longitudinal Gender Study. Front. Physiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Horobin, J.T.; Sabapathy, S.; Simmonds, M.J. Repetitive Supra-Physiological Shear Stress Impairs Red Blood Cell Deformability and Induces Hemolysis. Artif. Organs 2017, 41, 1017–1025. [Google Scholar] [CrossRef]
- Hardeman, M.R.; Goedhart, P.; Breederveld, D. Laser diffraction ellipsometry of erythrocytes under controlled shear stress using a rotational viscosimeter. Clin. Chim. Acta 1987, 165, 227–234. [Google Scholar] [CrossRef]
- Fischer, U.M.; Schindler, R.; Brixius, K.; Mehlhorn, U.; Bloch, W. Extracorporeal circulation activates endothelial nitric oxide synthase in erythrocytes. Ann. Thorac. Surg. 2007, 84, 2000–2003. [Google Scholar] [CrossRef]
- Curran, R.C.; Gregory, J. The unmasking of antigens in paraffin sections of tissue by trypsin. Experientia 1977, 33, 1400–1401. [Google Scholar] [CrossRef] [PubMed]
- Bogen, S.A.; Vani, K.; Sompuram, S.R. Molecular mechanisms of antigen retrieval: Antigen retrieval reverses steric interference caused by formalin-induced cross-links. Biotech. Histochem. 2009, 84, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.M.; van Noorden, S. Introduction to Immunocytochemistry, 2nd ed.; Springer: Oxford, UK, 1997. [Google Scholar]
- Kuck, L.; Grau, M.; Bloch, W.; Simmonds, M.J. Shear Stress Ameliorates Superoxide Impairment to Erythrocyte Deformability with Concurrent Nitric Oxide Synthase Activation. Front. Physiol. 2019, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.; Nordsletten, D.; Umeton, R.; Yankama, B.; Ayyadurai, S.; Garcia-Cardena, G.; Dewey, C.F., Jr. In silico modeling of shear-stress-induced nitric oxide production in endothelial cells through systems biology. Biophys. J. 2013, 104, 2295–2306. [Google Scholar] [CrossRef]
- Shin, S.; Mohan, S.; Fung, H.L. Intracellular L-arginine concentration does not determine NO production in endothelial cells: Implications on the “L-arginine paradox”. Biochem. Biophys. Res. Commun. 2011, 414, 660–663. [Google Scholar] [CrossRef]
- Omodeo-Sale, F.; Cortelezzi, L.; Vommaro, Z.; Scaccabarozzi, D.; Dondorp, A.M. Dysregulation of L-arginine metabolism and bioavailability associated to free plasma heme. Am. J. Physiol. Cell. Physiol. 2010, 299, C148–C154. [Google Scholar] [CrossRef]
- Fujiwara, T.; Kanazawa, S.; Ichibori, R.; Tanigawa, T.; Magome, T.; Shingaki, K.; Miyata, S.; Tohyama, M.; Hosokawa, K. L-arginine stimulates fibroblast proliferation through the GPRC6A-ERK1/2 and PI3K/Akt pathway. PLoS ONE 2014, 9, e92168. [Google Scholar] [CrossRef]
- Xiao, H.; Zeng, L.; Shao, F.; Huang, B.; Wu, M.; Tan, B.; Yin, Y. The role of nitric oxide pathway in arginine transport and growth of IPEC-1 cells. Oncotarget 2017, 8, 29976–29983. [Google Scholar] [CrossRef][Green Version]
- Kopincova, J.; Puzserova, A.; Bernatova, I. Biochemical aspects of nitric oxide synthase feedback regulation by nitric oxide. Interdis. Toxicol. 2011, 4, 63–68. [Google Scholar] [CrossRef]
- Ravi, K.; Brennan, L.A.; Levic, S.; Ross, P.A.; Black, S.M. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc. Natl. Acad. Sci. USA 2004, 101, 2619–2624. [Google Scholar] [CrossRef]
- Huang, B.; Chen, S.C.; Wang, D.L. Shear flow increases S-nitrosylation of proteins in endothelial cells. Cardiovasc. Res. 2009, 83, 536–546. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ischiropoulos, H. Biological tyrosine nitration: A pathophysiological function of nitric oxide and reactive oxygen species. Arch. Biochem. Biophys. 1998, 356, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Van der Vliet, A.; Hoen, P.A.; Wong, P.S.; Bast, A.; Cross, C.E. Formation of S-nitrosothiols via direct nucleophilic nitrosation of thiols by peroxynitrite with elimination of hydrogen peroxide. J. Biol. Chem. 1998, 273, 30255–30262. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.B.; Eiserich, J.P.; Chumley, P.H.; Jablonsky, M.J.; Krishna, N.R.; Kirk, M.; Barnes, S.; Darley-Usmar, V.M.; Freeman, B.A. Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem. Res. Toxicol. 1999, 12, 83–92. [Google Scholar] [CrossRef]
- Horobin, J.T.; Watanabe, N.; Hakozaki, M.; Sabapathy, S.; Simmonds, M.J. Shear-stress mediated nitric oxide production within red blood cells: A dose-response. Clin. Hemorheol. Microcirc. 2019, 71, 203–214. [Google Scholar] [CrossRef]
- Secomb, T.W. Theoretical models for regulation of blood flow. Microcirculation 2008, 15, 765–775. [Google Scholar] [CrossRef]
- Duling, B.R.; Berne, R.M. Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ. Res. 1970, 27, 669–678. [Google Scholar] [CrossRef]
- Popel, A.S.; Pittman, R.N.; Ellsworth, M.L.; Weerappuli, D.P. Measurements of oxygen flux from arterioles imply high permeability of perfused tissue to oxygen. Adv. Exp. Med. Biol. 1989, 248, 215–225. [Google Scholar]
- Torres Filho, I.P.; Kerger, H.; Intaglietta, M. pO2 measurements in arteriolar networks. Microvasc. Res. 1996, 51, 202–212. [Google Scholar] [CrossRef]
- Bauer, P.M.; Fulton, D.; Boo, Y.C.; Sorescu, G.P.; Kemp, B.E.; Jo, H.; Sessa, W.C. Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J. Biol. Chem. 2003, 278, 14841–14849. [Google Scholar] [CrossRef] [PubMed]
- Dudzinski, D.M.; Michel, T. Life history of eNOS: Partners and pathways. Cardiovasc. Res. 2007, 75, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Michell, B.J.; Harris, M.B.; Chen, Z.P.; Ju, H.; Venema, V.J.; Blackstone, M.A.; Huang, W.; Venema, R.C.; Kemp, B.E. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J. Biol. Chem. 2002, 277, 42344–42351. [Google Scholar] [CrossRef] [PubMed]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Fleming, I.; Fisslthaler, B.; Dimmeler, S.; Kemp, B.E.; Busse, R. Phosphorylation of Thr regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ. Res. 2001, 88, E68–E75. [Google Scholar] [CrossRef]
- Harris, M.B.; Ju, H.; Venema, V.J.; Liang, H.; Zou, R.; Michell, B.J.; Chen, Z.P.; Kemp, B.E.; Venema, R.C. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J. Biol. Chem. 2001, 276, 16587–16591. [Google Scholar] [CrossRef]
- Michell, B.J.; Chen, Z.; Tiganis, T.; Stapleton, D.; Katsis, F.; Power, D.A.; Sim, A.T.; Kemp, B.E. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J. Biol. Chem. 2001, 276, 17625–17628. [Google Scholar] [CrossRef]
- Thomas, S.R.; Chen, K.; Keaney, J.F., Jr. Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J. Biol. Chem. 2002, 277, 6017–6024. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horobin, J.T.; Sabapathy, S.; Kuck, L.; Simmonds, M.J. Shear Stress and RBC-NOS Serine1177 Phosphorylation in Humans: A Dose Response. Life 2021, 11, 36. https://doi.org/10.3390/life11010036
Horobin JT, Sabapathy S, Kuck L, Simmonds MJ. Shear Stress and RBC-NOS Serine1177 Phosphorylation in Humans: A Dose Response. Life. 2021; 11(1):36. https://doi.org/10.3390/life11010036
Chicago/Turabian StyleHorobin, Jarod T., Surendran Sabapathy, Lennart Kuck, and Michael J. Simmonds. 2021. "Shear Stress and RBC-NOS Serine1177 Phosphorylation in Humans: A Dose Response" Life 11, no. 1: 36. https://doi.org/10.3390/life11010036
APA StyleHorobin, J. T., Sabapathy, S., Kuck, L., & Simmonds, M. J. (2021). Shear Stress and RBC-NOS Serine1177 Phosphorylation in Humans: A Dose Response. Life, 11(1), 36. https://doi.org/10.3390/life11010036




