Circumnutation and Growth of Inflorescence Stems of Arabidopsis thaliana in Response to Microgravity under Different Photoperiod Conditions
Abstract
1. Introduction
2. Materials and Methods
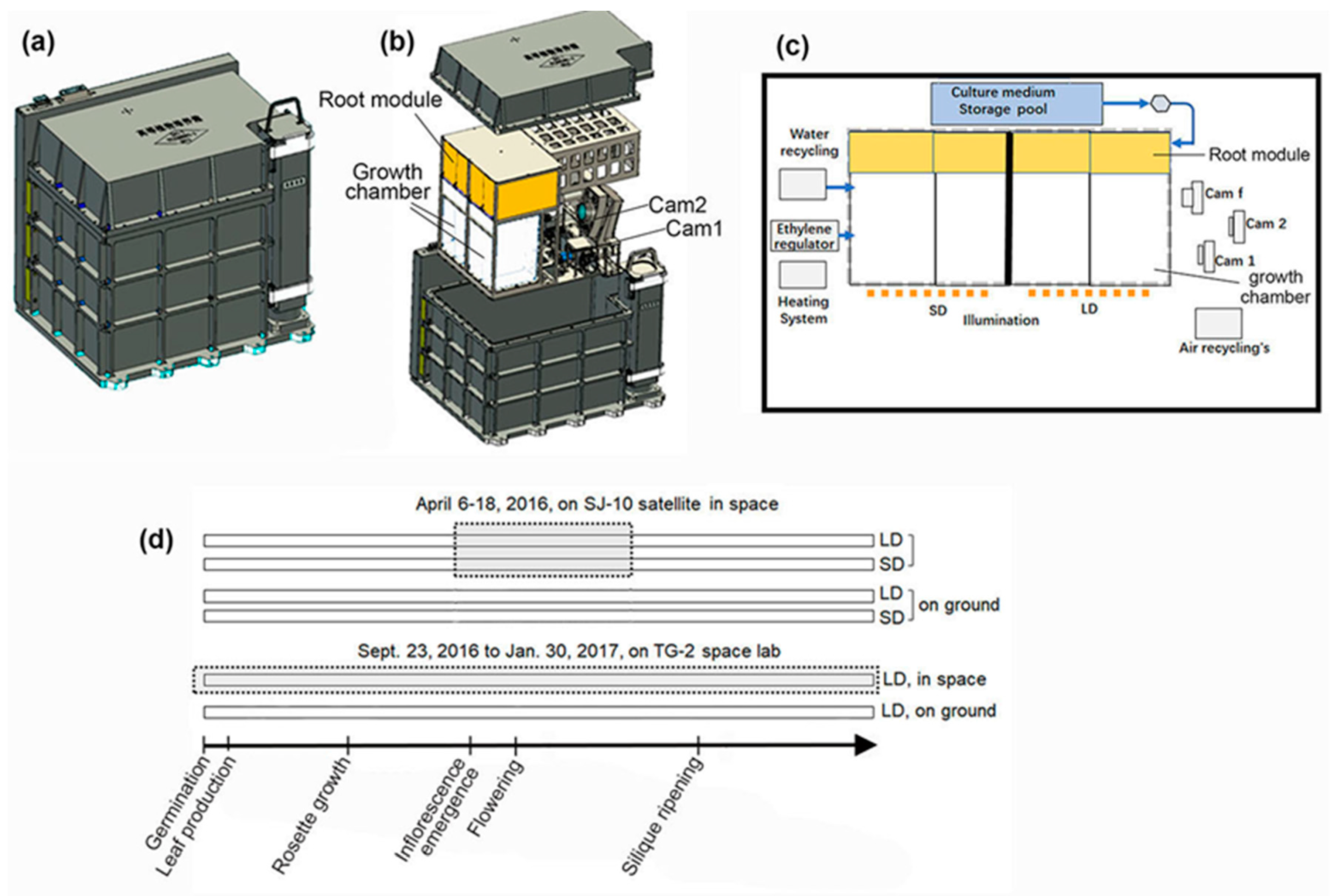
2.1. Space Experiments
2.2. Circumnutation Measurement and Data Collection
2.3. Data Analysis
3. Results
3.1. Floral Initiation Rates of Plants in Microgravity on Board the SJ-10 and the TG-2
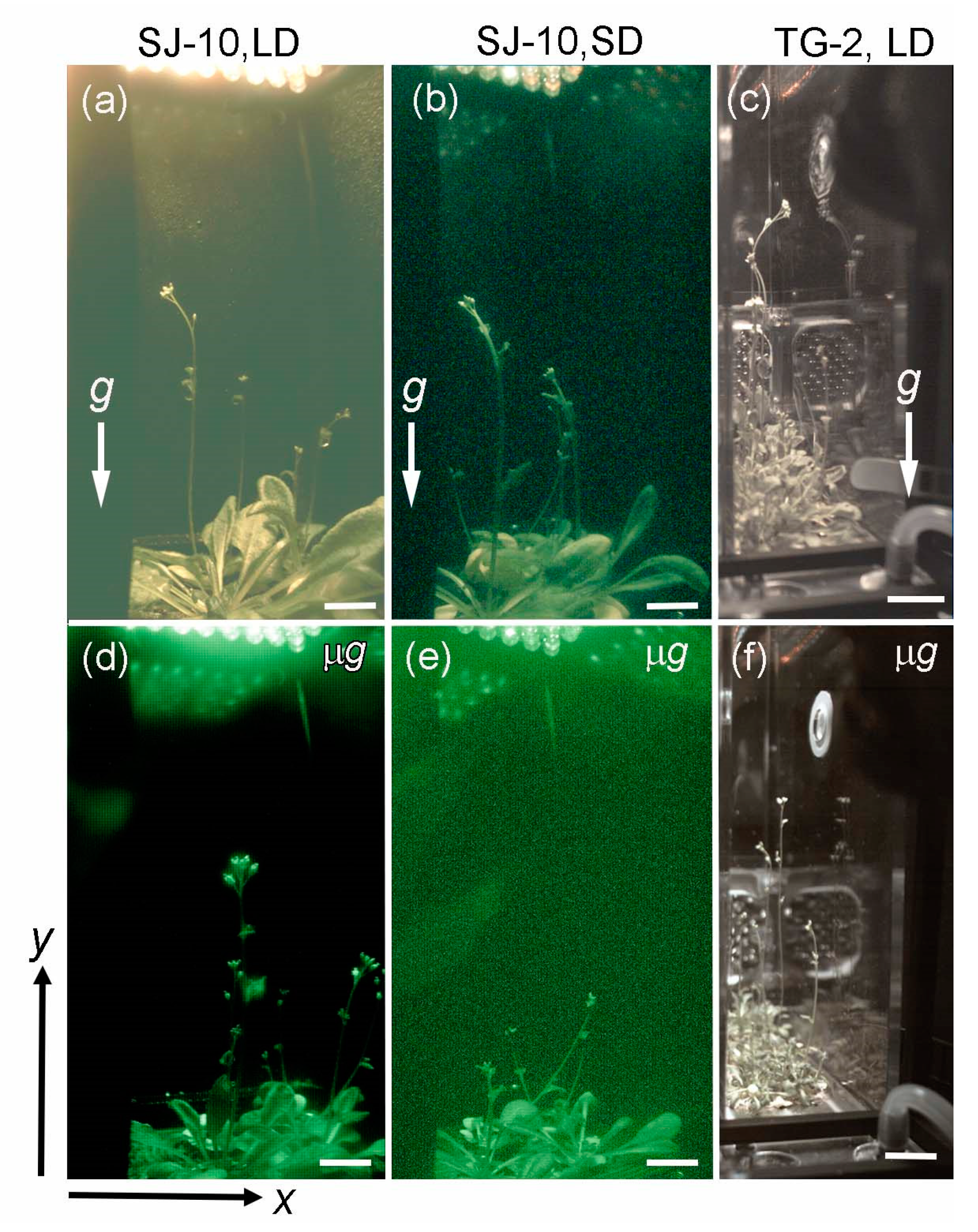
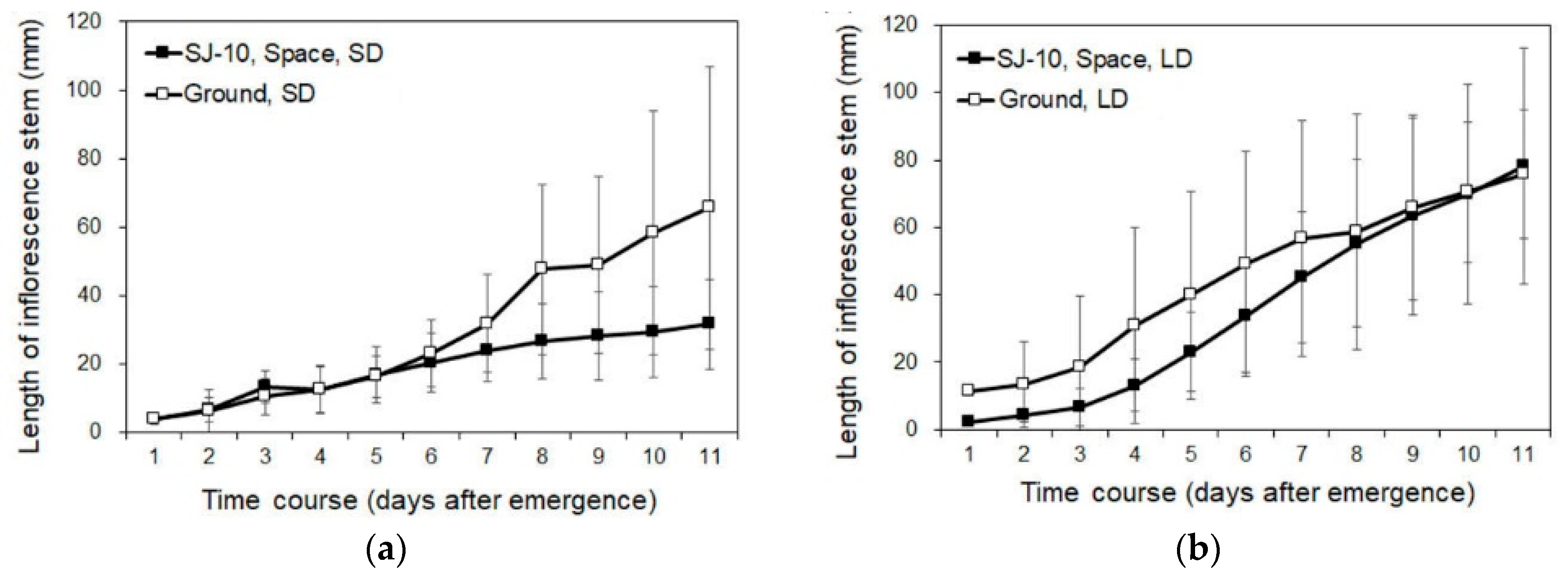
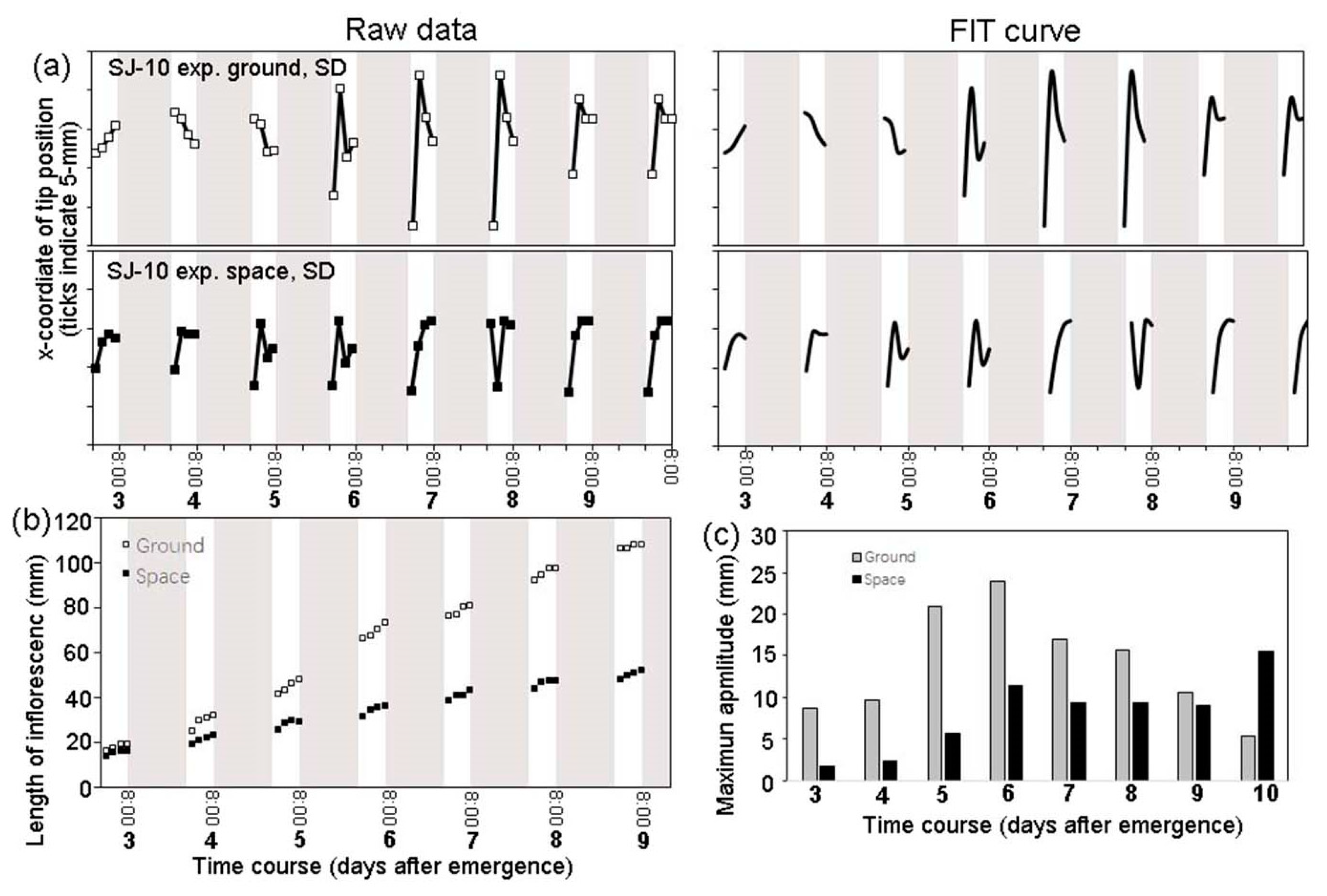
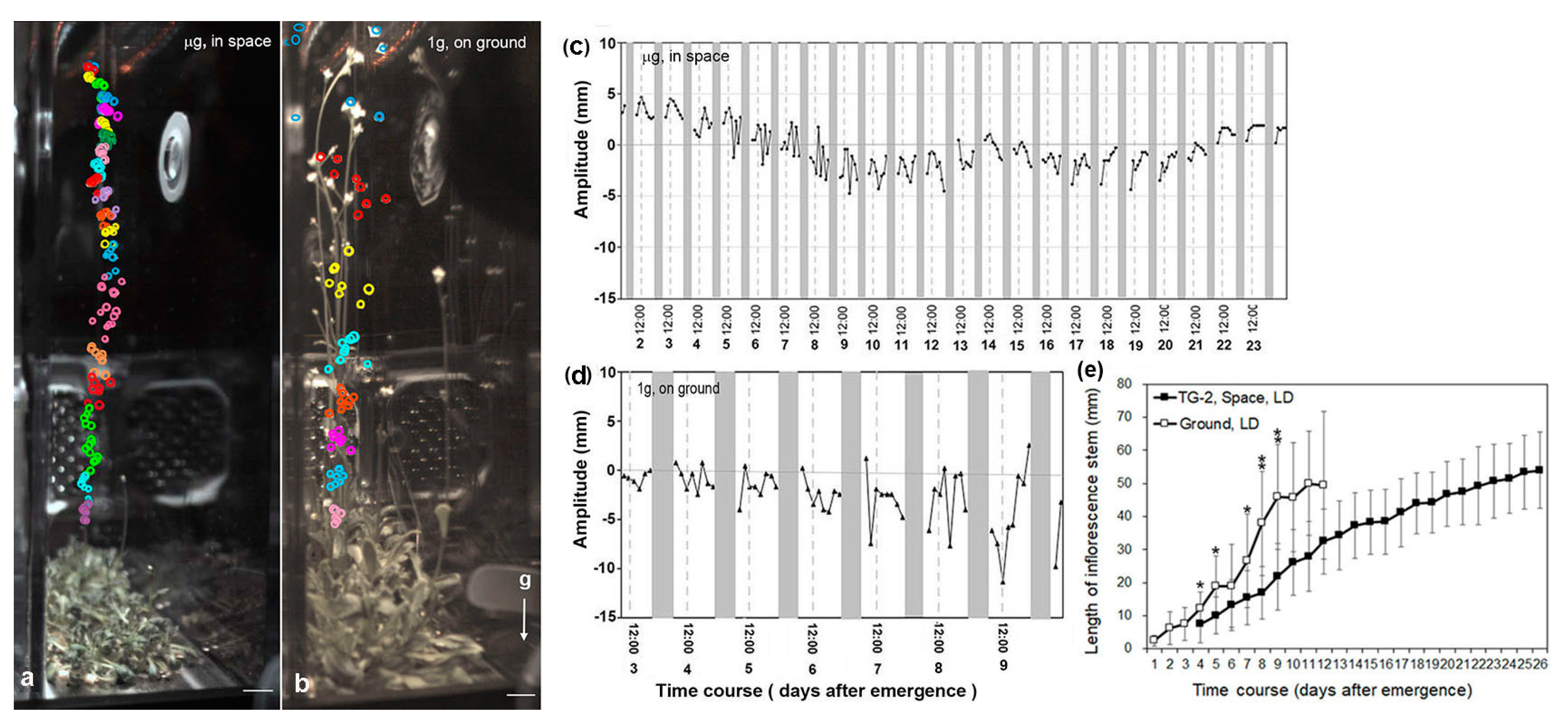
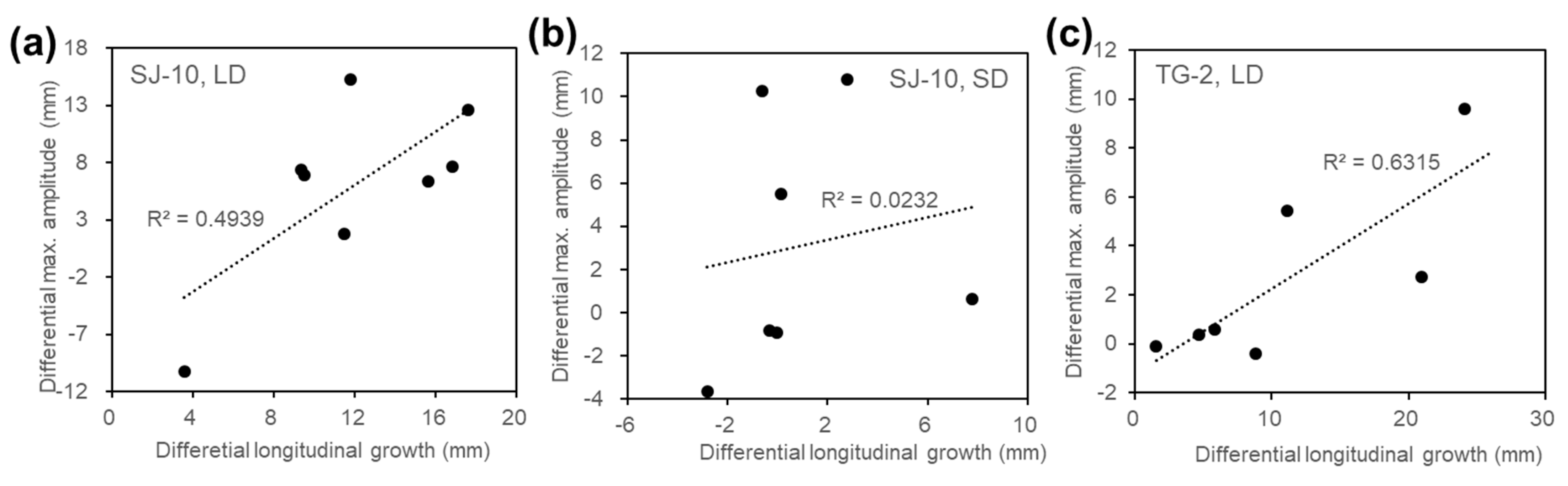
3.2. Circumnutation of Plants under Different Photoperiod Conditions in Response to Microgravity on the SJ-10
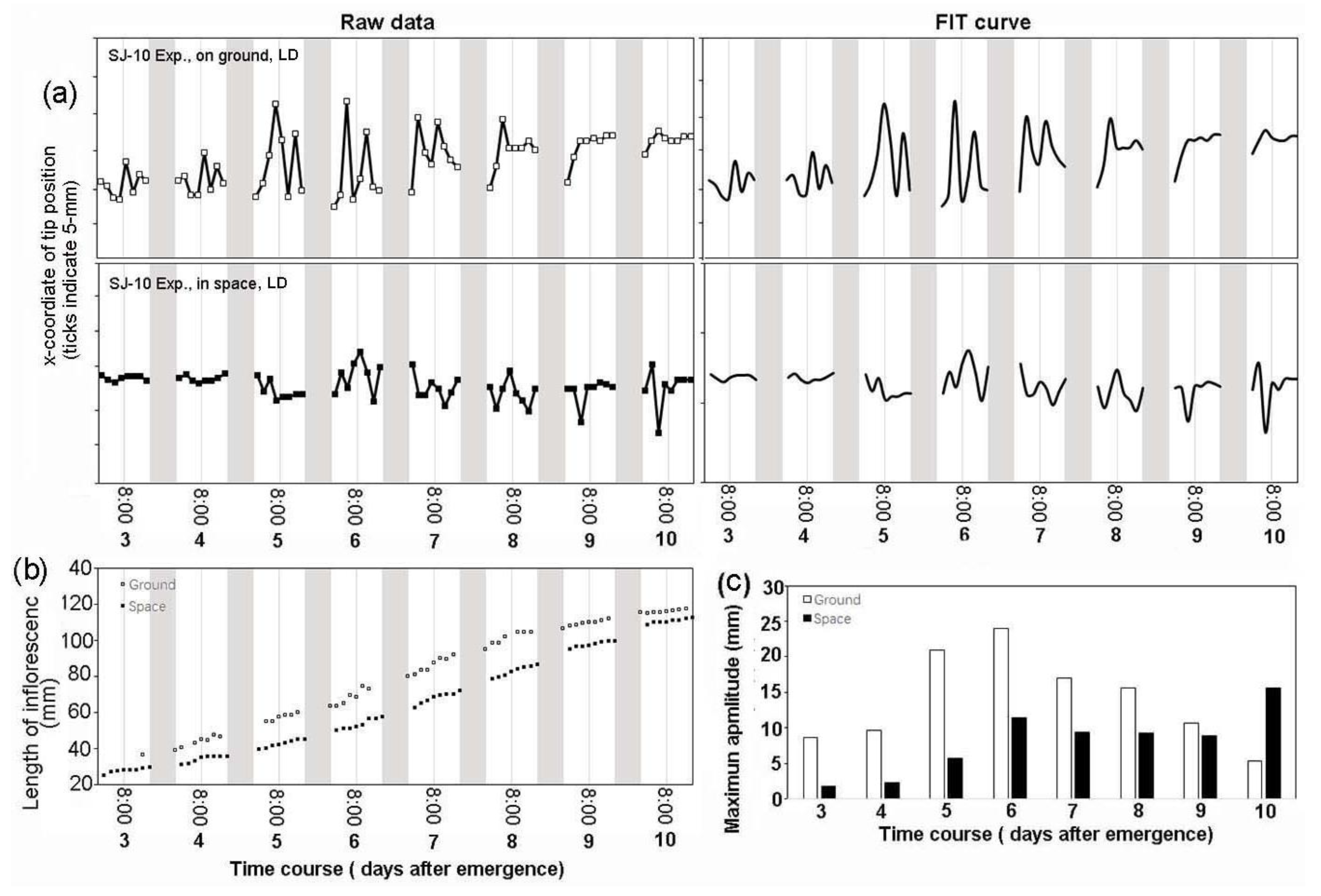
3.3. Circumnutation of Plants in Long-Term Microgravity Exposure on the TG-2
3.4. An Infradian Rhythm of Apex Movements of Plants Grown in Space
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niinuma, K.; Someya, N.; Kimura, M.; Yamaguchi, I.; Hamamoto, H. Circadian rhythm of circumnutation in inflorescence stems of Arabidopsis. Plant Cell Physiol. 2005, 46, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, A.; Solheim, B.G.B.; Iversen, T.H. Gravity amplifies and microgravity decreases circumnutations in Arabidopsis thaliana stems: Results from a space experiment. New Phytol. 2009, 182, 621–629. [Google Scholar] [CrossRef]
- Smyth, D.R. Helical growth in plant organs: Mechanisms and significance. Development 2016, 143, 3272–3282. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Raja, V.; Lee, D.N. Guidance of circumnutation of climbing bean stems: An ecological exploration. BioRxiv 2017, 122358. [Google Scholar] [CrossRef]
- Anderson-Bernadas, C.; Cornelissen, G.; Turner, C.M.; Koukkari, W.L. Rhythmic nature of thigmomorphogenesis and thermal stress of Phaseolus vulgaris L. shoots. J. Plant Physiol. 1997, 151, 575–580. [Google Scholar] [CrossRef]
- Buda, A.; Zawadzki, T.; Krupa, M.; Stolarz, M.; Okulski, W. Daily and infradian rhythms of circumnutation intensity in Helianthus annuus. Physiol. Plant. 2003, 119, 582–589. [Google Scholar] [CrossRef]
- Mugnai, S.; Azzarello, E.; Masi, E.; Pandolfi, C.; Mancuso, S. Nutation in plants. In Rhythms in Plants; Springer: Cham, Switzerland, 2015; pp. 19–34. [Google Scholar]
- Iida, M.; Takano, T.; Matsuura, T.; Mori, I.C.; Takagi, S. Circumnutation and distribution of phytohormones in Vigna angularis epicotyls. J. Plant Res. 2018, 131, 165–178. [Google Scholar] [CrossRef]
- Johnsson, A. Circumnutations: Results from recent experiments on Earth and in space. Planta 1997, 203, 147–158. [Google Scholar] [CrossRef]
- Millet, B.; Badot, P.M. The revolving movement mechanism. In Phaseolus: New approaches to old questions. In Vistas on Biorhythmicity; Greppin, H., Degli Agosti, R., Bonzon, M., Eds.; University of Geneva: Geneva, Switzerland, 1996; pp. 77–98. [Google Scholar]
- Schuster, J.; Engelmann, W. Circumnutations of Arabidopsis thaliana seedlings. Biol. Rhythm. Res. 1997, 28, 422–440. [Google Scholar] [CrossRef]
- Stolarz, M.; Krol, E.; Dziubinska, H.; Zawadzki, T. Complex relationship between growth and circumnutations in Helianthus annuus stem. Plant Signal. Behav. 2008, 3, 376–380. [Google Scholar] [CrossRef][Green Version]
- Stolarz, M.; Dziubinska, H. Spontaneous action potentials and circumnutation in Helianthus annuus. Acta Physiol. Pant. 2017, 39, 234. [Google Scholar] [CrossRef][Green Version]
- Darwin, C.A.; Darwin, F. The Power of Movement in Plants; John Murray: London, UK, 1880. [Google Scholar]
- Whippo, C.W.; Hangarter, R.P. The “sensational” power of movement in plants: A Darwinian system for studying the evolution of behavior. Am. J. Bot. 2009, 96, 2115–2127. [Google Scholar] [CrossRef] [PubMed]
- Ciszak, M.; Masi, K.E.; Baluska, F.; Mancuso, S. Plant shoots exhibit synchronized oscillatory motions. Commun. Integr. Biol. 2016, 9, e1238117. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, D.; Hatakeda, Y.; Kamada, M.; Fujii, N.; Miyazawa, Y.; Hoshino, A.; Iida, S.; Fukaki, H.; Morita, M.; Tasaka, M.; et al. Shoot circumnutation and winding movements require gravisensing cells. Proc. Natl. Acad. Sci. USA 2005, 102, 18742–18747. [Google Scholar] [CrossRef] [PubMed]
- Hatakeda, Y.; Kamada, M.; Goto, N.; Fukaki, H.; Tasaka, M.; Suge, H.; Takahashi, H. Gravitropic response plays an important role in the nutational movements of the shoots of Pharbitis nil and Arabidopsis thaliana. Physiol. Plant 2003, 118, 464–473. [Google Scholar] [CrossRef]
- Brown, A.H.; Chapman, D.K. Circumnutation observed without a significant gravitational force in spaceflight. Science 1984, 225, 230–232. [Google Scholar] [CrossRef]
- Solheim, B.G.B.; Johnsson, A.; Iversen, T.H. Ultradian rhythms in Arabidopsis thaliana leaves in microgravity. New Phytol. 2009, 183, 1043–1052. [Google Scholar] [CrossRef]
- Correll, M.J.; Kiss, J.Z. Space based research on plant tropisms. In Plant Tropisms; Gilroy, S., Masson, P.K., Eds.; Blackwell: New York, NY, USA, 2008; pp. 161–182. [Google Scholar]
- Paul, A.L.; Amalfitano, C.E.; Ferl, R.J. Plant growth strategies are remodeled by spaceflight. BMC Plant Biol. 2012, 12, 232. [Google Scholar] [CrossRef]
- Engelmann, W.; Johnsson, A. Rhythms in organ movement. In Biological Rhythms and Photoperiodism in Plants; Lumsden, P.J., Millar, A.J., Eds.; BIOS: Oxford, UK, 1998; pp. 35–50. [Google Scholar]
- Jouve, L.; Greppin, H.; Degli Agosti, R. Arabidopsis thaliana floral stem elongation: Evidence for an endogenous circadian rhythm. Plant Physiol. Biochem. 1998, 36, 469–472. [Google Scholar] [CrossRef]
- Dziubinska, H.; Trebacz, K.; Zawadzki, T. Circadian growth rhythm of Helianthus annuus stem. Folia Histochem. Cytol. 1999, 37, 28. [Google Scholar]
- Zhao, H.; Qiu, J.; Wang, Y. System design and flight results of China SJ-10 recoverable microgravity experimental satellite. In Life Science in Space: Experiments on Board the SJ-10 Recoverable Satellite; Duan, E., Long, M., Eds.; Science Press: Beijing, China, 2019; pp. 9–42. [Google Scholar]
- Wang, L.; Han, F.; Zheng, H.Q. Photoperiod-controlling guttation and growth of rice seedlings under microgravity on board Chinese spacelab TG-2. Microgravity Sci. Technol. 2018, 30, 834–847. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, L.; Xie, J. Flowering of Arabidopsis and rice in space. In Life Science in Space: Experiments on Board the SJ-10 Recoverable Satellite; Duan, E., Long, M., Eds.; Beijing, Science Press: Beijing, China, 2019; pp. 189–204. [Google Scholar]
- Boyes, D.C.; Zayed, A.M.; Ascenzi, R.; McCaskill, A.J.; Hoffman, N.E.; Davis, K.R.; Görlach, J. Growth stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell 2001, 13, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Plautz, J.D.; Straume, M.; Stanewsky, R.; Jamison, C.F.; Brandes, C.; Dowse, H.B.; Hall, J.C.; Kay, S.A. Quantitative analysis of Drosophila period gene transcription in living animals. J. Biol. Rhythm. 1997, 12, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Yanovsky, M.J.; Kay, S.A. Living by the calendar: How plants know when to flower. Nat. Rev. 2003, 4, 265–275. [Google Scholar] [CrossRef]
- Hoson, T.; Kamisaka, S.; Masuda, Y.; Yamashita, M.; Buchen, B. Evaluation of the three-dimensional clinostat as a simulator of weightlessness. Planta 1997, 203, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Hoson, T.; Soga, K.; Mori, R.; Saiki, M.; Wakabayashi, K.; Kamisakea, S.; Kamigaichi, S.; Aizawa, S.; Yoshizaki, I.; Mukai, C.; et al. Morphogenesis of rice and Arabidopsis seedlings in space. J. Plant Res. 1999, 112, 477–486. [Google Scholar] [CrossRef]
- Stanković, B.; Volkmann, D.; Sack, F.D. Autotropism, automorphogenesis, and gravity. Physiol. Plant 1998, 102, 328–335. [Google Scholar] [CrossRef]
- Babbick, M.; Barjaktarović, Ž.; Hampp, R. Alterations in the expression of transcription factors in Arabidopsis thaliana cell cultures during sounding rocket μG. In Proceedings of the 18th ESA Symposium on European Rocket and Balloon Programmes, Bad Reichenhall, Germany, 7–11 June 2007; pp. 473–477. [Google Scholar]
- Babbick, M.; Dijkstra, C.; Larkin, O.J.; Anthony, P.; Davey, M.R.; Power, J.B.; Lowe, K.C.; Cogoli-Greuter, M.; Hampp, R. Expression of transcription factors after short-term exposure of Arabidopsis thaliana cell cultures to hypergravity and simulated microgravity (2-D/3-D clinorotation, magnetic levitation). Adv. Space Res. 2007, 39, 1182–1189. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, H.Q. Changes in plastid and mitochondria protein expression in Arabidopsis thaliana callus on board Chinese spacecraft SZ-8. Microgravity Sci. Technol. 2015, 27, 387–401. [Google Scholar] [CrossRef]
- Link, B.M.; Busse, J.S.; Stankovic, B. Seed-to-seed-to-seed growth and development of Arabidopsis in microgravity. Astrobiology 2014, 14, 866–875. [Google Scholar] [CrossRef]
- Franke, H.D. On a clocklike mechanism timing lunar-rhythmic reproduction in Typosyllis prolifera (Polychaeta). J. Comp. Physiol. A 1985, 156, 553–561. [Google Scholar] [CrossRef]
- Spruyt, E.; Verbelen, J.P.; De Greef, J.A. Expression of circaseptan and circannual rhythmicity in the imbibition of dry stored bean seeds. Plant Physiol. 1987, 84, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Kautsky, L.; Kalvas, A. Circadian and lunar gamete release in Fucus vesiculosus in the atidal Baltic Sea. Mar. Ecol. Prog. Ser. 1994, 110, 195–201. [Google Scholar] [CrossRef]
- Olovnikov, A. Lunasensor, infradian rhythms, telomeres, and the chronomere program of aging. Ann. N. Y. Acad. Sci. 2005, 1057, 112–132. [Google Scholar] [CrossRef]
- Lüning, K.; Kadel, P.; Pang, S. Control of reproduction rhythmicity by environmental and endogenous signals in Ulva pseudocurvata (Chlorophyta). J. Phycol. 2008, 44, 866–873. [Google Scholar] [CrossRef]
| SJ-10 | TG-2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LD, 1 g | P(a.u.) | LD, μg | P(a.u.) | SD, 1 g | P(a.u.) | SD, μg | P(a.u.) | LD, 1 g | P(a.u.) | LD, μg | P(a.u.) | |
| 1 | 24h 00min | 123.0 | 20h 48min | 75.2 | 1d 02h 40min | 43.9 | 3d 08h 00min | 32 | 1d 09h 36min | 174.8 | 2d 16h 48min | 56.05 |
| 2 | 2d 02h 40min | 123.0 | 1d 16h 00min | 75.2 | 2d 00h 00min | 43.9 | 1d 08h 00min | 25.6 | 1d 16h 00min | 151.2 | 5d 16h 00min | 56.05 |
| 3 | 18h 42min | 84.5 | 3d 00h 00min | 75.2 | 1d 04h 48min | 27.1 | 2d 00h 00min | 25.6 | 1d 05h 20min | 151.2 | 1d 20h 36min | 53.9 |
| 4 | 1d 16h 00min | 84.5 | 5d 16h 00min | 64.0 | 1d 08h 00min | 8 | 1d 01h 09min | 23.4 | 20h 48min | 90.1 | 3d 00h 00min | 24.61 |
| 5 | 20h 48min | 61.2 | 24h 00min | 61.7 | 1d 13h 20min | 10.7 | 24h 00min | 90.1 | 4d 14h 24min | 24.11 | ||
| 6 | 1d 02h 17min | 60.1 | 17h 51min | 59.3 | 1d 04h 48min | 9.1 | 17h 51min | 66.7 | 2d 02h 36min | 19.49 | ||
| 7 | 1d 09h 36min | 50.5 | 18h 40min | 41.9 | 3d 00h 00min | 40.0 | 21d 16h 00min | 10.8 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Xie, J.; Wang, L.; Zheng, H. Circumnutation and Growth of Inflorescence Stems of Arabidopsis thaliana in Response to Microgravity under Different Photoperiod Conditions. Life 2020, 10, 26. https://doi.org/10.3390/life10030026
Wu Y, Xie J, Wang L, Zheng H. Circumnutation and Growth of Inflorescence Stems of Arabidopsis thaliana in Response to Microgravity under Different Photoperiod Conditions. Life. 2020; 10(3):26. https://doi.org/10.3390/life10030026
Chicago/Turabian StyleWu, Yuanyuan, Junyan Xie, Lihua Wang, and Huiqiong Zheng. 2020. "Circumnutation and Growth of Inflorescence Stems of Arabidopsis thaliana in Response to Microgravity under Different Photoperiod Conditions" Life 10, no. 3: 26. https://doi.org/10.3390/life10030026
APA StyleWu, Y., Xie, J., Wang, L., & Zheng, H. (2020). Circumnutation and Growth of Inflorescence Stems of Arabidopsis thaliana in Response to Microgravity under Different Photoperiod Conditions. Life, 10(3), 26. https://doi.org/10.3390/life10030026




