Magnetite Synthesis in the Presence of Cyanide or Thiocyanate under Prebiotic Chemistry Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
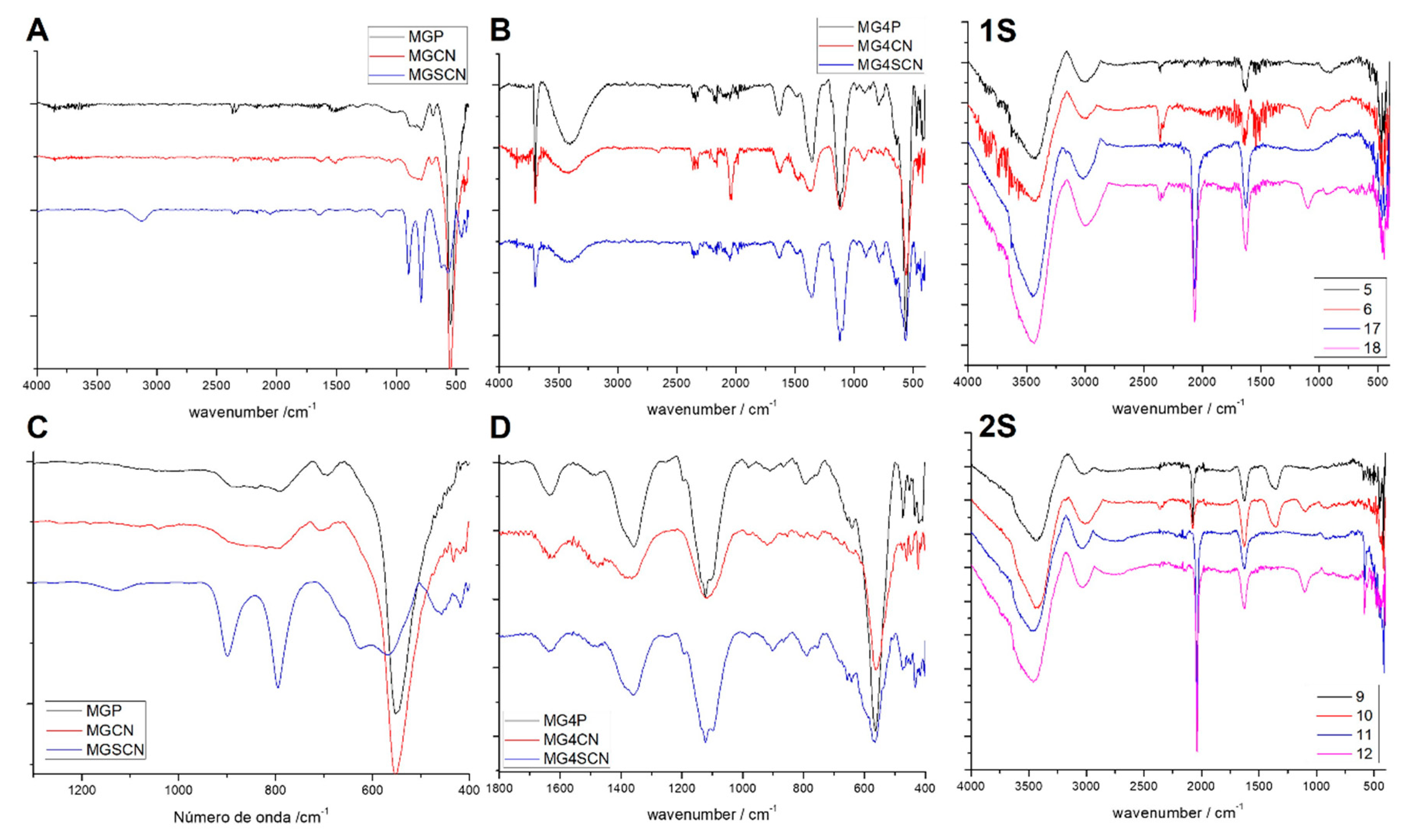
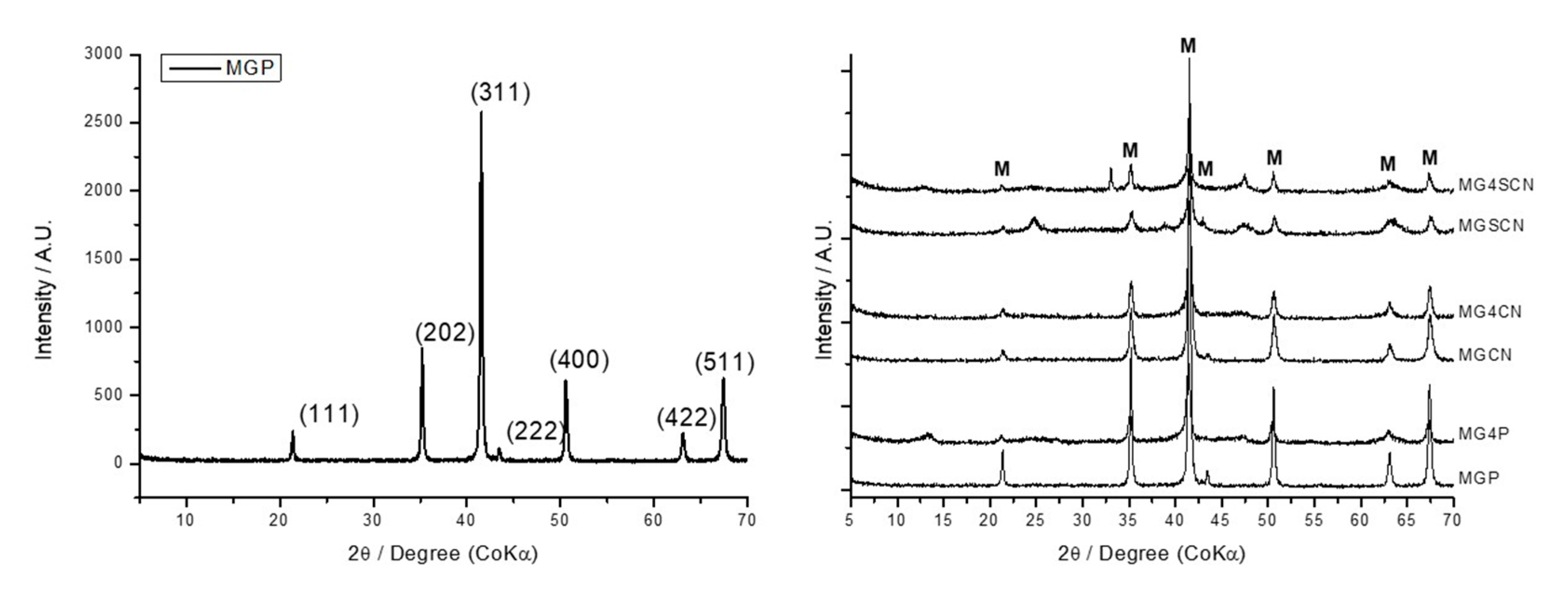
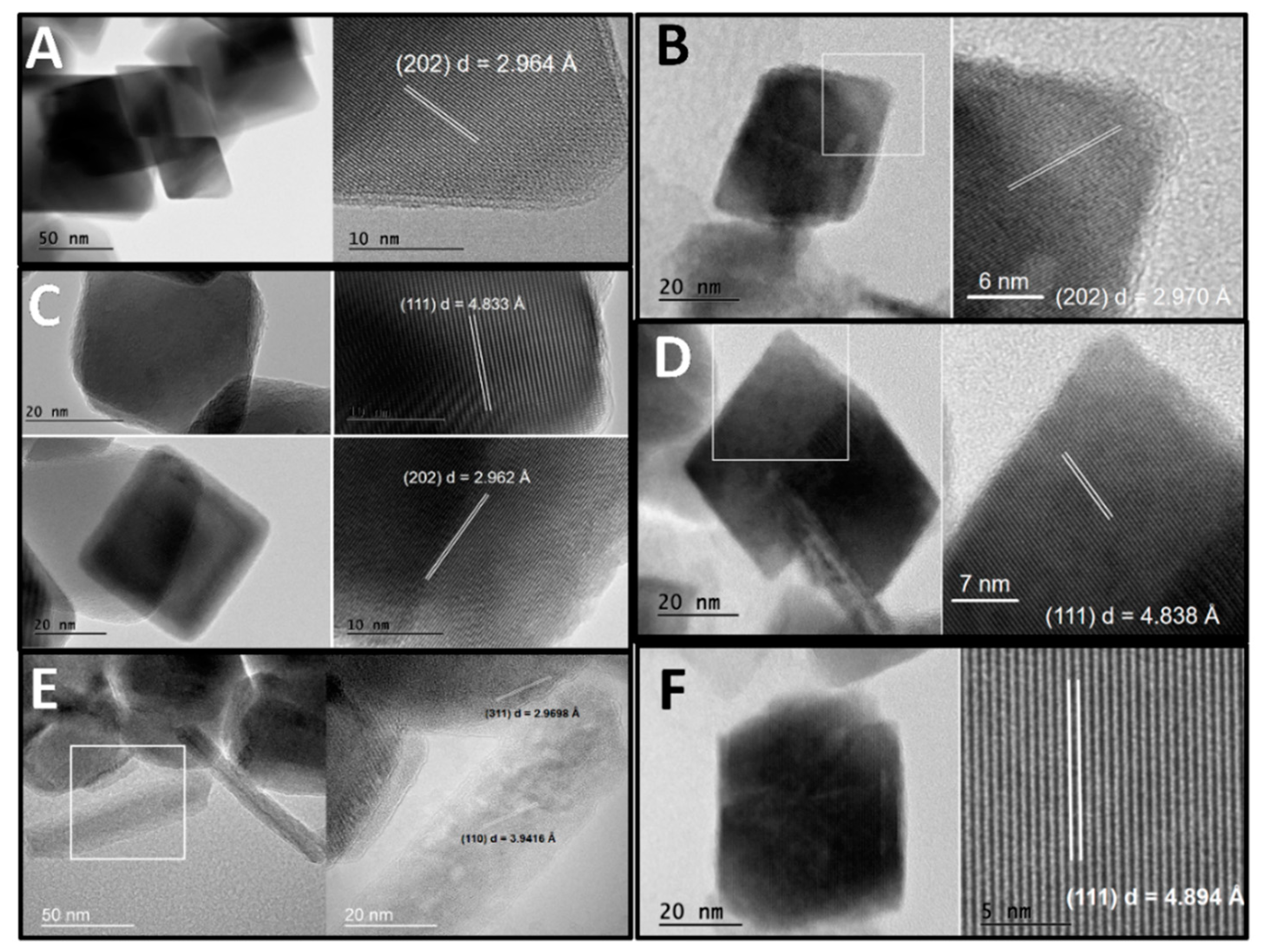
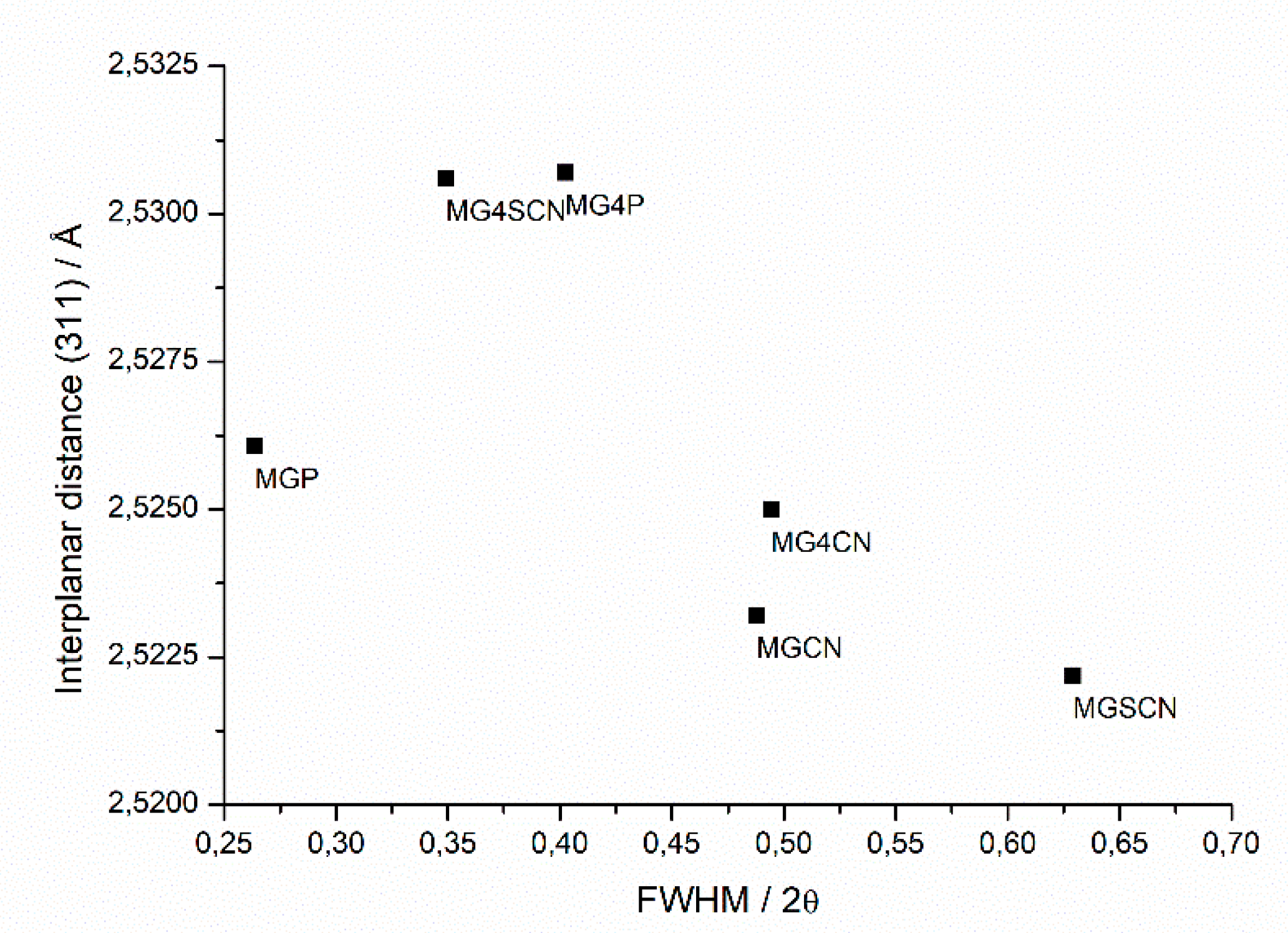
3. Results
4. Discussion
Implications for Prebiotic Chemistry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Erastova, V.; Degiacomi, M.T.; Fraser, D.G.; Greenwell, H.C. Mineral Surface Chemistry Control for Origin of Prebiotic Peptides. Nat. Commun. 2017, 8, 2033. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.-F. Adsorption and Polymerization of Amino Acids on Mineral Surfaces: A Review. Orig. Life Evol. Biosph. 2008, 38, 211–242. [Google Scholar] [CrossRef] [PubMed]
- Hazen, R.M.; Ferry, J.M. Mineral Evolution: Mineralogy in the Fourth Dimension. Elements 2010, 6, 9–12. [Google Scholar] [CrossRef]
- Hazen, R.M.; Papineau, D.; Bleeker, W.; Downs, R.T.; Ferry, J.M.; McCoy, T.J.; Sverjensky, D.A.; Yang, H. Mineral Evolution. Am. Mineral. 2008, 93, 1693–1720. [Google Scholar] [CrossRef]
- Bykova, E.; Dubrovinsky, L.; Dubrovinskaia, N.; Bykov, M.; McCammon, C.; Ovsyannikov, S.V.; Liermann, H.P.; Kupenko, I.; Chumakov, A.I.; Rüffer, R.; et al. Structural Complexity of Simple Fe2O3 at High Pressures and Temperatures. Nat. Commun. 2016, 7, 10661. [Google Scholar] [CrossRef]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal Vents and the Origin of Life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef]
- Schoonen, M.; Smirnov, A.; Cohn, C. A Perspective on the Role of Minerals in Prebiotic Synthesis. AMBIO A J. Hum. Environ. 2004, 33, 539–551. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Guth, T.D. Hydrogen Evolving Systems. 1. The Formation of Molecular Hydrogen from Aqueous Suspensions of Iron (II) Hydroxide and Reactions with Reducible Substrates, Including Molecular Nitrogen. J. Am. Chem. Soc. 1976, 98, 3508–3513. [Google Scholar] [CrossRef]
- Blesa, M.A.; Matijević, E. Phase Transformations of Iron Oxides, Oxohydroxides, and Hydrous Oxides in Aqueous Media. Adv. Colloid Interface Sci. 1989, 29, 173–221. [Google Scholar] [CrossRef]
- Evans, U.R.; Wanklyn, J.N. Evolution of Hydrogen from Ferrous Hydroxide. Nature 1948, 162, 27–28. [Google Scholar] [CrossRef]
- Géhin, A.; Ruby, C.; Abdelmoula, M.; Benali, O.; Ghanbaja, J.; Refait, P.; Génin, J.M.R. Synthesis of Fe (II-III) Hydroxysulphate Green Rust by Coprecipitation. Solid State Sci. 2002. [Google Scholar] [CrossRef]
- Schwertmann, U.; Fechter, H. The Formation of Green Rust and Its Transformation to Lepidocrocite. Clay Miner. 1994, 29, 87–92. [Google Scholar] [CrossRef]
- Braterman, P.S.; Cairns-Smith, A.G.; Sloper, R.W.; Truscott, T.G.; Craw, M. Photo-Oxidation of Iron (II) in Water between PH 7.5 and 4.0. J. Chem. Soc. Dalt. Trans. 1984, 7, 1441–1445. [Google Scholar] [CrossRef]
- Braterman, P.S.; Cairns-Smith, A.G.; Sloper, R.W. Photo-Oxidation of Hydrated Fe2+—Significance for Banded Iron Formations. Nature 1983, 303, 163–164. [Google Scholar] [CrossRef]
- Bassez, M.-P. Water near Its Supercritical Point and at Alkaline PH for the Production of Ferric Oxides and Silicates in Anoxic Conditions. A New Hypothesis for the Synthesis of Minerals Observed in Banded Iron Formations and for the Related Geobiotropic Chemistry Insi. Orig. Life Evol. Biosph. 2018, 48, 289–320. [Google Scholar] [CrossRef] [PubMed]
- Holm, N.G.; Andersson, E. Hydrothermal Simulation Experiments as a Tool for Studies of the Origin of Life on Earth and Other Terrestrial Planets: A Review. Astrobiology 2005, 5, 444–460. [Google Scholar] [CrossRef]
- Rao, K.R.P.M.; Huggins, F.E.; Mahajan, V.; Huffman, G.P.; Rao, V.U.S. The role of magnetite in Fischer-Tropsch synthesis. Hyperfine Interact. 1994, 93, 1745–1749. [Google Scholar] [CrossRef]
- Shroff, M.D.; Kalakkad, D.S.; Coulter, K.E.; Kohler, S.D.; Harrington, M.S.; Jackson, N.B.; Sault, A.G.; Datye, A.K. Activation of Precipitated Iron Fischer-Tropsch Synthesis Catalysts. J. Catal. 1995, 156, 185–207. [Google Scholar] [CrossRef]
- Taran, Y.A.; Kliger, G.A.; Sevastianov, V.S. Carbon isotope effects in the open-system Fischer–Tropsch synthesis. Geochim. Cosmochim. Acta 2007, 71, 4474–4487. [Google Scholar] [CrossRef]
- Mißbach, H.; Schmidt, B.C.; Duda, J.-P.; Lünsdorf, N.K.; Goetz, W.; Thiel, V. Assessing the diversity of lipids formed via Fischer-Tropsch-type reactions. Org. Geochem. 2018, 119, 110–121. [Google Scholar] [CrossRef]
- Wang, D.; Han, Y.; Han, H.; Li, K.; Xu, C.; Zhuang, H. New insights into enhanced anaerobic degradation of Fischer-Tropsch wastewater with the assistance of magnetite. Bioresour. Technol. 2018, 257, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Urey, H.C. On the Early Chemical History of the Earth and the Origin of Life. Proc. Natl. Acad. Sci. USA 1952, 38, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Joshi, P.C.; Edelson, E.H.; Lawless, J.G. HCN: A Plausible Source of Purines, Pyrimidines and Amino Acids on the Primitive Earth. J. Mol. Evol. 1978. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. A Production of Amino Acids under Possible Primitive Earth Conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Oró, J. Synthesis of Adenine from Ammonium Cyanide. Biochem. Biophys. Res. Commun. 1960, 2, 407–412. [Google Scholar] [CrossRef]
- Lahav, N.; Chang, S. The Possible Role of Solid Surface Area in Condensation Reactions during Chemical Evolution: Reevaluation. J. Mol. Evol. 1976, 8, 357–380. [Google Scholar] [CrossRef]
- Bartlett, P.D.; Davis, R.E. Reactions of Elemental Sulfur. II. The Reaction of Alkali Cyanides with Sulfur, and Some Single-Sulfur Transfer Reactions. J. Am. Chem. Soc. 1958. [Google Scholar] [CrossRef]
- De Santana, H.; Paesano, A.; da Costa, A.C.S.; di Mauro, E.; de Souza, I.G.; Ivashita, F.F.; de Souza, C.M.D.; Zaia, C.T.B.V.; Zaia, D.A.M. Cysteine, Thiourea and Thiocyanate Interactions with Clays: FT-IR, Mössbauer and EPR Spectroscopy and X-Ray Diffractometry Studies. Amino Acids 2010, 38, 1089–1099. [Google Scholar] [CrossRef]
- Zaia, D.A.M. Adsorption of Amino Acids and Nucleic Acid Bases onto Minerals: A Few Suggestions for Prebiotic Chemistry Experiments. Int. J. Astrobiol. 2012, 11, 229–234. [Google Scholar] [CrossRef]
- Izawa, M.; Nesbit, H.; MacRae, N.; Hoffman, E. Composition and evolution of the early oceans: evidence from the Tagish Lake meteorite. Earth Planet. Sci. Lett. 2010, 298, 443–449. [Google Scholar] [CrossRef]
- Higgins, J.H.; Schrag, D.P. The Mg isotopic composition of Cenozoic seawater evidence for a link between Mg-Clays, seawater Mg/Ca, and climate. Earth Planet. Sci. Lett. 2015, 416, 73–81. [Google Scholar] [CrossRef]
- Boehnke, P.; Harrison, T.M. Illusory late heavy bombardments. Proc. Natl. Acad. Sci. USA 2016, 113, 10802–10806. [Google Scholar] [CrossRef] [PubMed]
- Breuer, D. Early planetary atmospheres and surfaces: Origin of the Earth’s water, crust and atmosphere. Proc. IAU Symp. 2018, 345, 156–163. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in Laboratory; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Uehara, G. Mineral–Chemical properties of oxisols. In International Soil Classification Workshop, Volume 2; Soil Survey Division-Land Development Department: Bangkok, Thailand, 1979; pp. 45–46. [Google Scholar]
- Scoggins, M.W. Ultraviolet Spectrophotometric Determination of Cyanide Ion. Anal. Chem. 1972, 44, 1294–1296. [Google Scholar] [CrossRef]
- Suzuki, T.; Hioki, A.; Kurahashi, M. Development of a Method for Estimating an Accurate Equivalence Point in Nickel Titration of Cyanide Ions. Anal. Chim. Acta 2003, 476, 159–165. [Google Scholar] [CrossRef]
- Martins, F.G.; Andrade, J.F.; Pimenta, A.C.; Lourenço, L.M.; Castro, J.R.M.; Balbo, V.R. Spectrophotometric Study of Iron Oxidation in the Iron(II)/Thiocyanate/ Acetone System and Some Analytical Applications. Eclet. Quim. 2005. [Google Scholar] [CrossRef]
- Paterson, E. The Iron Oxides. Structure, Properties, Reactions, Occurrences and Uses. Clay Miner. 1999, 34, 209–210. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Burton, E.D.; Bush, R.T.; Sullivan, L.A.; Mitchell, D.R.G. Schwertmannite Transformation to Goethite via the Fe (II) Pathway: Reaction Rates and Implications for Iron–Sulfide Formation. Geochim. Cosmochim. Acta 2008, 72, 4551–4564. [Google Scholar] [CrossRef]
- Burton, E.D.; Bush, R.T.; Sullivan, L.A.; Mitchell, D.R.G. Reductive Transformation of Iron and Sulfur in Schwertmannite-Rich Accumulations Associated with Acidified Coastal Lowlands. Geochim. Cosmochim. Acta 2007, 71, 4456–4473. [Google Scholar] [CrossRef]
- Carneiro, C.E.A.; Ivashita, F.F.; De Souza, I.G.; De Souza, C.M.D.; Paesano, A.; Da Costa, A.C.S.; Di Mauro, E.; De Santana, H.; Zaia, C.T.B.V.; Zaia, D.A.M. Synthesis of Goethite in Solutions of Artificial Seawater and Amino Acids: A Prebiotic Chemistry Study. Int. J. Astrobiol. 2013. [Google Scholar] [CrossRef]
- Millero, F.J.; Sotolongo, S.; Izaguirre, M. The Oxidation Kinetics of Fe (II) in Seawater. Geochim. Cosmochim. Acta 1987, 51, 793–801. [Google Scholar] [CrossRef]
- Bernal, J.D. The Physical Basis of Life; Routledge and Kegan Paul Ltd.: London, UK, 1951. [Google Scholar]
- Prasad, P.S.R. Direct Formation of the CaSO4 Phase in Dehydration Process of Gypsum: In Situ FTIR Study. Am. Mineral. 2005, 90, 672–678. [Google Scholar] [CrossRef]
- Daniels, J.M.; Rosencwaig, A. Mössbauer Spectroscopy of Stoichiometric and Non-Stoichiometric Magnetite. J. Phys. Chem. Solids 1969, 30, 1561–1571. [Google Scholar] [CrossRef]
- Řezníček, R.; Chlan, V.; Štěpánková, H.; Novák, P.; Żukrowski, J.; Kozłowski, A.; Kąkol, Z.; Tarnawski, Z.; Honig, J.M. Understanding the Mössbauer Spectrum of Magnetite below the Verwey Transition: Ab Initio Calculations, Simulation, and Experiment. Phys. Rev. B 2017, 96, 195124. [Google Scholar] [CrossRef]
- Shipilin, M.A.; Zakharova, I.N.; Shipilin, A.M.; Bachurin, V.I. Mössbauer Studies of Magnetite Nanoparticles. J. Surf. Investig. X Ray Synchrotron Neutron Tech. 2014. [Google Scholar] [CrossRef]
- Silva, N.J.O.; Amaral, V.S.; Carlos, L.D.; Rodríguez-González, B.; Liz-Marzán, L.M.; Millan, A.; Palacio, F.; de Zea Bermudez, V. Structural and Magnetic Studies in Ferrihydrite Nanoparticles Formed within Organic-Inorganic Hybrid Matrices. J. Appl. Phys. 2006, 100, 054301. [Google Scholar] [CrossRef]
- Silva, N.J.O.; Amaral, V.S.; Carlos, L.D.; Fu, L.S.; Nunes, S.C.; de Zea Bermudez, V. Magnetic Behavior of Iron (III) Oxyhydroxy Nanoparticles in Organic–Inorganic Hybrid Matrices. J. Magn. Magn. Mater. 2005, 290–291, 962–965. [Google Scholar] [CrossRef]
- Pósfai, M.; Lefèvre, C.T.; Trubitsyn, D.; Bazylinski, D.A.; Frankel, R.B. Phylogenetic Significance of Composition and Crystal Morphology of Magnetosome Minerals. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef]
- Ranjan, S.; Todd, Z.R.; Rimmer, P.B.; Sasselov, D.D.; Babbin, A.R. Nitrogen oxide concentrations in natural waters on early Earth. Geochem. Geophys. Geosystem. 2019, 20, 2021–2039. [Google Scholar] [CrossRef]
- Ewers, W.E. Chemical Conditions for the Precipitation of Banded Iron-Formations. In Biogeochemistry of Ancient and Modern Environments; Springer: Berlin/Heidelberg, Germany, 1980; pp. 83–92. [Google Scholar] [CrossRef]
- Holland, H.D. The Oceans: A possible source of iron in iron-formations. Econ. Geol. 1973, 68, 1169–1172. [Google Scholar] [CrossRef]
- Walker, J.C.G.; Brimblecombe, P. Iron and sulfur in the pre-biologic ocean. Precambrian Res. 1985, 28, 205–222. [Google Scholar] [CrossRef]
| Sample Code | SOLUTION 1 Reagents |
|---|---|
| MGP | 5.72 g (30 mmol) of FeCl2·7H2O/60 mL ultrapure water |
| MG4P | 5.72 g (30 mmol) of FeCl2·7H2O/60 mL of seawater 4.0 Gy |
| MGCN | 5.72 g (30 mmol) of FeCl2·7H2O)/60 mL ultrapure water/3.9 g (60 mmol) of KCN |
| MG4CN | 5.72 g (30 mmol) of FeCl2·7H2O/60 mL of seawater 4.0 Gy/3.9 g (60 mmol) of KCN |
| MGSCN | 5.72 g (30 mmol) of FeCl2·7H2O)/60 mL ultra-pure water/5.82 g (60 mmol) of KSCN |
| MG4SCN | 5.72 g (30 mmol) of FeCl2·7H2O/60 mL of seawater 4.0 Gy/5.82 g (60 mmol) of KSCN |
| Sample | Mineral Phase Found | % = (Mineral Phase Mass/Total Mass) × 100 | Rwp/% | χ2 |
|---|---|---|---|---|
| MGP | Magnetite | 100 | 22.16196 | 2.59452 |
| MG4P | Magnetite | 53.7 | 22.28601 | 2.46596 |
| Goethite | 26.9 | |||
| Gypsum | 19.4 | |||
| MGCN | Magnetite | 100 | 20.11383 | 1.70119 |
| MG4CN | Magnetite | 100 | 24.34639 | 2.90163 |
| MGSCN | Magnetite | 59.4 | 23.78344 | 3.03894 |
| Goethite | 40.6 | |||
| MG4SCN | Magnetite | 49.5 | 19.47453 | 2.09246 |
| Goethite | 36.2 | |||
| Gypsum | 10.1 | |||
| Sylvite | 4.3 |
| Sample | Sub-Spectra | IS/mm s−1 | QS/mm s−1 | Bhf/T | Mineral Correspondence |
|---|---|---|---|---|---|
| MGP | Sextet | 0.34 | −0.08 | 48.5 | Magnetite |
| Sextet | 0.65 | −0.01 | 45.1 | ||
| MG4P | Sextet | 0.31 | −0.00 | 47.8 | Magnetite |
| * Dist. | 0.63 | −0.12 | 42.8 | Magnetite/Goethite | |
| Sextet | |||||
| Doublet | 0.38 | 0.77 | ----- | Ferrihydrite | |
| MGCN | Sextet | 0.34 | −0.08 | 51.2 | Magnetite |
| Sextet | 0.67 | −0.10 | 47.9 | ||
| MG4CN | Sextet | 0.29 | −0.02 | 49.1 | Magnetite |
| Sextet | 0.58 | 0.08 | 44.8 | Magnetite | |
| Doublet | 0.36 | 0.71 | ----- | Ferrihydrite | |
| MGSCN | Sextet | 0.34 | −0.04 | 51.2 | Magnetite |
| Sextet | 0.67 | −0.06 | 47.4 | ||
| * Dist. | 0.34 | −0.02 | 33.6 | Goethite | |
| Sextet | |||||
| Doublet | 0.37 | 0.70 | ----- | Ferrihydrite | |
| Sextet | 0.39 | −0.23 | 39.6 | Goethite | |
| MG4SCN | Sexto | 0.28 | −0.03 | 49.2 | Magnetite |
| * Dist. | 0.51 | 0.10 | 44.1 | Magnetite/Goethite | |
| Sextet | |||||
| Doublet | 0.36 | 0.69 | ----- | Ferrihydrite |
| Sample | pHPZC |
|---|---|
| MGP | 7.34 ± 0.04 |
| MG4P | 8.97 ± 0.07 |
| MGCN | 6.15 ± 0.08 |
| MG4CN | 9.14 ± 0.04 |
| MGSCN | 8.35 ± 0.06 |
| MG4SCN | 8.79 ± 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samulewski, R.B.; Gonçalves, J.M.; Urbano, A.; da Costa, A.C.S.; Ivashita, F.F.; Paesano, A., Jr.; Zaia, D.A.M. Magnetite Synthesis in the Presence of Cyanide or Thiocyanate under Prebiotic Chemistry Conditions. Life 2020, 10, 34. https://doi.org/10.3390/life10040034
Samulewski RB, Gonçalves JM, Urbano A, da Costa ACS, Ivashita FF, Paesano A Jr., Zaia DAM. Magnetite Synthesis in the Presence of Cyanide or Thiocyanate under Prebiotic Chemistry Conditions. Life. 2020; 10(4):34. https://doi.org/10.3390/life10040034
Chicago/Turabian StyleSamulewski, Rafael Block, Josué Martins Gonçalves, Alexandre Urbano, Antônio Carlos Saraiva da Costa, Flávio F. Ivashita, Andrea Paesano, Jr., and Dimas Augusto Morozin Zaia. 2020. "Magnetite Synthesis in the Presence of Cyanide or Thiocyanate under Prebiotic Chemistry Conditions" Life 10, no. 4: 34. https://doi.org/10.3390/life10040034
APA StyleSamulewski, R. B., Gonçalves, J. M., Urbano, A., da Costa, A. C. S., Ivashita, F. F., Paesano, A., Jr., & Zaia, D. A. M. (2020). Magnetite Synthesis in the Presence of Cyanide or Thiocyanate under Prebiotic Chemistry Conditions. Life, 10(4), 34. https://doi.org/10.3390/life10040034






