Spectrum of Light as a Determinant of Plant Functioning: A Historical Perspective
Abstract
1. The Milestones of Plant Photobiology
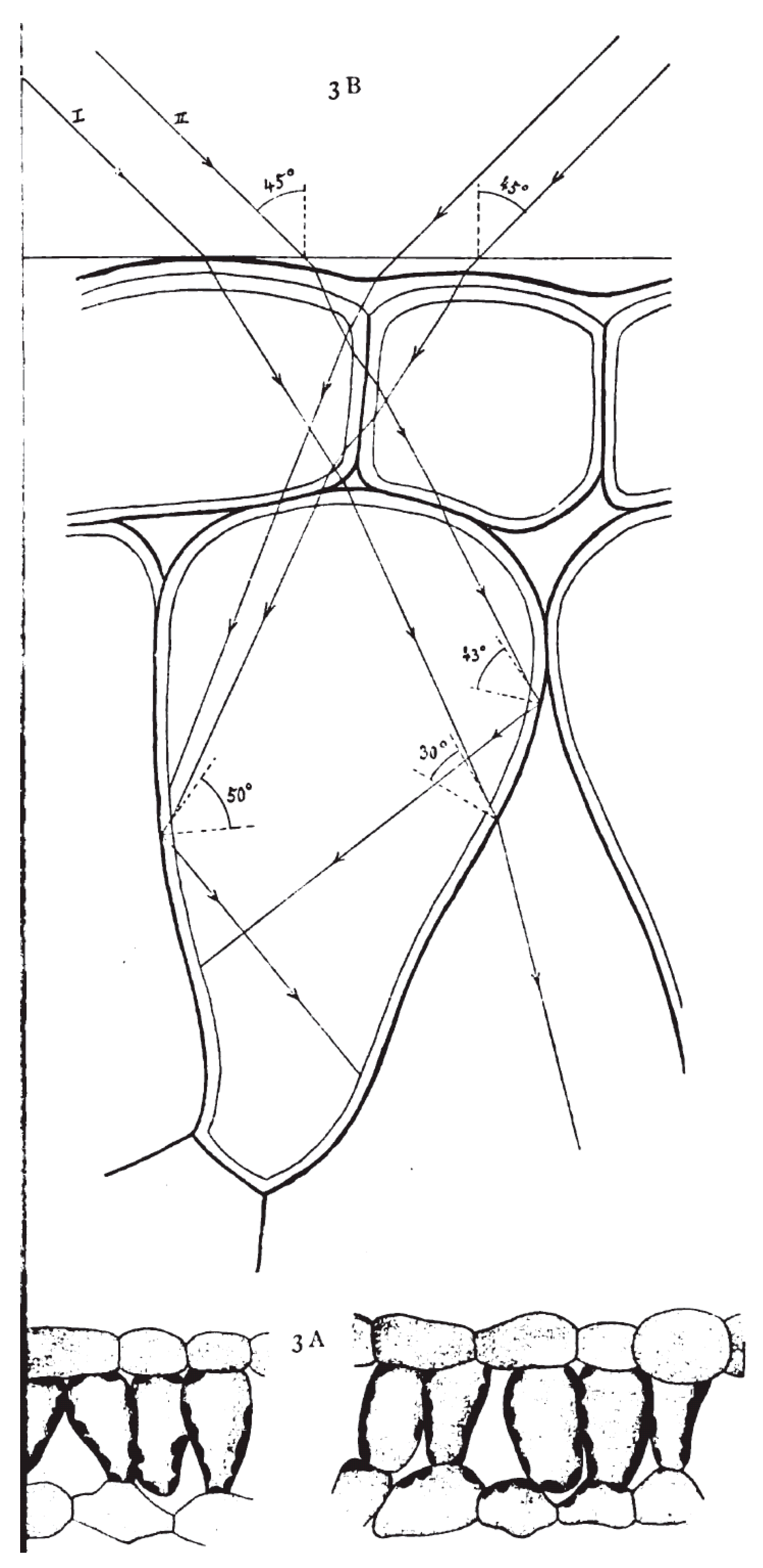
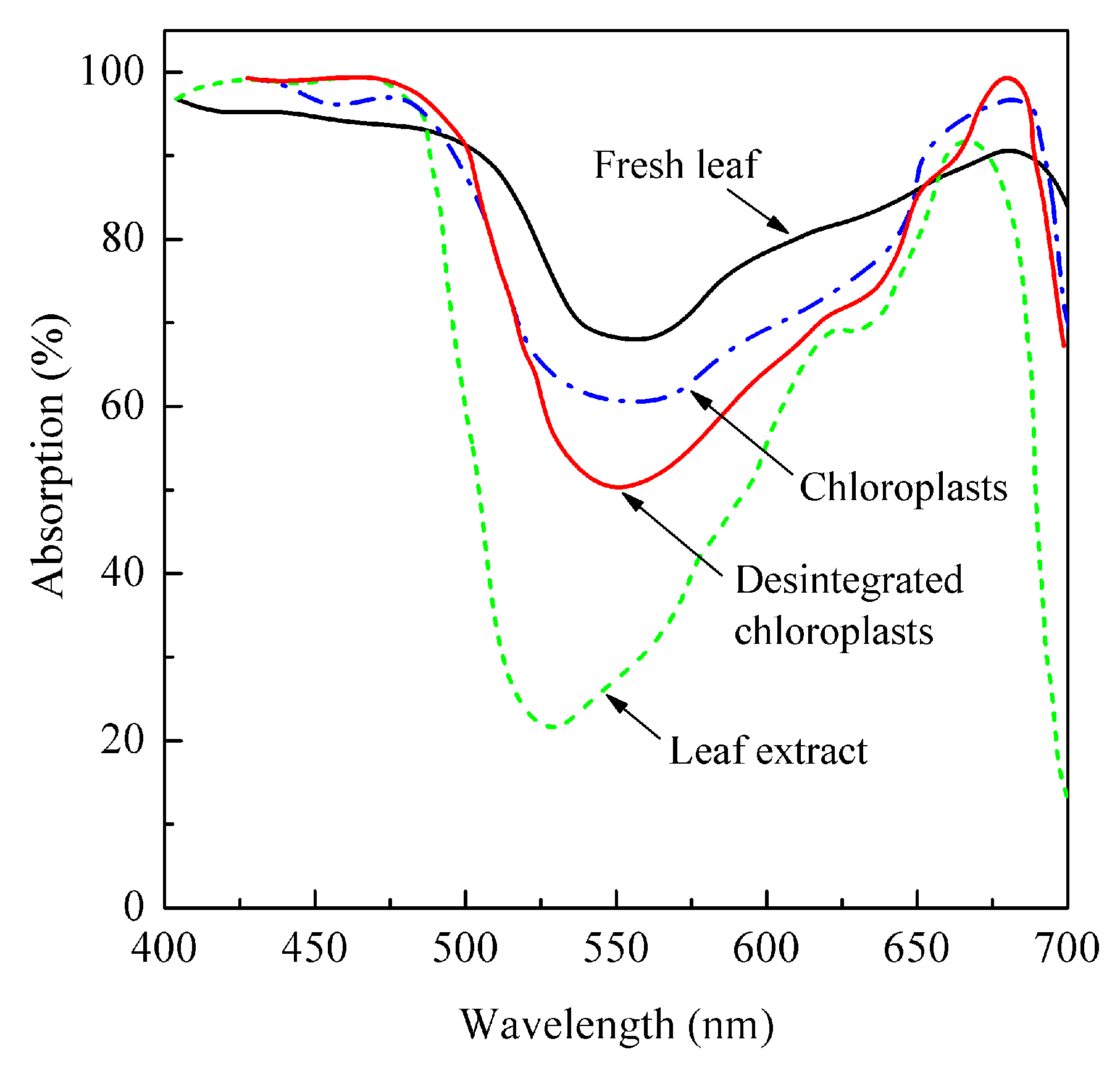
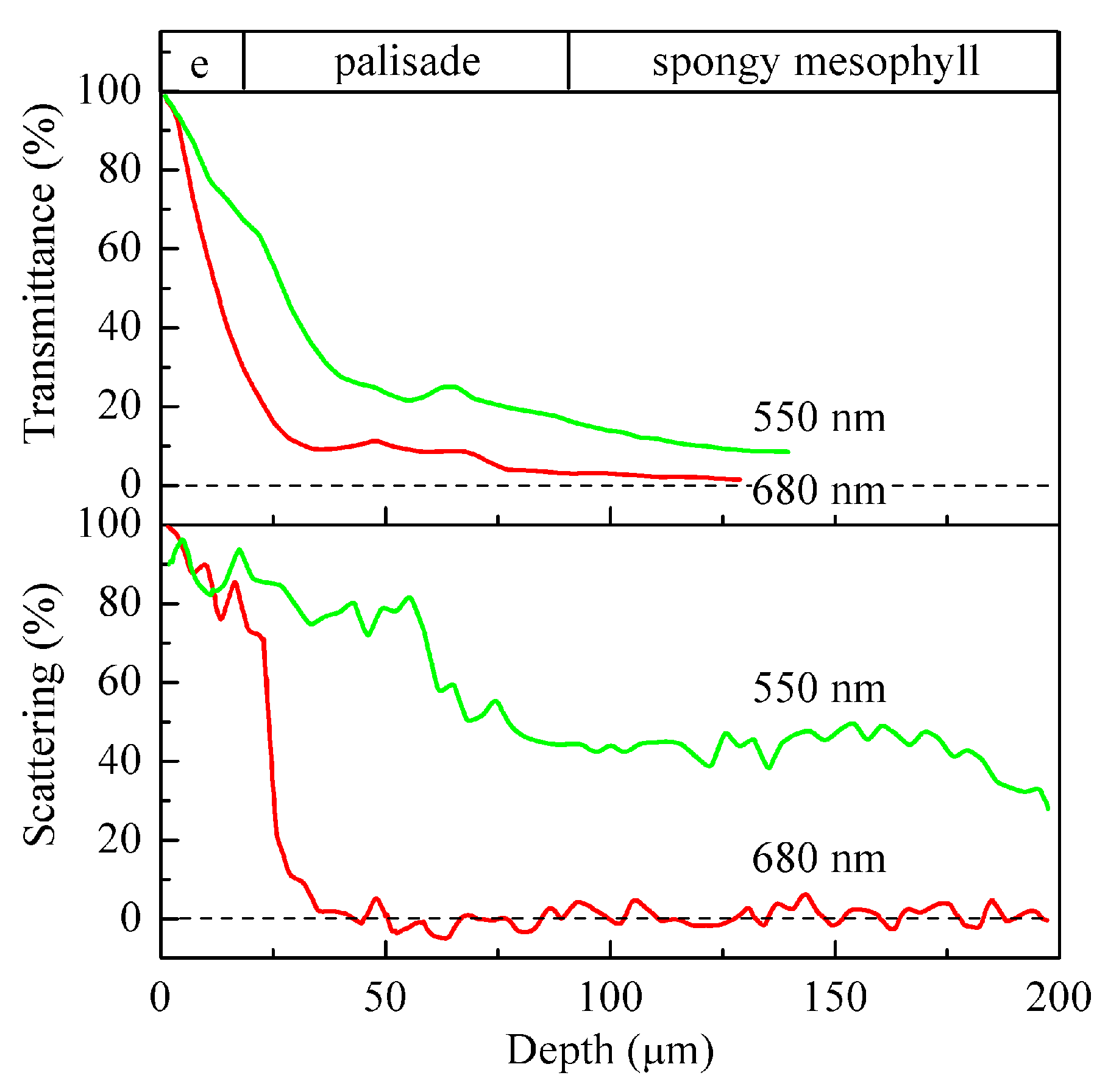
2. Energy-Supplying Function of Light in Photosynthesis
3. Spectrum-Dependent Light Regulation of Photosynthesis
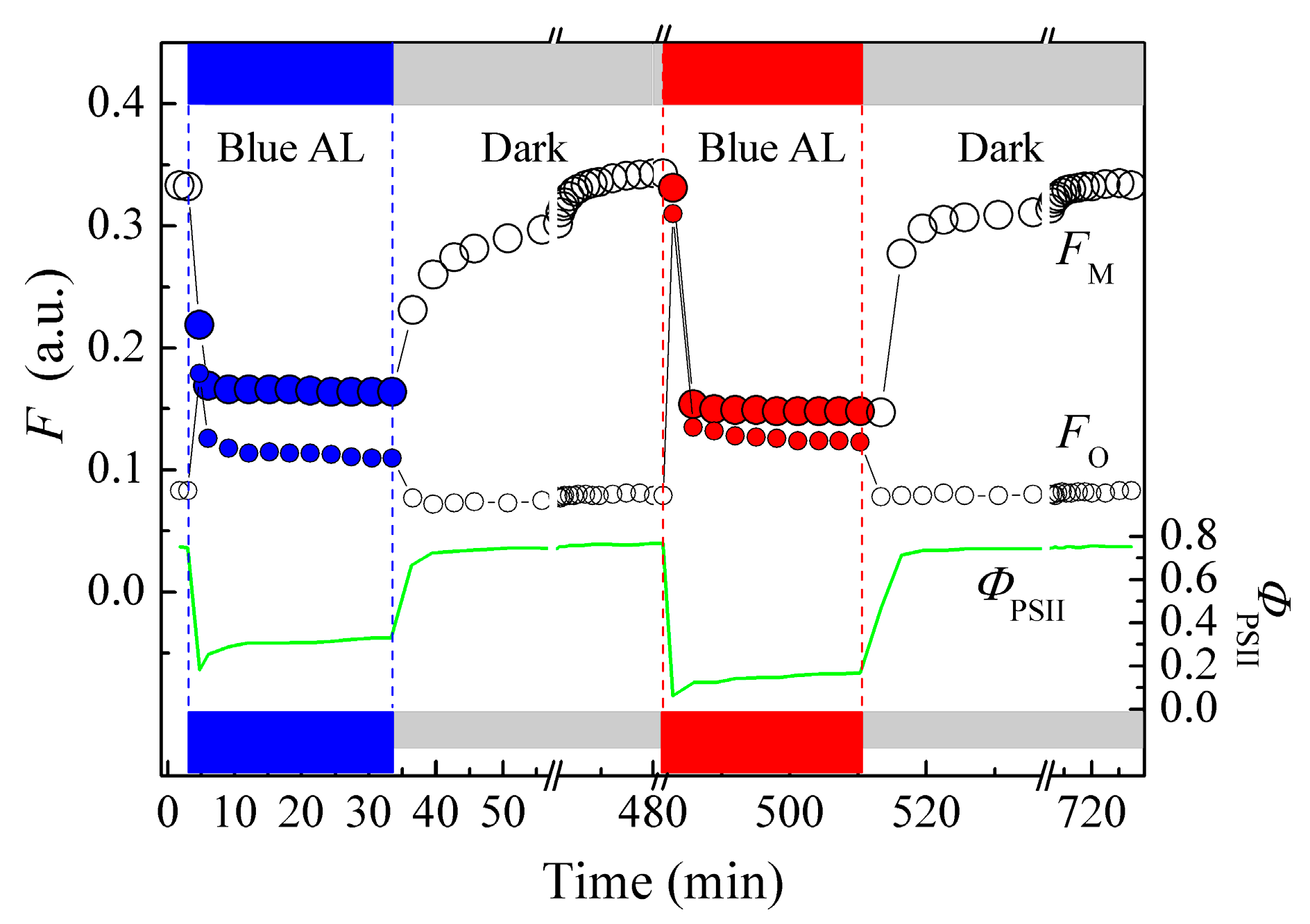
3.1. Spectral Dependency of and Photoreceptors for Chloroplast Photorelocation Movement
3.2. Chloroplast Photorelocation Movement in Acclimation to Environmental Stimuli
4. The Spectral Effects on a Whole-Plant Scale
5. The Promise for Improving the Spectral Response of Photosynthesis in Plants
6. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| A | B | C | D | E | F | G | H | I | J | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cucumis sativus L. | 7–50% | 100 | 16 | 17–22 | RP | 3 | 1.4 | [86] | ||
| 2 | Raphanus sativus L. | 5–10% | 200 | 16 | 25, 50 | RP DM | 1.8–2.2 1.6 | [83] | |||
| 3 | Raphanus sativus L., Lactuca sativa L., Spinacea oleracea L. | 50% | 300 | 18, 18, 12 1) | 21 | RP DM | 2.4, 0.93, 0.94 1) 1.6–2.4, up to 8 3) | 0.97, 1.11, 0.82 1) 0.5–0.95 | CWF | [112] | |
| 4 | Raphanus sativus L., Lactuca sativa L., Capsicum annuum L. | 0–12% | 200 | 16 | 21, 21, 21 3) | DM | 1.1–2.0 | 1.15–1.75 | [81] | ||
| 5 | —“— | –“– | 500 | 16 | –“– | DM | 1.2–1.65 | 1.15–1.8 | —“— | ||
| 6 | Brassica campestris L. | 14% | 150 | 12 | 28 3) | FM DM SS SP | 1.8 1.15 0.71 1.15 | 1.8 1.19 0.51 1.08 | 2.0 1.23 1.32 1.05 | DyL | [113] |
| 7 | Triticum aestivum L. | 10% | 350 | 24 | 15–70 | RP DM SM | 2.1–2.3 1.4–1.7 1.9 | 0.7–0.1 (0.55–0.67) 4) 0.6–1.0 (0.4–0.9) 4) 0.8 (0.7) 4) | CWF | [84] | |
| 8 | Doritaenopsis hort., in vitro | 50% | 70 | 16 | 14–56 | FM, DM | 1.3–1.7 | 1.3–2 | [114] | ||
| 9 | Withania somnifera (L) Dunal., in vitro | 50% | 30 | 16 | 35 3) | FM, DM | 2.6 | 2.8 | [115] | ||
| 10 | Lactuca sativa L. | 0–59% | 170 | 12 | 28 3) | FM DM | 0.25 0.3 | [116] | |||
| 11 | Brassica campestris L. | 11% | 80 | 12 | 60 | FM DM | 0.9 0.7 | 0.8 1.1 | 3.8 2.3 | FL | [117] |
| 12 | Lactuca sativa L. | 10% | 325 | 16 | 21 | FM DM | 1.17 1.14 | CWF + Inc | [77] | ||
| 13 | Capsicum annuum L. | 1% | 300 | 12 | 21 3) | DM | 1.0–1.7 | 0.75–0.9 | MH | [118] | |
| 14 | Brassica chinensis L. | 14% | 100 | 24 | 15, 27 | DM SS | 0.5–1.08 0.2 | HPSL | [119] | ||
| 15 | —“— | –“– | 400 | –“– | –“– | DM SS | 0.76–0.86 0.4–0.6 | HPSL | —“— |
References
- Priestley, J. XIX. Observations on different kinds of air. Philos. Trans. R. Soc. Lond. 1772, 62, 147–264. [Google Scholar]
- Ingenhousz, J. Experiments upon Vegetables: Discovering Their Great Power of Purifying the Common Air in the Sun-Shine, and of Injuring It in the Shade and at Night. To Which Is Joined, a New Method of Examining the Accurate Degree of Salubrity of the Atmosphere; Elmsly, P.& Payne, H.: London, UK, 1779. [Google Scholar]
- Govindjee Krogmann, D. Discoveries in oxygenic photosynthesis (1727–2003): A perspective. In Discoveries in Photosynthesis; Govindjee Beatty, J.T., Gest, H., Allen, J.F., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 63–105. [Google Scholar]
- Daubeny, C.G.B. On the action of light upon plants, and of plants upon the atmosphere. Philos. Trans. R. Soc. Lond. 1836, 126, 149–175. [Google Scholar]
- Senebier, J. Expériences sur L’action de la Lumière Solaire Dans la Végétation; Barde, Magnet & Compagnie: Geneva, Switzerland, 1788. [Google Scholar]
- Hunt, R. XLVIII. Experiments and observations on light which has permeated coloured media, and on the chemical action of the solar spectrum. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1840, 16, 267–275. [Google Scholar] [CrossRef][Green Version]
- Gardner, D.P.I. On the action of yellow light in producing the green colour, and indigo light the movements of plants. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1844, 24, 1–15. [Google Scholar] [CrossRef]
- Pringsheim, M. VI. On the action of light and the function of chlorophyll in plants. J. Nat. Hist. 1880, 5, 62–74. [Google Scholar] [CrossRef][Green Version]
- Draper, J.W. XXV. Note on the decomposition of carbonic acid by the leaves of plants under the influence of yellow light. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1844, 25, 169–173. [Google Scholar] [CrossRef]
- Draper, J.W. XXI. On the decomposition of carbonic ac id gas and the alkaline carbonates, by the light of the sun; and on the tithonotype. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1843, 23, 161–176. [Google Scholar] [CrossRef]
- Pfeffer, W. Die Wirkung Farbigen Lichtes Auf Die Zersetzung der Kohlens{ä}ure in Pflanzen: Habilitationsschrift; Wilhelm Engelmann: Leipzig, Germany, 1871. [Google Scholar]
- Von Mayer, R. Die Organische Bewegung in Ihrem Zusammenhange Mit dem Stoffwechsel: Ein Beitrag zur Naturkunde; Verlag der C. Drechsler ҆schen Buchhandlung: Heilbronn, Germany, 1845. [Google Scholar]
- Tyndall, J. Heat Considered as a Mode of Motion: Being a Course of Twelve Lectures Delivered at the Royal Institution of Great Britain in the Season of 1862; Longman, Green, Longman, Roberts & Green: London, UK, 1863. [Google Scholar]
- Kirchhoff, P.; Bunsen, P. IX. Chemical analysis by spectrum-observations. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1860, 20, 88–109. [Google Scholar] [CrossRef]
- Stokes, G.G. XXXIV. On the application of the optical properties of bodies to the detection and discrimination of organic substances. J. Chem. Soc. 1864, 17, 304–318. [Google Scholar] [CrossRef]
- Stokes, G.G. II. On the supposed identity of biliverdin with chlorophyll, with remarks on the constitution of chlorophyll. Proc. R. Soc. Lond. 1864, 13, 144–145. [Google Scholar]
- Timiriazeff, C.A. Recherches sur la décomposition de l’acide carbonique dans le spectre solaire par les parties vertes des végetaux. Ann. Chim. Phys. Ser. 1877, 12, 355–396. [Google Scholar]
- Timiriazeff, C.A. Croonian lecture. The cosmical function of the green plant. Proc. R. Soc. Lond. 1904, 72, 424–461. [Google Scholar]
- Böhm, J.A. Beiträge zur näheren Kenntniss des Chlorophylls. Sitz. Kais Akad. Wiss. Math. Nat. Cl. 1856, Bd. XXII, 479–498. [Google Scholar]
- Frank, B. Über lichtwärts sich bewegende Chlorophyllkörner. Bot. Ztg. 1871, 29, 209. [Google Scholar]
- Kataoka, H. Gustav Senn (1875–1945): The pioneer of chloroplast movement research. J. Integr. Plant Biol. 2015, 57, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Senn, G. Die Gestalts-Und Lagever{ä}nderung der Pflanzen-Chromatophoren: Mit Einer Beilage: Die Lichtbrechung der Lebenden Pflanzenzelle; Verlag von Wilhelm Engelmann: Leipzig, Germany, 1908. [Google Scholar]
- Wada, M. Chloroplast movement. Plant Sci. 2013, 210, 177–182. [Google Scholar] [CrossRef]
- Butler, W.L.; Norris, K.H.; Siegelman, H.W.; Hendricks, S.B. Detection, assay, and preliminary purification of the pigment controlling photoresponsive development of plants. Proc. Natl. Acad. Sci. USA 1959, 45, 1703. [Google Scholar] [CrossRef]
- Borthwick, H.A.; Hendricks, S.B.; Parker, M.W.; Toole, E.H.; Toole, V.K. A reversible photoreaction controlling seed germination. Proc. Natl. Acad. Sci. USA 1952, 38, 662. [Google Scholar] [CrossRef]
- Engelmann, T.W. Farbe und assimilation. Bot. Ztg. 1883, 41, 1–13. [Google Scholar]
- Haxo, F.T.; Blinks, L.R. Photosynthetic action spectra of marine algae. J. Gen. Physiol. 1950, 33, 389–422. [Google Scholar] [CrossRef]
- Chen, S.L. The action spectrum for the photochemical evolution of oxygen by isolated chloroplasts. Plant Physiol. 1952, 27, 35. [Google Scholar] [CrossRef] [PubMed]
- Nishio, J.N. Why are higher plants green? Evolution of the higher plant photosynthetic pigment complement. Plant Cell Environ. 2000, 23, 539–548. [Google Scholar] [CrossRef]
- Rabideau, G.S.; French, C.S.; Holt, A.S. The absorption and reflection spectra of leaves, chloroplast suspensions, and chloroplast fragments as measured in an Ulbricht sphere. Am. J. Bot. 1946, 769–777. [Google Scholar] [CrossRef]
- Moss, R.A.; Loomis, W.E. Absorption spectra of leaves. I. The visible spectrum. Plant Physiol. 1952, 27, 370. [Google Scholar] [CrossRef]
- Strain, H.H. Cellular opacity and the activity of chloroplast pigments in photosynthesis. Science 1950, 112, 161–164. [Google Scholar] [CrossRef]
- Strain, H.H. Functions and properties of the chloroplast pigments. In Photosynthesis in Plants; Franck, J., Loomis, W.E., Eds.; Iowa State College Press: Ames, IA, USA, 1949; pp. 133–178. [Google Scholar]
- Jeje, A.; Zimmermann, M. The anisotropy of the mesophyll and CO2 capture sites in Vicia faba L. leaves at low light intensities. J. Exp. Bot. 1983, 34, 1676–1694. [Google Scholar] [CrossRef]
- Nishio, J.N.; Sun, J.; Vogelmann, T.C. Carbon fixation gradients across spinach leaves do not follow internal light gradients. Plant Cell 1993, 5, 953–961. [Google Scholar] [CrossRef]
- Terashima, I.; Saeki, T. A new model for leaf photosynthesis incorporating the gradients of light environment and of photosynthetic properties of chloroplasts within a leaf. Ann. Bot. 1985, 56, 489–499. [Google Scholar] [CrossRef]
- Vogelmann, T.C.; Bornman, J.F.; Josserand, S. Photosynthetic light gradients and spectral regime within leaves of Medicago sativa. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1989, 323, 411–421. [Google Scholar]
- Cui, M.; Vogelmann, T.C.; Smith, W.K. Chlorophyll and light gradients in sun and shade leaves of Spinacia oleracea. Plant Cell Environ. 1991, 14, 493–500. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.-L.; Neilan, B.A.; Scheer, H. A red-shifted chlorophyll. Science 2010, 329, 1318–1319. [Google Scholar] [CrossRef] [PubMed]
- Karapetyan, N.V.; Schlodder, E.; van Grondelle, R.; Dekker, J.P. The long wavelength chlorophylls of photosystem I. In Photosystem I.; Golbeck, J.H., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 177–192. [Google Scholar]
- Kono, M.; Kawaguchi, H.; Mizusawa, N.; Yamori, W.; Suzuki, Y.; Terashima, I. Far-Red Light Accelerates Photosynthesis in the Low-Light Phases of Fluctuating Light. Plant Cell Physiol. 2019, 61, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-G.; Ort, D.R.; Whitmarsh, J.; Long, S.P. The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: A theoretical analysis. J. Exp. Bot. 2004, 55, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Haupt, W. Phototaxis in plants. In International Review of Cytology; Bourne, G.H., Danielli, J.F., Eds.; Academic Press: New York, NY USA; London, UK, 1966; Volume 19, pp. 267–299. [Google Scholar]
- Babushkin, L.N. The phototactic action spectrum of chloroplasts. Dokl. Akad. Nauk SSSR 1955, 103, 333–335. [Google Scholar]
- Zurzycki, J. The action spectrum for the light depended movements of chloroplasts in Lemna trisulca L. Acta Soc. Bot. Pol. 1962, 31, 489–538. [Google Scholar] [CrossRef]
- Zurzycki, J. Properties and localization of the photoreceptor active in displacements of chloroplasts in Funaria hygrometrica. I. Action spectrum. Acta Soc. Bot. Pol. 1967, 36, 133–142. [Google Scholar] [CrossRef][Green Version]
- Lechowski, Z. The action spectrum in chloroplast translocation in multilayer leaf cells. Acta Soc. Bot. Pol. 1973, 42, 461–472. [Google Scholar] [CrossRef][Green Version]
- Seitz, K. Wirkungsspektren fur die Starklichtb-ewegung der Chloroplasten, die Photodinese und die lichtabhangige Viskositatsanderung bei Vallisneria spiralis ssp. torta. Z. Pflanzenphysiol. 1967, 56, 246–261. [Google Scholar]
- Bock, G.; Haupt, W. Die Chloroplastendrehung bei Mougeotia: III. Die Frage der Lokalisierung des Hellrot-Dunkelrot-Pigmentsystems in der Zelle. Planta 1961, 57, 518–530. [Google Scholar] [CrossRef]
- Fischer-Arnold, G. Untersuchungen über die Chloroplastenbewegung beiVaucheria sessilis. Protoplasma 1963, 56, 495–520. [Google Scholar] [CrossRef]
- Galston, A.W.; Baker, R.S. Studies on the physiology of light action. II. The photodynamic action of riboflavin. Am. J. Bot. 1949, 773–780. [Google Scholar] [CrossRef]
- Haupt, W. Role of light in chloroplast movement. BioScience 1973, 23, 289–296. [Google Scholar] [CrossRef]
- Zurzycki, J. Blue light-induced intracellular movements. In The Blue Light Syndrome. Proceedings in Life Sciences; Senger, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1980; pp. 50–68. [Google Scholar]
- Huala, E.; Oeller, P.W.; Liscum, E.; Han, I.-S.; Larsen, E.; Briggs, W.R. Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science 1997, 278, 2120–2123. [Google Scholar] [CrossRef] [PubMed]
- Hermanowicz, P.; Banas, A.K.; Sztatelman, O.; Gabrys, H.; Łabuz, J.M. UV-B induces chloroplast movements in a phototropin-dependent manner. Front. Plant Sci. 2019, 10, 1279. [Google Scholar] [CrossRef]
- Suetsugu, N.; Wada, M. Chloroplast photorelocation movement. In Plant Cell Monographs. The Chloroplas; Sandelius, A.S., Aronsson, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 235–266. [Google Scholar]
- Stahl, E. Über den Einfluß von Richtung und Stärke der Beleuchtung auf einige Bewegungserscheinungen im Pflanzenreich. Bot. Ztg. 1880, 38, 297–413. [Google Scholar]
- Schanderl, H.; Kaempfert, W. Über die Strahlungsdurchlässigkeit von Blättern und Blattgeweben. Z. Wiss. Biol. Abt. E Planta 1933, 18, 700–750. [Google Scholar] [CrossRef]
- Zurzycki, J. Chloroplasts arrangement as a factor in photosynthesis. Acta Soc. Bot. Pol. 1955, 24, 27–63. [Google Scholar] [CrossRef]
- Kasahara, M.; Kagawa, T.; Oikawa, K.; Suetsugu, N.; Miyao, M.; Wada, M. Chloroplast avoidance movement reduces photodamage in plants. Nature 2002, 420, 829–832. [Google Scholar] [CrossRef]
- Pfündel, E.E.; Latouche, G.; Meister, A.; Cerovic, Z.G. Linking chloroplast relocation to different responses of photosynthesis to blue and red radiation in low and high light-acclimated leaves of Arabidopsis thaliana (L.). Photosynth. Res. 2018, 137, 105–128. [Google Scholar] [CrossRef]
- Higa, T.; Wada, M. Chloroplast avoidance movement is not functional in plants grown under strong sunlight. Plant Cell Environ. 2016, 39, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, E.; Suetsugu, N.; Yamori, W.; Ishishita, K.; Kiyabu, R.; Fukuda, M.; Higa, T.; Shirouchi, B.; Wada, M. Chloroplast accumulation response enhances leaf photosynthesis and plant biomass production. Plant Physiol. 2018, 178, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.M.; Bae, A.; Königer, M. The importance of chloroplast movement, nonphotochemical quenching, and electron transport rates in light acclimation and tolerance to high light in Arabidopsis thaliana. Am. J. Bot. 2019, 106, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-I.; Chow, W.S.; Anderson, J.M. Chloroplast movement in the shade plant Tradescantia albiflora helps protect photosystem II against light stress. Plant Physiol. 1996, 111, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Ptushenko, V.V.; Ptushenko, O.S.; Samoilova, O.P.; Solovchenko, A.E. An exceptional irradiance-induced decrease of light trapping in two Tradescantia species: An unexpected relationship with the leaf architecture and zeaxanthin-mediated photoprotection. Biol. Plant 2016, 60, 385–393. [Google Scholar] [CrossRef]
- Davis, P.A.; Caylor, S.; Whippo, C.W.; Hangarter, R.P. Changes in leaf optical properties associated with light-dependent chloroplast movements. Plant Cell Environ. 2011, 34, 2047–2059. [Google Scholar] [CrossRef] [PubMed]
- Königer, M.; Bollinger, N. Chloroplast movement behavior varies widely among species and does not correlate with high light stress tolerance. Planta 2012, 236, 411–426. [Google Scholar] [CrossRef]
- Davis, P.A.; Hangarter, R.P. Chloroplast movement provides photoprotection to plants by redistributing PSII damage within leaves. Photosynth. Res. 2012, 112, 153–161. [Google Scholar] [CrossRef]
- Ptushenko, O.S.; Ptushenko, V.V. The effect of photoinduced relocation of chloroplasts on the protection against photodamage in Tradescantia fluminensis leaves. In Proceedings of the 10th International Meeting Photosynthesis and Hydrogen Energy Research for Sustainability—2019, Petersburg, Russia, 23–28 June 2019; Allakhverdiev, S., Naydov, I., Eds.; Komarov Botanical Institute: Saint Petersburg, Russia, 2019; p. 138. [Google Scholar]
- Kalmatskaya, O.A.; Karavaev, V.A.; Tikhonov, A.N. Slow induction of chlorophyll a fluorescence excited by blue and red light in Tradescantia leaves acclimated to high and low light. Photosynth. Res. 2019, 142, 265–282. [Google Scholar] [CrossRef]
- Sarvikas, P.; Hakala, M.; Pätsikkä, E.; Tyystjärvi, T.; Tyystjärvi, E. Action spectrum of photoinhibition in leaves of wild type and npq1-2 and npq4-1 mutants of Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 391–400. [Google Scholar] [CrossRef]
- Islam, M.S.; Van Nguyen, T.; Sakamoto, W.; Takagi, S. Phototropin-and photosynthesis-dependent mitochondrial positioning in Arabidopsis thaliana mesophyll cells. J. Integr. Plant Biol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Famintzin, A.S. Die Wirkung des Lichtes Wachsen der keimenden Kresse. Mémoires L ҆Academie Imperiale Des Sciences De St.-Petersbourg, VII Serie 1865, 15, 1–19. [Google Scholar]
- Barta, D.J.; Tibbitts, T.W.; Bula, R.J.; Morrow, R.C. Evaluation of light emitting diode characteristics for a space-based plant irradiation source. Adv. Space Res. 1992, 12, 141–149. [Google Scholar] [CrossRef]
- Bula, R.J.; Morrow, R.C.; Tibbitts, T.W.; Barta, D.J.; Ignatius, R.W.; Martin, T.S. Light-emitting diodes as a radiation source for plants. HortScience 1991, 26, 203–205. [Google Scholar] [CrossRef]
- Olle, M.; Viršile, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Tennessen, D.J.; Singsaas, E.L.; Sharkey, T.D. Light-emitting diodes as a light source for photosynthesis research. Photosynth. Res. 1994, 39, 85–92. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Cope, K.R.; Snowden, M.C.; Bugbee, B. Photobiological Interactions of Blue Light and Photosynthetic Photon Flux: Effects of Monochromatic and Broad-Spectrum Light Sources. Photochem. Photobiol. 2014, 90, 574–584. [Google Scholar] [CrossRef]
- Hoenecke, M.E.; Bula, R.J.; Tibbitts, T.W. Importance ofBlue’Photon Levels for Lettuce Seedlings Grown under Red-light-emitting Diodes. HortScience 1992, 27, 427–430. [Google Scholar] [CrossRef]
- Samuolienė, G.; Sirtautas, R.; Brazaitytė, A.; Sakalauskaitė, J.; Sakalauskienė, S.; Duchovskis, P. The impact of red and blue light-emitting diode illumination on radish physiological indices. Cent. Eur. J. Biol. 2011, 6, 821–828. [Google Scholar] [CrossRef]
- Goins, G.D.; Yorio, N.C.; Sanwo, M.M.; Brown, C.S. Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting. J. Exp. Bot. 1997, 48, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Lee, C.; Chakrabarty, D.; Paek, K. Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a Light-Emitting Diode (LED). Plant Growth Regul. 2002, 38, 225–230. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kakizono, T.; Nishio, N.; Nagai, S. Effects of light intensity, light quality, and illumination cycle on astaxanthin formation in a green alga, Haematococcus pluvialis. J. Ferment. Bioeng. 1992, 74, 61–63. [Google Scholar] [CrossRef]
- Suyono, E.A.; Pradani, L.; Mu’avatun, U.; Habiba, R.N.; Rohma, E.F. Combination of blue, red, white, and ultraviolet lights for increasing carotenoids and biomass of microalga Haematococcus pluvialis. Procedia Environ. Sci. 2015, 28, 399–405. [Google Scholar] [CrossRef]
- McGee, D.; Archer, L.; Fleming, G.T.A.; Gillespie, E.; Touzet, N. Influence of spectral intensity and quality of LED lighting on photoacclimation, carbon allocation and high-value pigments in microalgae. Photosynth. Res. 2020, 143, 67–80. [Google Scholar] [CrossRef]
- Ahmad, M.; Cashmore, A.R. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 1993, 366, 162–166. [Google Scholar] [CrossRef]
- Deininger, W.; Kröger, P.; Hegemann, U.; Lottspeich, F.; Hegemann, P. Chlamyrhodopsin represents a new type of sensory photoreceptor. EMBO J. 1995, 14, 5849–5858. [Google Scholar] [CrossRef]
- Kagawa, T.; Sakai, T.; Suetsugu, N.; Oikawa, K.; Ishiguro, S.; Kato, T.; Tabata, S.; Okada, K.; Wada, M. Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 2001, 291, 2138–2141. [Google Scholar] [CrossRef]
- Jarillo, J.A.; Gabrys, H.; Capel, J.; Alonso, J.M.; Ecker, J.R.; Cashmore, A.R. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 2001, 410, 952–954. [Google Scholar] [CrossRef]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. In Current Topics in Developmental Biology; Timmermans, M.C.P., Ed.; Academic Press: New York, NY, USA, 2010; Volume 91, pp. 29–66. [Google Scholar]
- Petroutsos, D.; Tokutsu, R.; Maruyama, S.; Flori, S.; Greiner, A.; Magneschi, L.; Cusant, L.; Kottke, T.; Mittag, M.; Hegemann, P.; et al. A blue-light photoreceptor mediates the feedback regulation of photosynthesis. Nature 2016, 537, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Nozue, K.; Kanegae, T.; Imaizumi, T.; Fukuda, S.; Okamoto, H.; Yeh, K.-C.; Lagarias, J.C.; Wada, M. A phytochrome from the fern Adiantum with features of the putative photoreceptor NPH1. Proc. Natl. Acad. Sci. USA 1998, 95, 15826–15830. [Google Scholar] [CrossRef] [PubMed]
- Gomelsky, M.; Klug, G. BLUF: A novel FAD-binding domain involved in sensory transduction in microorganisms. Trends Biochem. Sci. 2002, 27, 497–500. [Google Scholar] [CrossRef]
- Takahashi, F.; Yamagata, D.; Ishikawa, M.; Fukamatsu, Y.; Ogura, Y.; Kasahara, M.; Kiyosue, T.; Kikuyama, M.; Wada, M.; Kataoka, H. Aureochrome, a photoreceptor required for photomorphogenesis in stramenopiles. Proc. Natl. Acad. Sci. USA 2007, 104, 19625–19630. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hu, Q.; Yan, Z.; Chen, W.; Yan, C.; Huang, X.; Zhang, J.; Yang, P.; Deng, H.; Wang, J.; et al. Structural basis of ultraviolet-B perception by UVR8. Nature 2012, 484, 214–219. [Google Scholar]
- Golovatskaya, I.F.; Karnachuk, R.A. Role of green light in physiological activity of plants. Russ. J. Plant Physiol. 2015, 62, 727–740. [Google Scholar] [CrossRef]
- Lin, C.; Ahmad, M.; Gordon, D.; Cashmore, A.R. Expression of an Arabidopsis cryptochrome gene in transgenic tobacco results in hypersensitivity to blue, UV-A, and green light. Proc. Natl. Acad. Sci. USA 1995, 92, 8423–8427. [Google Scholar] [CrossRef]
- Sellaro, R.; Crepy, M.; Trupkin, S.A.; Karayekov, E.; Buchovsky, A.S.; Rossi, C.; Casal, J.J. Cryptochrome as a sensor of the blue/green ratio of natural radiation in Arabidopsis. Plant Physiol. 2010, 154, 401–409. [Google Scholar] [CrossRef]
- Ptushenko, V.V.; Avercheva, O.V.; Bassarskaya, E.M.; Berkovich, Y.A.; Erokhin, A.N.; Smolyanina, S.O.; Zhigalova, T.V. Possible reasons of a decline in growth of Chinese cabbage under a combined narrowband red and blue light in comparison with illumination by high-pressure sodium lamp. Sci. Hortic. 2015, 194, 267–277. [Google Scholar] [CrossRef]
- Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A.; et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 2011, 332, 805–809. [Google Scholar] [CrossRef]
- Orr, D.J.; Pereira, A.M.; da Fonseca Pereira, P.; Pereira-Lima, Í.A.; Zsögön, A.; Araújo, W.L. Engineering photosynthesis: Progress and perspectives. F1000Research 2017, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhou, Z.; Li, Z.; Chen, Y.; Wang, Z.; Zhang, H. Rice (Oryza sativa L.) with reduced chlorophyll content exhibit higher photosynthetic rate and efficiency, improved canopy light distribution, and greater yields than normally pigmented plants. Field Crop. Res. 2017, 200, 58–70. [Google Scholar] [CrossRef]
- Kirst, H.; Gabilly, S.T.; Niyogi, K.K.; Lemaux, P.G.; Melis, A. Photosynthetic antenna engineering to improve crop yields. Planta 2017, 245, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.E.; Sullivan, S.; Hermanowicz, P.; Petersen, J.; Diaz-Ramos, L.A.; Hoey, D.J.; Łabuz, J.; Christie, J.M. Engineering the phototropin photocycle improves photoreceptor performance and plant biomass production. Proc. Natl. Acad. Sci. USA 2019, 116, 12550–12557. [Google Scholar] [CrossRef] [PubMed]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef]
- Kondrashov, A.S. Comparative genomics and evolutionary biology. Curr. Opin. Genet. Dev. 1999, 9, 624–629. [Google Scholar] [CrossRef]
- Kiang, N.Y.; Siefert, J.; Blankenship, R.E. Spectral signatures of photosynthesis. I. Review of Earth organisms. Astrobiology 2007, 7, 222–251. [Google Scholar] [CrossRef]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef]
- Fan, X.; Zang, J.; Xu, Z.; Guo, S.; Jiao, X.; Liu, X.; Gao, Y. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading Chinese cabbage (Brassica campestris L.). Acta Physiol. Plant. 2013, 35, 2721–2726. [Google Scholar] [CrossRef]
- Shin, K.S.; Murthy, H.N.; Heo, J.W.; Hahn, E.J.; Paek, K.Y. The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiol. Plant. 2008, 30, 339–343. [Google Scholar] [CrossRef]
- Lee, S.-H.; Tewari, R.K.; Hahn, E.-J.; Paek, K.-Y. Photon flux density and light quality induce changes in growth, stomatal development, photosynthesis and transpiration of Withania somnifera (L.) Dunal. plantlets. Plant Cell Tissue Organ Cult. 2007, 90, 141–151. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, M.-M. Leaf Shape, Growth, and Antioxidant Phenolic Compounds of Two Lettuce Cultivars Grown under Various Combinations of Blue and Red Light-emitting Diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z.; Liu, X.; Han, X. Effects of different light sources on the growth of non-heading Chinese cabbage (Brassica campestris L.). J. Agric. Sci. 2012, 4, 262. [Google Scholar] [CrossRef]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and photomorphogenesis of pepper plants under red light-emitting diodes with supplemental blue or far-red lighting. J. Am. Soc. Hortic. Sci. 1995, 120, 808–813. [Google Scholar] [CrossRef]
- Avercheva, O.V.; Berkovich, Y.A.; Erokhin, A.N.; Zhigalova, T.V.; Pogosyan, S.I.; Smolyanina, S.O. Growth and photosynthesis of Chinese cabbage plants grown under light-emitting diode-based light source. Russ. J. Plant Physiol. 2009, 56, 14–21. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ptushenko, O.S.; Ptushenko, V.V.; Solovchenko, A.E. Spectrum of Light as a Determinant of Plant Functioning: A Historical Perspective. Life 2020, 10, 25. https://doi.org/10.3390/life10030025
Ptushenko OS, Ptushenko VV, Solovchenko AE. Spectrum of Light as a Determinant of Plant Functioning: A Historical Perspective. Life. 2020; 10(3):25. https://doi.org/10.3390/life10030025
Chicago/Turabian StylePtushenko, Oxana S., Vasily V. Ptushenko, and Alexei E. Solovchenko. 2020. "Spectrum of Light as a Determinant of Plant Functioning: A Historical Perspective" Life 10, no. 3: 25. https://doi.org/10.3390/life10030025
APA StylePtushenko, O. S., Ptushenko, V. V., & Solovchenko, A. E. (2020). Spectrum of Light as a Determinant of Plant Functioning: A Historical Perspective. Life, 10(3), 25. https://doi.org/10.3390/life10030025





