Suppression of the Swallowing Reflex during Rhythmic Jaw Movements Induced by Repetitive Electrical Stimulation of the Dorsomedial Part of the Central Amygdaloid Nucleus in Rats
Abstract
1. Introduction
2. Materials and Methods
3. Results
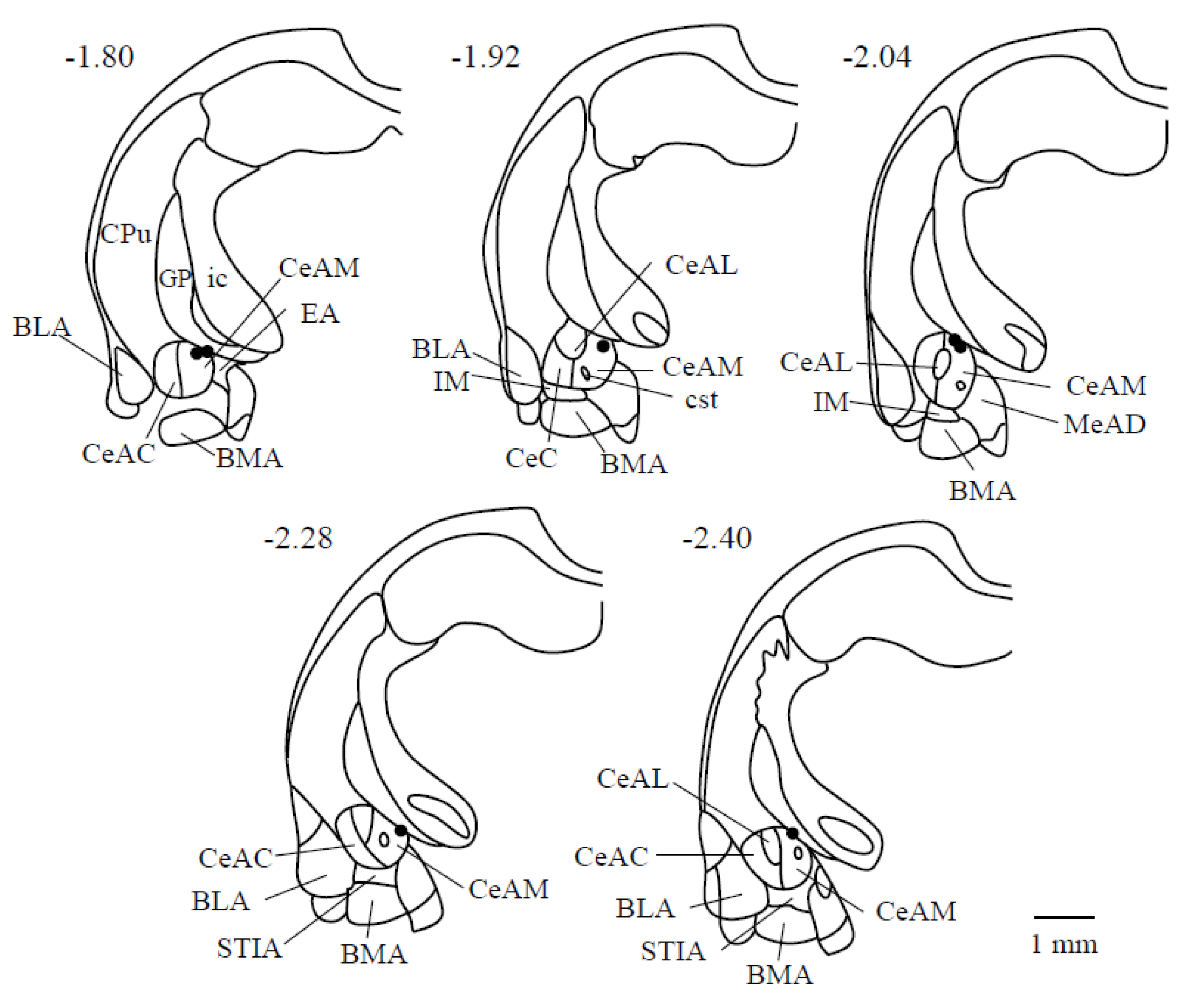
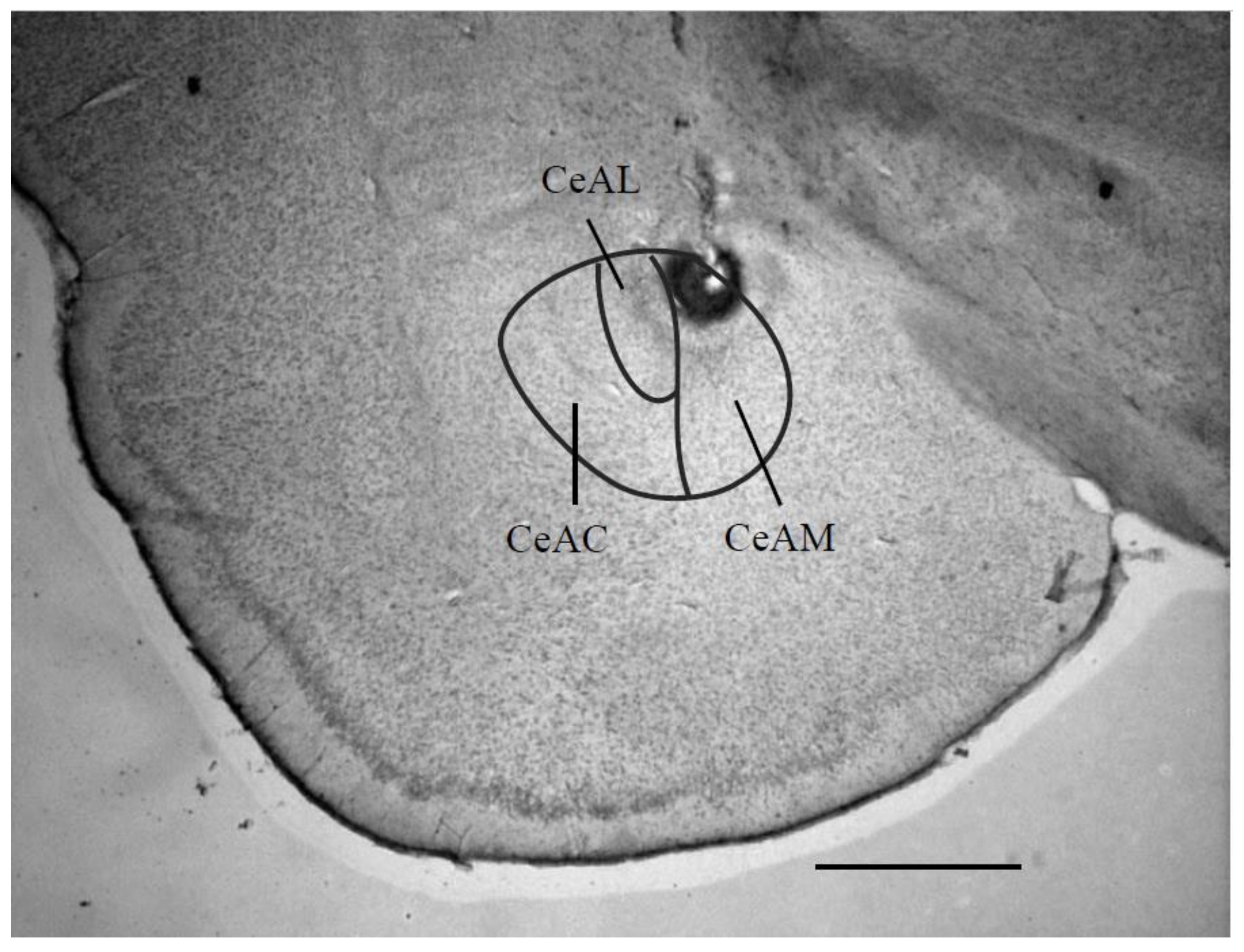
3.1. Electrical Stimulation Sites in the CeA
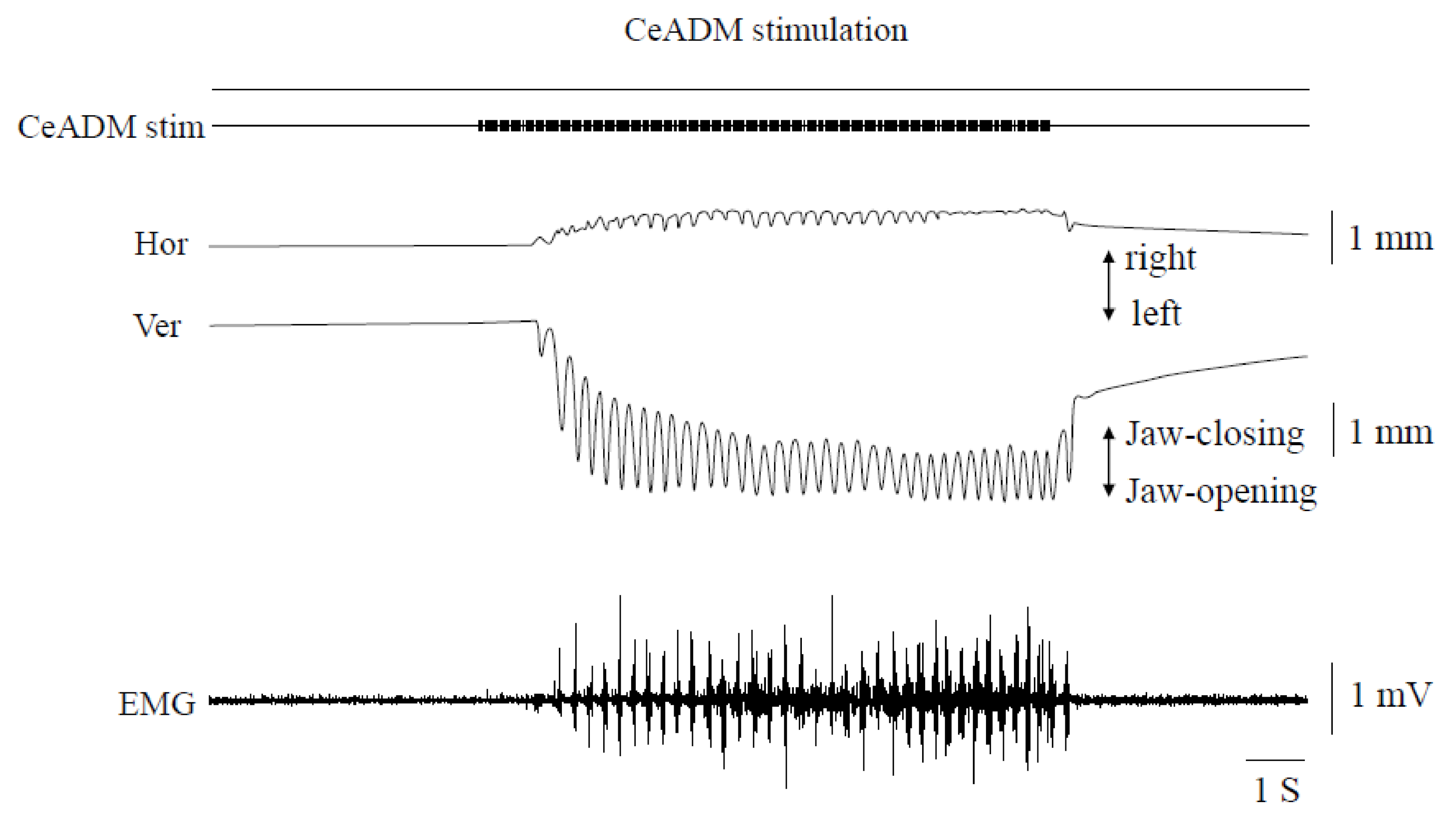
3.2. Rhythmic Jaw Movements
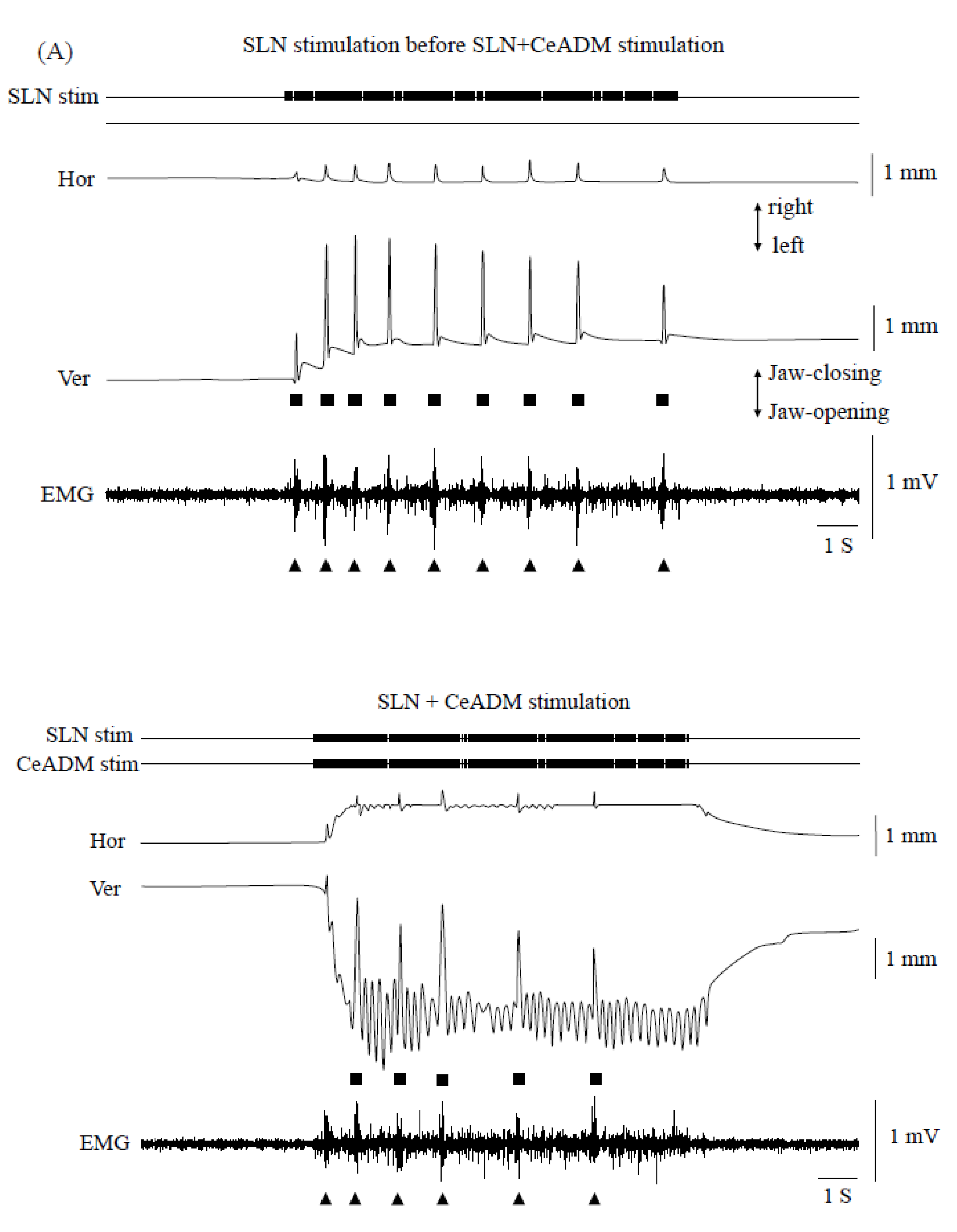
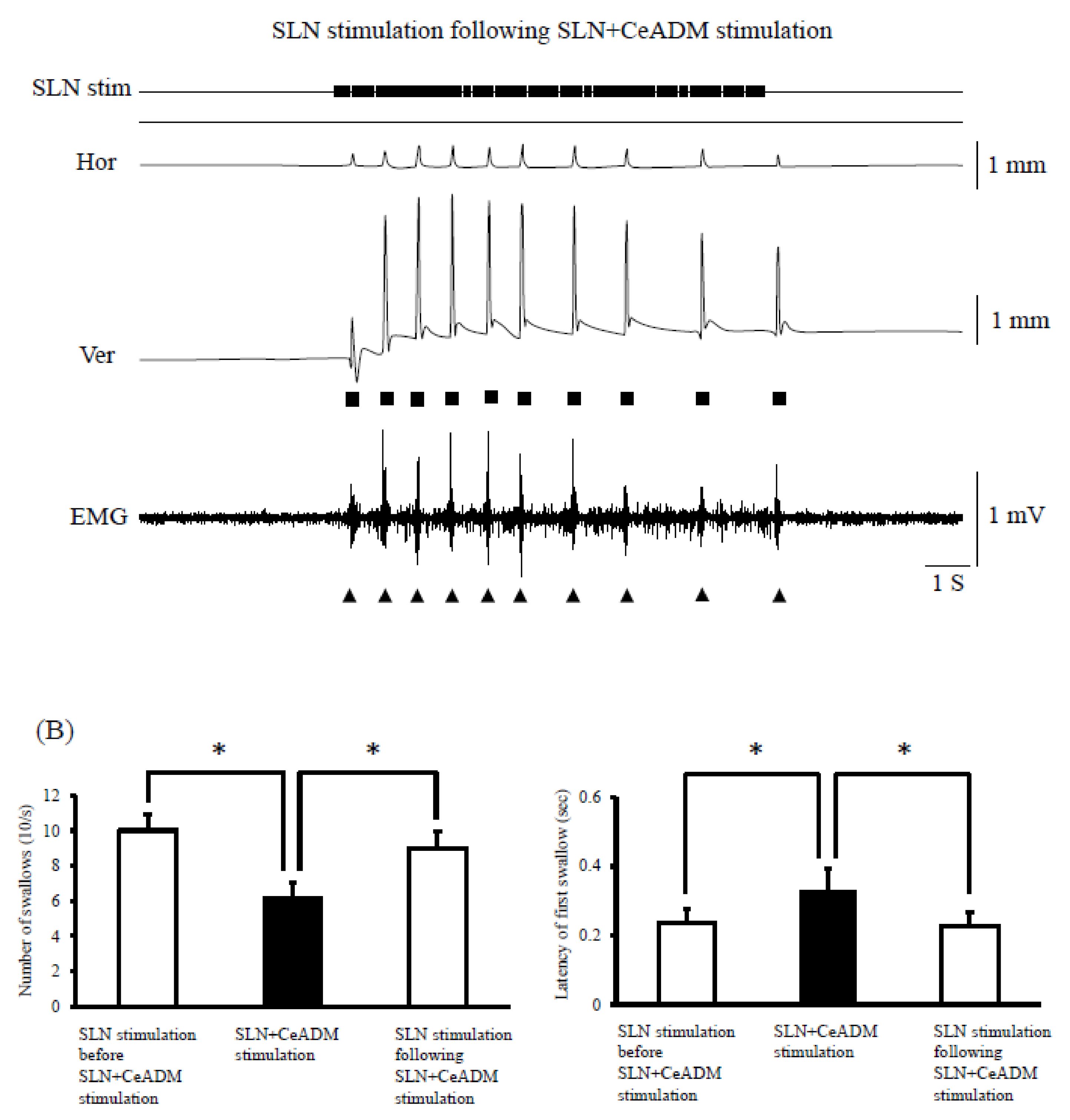
3.3. Swallowing Reflex
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Sasamoto, K.; Zhang, G.; Iwasaki, M. Two types of rhythmical jaw movements evoked by stimulation of the rat cortex. Jpn. J. Oral Biol. 1990, 32, 57–68. [Google Scholar] [CrossRef]
- Satoh, Y.; Ishizuka, K.; Murakami, T. Modulation of cortically induced rhythmical jaw movements by stimulation of the red nucleus in the rat. Brain Res. 2006, 1087, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sasamoto, K. Projections of two separate cortical areas for rhythmical jaw movements in the rat. Brain Res. Bull. 1990, 24, 221–230. [Google Scholar] [CrossRef]
- Nakamura, Y.; Katakura, N. Generation of masticatory rhythm in the brainstem. Neurosci. Res. 1995, 23, 1–19. [Google Scholar] [CrossRef]
- Lund, J.P.; Kolta, A. Generation of the central masticatory pattern and its modification by sensory feedback. Dysphagia 2006, 21, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Hiiemae, K.M.; Palmer, J.B. Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia 1999, 14, 31–42. [Google Scholar] [CrossRef]
- Palmer, J.B.; Rudin, N.J.; Lara, G.; Crompton, A.W. Coordination of mastication and swallowing. Dysphagia 1992, 7, 187–200. [Google Scholar] [CrossRef]
- Jean, A. Brainstem organization of the swallowing network. Brain Behav. Evol. 1984, 25, 109–116. [Google Scholar] [CrossRef]
- Jean, A. Control of the central swallowing program by inputs from the peripheral receptors, a review. J. Auton. Nerv. Syst. 1984, 10, 225–233. [Google Scholar] [CrossRef]
- Jean, A. Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiol. Rev. 2001, 81, 929–969. [Google Scholar] [CrossRef]
- Tsujimura, T.; Tsuji, K.; Ariyasinghe, S.; Fukuhara, T.; Yamada, A.; Hayashi, H.; Nakamura, Y.; Iwata, K.; Inoue, M. Differential involvement of two cortical masticatory areas in modulation of the swallowing reflex in rats. Neurosci. Lett. 2012, 528, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Ciocchi, S.; Herry, C.; Grenier, F.; Wolff, S.B.; Letzkus, J.J.; Vlachos, I.; Ehrlich, I.; Sprengel, R.; Deisseroth, K.; Stadler, M.B.; et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 2010, 468, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Penzo, M.A.; Taniguchi, H.; Kopec, C.D.; Huang, Z.; Li, B. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci. 2013, 16, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Botta, P.; Demmou, L.; Kasugai, Y.; Markovic, M.; Xu, C.; Fadok, J.P.; Lu, T.; Poe, M.M.; Xu, L.; Cook, J.M.; et al. Regulating anxiety with extrasynaptic inhibition. Nat. Neurosci. 2015, 18, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.O.; Funderburk, S.C.; Bhatti, D.L.; Motard, L.E.; Newbold, D.; Girven, K.S.; McCall, J.G.; Krashes, M.; Sparta, D.R.; Bruchas, M.R. A GABAergic projection from the centromedial nuclei of the amygdala to ventromedial prefrontal cortex modulates reward behavior. J. Neurosci. 2016, 36, 10831–10842. [Google Scholar] [CrossRef]
- Mahler, S.V.; Berridge, K.C. Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J. Neurosci. 2009, 29, 6500–6513. [Google Scholar] [CrossRef]
- Cai, H.; Haubensak, W.; Anthony, T.E.; Anderson, D.J. Central amygdala PKC-δ (+) neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci. 2014, 17, 1240–1248. [Google Scholar] [CrossRef]
- Fortaleza, E.A.T.; Scopinho, A.A.; de Aguiar Corrêa, F.M. Cardiovascular responses to microinjection of noradrenaline into the medial amygdaloid nucleus of conscious rats result from α2-receptor activation and vasopressin release. Eur. J. Neurosci. 2011, 33, 1677–1684. [Google Scholar] [CrossRef]
- Kubo, T.; Okatani, H.; Nishigori, Y.; Hagiwara, Y.; Fukumori, R.; Goshima, Y. Involvement of the medial amygdaloid nucleus in restraint stress-induced pressor responses in rats. Neurosci. Lett. 2004, 354, 84–86. [Google Scholar] [CrossRef]
- Morilak, D.A.; Barrera, G.; Echevarria, D.J.; Garcia, A.S.; Hernandez, A.; Ma, S.; Petre, C.O. Role of brain norepinephrine in the behavioral response to stress. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 1214–1224. [Google Scholar] [CrossRef]
- Kaku, T. Functional differentiation of hypoglossal motoneurons during the amygdaloid or cortically induced rhythmical jaw and tongue movements in the rat. Brain Res. Bull. 1984, 13, 147–154. [Google Scholar] [CrossRef]
- Ohta, M. Amygdaloid and cortical facilitation or inhibition of trigeminal motoneurons in the rat. Brain Res. 1984, 291, 39–48. [Google Scholar] [CrossRef]
- Sasamoto, K.; Ohta, M. Amygdaliod-induced jaw opening and facilitation or inhibition of the trigeminal motoneurons in the rat. Comp. Biochem. Physiol. 1982, 73A, 349–354. [Google Scholar] [CrossRef]
- Petrov, T.; Jhamandas, J.H.; Krukoff, T.L. Connectivity between brainstem autonomic structures and expression of c-fos following electrical stimulation of the central nucleus of the amygdala in rat. Cell Tissue Res. 1996, 283, 367–374. [Google Scholar] [CrossRef]
- Rogers, R.C.; Fryman, D.L. Direct connections between the central nucleus of the amygdala and the nucleus of the solitary tract: An electrophysiological study in the rat. J. Auton. Nerv. Syst. 1988, 22, 83–87. [Google Scholar] [CrossRef]
- Saha, S.; Batten, T.F.; Henderson, Z. A GABAergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: A combined anterograde tracing and electron microscopic immunohistochemical study. Neuroscience 2000, 99, 613–626. [Google Scholar] [CrossRef]
- Gasparini, S.; Howland, J.M.; Thatcher, A.J.; Geerling, J.C. Central afferents to the nucleus of the solitary tract in rats and mice. J. Comp. Neurol. 2020. [Google Scholar] [CrossRef]
- Thompson, R.L.; Cassell, M.D. Differential distribution and non-collateralization of central amygdaloid neurons projecting to different medullary regions. Neurosci. Lett. 1989, 97, 245–251. [Google Scholar] [CrossRef]
- Hopkins, D.A.; Holstege, G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp. Brain Res. 1978, 32, 529–547. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Mostafeezur, R.M.; Zakir, H.M.; Takatsuji, H.; Yamada, Y.; Yamamura, K.; Kitagawa, J. Cannabinoids facilitate the swallowing reflex elicited by the superior laryngeal nerve stimulation in rats. PLoS ONE 2012, 7, e50703. [Google Scholar] [CrossRef]
- Tsuji, K.; Tsujimura, T.; Magara, J.; Sakai, S.; Nakamura, Y.; Inoue, M. Changes in the frequency of swallowing during electrical stimulation of superior laryngeal nerve in rats. Brain Res. Bull. 2015, 111, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Takakusaki, K.; Kita, S.; Matsuda, M.; Nonaka, S.; Sakamoto, T. Effects of injecting GABAergic agents into the medullary reticular formation upon swallowing induced by the superior laryngeal nerve stimulation in decerebrate cats. Neurosci. Res. 2005, 51, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Marowsky, A.; Fritschy, J.-M.; Vogt, K.E. Functional mapping of GABAA receptor subtypes in the amygdala. Eur. J. Neurosci. 2004, 20, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, N.E.; Usunoff, K.G.; Schmitt, O.; Itzev, D.E.; Rolfs, A.; Wree, A. Amygdalotrigeminal projection in the rat: An anterograde tracing study. Ann. Anat. 2011, 193, 118–126. [Google Scholar] [CrossRef]
- Shirasu, M.; Takahashi, T.; Yamamoto, T.; Itoh, K.; Sato, S.; Nakamura, H. Direct projections from the central amygdaloid nucleus to the mesencephalic trigeminal nucleus in rats. Brain Res. 2011, 1400, 19–30. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Satoda, T.; Tashiro, T.; Matsuyama, R.; Uemura-Sumi, M. Amygdaloid pathway to the trigeminal motor nucleus via the pontine reticular formation in the rat. Brain Res. Bull. 1988, 21, 829–833. [Google Scholar] [CrossRef]
- Rokx, J.T.M.; Jüch, P.J.W.; van Willigen, J.D. Arrangement and connections of mesencephalic trigeminal neurons in the rat. Cells Tissues Organs 1986, 127, 7–15. [Google Scholar] [CrossRef]
- Rokx, J.T.M.; van Willigen, J.D.; Jüch, P.J.W. 1986b. Bilateral brainstem connections of the rat supratrigeminal region. Cells Tissues Organs 1986, 127, 16–21. [Google Scholar] [CrossRef]
- Ruggiero, D.A.; Ross, C.A.; Kumada, M.; Reis, D.J. Reevaluation of projections from the mesencephalic trigeminal nucleus to the medulla and spinal cord: New projections. A combined retrograde and anterograde horseradish peroxidase study. J. Comp. Neurol. 1986, 206, 278–292. [Google Scholar] [CrossRef]
- Guan, Z.L.; Ding, Y.Q.; Li, J.L.; Lu, B.Z. Substance P receptor-expressing neurons in the medullary and spinal dorsal horns projecting to the nucleus of the solitary tract in the rat. Neurosci. Res. 1998, 30, 213–218. [Google Scholar] [CrossRef]
- Menétrey, D.; Basbaum, A.I. Spinal and trigeminal projections to the nucleus of the solitary tract: A possible substrate for somatovisceral and viscerovisceral reflex activation. J. Comp. Neurol. 1987, 255, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Yamamoto, M.; Takeda, R.; Ohara, H.; Sato, F.; Akhter, F.; Haque, T.; Kato, T.; Sessle, B.J.; Takada, K.; et al. Jaw-opening and -closing premotoneurons in the nucleus of the solitary tract making contacts with laryngeal and pharyngeal afferent terminals in rats. Brain Res. 2013, 1540, 48–63. [Google Scholar] [CrossRef]
- Félix, B.; Jean, A.; Roman, C. Leptin inhibits swallowing in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R657–R663. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.T.; Bieger, D. Role of solitarial GABAergic mechanisms in control of swallowing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1991, 261, R639–R646. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, T.; Tsujimura, T.; Kajii, Y.; Yamamura, K.; Inoue, M. Effects of electrical stimulation of the superior laryngeal nerve on the jaw-opening reflex. Brain Res. 2011, 1391, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Appenteng, K.; Donga, R.; Williams, R.G. Morphological and electrophysiological determination of the projections of jaw-elevator muscle spindle afferents in rats. J. Physiol. 1985, 369, 93–113. [Google Scholar] [CrossRef]
- Chandler, S.H. Evidence for excitatory amino acid transmission between mesencephalic nucleus of V afferents and jaw-closer motoneurons in the guinea pig. Brain Res. 1989, 477, 252–264. [Google Scholar] [CrossRef]
- Kidokoro, Y.; Kubota, K.; Shuto, S.; Sumino, R. Possible interneurons responsible for reflex inhibition of motoneurons of jaw-closing muscles from the inferior dental nerve. J. Neurophysiol. 1968, 31, 709–716. [Google Scholar] [CrossRef]
- Goldberg, L.J.; Nakamura, Y. Lingually induced inhibition of masseteric motoneurones. Experientia 1978, 24, 371–373. [Google Scholar] [CrossRef]
- Rowlerson, A.M. Specialization of mammalian jaw muscles: Fibre type compositions and the distribution of muscle spindles. In Neurophysiology of the Jaws and Teeth; Taylor, A., Ed.; Macmillan: London, UK, 1990; pp. 1–51. [Google Scholar]
- Luo, P.F.; Li, J.S. Monosynaptic connections between neurons of trigeminal mesencephalic nucleus and jaw-closing motoneurons in the rat: An intracellular horseradish peroxidase labelling study. Brain Res. 1991, 559, 267–275. [Google Scholar] [CrossRef]
- Dessem, D.; Donga, R.; Luo, P. Primary- and secondary-like jaw-muscle spindle afferents have characteristic topographic distributions. J. Neurophysiol. 1997, 77, 2925–2944. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satoh, Y.; Tsuji, K. Suppression of the Swallowing Reflex during Rhythmic Jaw Movements Induced by Repetitive Electrical Stimulation of the Dorsomedial Part of the Central Amygdaloid Nucleus in Rats. Life 2020, 10, 190. https://doi.org/10.3390/life10090190
Satoh Y, Tsuji K. Suppression of the Swallowing Reflex during Rhythmic Jaw Movements Induced by Repetitive Electrical Stimulation of the Dorsomedial Part of the Central Amygdaloid Nucleus in Rats. Life. 2020; 10(9):190. https://doi.org/10.3390/life10090190
Chicago/Turabian StyleSatoh, Yoshihide, and Kojun Tsuji. 2020. "Suppression of the Swallowing Reflex during Rhythmic Jaw Movements Induced by Repetitive Electrical Stimulation of the Dorsomedial Part of the Central Amygdaloid Nucleus in Rats" Life 10, no. 9: 190. https://doi.org/10.3390/life10090190
APA StyleSatoh, Y., & Tsuji, K. (2020). Suppression of the Swallowing Reflex during Rhythmic Jaw Movements Induced by Repetitive Electrical Stimulation of the Dorsomedial Part of the Central Amygdaloid Nucleus in Rats. Life, 10(9), 190. https://doi.org/10.3390/life10090190



