1. Introduction
Obstructive sleep apnea syndrome (OSAS) is a common sleep-related breathing disorder. It is estimated that 4% of both female and male adults suffer from OSAS [
1]. Specifically, about 2% middle-aged individuals (30–60 years) suffer from OSAS, and prevalence rates increased by 30% to 50% in adults with overweight and obesity [
2,
3]. Typically, in individuals with OSAS, the repetitive obstruction of the upper airway during sleep leads to hypoxemia, arousals, intermittent snoring, episodes of complete apnea, and to increased daytime sleepiness as a result of non-restoring sleep [
4]. To define OSAS, both objective and subjective measurements are applied: While self-rating questionnaires such as the STOP-Bang [
5,
6] allow a rough screening, polysomnography is the gold standard: OSAS are defined as the Apnea–Hypopnea Index (AHI) > 5 events per hour [
7].
People with objectively assessed OSAS are at increased risk to suffer from atherogenesis [
8], vascular dysfunction [
9], congestive heart failure, hypertension, atrial fibrillation, nocturnal arrhythmias, stroke, pulmonary hypertension, and metabolic syndrome [
10,
11].
As regards the immune system, the tumor necrosis factor alpha (TNF-α) is a common cytokine secreted by adipocytes and the mononuclear-macrophage system [
12], which regulates the immune system, induces inflammation, and participates in the regulation of fat metabolism [
13]. More specifically, several inflammatory cells secrete proinflammatory cytokines such as TNF-α. Increased TNF-α concentrations can cause inflammatory responses such as neutrophil recruitment, tissue destruction, neovascularization [
14], oxidative stress, systemic inflammation, autonomic nervous system dysfunction, atherosclerosis, and aging [
15]. Further, highly increased TNF-α concentrations appear to be causally associated with the pathogenesis of diseases such as metabolic diseases, cardiovascular diseases, and OSAS [
16]. Further, higher TNF-α concentrations were observed in individuals with OSAS, compared to healthy controls. In 2013, Nadeem et al. [
17] showed in their meta-analysis of 15 case-control studies that compared to controls, individuals with OSAS had significantly higher TNF-α concentrations. Similarly, in 2015, Wang et al. [
18] also reported in their meta-analysis that compared to healthy controls, individuals with OSAS had significantly higher serum TNF-α concentrations. With the present meta-analysis, we expand upon the previous two meta-analyses in that we focused on (a) both on serum and on plasma TNF-α, (b) on samples with adults and children with OSAS, and in that (c) we performed a meta-regression, and (d) we ran sub-group analyses.
2. Materials and Methods
The meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [
19].
2.1. Search Strategy
One of the authors (M. Sadeghi) systematically searched four databases, namely PubMed/Medline, Scopus, Cochrane Library, and Web of Science, for articles published in English up to 1 February 2020, with no restrictions, and with the following expressions: “sleep apnea” or “OSA” or “obstructive sleep apnea” or “obstructive sleep apnea syndrome” or “OSAS“, with “TNF-alpha” or “TNF-α” or “TNF” or “tumor necrosis factor alpha”; and with “blood” or “plasma” or “serum”. We also manually inspected the reference lists of the articles (original and review articles) for publications related to our topic.
2.2. Eligibility Criteria
Inclusion criteria were as follows: (1) studies evaluating the association between plasma or serum TNF-α levels and OSAS with case-control design without age (≥18 years as adults and <18 years as children), sex or BMI restrictions; (2) OSAS was defined as AHI > 5 events/h diagnosed by polysomnography; (3) participants with OSAS and controls had no other systematic diseases such as diabetes, asthma, neurological disorders such as multiple sclerosis, neurodegenerative disorders such as Alzheimer’s disease, or oral diseases; (4) studies reporting pretreatment morning serum and/or plasma levels of TNF-α (around 6:00–10:00 a.m.); (5) studies reporting sufficient data to compute the MD and 95% confidence interval (CI) in participants with OSAS and controls; (6) studies with more than 10 cases included as individuals with OSAS and controls.
Exclusion criteria were: (1) studies with irrelevant or insufficient data or without clinical data; (2) Letter to the editors, review articles, conference papers, meeting abstracts, and book chapters; (3) studies without a control group; (4) studies reporting controls with AHI > 5 events/h; (5) studies with overlapped data with other studies; (6) studies without reporting cut-off AHI values for OSAS; (7) studies mixing pediatric and adult participants.
2.3. Study Selection
Two authors (M.M.I. and M.E.) read independently the titles and abstracts of the retrieved studies. They then selected the relevant studies, while another author (M.S.) retrieved the full-texts of the articles and excluded several full-texts based on the exclusion criteria mentioned above. If two studies had overlapped data, we selected the study with the most recent publication year.
2.4. Data Extraction
Two authors (S.B. and M.S.) independently extracted the data from each study included in the meta-analysis. If there was a disagreement between the two authors, the third author (M.M.I.) helped to find a final decision. The data extracted for the meta-analysis included basic information including the first author, publication year, country of study, ethnicity of subjects in each study, age, BMI, and AHI of both groups, and mean and standard deviation (SD) of TNF-α levels in plasma and serum. Since some studies considered patients with mild OSAS (AHI: 1–5 events/h) as a control group condition, and since we had to use this protocol for other studies, reporting the quality of studies as for usual case-control studies did not seem to be reasonable.
2.5. Quality Assessment
One author (M.S) evaluated the quality of the studies included in the meta-analysis using the Newcastle-Ottawa Scale (NOS) with a total score of nine for each study [
20].
2.6. Statistical Analyses
The data were analyzed by one author. Review Manager 5.3 software (RevMan 5.3, (RevMan 5.3, The Cochrane Collaboration, Oxford, UK) was used to calculate the crude MD and 95% CI, which evaluated the significance of the pooled MD by Z-test. Heterogeneity across the studies was evaluated using both Cochran Q [
21] and I2 metrics with scores ranging from 0% to 100% [
22]. In addition, when
p-value < 0.1 and I2 > 50% showed a statistically significant heterogeneity, the analysis was performed by the random-effect model to evaluate the pooled ORs and CI values. Otherwise, we used the fixed-effect model. The weight (%) represents the effect of an individual study on the pooled results obtained. In general, the larger the sample size and the narrower CI are, the higher is the percentage of weight.
The results of the Begg’s and Egger’s tests were analyzed by the Comprehensive Meta-Analysis version 2.0 software (CMA 2.0). The Begg’s funnel plot shows the standard error (SE) of the log (MD), and the precision of each study is plotted against its log (MD) [
23]. In addition, the Egger’s test shows the linear regression between the precision of the studies and the standardized effect [
24].
Subgroup analyses were performed based on ethnicity, AHI, BMI, and number of participants. The sensitivity analyses, namely the “cumulative analysis” and “one study removed”, were applied to estimate the consistency/stability of the results. If the
p-value (two-tailed) was less than 0.05, then there was a statistically significant difference. The meta-regression is a quantitative method used in meta-analyses to estimate the impact of moderator variables on study effect size. The trim-and-fill method was used to estimate potentially missing studies due to publication bias in the funnel plot and adjusting the overall effect estimate [
25].
Some studies reported the values of TNF-α in standard errors (SE); SEs were transformed into standard deviation (SD) (SE = SD/√
N;
N = number of individuals). Some studies reported median and interquartile values, which were transformed into mean and SD [
26]. The serum and plasma TNF-α levels were reported in picograms per milliliter (pg/mL). Participants with BMI >30 kg/m
2 were considered obese [
27].
3. Results
3.1. Search Strategies
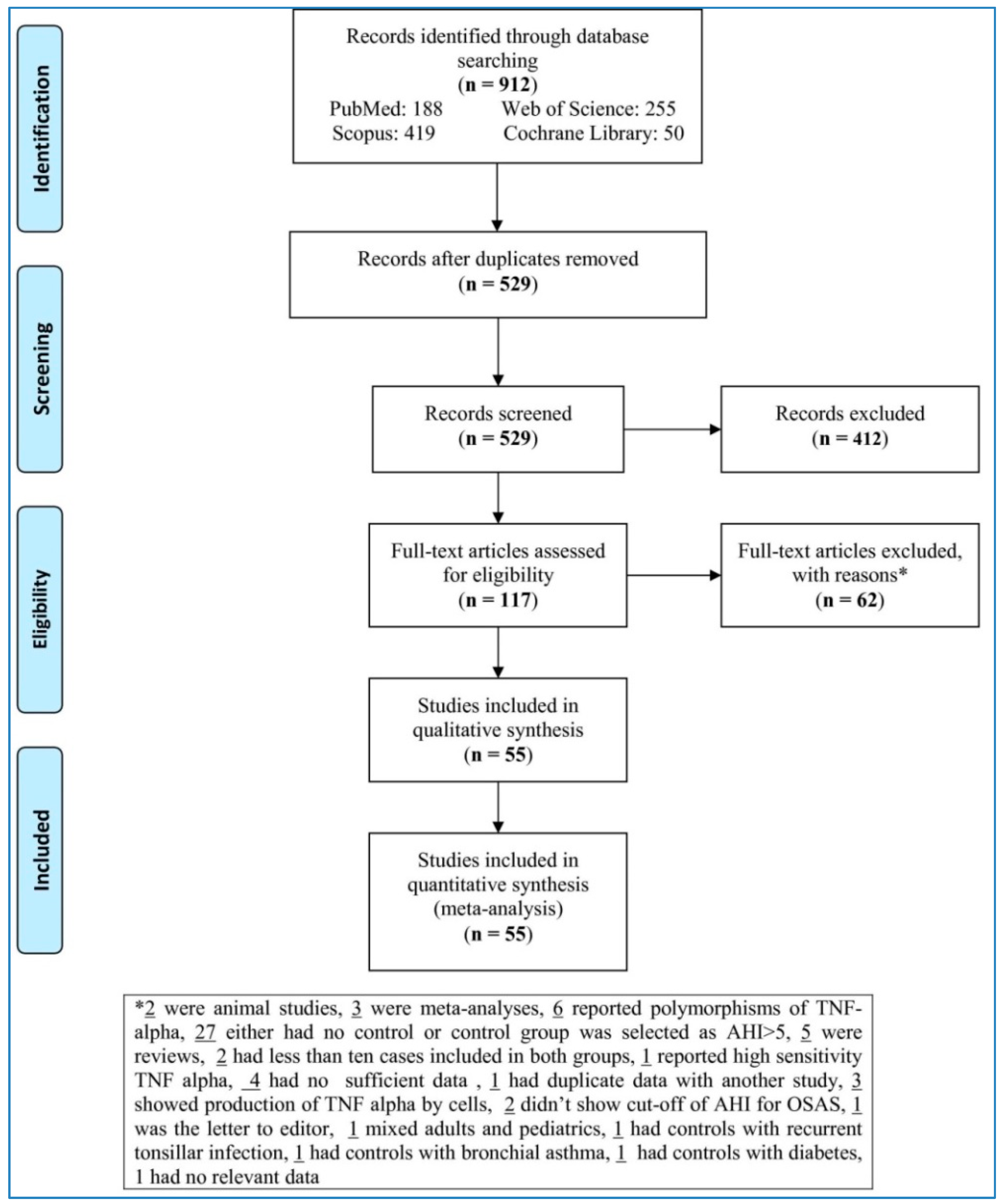
The flow chart (
Figure 1) sets out the selection process. The search databases yielded 912 articles; of these, 117 full-texts met eligibility criteria after removing duplicates and irrelevant studies. Next, 62 articles were removed with reasons (two were animal studies, three were meta-analyses, six reported polymorphisms of TNF-alpha, twenty-seven either had no control or control group was selected as AHI > 5, five were reviews, two had less than ten cases included in both groups, one reported high sensitivity TNF alpha, four had no sufficient data, one had duplicate data with another study, three showed production of TNF alpha by cells, two did not show cut-off of AHI for OSAS, one was the letter to editor, one mixed adults and pediatric samples, one had controls with recurrent tonsillar infection, one had controls with bronchial asthma, one had controls with diabetes, one had no relevant data). This left 55 studies to be analyzed in the meta-analysis [
14,
16,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73,
74,
75,
76,
77,
78,
79,
80].
3.2. Characteristics of Studies
Table 1 presents the characteristics of the 55 studies included in the meta-analysis. Forty-six studies [
16,
28,
29,
30,
31,
32,
33,
34,
35,
36,
38,
39,
40,
41,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
54,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65,
67,
68,
69,
70,
71,
72,
75,
76,
78,
79,
80] reported serum or plasma TNF-α concentrations in adults, and nine studies [
14,
37,
42,
53,
55,
66,
73,
74,
77] reported serum or plasma TNF-α concentrations in children and adolescents. Among adult participants, eighteen studies were performed with Caucasians [
31,
34,
36,
39,
40,
41,
46,
47,
48,
49,
59,
61,
63,
65,
68,
69,
75,
80], nineteen sampled Asians [
16,
29,
32,
33,
35,
43,
45,
52,
54,
58,
60,
62,
64,
67,
71,
72,
76,
78,
79], and nine samples mixed [
28,
30,
38,
44,
50,
51,
56,
57,
70] ethnicities. For studies with adult samples, 29 out of 46 studies reported serum TNF-α concentrations [
16,
31,
32,
33,
34,
35,
36,
38,
40,
41,
45,
46,
48,
50,
51,
52,
56,
60,
61,
63,
64,
65,
67,
68,
70,
76,
78,
79,
80] and 17 reported plasma TNF-α concentrations [
28,
29,
30,
39,
43,
44,
47,
49,
54,
57,
58,
59,
62,
69,
71,
72,
75].
3.3. Overall Analysis
3.3.1. Serum Levels of TNF-α in Adults
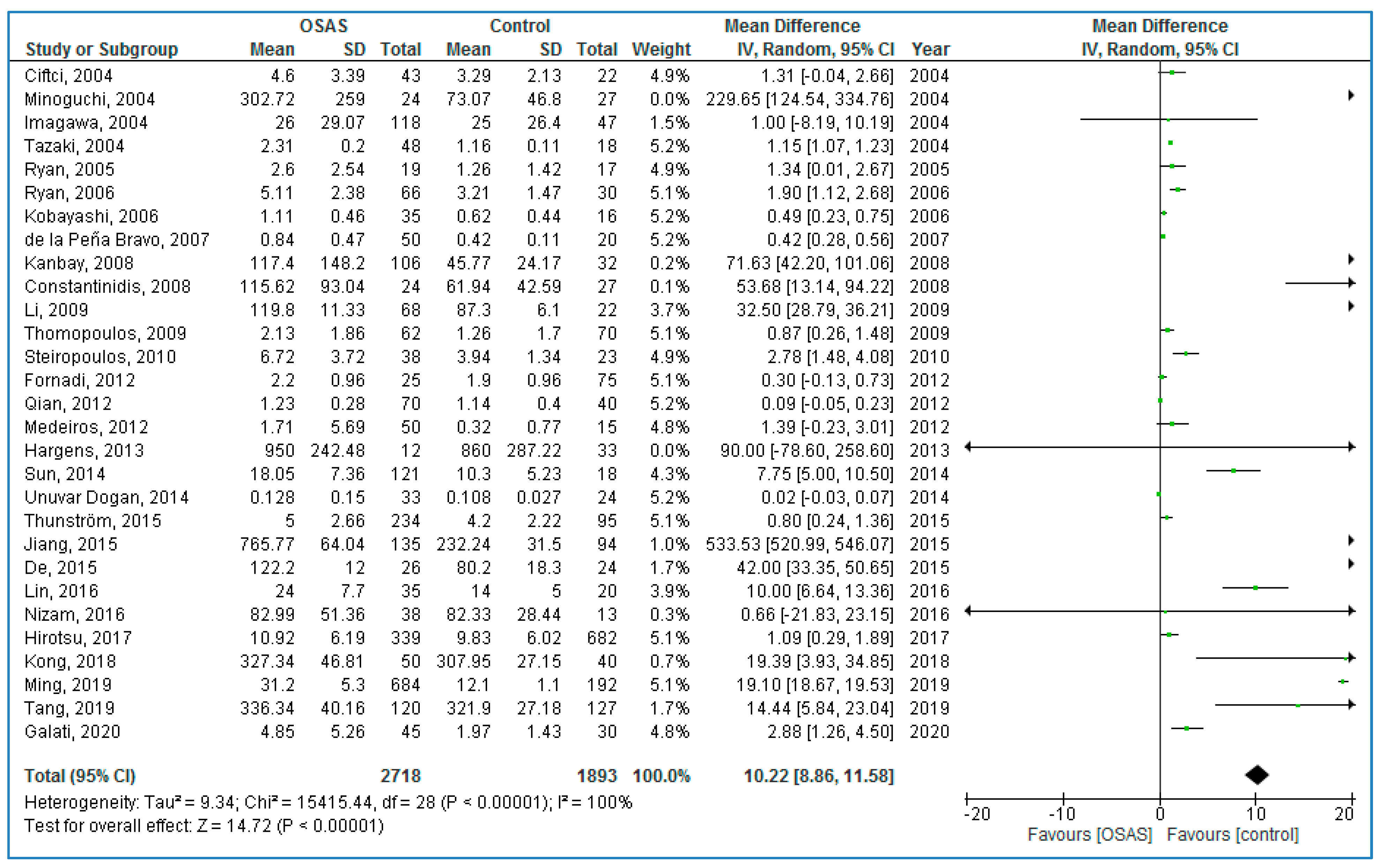
Twenty-nine studies compared serum TNF-α concentrations between adults with OSAS and healthy controls (
Figure 2); adults with OSAS had significantly higher TNF-α concentrations compared to healthy controls: 10.22 pg/mL (95% CI = 8.86, 11.58;
p < 0.00001; I
2 = 100% (P
h or P
heterogeneity < 0.00001)).
3.3.2. Plasma Levels of TNF-α in Adults
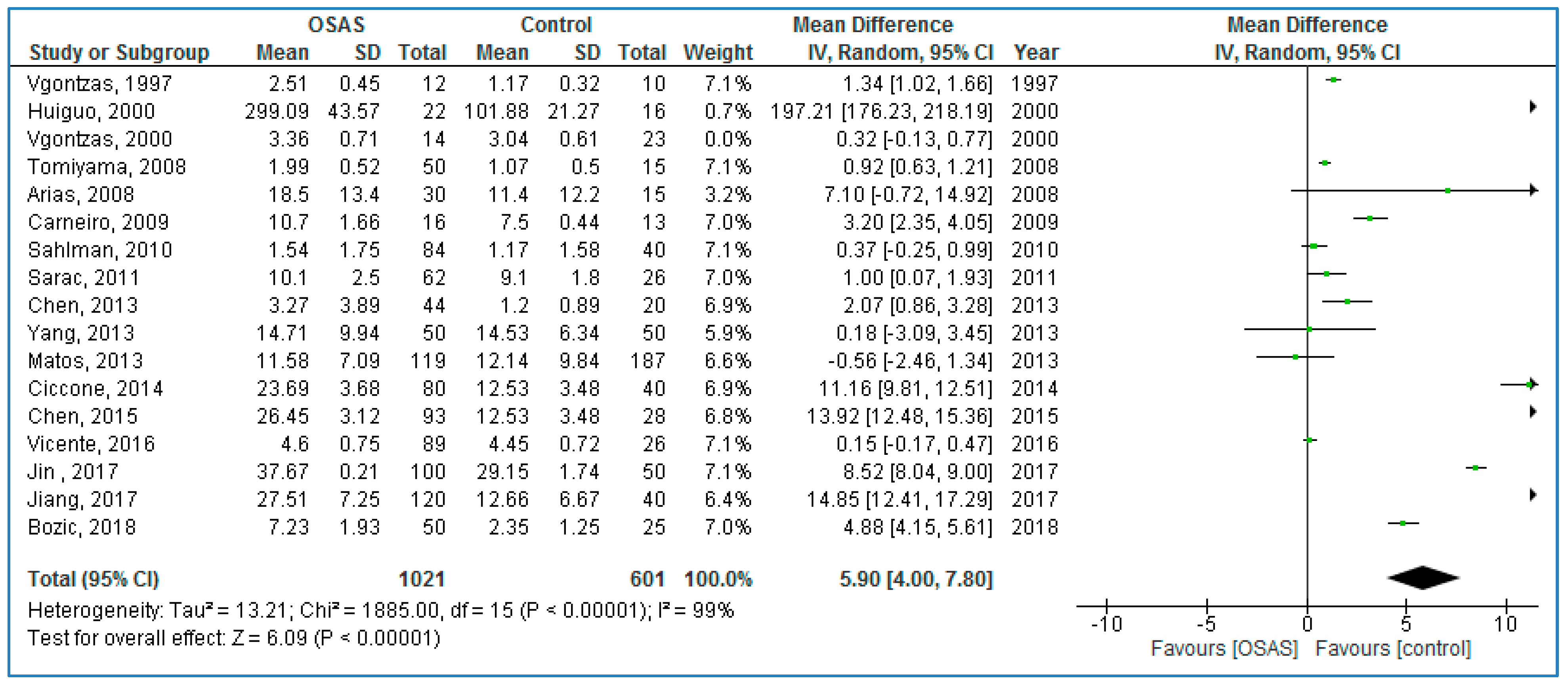
Seventeen studies compared plasma TNF-α concentrations between adults with OSAS and healthy controls (
Figure 3) and found significantly higher TNF-α concentrations in those with OSAS: 5.90 pg/mL (95% CI = 4.00, 7.80;
p < 0.00001; I
2 = 99% (P
h < 0.00001)).
3.3.3. Serum TNF-α Levels in Children
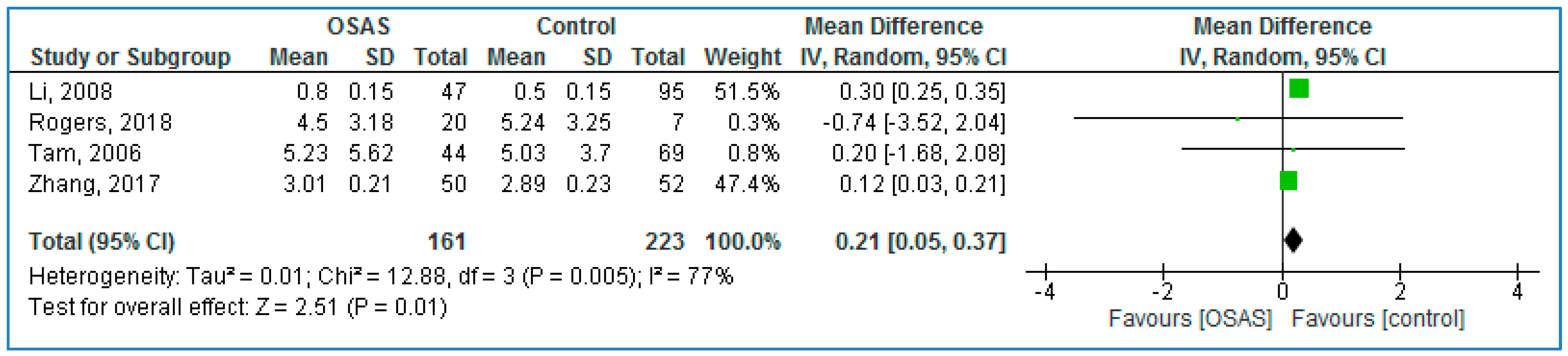
Four studies compared serum TNF-α concentrations between children with OSAS and without OSAS (
Figure 4) and found significantly higher TNF-α concentrations in those with OSAS: 0.21 pg/mL (95% CI = 0.05, 0.37;
p = 0.01; I
2 = 77% [P
h = 0.005].
3.3.4. Plasma TNF-α Levels in Children
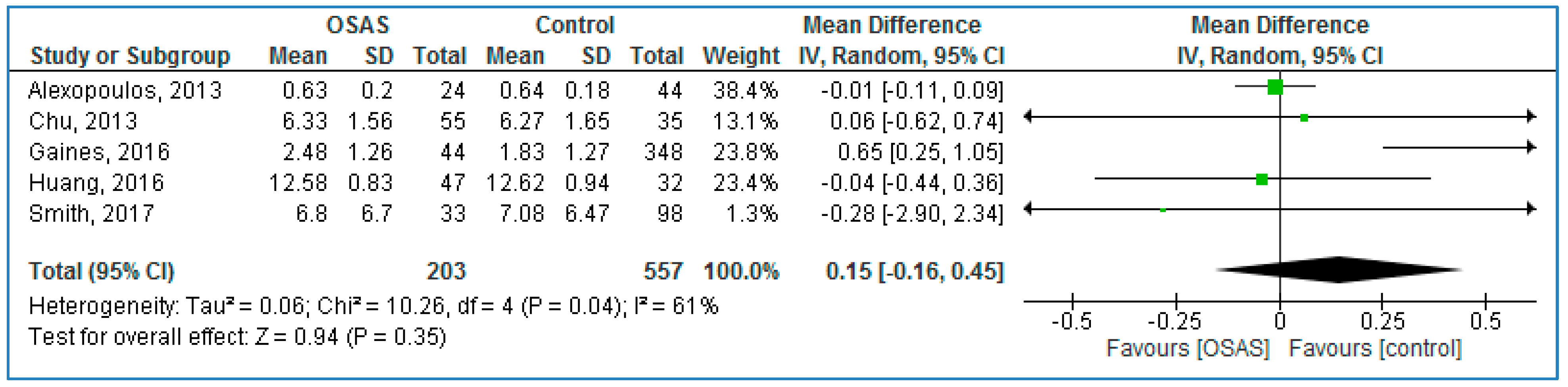
Five studies compared serum TNF-α concentrations between children with OSAS and without OSAS (
Figure 5) and found no statistically significant MDs: 0.15 pg/mL (95% CI = −0.16, 0.45;
p = 0.35; I
2 = 61% [P
h = 0.04]).
3.4. Overall Subgroup Analysis of Serum and Plasma TNF-α Levels
3.4.1. Ethnicity
Subgroup analysis of serum TNF-α concentrations in adult samples are reported in
Table 2. The pooled analysis showed that for those with OSAS, serum TNF-α concentrations in Asians (MD = 37.11 pg/mL (95% CI = 33.27, 40.95;
p < 0.00001), in Caucasians (MD = 2.20 pg/mL; 95% CI = 1.06, 3.34;
p = 0.0002), and in mixed ethnicities (MD = 0.43; 95% CI = 0.30, 0.56;
p < 0.00001) were significantly higher, compared to healthy controls. Serum TNF-α concentrations in Asians were 16.9 and 86.3 times higher than serum TNF-α concentrations in Caucasian and mixed ethnicities with OSAS, and always compared to controls.
For plasma TNF-α concentrations, for those adults with OSAS, plasma TNF-α concentrations in Asians (MD = 14.92 pg/mL; 95% CI = 9.86, 19.98; p < 0.00001), in Caucasians (MD = 3.72 (95% CI = 1.04, 6.40; p = 0.007)), and in mixed ethnicities (MD = 1.22; 95% CI = 0.10, 2.34; p = 0.03) were significantly higher, compared to healthy controls. Next, in Asians, plasma TNF-α concentrations were 4 and 12.2 times higher than in Caucasian and mixed ethnicities with OSAS, and always compared to controls.
3.4.2. Mean BMI of Individuals with OSAS
For serum TNF-α concentrations, and compared to healthy controls, adult participants with OSAS and a BMI > 30 km/m2 showed a pooled MD of 1.27 (95% CI: 0.67, 1.86; p < 0.0001). For adult participants with OSAS and a BMI ≤ 30 km/m2, the pooled MD was 23.07 pg/mL (95% CI: 20.18, 25.96; p < 0.00001). Thus, results showed that serum TNF-α concentrations were 18.2 times higher participants with OSAS and a BMI > 30 kg/m2, when compared to those participants with a mean BMI ≤30 kg/m2.
For plasma TNF-α concentrations, and compared to healthy controls, adult participants with OSAS and a BMI > 30 km/m2 showed a pooled MD of 1.06 pg/mL (95% CI: 0.34, 1.78; p = 0.004). For adult participants with OSAS and a BMI ≤ 30 km/m2, the pooled MD was 10.82 pg/mL (95% CI: 7.14, 14.49; p < 0.00001). Thus, results showed that plasma TNF-α concentrations were 10.2 times higher in adults with OSAS and a BMI > 30 kg/m2 than plasma TNF-α concentrations of adults with OSAS and a BMI ≤ 30 kg/m2 and always compared to healthy controls.
3.4.3. Mean BMI of Controls
For serum TNF-α levels, compared to controls, individuals with OSAS and with a BMI > 30 kg/m2 showed a pooled MD of 2.28 pg/mL (95% CI: 0.86, 3.70; p = 0.002); while compared to controls, individuals with OSAS and with a BMI ≤ 30 kg/m2 showed a pooled MD of 18.64 pg/mL (95% CI: 16.33, 20.95; p < 0.00001). Thus, serum TNF-α levels were 8.2 times higher in studies with participants with OSAS and a BMI >30 kg/m2 than serum TNF-α levels with participants with OSAS and a BMI ≤ 30 kg/m2.
For plasma TNF-α levels, compared to controls, the pooled MD was 7.32 pg/mL (95% CI: 5.02, 9.62; p < 0.00001) for participants with OSAS and a BMI > 30 kg/m2; for participants with OSAS and a BMI ≤ 30 kg/m2, the pooled MD was not statistically significant (MD = 1.19 pg/mL; 95% CI: 0.00, 2.38; p = 0.05).
3.4.4. Total Number of Participants
For serum TNF-α levels, the pooled MD was 55.47 pg/mL (95% CI: 47.21, 63.72; p < 0.00001) for studies with more than 100 cases, and 2.15 pg/mL (95% CI: 1.57, 2.72; p < 0.00001) for studies with less than 100 cases. Thus, serum TNF-α levels were 25.8 times higher in the studies with more 100 cases, compared to studies with less than 100 cases.
For plasma TNF-α levels, the pooled MD was 6.91 pg/mL (95%CI: 2.20, 11.63; p = 0.004) for studies with more than 100 cases, while the pooled MD was 2.87 pg/mL (95%CI: 1.37, 4.37; p = 0.0002) for studies with less than 100 cases. Thus, plasma TNF-α levels were 2.4 times higher in the studies with more than 100 cases, compared to studies with less than 100 cases.
3.4.5. Mean AHI of Individuals with OSAS
For serum TNF-α levels, in individuals with OSAS and a AHI > 30 events/h, the pooled MD was 5.72 pg/mL (95% CI: 4.32, 7.12; p < 0.00001), while the pooled MD was 127.01 pg/mL (95%CI: 100.56, 153.47; p < 0.00001) in individuals with OSAS and a AHI ≤ 30 events/h. Thus, serum TNF-α levels were 22.2 times higher, when participants with OSAS had AHI ≤ 30 events/h, compared to AHI > 30 events/h.
For plasma TNF-α levels, in individuals with OSAS and AHI > 30 events/h, the pooled MD was 8.15 pg/mL (95% CI: 5.59, 10.71; p < 0.00001), while in individuals with OSAS and AHI ≤ 30 events/h, the pooled MD was 2.97 pg/mL (95% CI: 0.21, 5.73; p = 0.03). Thus, serum TNF-α levels were 2.7 times higher in participants with OSAS and AHI > 30 events/h, compared to serum TNF-α levels in participants with OSAS and AHI ≤ 30 events/h.
3.5. Adult Caucasian Participants
3.5.1. Mean BMI of Individuals with OSAS
For serum TNF-α levels, studies with Caucasian participants with OSAS and a BMI > 30 kg/m
2 showed the pooled MD of 2.58 pg/mL (95% CI: 1.07, 4.10;
p = 0.0008), whereas for studies with participants with a BMI ≤ 30 kg/m
2 serum TNF-α levels did not statistically differ between participants with or without OSAS (
Table 3).
For plasma TNF-α levels, studies with Caucasian participants with OSAS and a BMI ≤ 30 kg/m2 showed the pooled MD of 7.99 pg/mL (95% CI: 1.84, 14.15; p = 0.01), whereas no significant MDs were observed in studies including Caucasian participants with OSAS and a BMI > 30 kg/m2.
3.5.2. Mean BMI of Controls
For studies with serum TNF-α levels and Caucasian controls with a mean BMI > 30 kg/m2, the pooled MD was 2.58 pg/mL (95% CI: 1.07, 4.10; p = 0.0008). There was no statistically significant MD for serum TNF-α levels between Caucasians with or without OSAS and a mean ≤ 30 kg/m2.
For studies with plasma TNF-α levels and controls with a BMI > 30 kg/m2, the pooled MD was 0.56 pg/mL (95% CI: 0.05, 1.08; p = 0.03). Studies with controls with a BMI ≤ 30 kg/m2 showed a pooled MD of 5.64 pg/mL (95% CI: 0.77, 10.52; 0.02). Thus, plasma TNF-α levels were 10.1 times higher in controls with a BMI ≤ 30 kg/m2, compared to plasma TNF-α levels of controls with a BMI > 30 kg/m2
3.5.3. Total Number of Participants
Serum TNF-α levels had a significant difference in studies with ≤ 100 cases (MD = 3.21 pg/mL; 95% CI: 1.37, 5.05; p = 0.0006), whereas there was no significant difference for studies with >100 cases.
3.5.4. Mean AHI of Individuals with OSAS
In studies with individuals with OSAS and AHI of > 30 events/h, serum TNF-α levels were 1.40 pg/mL (95% CI: 0.34, 2.46; p = 0.010), and plasma TNF-α levels were 7.80 pg/mL (95% CI: 2.47, 13.13; p = 0.004).
3.6. Adult Asian Participants
3.6.1. Mean BMI of Individuals with OSAS
For serum and plasma TNF-α levels, there were no Asian participants with OSAS and a mean BMI > 30 kg/m
2. For studies with Asian participants with OSAS and a mean BMI ≤ 30 kg/m
2, serum (MD = 37.11 pg/mL; 95% CI: 33.27, 25.96;
p < 0.0001) and plasma (MD = 14.92 pg/mL; 95% CI: 9.86, 19.98;
p < 0.00001) TNF-α levels differed statistically significantly (
Table 4).
3.6.2. Mean BMI of Controls
For serum and plasma TNF-α levels, there were no studies including controls with a BMI > 30 kg/m2. The serum TNF-α levels were 37.11 pg/mL (95% CI: 33.27, 25.96; p < 0.0001) for studies including OSAS patients with a mean BMI of ≤30 kg/m2, and plasma TNF-α levels were 14.92 pg/mL (CI: 9.86, 19.98; p < 0.00001) for studies including OSAS patients with a mean BMI of ≤30 kg/m2.
3.6.3. Total Number of Participants
For serum TNF-α levels, the pooled MD was 89.88 pg/mL (95% CI: 74.59, 105.17; p < 0.00001) for studies with 100 or more participants, and the pooled MD was 7.80 pg/mL (95% CI: 5.76, 9.85; p < 0.00001) for studies less than 100 participants. Thus, serum TNF-α levels were 11.5 times higher in studies with more than 100 participants, compared to studies with less than 100 participants.
For plasma TNF-α levels, the pooled MD was 12.34 pg/mL (95%CI: 7.84, 16.84; p < 0.00001) for studies with 100 or more participants, and the pooled MD was 21.51 pg/mL (95%CI: 13.15, 29.87; p < 0.00001) for studies less than 100 participants. Thus, plasma TNF-α levels were 1.7 times higher in studies with less than 100 participants, compared to studies with more than 100 participants.
3.6.4. Mean AHI of Individuals with OSAS
No study investigated serum and plasma TNF-α levels in participants with OSAS with a mean AHI ≤ 30 events/h.
For studies including AHI > 30 events/h, mean serum TNF-α levels were 12.94 (pg/mL; 95% CI: 7.74, 18.13; p < 0.00001); plasma TNF-α levels were 12.48 pg/mL (95% CI: 17.04, 31.52; p < 0.00001).
3.7. Adult Participants Including Mean BMI of Individuals with OSAS > 30 kg/m2
3.7.1. Mean BMI of Controls
For studies with serum TNF-α levels and controls with BMI of controls >30 kg/m
2, there was a significant difference for serum TNF-α levels (MD = 2.28 pg/mL; 95% CI: 0.86, 3.70;
p = 0.002), compared to studies with controls with BMI of controls ≤ 30 kg/m
2 (
Table 5).
3.7.2. Total Number of Participants
For studies with 100 or less participants, there was a significant difference for serum TNF-α levels (MD = 1.27 pg/mL; 95% CI: 0.65, 1.89; p < 0.0001) and for plasma TNF-α levels (MD = 1.51 pg/mL; 95% CI: 0.53, 2.49; p = 0.003), compared to studies with more than 100 participants.
3.7.3. Mean AHI of Participants with OSAS
For serum TNF-α levels, the pooled MD for studies including participants with OSAS with AHI > 30 events/h was 0.96 pg/mL (95% CI: 0.50, 1.42; p < 0.0001); the pooled MD for studies with participants with OSAS with AHI ≤ 30 events/h was 42.13 pg/mL (95%CI: 33.48, 50.77; p < 0.00001). Thus, serum TNF-α levels were 42.9 times higher in studies with participants with OSAS and AHI ≤ 30 events/h, compared to the serum TNF-α levels in studies including participants with OSAS and AHI > 30 events/h.
For plasma TNF-α levels, the pooled MD for studies with participants with OSAS with AHI > 30 events/h was 1.69 pg/mL (95% CI: 0.47, 2.90; p = 0.007). When studies included participants with OSAS and an AHI ≤ 30 events/h, MDs between studies with participants with or without OSAS were not significant.
3.8. Adult Participants with a Mean BMI ≤ 30 kg/m2 in Participants with OSAS
3.8.1. Mean BMI of Controls
No study investigated serum and plasma TNF-α levels in participants with OSAS with a mean BMI >30 kg/m2.
For studies including BMI ≤ 30 kg/m
2 in controls, TNF-α levels were 23.07 pg/mL (95% CI: 20.18, 4.50;
p < 0.00001) for serum, and MD = 10.82 pg/mL (95% CI: 7.14, 14.49;
p < 0.00001) for plasma (
Table 6).
3.8.2. Total Number of Participants
For serum TNF-α levels and in studies with more than 100 participants, the pooled MD was 63.51 pg/mL (95% CI: 53.65, 73.45; p < 0.00001), while in studies with ≤ 100 participants, the pooled MD was 4.42 pg/mL (95% CI: 3.10, 5.74; p < 0.00001). Thus, serum TNF-α levels were 14.4 times higher in studies with more than 100 participants, compared to serum TNF-α levels in studies with ≤100 participants.
For plasma TNF-α levels and in studies with more than 100 participants, the pooled MD was 8.12 pg/mL (95%CI: 4.46, 11.79; p < 0.0001) while in studies with ≤100 participants, the pooled MD 13.79 pg/mL (95%CI: 8.24, 19.35; p < 0.00001). Thus, plasma TNF-α levels were 1.7 times higher in studies with more than 100 participants, compared to plasma TNF-α levels in studies with ≤100 participants.
3.8.3. Mean AHI of Participants with OSAS
For serum TNF-α levels, the pooled MD for studies including participants with OSAS and an AHI > 30 events/h was 12.94 pg/mL (95% CI: 7.74, 18.13; p < 0.00001); the pooled MD for studies including participants with OSAS and an AHI ≤ 30 events/h was 173.00 pg/mL (95%CI: 139.66, 206.34; p < 0.00001). Thus, serum TNF-α levels were 13.4 times higher in participants with OSAS and AHI ≤ 30 events/h, compared to the serum TNF-α levels in participants with OSAS and AHI > 30 events/h.
For plasma TNF-α levels, the pooled MD for studies including participants with OSAS and an AHI > 30 events/h was 16.13 pg/mL (95% CI: 11.20, 21.06; p < 0.00001). When studies included participants with OSAS had an AHI ≤ 30 events/h, MDs between studies including participants with or without OSAS were not significant.
3.9. Meta-Regression
The results of meta-regression showed that age, the publication year, the mean BMI, the mean AHI, and the number of participants did not systematically change serum and plasma TNF-α levels (
Table 7).
3.10. Quality Assessment
The quality score of each study included in the meta-analysis is illustrated in
Table 8.
3.11. Sensitivity Analysis
The “cumulative analysis” and the “one study removed” as two sensitivity analyses showed the stability of the results. In addition, excluding statistically the studies with outliers data did not change pooled analysis of serum (MD = 4.53 pg/mL,
p < 0.00001) and plasma (MD = 4.18 pg/mL,
p < 0.00001) TNF-α levels (
Table 9).
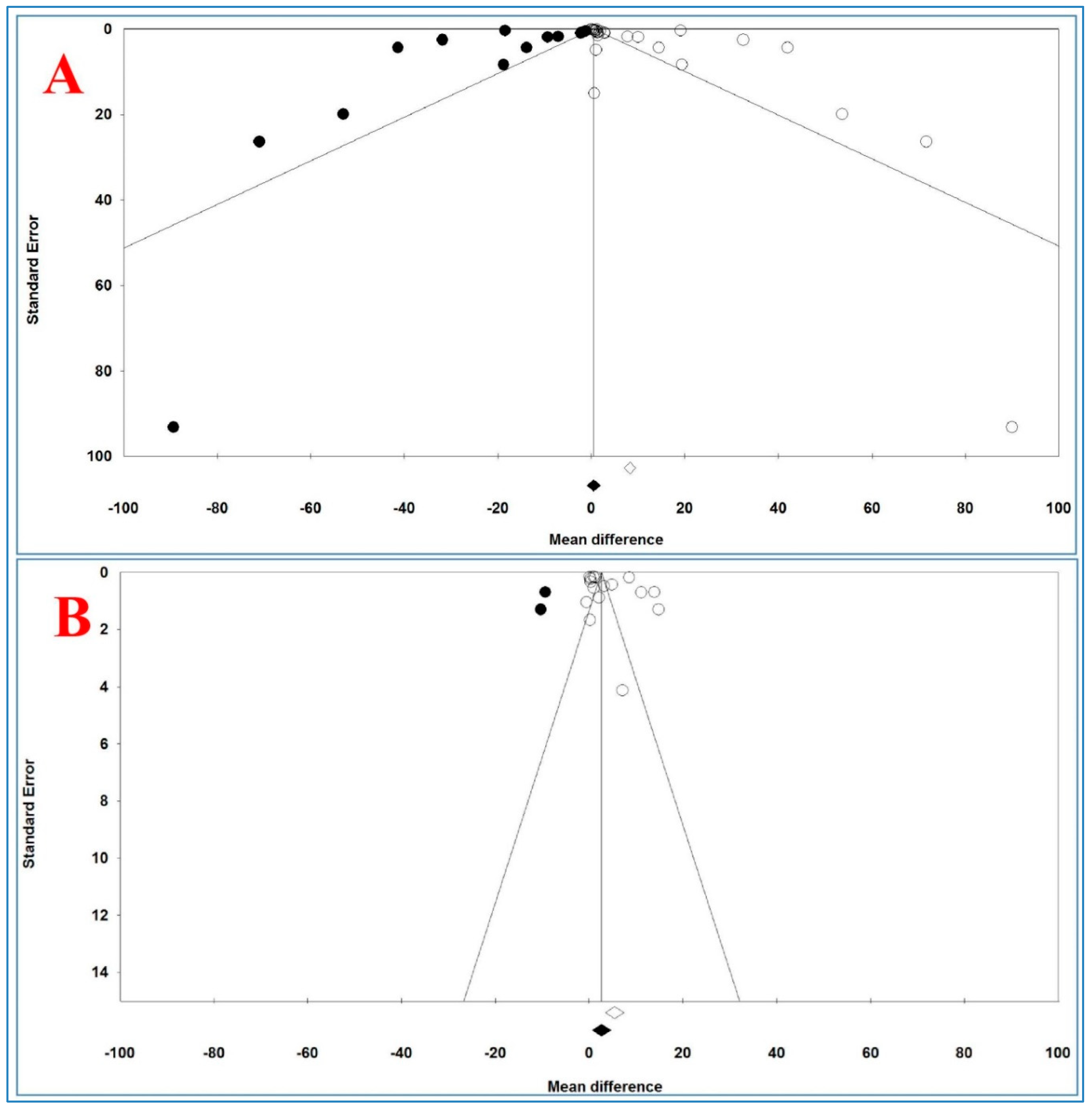
3.12. Publication Bias
Figure 6 shows the funnel plots of the analysis of serum and plasma TNF-α levels and
Table 10 illustrates the results of trim-and-fill method on bias.
For serum levels, Egger’s and Begg’s tests (p = 0.02431 and p = 0.04677, respectively) revealed a publication bias. For plasma TNF-α levels, Begg’s test (p = 0.03943) revealed a publication bias, but Egger’s test (p = 0.19315) did not show any bias between and across the studies.
For serum TNF-α levels and 15 imputed studies, under the fixed-effects model, the point estimate and pseudo 95% CI for the combined studies was 0.410 (0.365, 0.455); using the trim-fill method, the imputed point estimate was 0.323 (0.278, 0.368). In addition, under the random-effects model, the point estimate and 95% CI for the combined studies was 8.245 (7.006, 9.483); using the trim-fill method, the imputed point estimate was 0.523 (−0.889, 1.935).
For plasma TNF-α levels and 3 imputed studies, under the fixed-effects model, the point estimate and 95% CI for the combined studies was 2.463 (2.323, 2.602), and using trim-fill method, the imputed point estimate was 2.294 (2.156, 2.433). In addition, under the random-effects model, the point estimate and pseudo 95% CI for the combined studies was 5.452 (3.501, 7.403); using the trim-fill method, the imputed point estimate was 2.668 (0.638, 4.698).
The overall effect sizes on serum and plasma TNF-α levels reported in the forest plot appeared valid, with trivial publication bias effect based on fixed-effects model, because the observed estimates were similar to the adjusted estimates. In contrast, the overall effect sizes on serum and plasma TNF-α levels reported in the forest plot appeared invalid, with significant publication bias effect based on random-effects model, because the observed estimates had high difference with the adjusted estimates.
4. Discussion
The present meta-analysis with 55 studies evaluated the serum TNF-α levels (29 studies of adults and 4 studies of children) and plasma TNF-α levels (17 studies of adults and 5 studies of children) in individuals with OSAS, compared to controls. The results showed that plasma and serum TNF-α levels in adult individuals with OSAS were significantly higher than the corresponding levels of control. For children with OSAS serum TNF-α levels, but not plasma TNF-α levels, were statistically significantly higher than those of controls. The present results have clinical importance because elevated serum and plasma TNF-α levels can be a risk factor for the development of further systemic diseases in adults with OSAS. Further, more generally, inflammation may play a crucial role in the pathogenesis of OSAS, and there is evidence that individuals with OSAS have elevated interleukin TNF-α [
16,
29,
40,
41,
45,
48,
62,
78]. These conclusions also have practical importance because, in addition to routine checks on sleep and sleep-disordered breathing, both children and adults with OSAS need a thorough monitoring of their immune systems.
Elevated TNF-α levels have been associated with the pathophysiology of reoxygenation injury, myocarditis, cardiac allograft vasculopathy, heart failure progression [
81], arthritis, diabetes, Crohn’s disease, and also cachexia which correlated with terminal malignancy and AIDS [
82]. Further, TNF-α is a well-known inflammatory marker, besides being related to atherosclerosis in males [
83]. It is also confirmed that hypoxia raises the expression of proinflammatory molecules such TNF-α [
84,
85].
The present pattern of results also sheds some light on further new findings: More specifically, for adults, it turned out that compared to healthy controls, TNF-α levels were higher both in blood serum and blood plasma. It follows that at least for adults with OSAS, assessing either blood plasma or blood serum does not appear to be of clinical importance. In contrast, for pediatric samples and compared to healthy controls, higher TNF-α levels were observed for blood serum but not for blood plasma. The quality of the data at hand does not allow a deeper understanding of the underlying physiological mechanisms. Thus, for want of such data, the following admittedly speculative assumptions are advanced. First, it is conceivable that for pediatric samples, the number of four studies on plasma TNF-α was too small, and therefore, the statistical variance of plasma TNF-α was blurred by sample size inconsistencies. Similarly, second, at least among adults, Hirotsu et al. [
70] showed that OSAS and inflammatory markers such as IL-6 and TNF-α were associated with gender in a very complex fashion. A closer inspection of the four studies on plasma TNF-α among pediatric samples showed that gender was not introduced as a specific factor or confounder. Given this lack of analyses in these publications, possible gender effects could neither be confirmed, nor declined. Third, Imani et al. [
86] showed that for pediatric samples, the definition of OSAS was not applied as strict and consistent as among adult samples. It follows that the zero-differences of plasma TNF-α between children with and without OSAS might mirror inconsistencies as regards the definition of control samples. Fourth, Cameron et al. [
87] observed that besides the disease status, in pediatric samples with Inflammatory Bowel Disease, the outcome of an antitumor necrosis factor (TNF) therapy depended from participants’ pubertal stage. Given this, it is conceivable that the zero-difference of plasma TNF-α between pediatric samples with and without OSAS might have been blurred by the pubertal stage. In the same vein, fifth, it is conceivable that pubertal stage and its implicit growth spurt in height and weight might have obscured a clear-cut pattern of results as regards TNF-α levels between children with and without OSAS. Last, even if highly unlikely, one cannot rule out that the pattern of results is an accidental finding.
The pattern of results described in the present meta-analysis and systematic review consistently showed that higher TNF-α levels were associated with pathologically higher BMI scores as a proxy of obesity. Given that above all childhood overweight and childhood obesity is increasing worldwide and is a major concern of public health [
88], the association between overweight/obesity, TNF-α levels and OSAS demand particular attention. Following Popa et al. [
13], TNF-alpha appeared to be of upmost importance in the development and maintenance of metabolic diseases in which a shift toward a proatherogenic lipid profile and impaired glucose tolerance appeared to occur. Further, investigations assessing the impact of anti-TNF agents on intermediary metabolism seemed to show that a TNF-alpha blockade may improve insulin resistance and lipid profiles, which in turn appeared to be associated with overweight and obesity. Likewise, Ciftci et al. [
31] showed in their study that compared to obese males with no OSAS, in obese males with OSAS TNF-alpha levels were significantly higher. Ciftci et al. [
31] concluded from their results, that the association between cardiovascular morbidity and OSAS appeared to be best described by the coexistence of other cardiovascular risk factors such as circulating IL-6 and TNF-alpha levels. In the same vein, Sahlman et al. [
47] showed in their study that pro-inflammatory markers such as TNF-alpha were markedly increased in patients with mild OSAS. To find a physiological explanation for such processes, Steiropoulos et al. [
48] summarized in their introduction that besides the role of energy depot adipose tissue is also an active endocrine organ, releasing proinflammatory cytokines such as Tumor Necrosis Factor-α (TNF-α) and Interleukin-6 (IL-6) which modulate blood pressure, and lipid- and glucose-metabolism. Further, the proinflammatory transcription factor NF-κB appears to be upregulated in OSAS as a result of alterations between hypoxia and reoxygenation and as a result of sleep deprivation. Additionally, NF-κB regulates the expression of inflammatory genes. Given these physiological processes, it appears plausible that OSAS, obesity and poor sleep appear to be highly interrelated.
The results of one study [
70] showed that IL-6, triglycerides, and AHI were positively associated with TNF-α, while sex, ghrelin and total cholesterol had a negative association. Further, postmenopausal women had higher TNF-α levels than those of premenopausal women [
70]. One study showed that higher TNF-α levels were significantly related to some neurocognitive deficits in children with OSAS [
14].
Next, TNF-α appears to mediate both a somnogenic activity and fatigue related to excessive daytime somnolence in obese patients with OSAS [
14,
28,
30]. TNF-α levels were related to the severity of OSAS [
31]. One study reported that BMI was weakly related to TNF-α levels [
32]. However, Kanbay et al. [
41] showed that in individuals with OSAS and obesity serum TNF-α levels were significantly higher than those without obesity. Not surprisingly, there was also a positive albeit modest correlation (r: 0.181;
p: 0.034) between higher BMI scores and higher TNF-α levels [
16].
As regards AHI and TNF-α levels, Kanbay et al. [
41] and others [
16,
78] reported a significant positive correlation between a higher AHI and higher serum TNF-α levels.
Next, Minoguchi et al. [
16] reported in their meta-analysis higher plasma and serum TNF-α levels in adults, compared to non-adults.
Despite the novelty of the results, the following limitations should be considered: (1) In all studies, results have not been adjusted for possible confounding factors such as obesity, smoking, or alcohol consumption. (2) The results of the funnel plots showed a publication bias across the studies; it follows that a systematic bias in the data presentation cannot be ruled out. (3) Studies with a small sample size (less than100 cases) had an inadequate power to detect possibly meaningful associations. (4) There was a high heterogeneity among studies in some analyses. (5) Studies reported different cut-off AHI values, which made comparisons between the studies difficult. (6) In some studies, TNF-α levels were considered as secondary outcome. (7) In some studies, the existence of mixed ethnicities might have blurred the associations between the ethnicity and TNF-α levels.
By contrast, the strengths of the meta-analysis were as follows: (1) There were sufficient studies to allow the subgroup analyses. (2) The sensitivity analysis showed a stability of the results. (3) The studies written in other languages than English were included in the meta-analysis.