Mapping of Photochemically-Derived Dityrosine across Fe-Bound N-Acetylated α-Synuclein
Abstract
1. Introduction
2. Materials and Methods
2.1. Transformation of Expression Strains for NAcαSyn Variants
2.2. Expression and Protein Purification
2.3. Photochemical Crosslinking of Unmodified Proteins (PICUP)
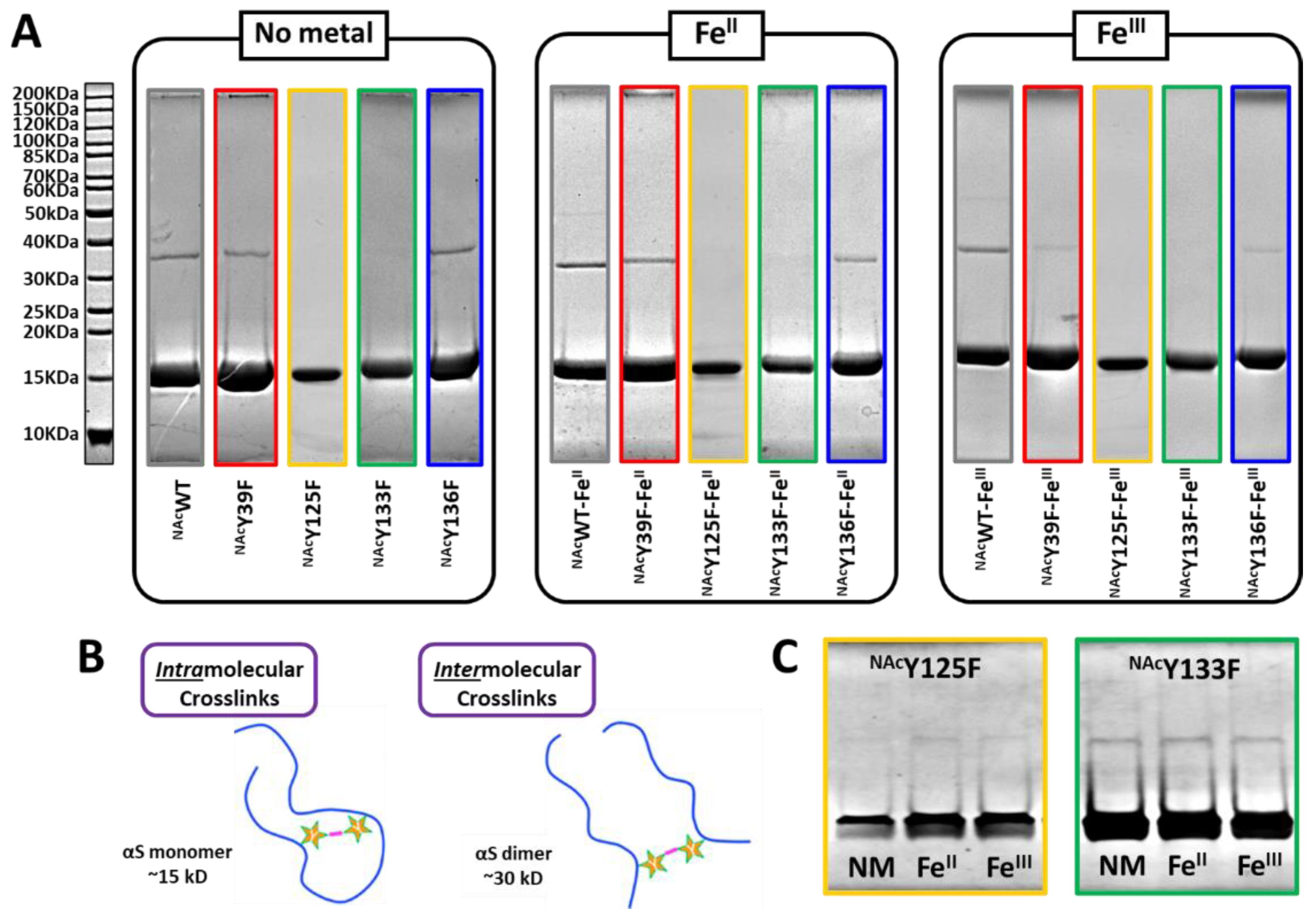
3. Results and Discussion
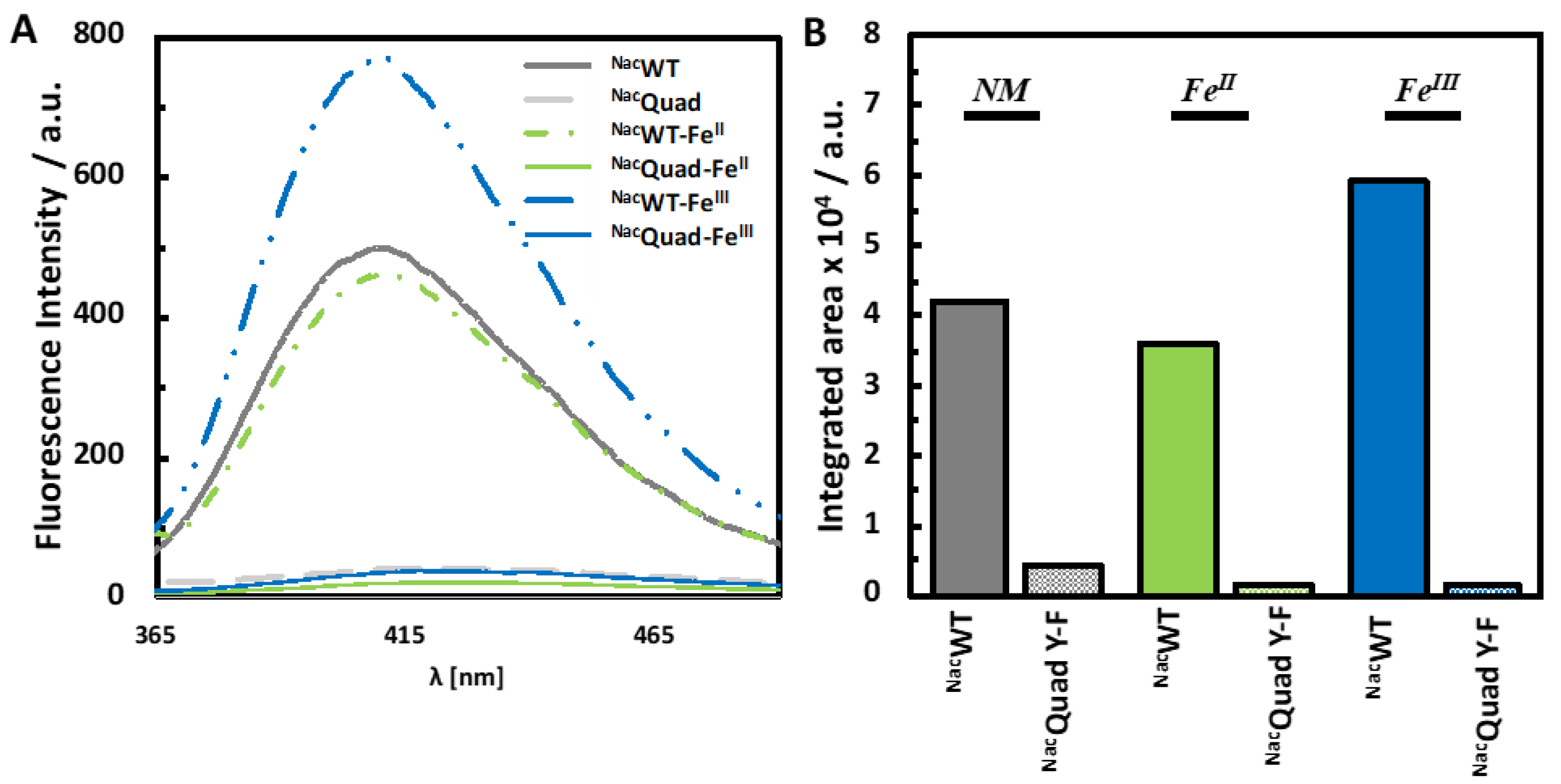
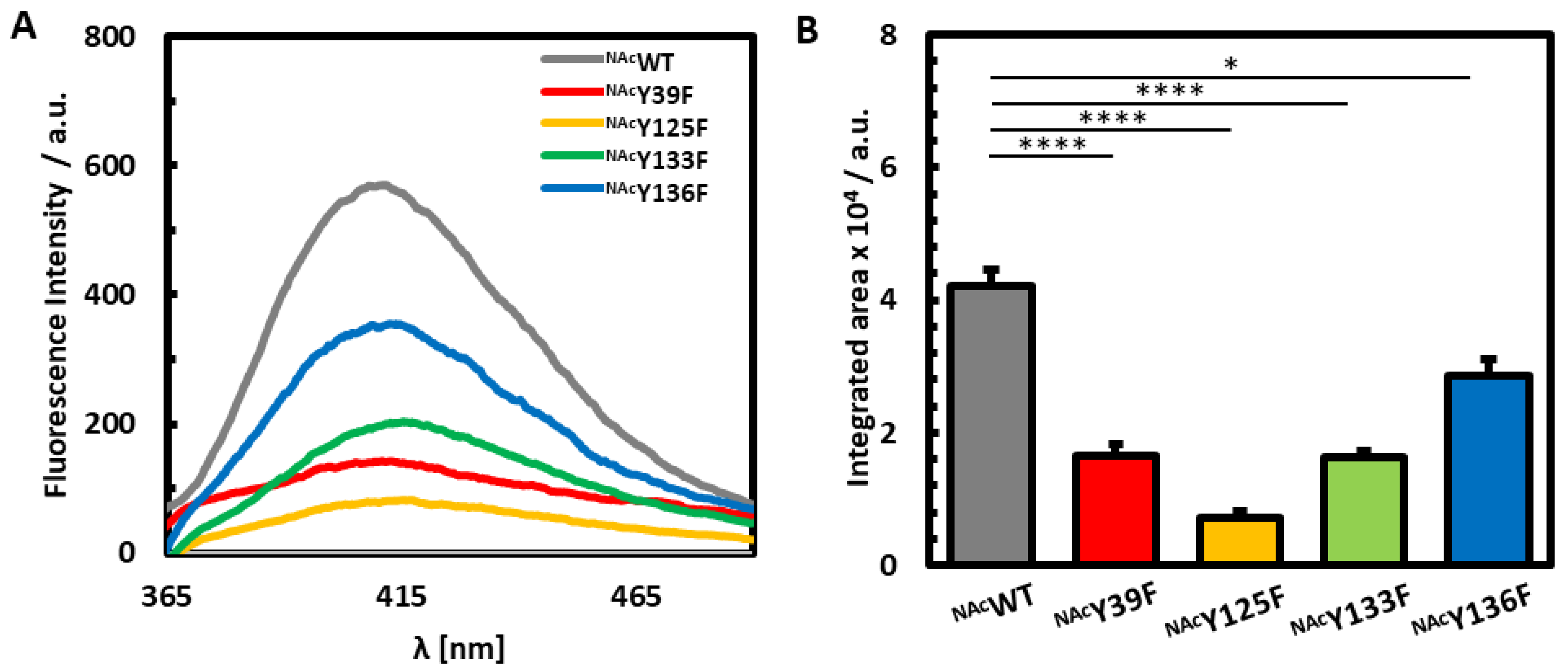
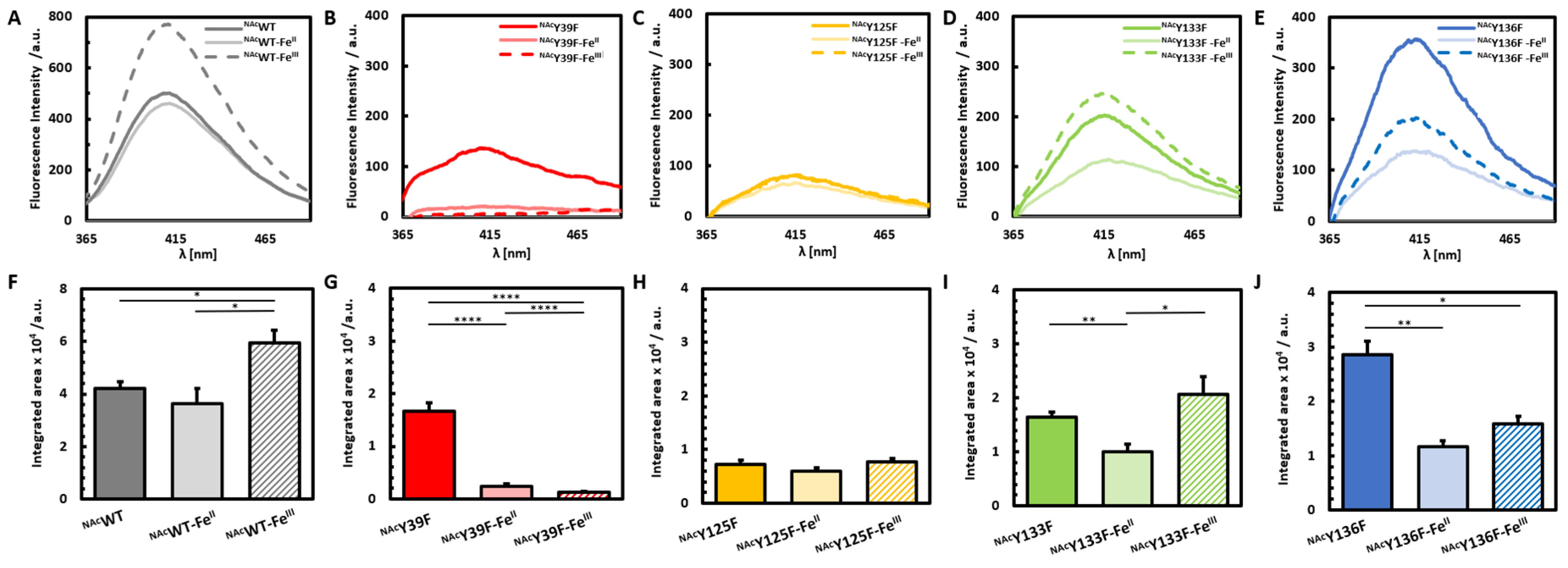
3.1. Photoinduced Dityrosine Formation of NAcαSyn Variants in the Absence of Iron
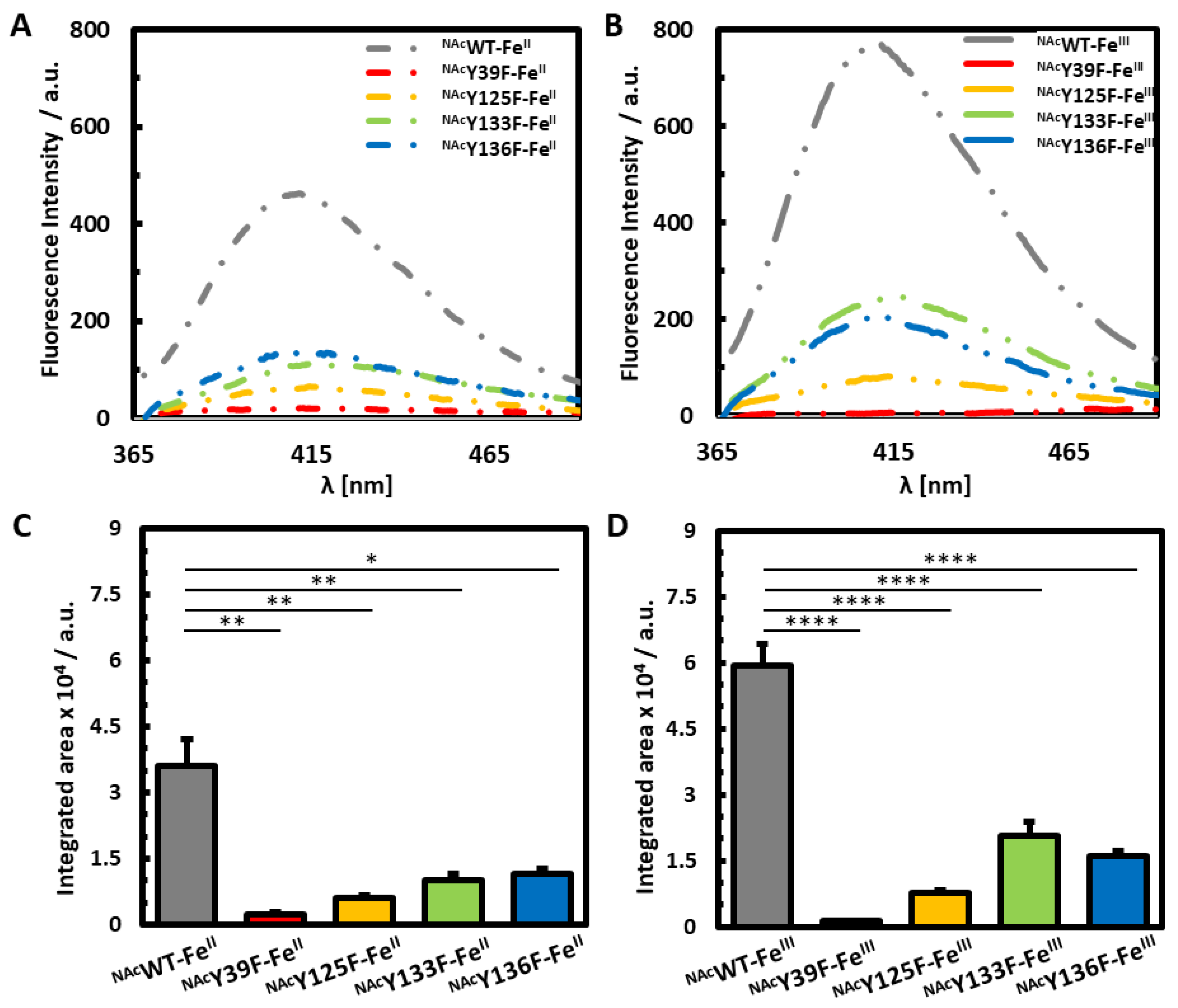
3.2. Photoinduced Dityrosine Formation of Fe-Bound NAcαSyn Variants
3.3. Gel Analysis of PICUP Reaction Products
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Brás, I.C.; Dominguez-Meijide, A.; Gerhardt, E.; Koss, D.; Lázaro, D.F.; Santos, P.I.; Vasili, E.; Xylaki, M.; Outeiro, T.F. Synucleinopathies: Where we are and where we need to go. J. Neurochem. 2020, 153, 433–454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xia, Y.; Wan, F.; Ma, K.; Guo, X.; Kou, L.; Yin, S.; Han, C.; Liu, L.; Huang, J.; et al. New Perspectives on Roles of Alpha-Synuclein in Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Rochet, J.-C.; Hay, B.A.; Guo, M. Chapter 5—Molecular Insights into Parkinson’s Disease. In Progress in Molecular Biology and Translational Science; Teplow, D.B., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 107, pp. 125–188. [Google Scholar]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Nakata, Y.; Mochizuki, H. α-Synuclein and Neuronal Cell Death. Mol. Neurobiol. 2013, 47, 466–483. [Google Scholar] [CrossRef]
- Coskuner, O.; Uversky, V.N. Chapter Six—Intrinsically disordered proteins in various hypotheses on the pathogenesis of Alzheimer’s and Parkinson’s diseases. In Progress in Molecular Biology and Translational Science; Uversky, V.N., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 166, pp. 145–223. [Google Scholar]
- Fauvet, B.; Mbefo, M.K.; Fares, M.B.; Desobry, C.; Michael, S.; Ardah, M.T.; Tsika, E.; Coune, P.; Prudent, M.; Lion, N.; et al. α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J. Biol. Chem. 2012, 287, 15345–15364. [Google Scholar] [CrossRef]
- Wu, K.-P.; Baum, J. Detection of Transient Interchain Interactions in the Intrinsically Disordered Protein α-Synuclein by NMR Paramagnetic Relaxation Enhancement. J. Am. Chem. Soc. 2010, 132, 5546–5547. [Google Scholar] [CrossRef]
- González, N.; Arcos-López, T.; König, A.; Quintanar, L.; Menacho Márquez, M.; Outeiro, T.F.; Fernández, C.O. Effects of alpha-synuclein post-translational modifications on metal binding. J. Neurochem. 2019, 150, 507–521. [Google Scholar] [CrossRef]
- Abeyawardhane, D.L.; Fernández, R.D.; Murgas, C.J.; Heitger, D.R.; Forney, A.K.; Crozier, M.K.; Lucas, H.R. Iron Redox Chemistry Promotes Antiparallel Oligomerization of α-Synuclein. J. Am. Chem. Soc. 2018, 140, 5028–5032. [Google Scholar] [CrossRef]
- Kang, L.; Moriarty, G.M.; Woods, L.A.; Ashcroft, A.E.; Radford, S.E.; Baum, J. N-terminal acetylation of α-synuclein induces increased transient helical propensity and decreased aggregation rates in the intrinsically disordered monomer. Protein Sci. 2012, 21, 911–917. [Google Scholar] [CrossRef]
- Bu, B.; Tong, X.; Li, D.; Hu, Y.; He, W.; Zhao, C.; Hu, R.; Li, X.; Shao, Y.; Liu, C.; et al. N-Terminal Acetylation Preserves α-Synuclein from Oligomerization by Blocking Intermolecular Hydrogen Bonds. ACS Chem. Neurosci. 2017, 8, 2145–2151. [Google Scholar] [CrossRef]
- Bendor, J.T.; Logan, T.P.; Edwards, R.H. The function of α-synuclein. Neuron 2013, 79, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.N.; Hirpa, D.; Zheng, K.H.; Banerjee, R.; Gunawardena, S. The Non-amyloidal Component Region of α-Synuclein Is Important for α-Synuclein Transport Within Axons. Front. Cell. Neurosci. 2020, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.D.; Paik, S.R.; Yang, C.-H. Structural and Functional Implications of C-Terminal Regions of α-Synuclein. Biochemistry 2002, 41, 13782–13790. [Google Scholar] [CrossRef] [PubMed]
- Lucas, H.R.; Fernández, R.D. Navigating the Dynamic Landscape of Alpha-Synuclein Morphology: A Review of the Physiologically Relevant Tetrameric Conformation. Neural Regener. Res. 2020, 15, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, C.J.; Dunker, A.K. Intrinsically Disordered Proteins and Intrinsically Disordered Protein Regions. Annu. Rev. Biochem. 2014, 83, 553–584. [Google Scholar] [CrossRef] [PubMed]
- Abeyawardhane, D.L.; Lucas, H.R. Iron Redox Chemistry and Implications in the Parkinson’s Disease Brain. Oxidative Med. Cell. Longev. 2019, 2019, 11. [Google Scholar] [CrossRef]
- Joppe, K.; Roser, A.-E.; Maass, F.; Lingor, P. The Contribution of Iron to Protein Aggregation Disorders in the Central Nervous System. Front. Neurosci. 2019, 13, 15. [Google Scholar] [CrossRef]
- Davies, K.M.; Mercer, J.F.; Chen, N.; Double, K.L. Copper dyshomoeostasis in Parkinson’s disease: Implications for pathogenesis and indications for novel therapeutics. Clin. Sci. 2016, 130, 565–574. [Google Scholar] [CrossRef]
- Abeyawardhane, D.L.; Fernández, R.D.; Heitger, D.R.; Crozier, M.K.; Wolver, J.C.; Lucas, H.R. Copper Induced Radical Dimerization of α-Synuclein Requires Histidine. J. Am. Chem. Soc. 2018, 140, 17086–17094. [Google Scholar] [CrossRef]
- Abeyawardhane, D.L.; Heitger, D.R.; Fernández, R.D.; Forney, A.K.; Lucas, H.R. C-Terminal CuII Coordination to α-Synuclein Enhances Aggregation. ACS Chem. Neurosci. 2019, 10, 1402–1410. [Google Scholar] [CrossRef]
- Abeyawardhane, D.L.; Curry, A.M.; Forney, A.K.; Roberts, J.W.; Lucas, H.R. Biometals as conformational modulators of α-synuclein photochemical crosslinking. JBIC J. Biol. Inorg. Chem. 2019, 24, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Carboni, E.; Lingor, P. Insights on the interaction of alpha-synuclein and metals in the pathophysiology of Parkinson’s disease. Metallomics 2015, 7, 395–404. [Google Scholar] [CrossRef]
- McDowall, J.S.; Brown, D.R. Alpha-synuclein: Relating metals to structure, function and inhibition. Metallomics 2016, 8, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Fancy, D.A.; Denison, C.; Kim, K.; Xie, Y.; Holdeman, T.; Amini, F.; Kodadek, T. Scope, limitations and mechanistic aspects of the photo-induced cross-linking of proteins by water-soluble metal complexes. Chem. Biol. 2000, 7, 697–708. [Google Scholar] [CrossRef]
- Fancy, D.A.; Kodadek, T. Chemistry for the analysis of protein-protein interactions: Rapid and efficient cross-linking triggered by long wavelength light. Proc. Natl. Acad. Sci. USA 1999, 96, 6020–6024. [Google Scholar] [CrossRef]
- Rahimi, F.; Maiti, P.; Bitan, G. Photo-induced cross-linking of unmodified proteins (PICUP) applied to amyloidogenic peptides. J. Vis. Exp. 2009, e1071. [Google Scholar] [CrossRef]
- Al-Hilaly, Y.K.; Biasetti, L.; Blakeman, B.J.; Pollack, S.J.; Zibaee, S.; Abdul-Sada, A.; Thorpe, J.R.; Xue, W.F.; Serpell, L.C. The involvement of dityrosine crosslinking in α-synuclein assembly and deposition in Lewy Bodies in Parkinson’s disease. Sci. Rep. 2016, 6, 39171. [Google Scholar] [CrossRef]
- Johnson, M.; Coulton, A.T.; Geeves, M.A.; Mulvihill, D.P. Targeted amino-terminal acetylation of recombinant proteins in E. coli. PLoS ONE 2010, 5, e15801. [Google Scholar] [CrossRef] [PubMed]
- Expasy Bioinformatics Resource Portal. Available online: https://web.expasy.org/compute_pi/ (accessed on 16 June 2020).
- Takahashi, T.; Yamashita, H.; Nakamura, T.; Nagano, Y.; Nakamura, S. Tyrosine 125 of alpha-synuclein plays a critical role for dimerization following nitrative stress. Brain Res. 2002, 938, 73. [Google Scholar] [CrossRef]
- Chen, L.; Periquet, M.; Wang, X.; Negro, A.; McLean, P.J.; Hyman, B.T.; Feany, M.B. Tyrosine and serine phosphorylation of alpha-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J. Clin. Investig. 2009, 119, 3257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Izawa, Y.; Tateno, H.; Kameda, H.; Hirakawa, K.; Hato, K.; Yagi, H.; Hongo, K.; Mizobata, T.; Kawata, Y. Role of C-terminal negative charges and tyrosine residues in fibril formation of α-synuclein. Brain Behav. 2012, 2, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Sanjeev, A.; Kumar Mattaparthi, V.S. Computational Investigation on Tyrosine to Alanine Mutations Delaying the Early Stage of α-Synuclein Aggregation. Curr. Proteom. 2017, 14, 31–41. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curry, A.M.; Fernàndez, R.D.; Pagani, T.D.; Abeyawardhane, D.L.; Trahan, M.L.; Lucas, H.R. Mapping of Photochemically-Derived Dityrosine across Fe-Bound N-Acetylated α-Synuclein. Life 2020, 10, 124. https://doi.org/10.3390/life10080124
Curry AM, Fernàndez RD, Pagani TD, Abeyawardhane DL, Trahan ML, Lucas HR. Mapping of Photochemically-Derived Dityrosine across Fe-Bound N-Acetylated α-Synuclein. Life. 2020; 10(8):124. https://doi.org/10.3390/life10080124
Chicago/Turabian StyleCurry, Alyson M., Ricardo D. Fernàndez, Talita D. Pagani, Dinendra L. Abeyawardhane, Morgan L. Trahan, and Heather R. Lucas. 2020. "Mapping of Photochemically-Derived Dityrosine across Fe-Bound N-Acetylated α-Synuclein" Life 10, no. 8: 124. https://doi.org/10.3390/life10080124
APA StyleCurry, A. M., Fernàndez, R. D., Pagani, T. D., Abeyawardhane, D. L., Trahan, M. L., & Lucas, H. R. (2020). Mapping of Photochemically-Derived Dityrosine across Fe-Bound N-Acetylated α-Synuclein. Life, 10(8), 124. https://doi.org/10.3390/life10080124




