A Biomarker Panel of Radiation-Upregulated miRNA as Signature for Ionizing Radiation Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Human Peripheral Blood Samples
2.3. miRNA Extraction and μParaflo™ MicroRNA Microarray Assay
2.4. Real-Time Quantitative PCR (RT–qPCR)
2.5. Bioinformatical Analyses
2.5.1. Prediction of Target Genes
2.5.2. Gene Ontology (GO) Analysis
2.5.3. Enrichment Analyses of Pathways
2.5.4. Construction of the miRNA-Gene Network and Target Gene-Pathway Network
2.6. Statistical Analysis
3. Results
3.1. Identification of Radiation-Induced miRNAs and Target Gene Prediction in HUVEC Cells
3.2. Annotation and Enrichment Pathways of Predicted miRNAs Target Genes
3.3. miRNA-Gene Network and Target Gene-Pathway Network
3.4. Confirmation of Differentially Expressed miRNAs Signature in Human Lymphoblast Cells AHH-1
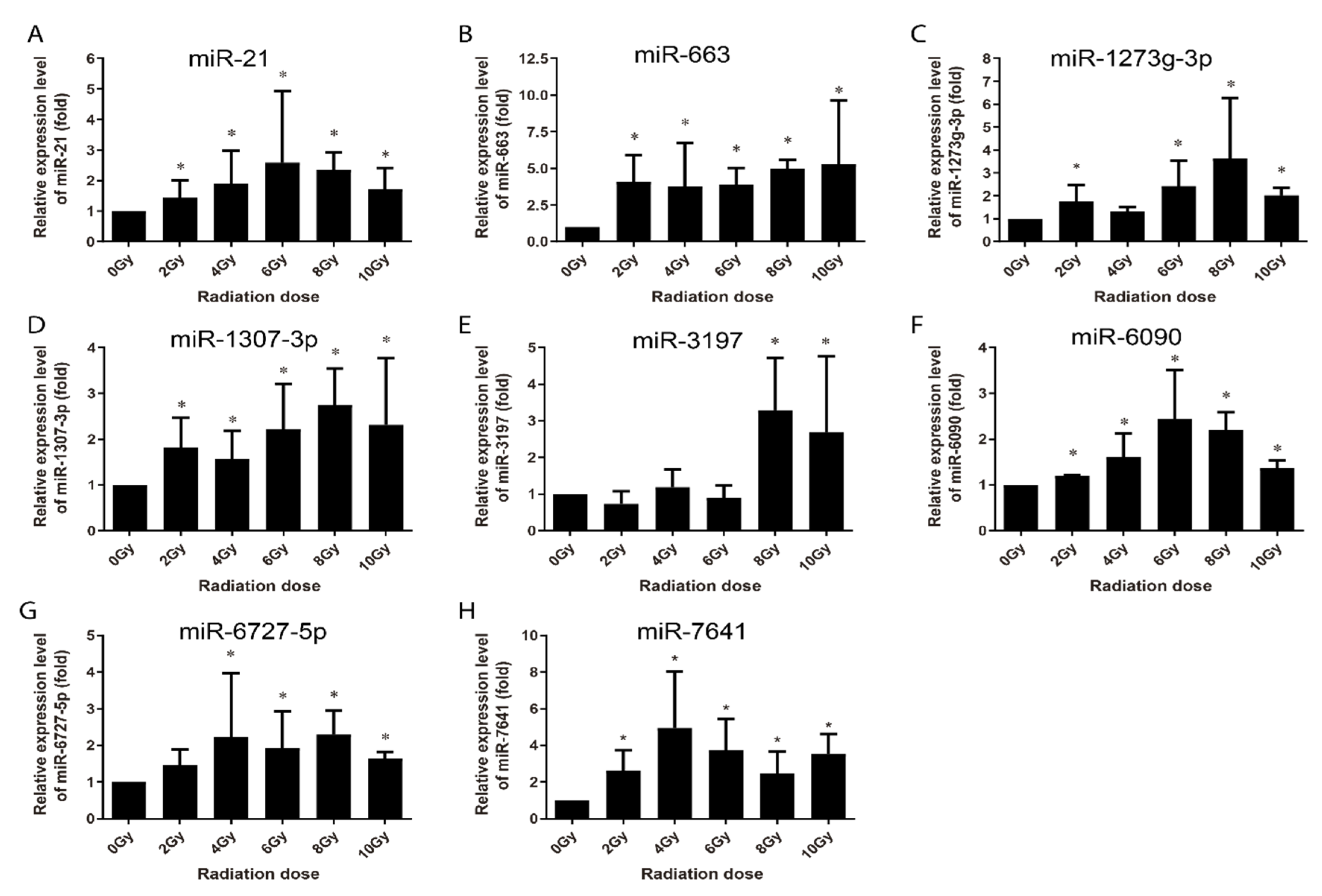
3.5. Validation of Radiation-Upregulated miRNAs in Human Peripheral Blood Cells as Radiation Exposure Biomarkers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Takamura, N.; Orita, M.; Saenko, V.; Yamashita, S.; Nagataki, S.; Demidchik, Y. Radiation and risk of thyroid cancer: Fukushima and Chernobyl. Lancet Diabetes Endocrinol. 2016, 4, 647. [Google Scholar] [CrossRef]
- Mettler, F.A.; Gus’Kova, A.K.; Gusev, I. Health effects in those with acute radiation sickness from the Chernobyl accident. Health Phys. 2007, 93, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Ozasa, K.; Akiba, S.; Niwa, O.; Kodama, K.; Takamura, N.; Zaharieva, E.K.; Kimura, Y.; Wakeford, R. Long-term effects of radiation exposure on health. Lancet 2015, 386, 469–478. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, P.-K.; Zhang, X.-Q.; Wang, Z.-D.; Wang, Y.; Darroudi, F. Cytogenetic studies for a group of people living in Japan 1 year after the Fukushima nuclear accident. Radiat. Prot. Dosim. 2014, 159, 20–25. [Google Scholar] [CrossRef]
- Chancellor, J.C.; Scott, G.B.I.; Sutton, J.P. Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life 2014, 4, 491–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obe, G.; Johannes, I.; Johannes, C.; Hallman, K.; Reitz, G.; Facius, R. Chromosomal aberrations in blood lymphocytes of astronauts after long-term space flights. Int. J. Radiat. Biol. 1997, 72, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.J.; Buckton, K.E.; Hamilton, G.E.; Carothers, A. Radiation-induced chromosome aberrations in nuclear-dockyard workers. Nat. Cell Biol. 1979, 277, 531–534. [Google Scholar] [CrossRef]
- Bianchi, M.; Bianchi, N.; Brewen, J.; Buckton, K.; Fabry, L.; Fischer, P.; Gooch, P.; Kučerová, M.; Léonard, A.; Mukherjee, R.; et al. Evaluation of radiation-induced chromosomal aberrations in human peripheral blood lymphocytes in vitro results of an IAEA-coordinated programme. Mutat. Res. Mol. Mech. Mutagen. 1982, 96, 233–242. [Google Scholar] [CrossRef]
- Andrei, A.; Wilkins, R.C. The response ofgamma-H2AX in human lymphocytes and lymphocytes subsets measured in whole blood cultures. Int. J. Radiat. Biol. 2009, 85, 369–376. [Google Scholar]
- Huang, R.; Zhou, P.-K. Double-edged effects of noncoding RNAs in responses to environmental genotoxic insults: Perspectives with regards to molecule-ecology network. Environ. Pollut. 2019, 247, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; He, J.; Wang, J.; Ding, N.; Wang, B.; Lin, S.; Zhang, X.; Hua, J.; Li, H.; Hu, B. Serum microRNAs as Early Indicators for Estimation of Exposure Degree in Response to Ionizing Irradiation. Radiat. Res. 2017, 188, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, J.; He, J.; Xie, X.D. Serum microRNA as noninvasive indicator for space radiation. Acta Astronaut. 2018, 152, 101–104. [Google Scholar] [CrossRef]
- Huang, R.; Yu, T.; Li, Y.; Huang, R. Upregulated has-miR-4516 as a potential biomarker for early diagnosis of dust-induced pulmonary fibrosis in patients with pneumoconiosis. Toxicol. Res. 2018, 7, 415–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Liang, X.; Li, X.; Liu, X.; Zhu, M.; Gu, Y.; Zhou, P. MiRNA-21 functions in ionizing radiation-induced epithelium-to-mesenchymal transition (EMT) by downregulating PTEN. Toxicol. Res. 2019, 8, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Rossetto, I.M.U.; Arantes, R.M.S.; Lizarte, F.S.N.; Tirapelli, L.F.; Tirapelli, D.P.C.; Chuffa, L.G.A.; Martinez, F.E. Serum miRNAs are differentially altered by ethanol and caffeine consumption in rats. Toxicol. Res. 2019, 8, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Małachowska, B.; Tomasik, B.; Stawiski, K.; Kulkarni, S.; Guha, C.; Chowdhury, D.; Fendler, W. Circulating microRNAs as Biomarkers of Radiation Exposure: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. 2020, 106, 390–402. [Google Scholar] [CrossRef] [Green Version]
- Ménard, C.; Johann, D.; Lowenthal, M.; Muanza, T.; Sproull, M.; Ross, S.; Gulley, J.; Petricoin, E.; Coleman, C.N.; Whiteley, G.; et al. Discovering Clinical Biomarkers of Ionizing Radiation Exposure with Serum Proteomic Analysis. Cancer Res. 2006, 66, 1844–1850. [Google Scholar] [CrossRef] [Green Version]
- Sproull, M.; Kramp, T.; Tandle, A.; Shankavaram, U.; Camphausen, K. Serum Amyloid A as a Biomarker for Radiation Exposure. Radiat. Res. 2015, 184, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Neto, J.D.O.V.; Da Silva, C.A.; Meneses, G.C.; Pinto, D.V.; Brito, L.C.; Fonseca, S.G.D.C.; Alves, R.D.S.; Martins, A.M.C.; Assumpção, C.D.O.; Daher, E.D.F. Novel renal biomarkers show that creatine supplementation is safe: A double-blind, placebo-controlled randomized clinical trial. Toxicol. Res. 2020, 9, 263–270. [Google Scholar] [CrossRef]
- Qiu, X.; Miao, Y.; Geng, X.; Zhou, X.; Li, B. Evaluation of biomarkers for in vitro prediction of drug-induced nephrotoxicity in RPTEC/TERT1 cells. Toxicol. Res. 2020, 9, 91–100. [Google Scholar] [CrossRef]
- Wei, W.; Bai, H.; Feng, X.; Hua, J.; Long, K.; He, J.; Zhang, Y.; Ding, N.; Wang, J.; Zhou, H. Serum Proteins as New Biomarkers for Whole-Body Exposure to High- and Low-LET Ionizing Radiation. Dose-Response 2020, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chipman, L.B.; Pasquinelli, A.E. miRNA Targeting: Growing beyond the Seed. Trends Genet. 2019, 35, 215–222. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Shi, J.; Li, J.; Wang, S.; Liu, H.; Zhao, S.; Duan, K.; Pan, X.; Yi, Z. The Role of miRNA in the Diagnosis, Prognosis, and Treatment of Osteosarcoma. Cancer Biother. Radiopharm. 2019, 34, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Pasi, F.; Corbella, F.; Baio, A.; Capelli, E.; De Silvestri, A.; Tinelli, C.; Nano, R. Radiation-induced circulating miRNA expression in blood of head and neck cancer patients. Radiat. Environ. Biophys. 2020, 59, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Norbury, J.W.; Slaba, T.C.; Aghara, S.; Badavi, F.F.; Blattnig, S.R.; Clowdsley, M.S.; Heilbronn, L.H.; Lee, K.; Maung, K.M.; Mertens, C.J.; et al. Advances in space radiation physics and transport at NASA. Life Sci. Space Res. 2019, 22, 98–124. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wang, Y.; Shang, Z.-F.; Liu, X.-D.; Xie, D.-F.; Wang, Q.; Guan, H.; Zhou, P.-K. Bystander autophagy mediated by radiation-induced exosomal miR-7-5p in non-targeted human bronchial epithelial cells. Sci. Rep. 2016, 6, 30165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, S.; Shi, G.-S.; Cheng, H.-Y.; Zeng, Y.-N.; Li, G.; Zhang, M.; Song, M.; Zhou, P.-K.; Tian, Y.; Cui, F.-M.; et al. Exosomal miR-7 Mediates Bystander Autophagy in Lung after Focal Brain Irradiation in Mice. Int. J. Biol. Sci. 2017, 13, 1287–1296. [Google Scholar] [CrossRef] [Green Version]
- Mo, L.-J.; Song, M.; Huang, Q.-H.; Guan, H.; Liu, X.-D.; Xie, D.-F.; Huang, B.; Huang, R.-X.; Zhou, P.-K. Exosome-packaged miR-1246 contributes to bystander DNA damage by targeting LIG4. Br. J. Cancer 2018, 119, 492–502. [Google Scholar] [CrossRef] [Green Version]
- Hamdi, Y.; The PEC Consortium; Boujemaa, M.; Ben Rekaya, M.; Ben Hamda, C.; Mighri, N.; El Benna, H.; Mejri, N.; Labidi, S.; Daoud, N.; et al. Family specific genetic predisposition to breast cancer: Results from Tunisian whole exome sequenced breast cancer cases. J. Transl. Med. 2018, 16, 158. [Google Scholar] [CrossRef] [Green Version]
- Asante, D.; MacCarthy-Morrogh, L.J.; Townley, A.K.; Weiss, M.A.; Katayama, K.; Palmer, K.J.; Suzuki, H.; Westlake, C.J.; Stephens, D.J. A role for the Golgi matrix protein giantin in ciliogenesis through control of the localization of dynein-2. J. Cell Sci. 2013, 126, 5189–5197. [Google Scholar] [CrossRef] [Green Version]
- Bergen, D.J.; Stevenson, N.L.; Skinner, R.E.H.; Stephens, D.J.; Hammond, C.L. The Golgi matrix protein giantin is required for normal cilia function in zebrafish. Biol. Open 2017, 6, 1180–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farber-Katz, S.E.; Dippold, H.C.; Buschman, M.D.; Peterman, M.C.; Xing, M.; Noakes, C.J.; Tat, J.; Ng, M.M.; Rahajeng, J.; Cowan, D.M.; et al. DNA Damage Triggers Golgi Dispersal via DNA-PK and GOLPH3. Cell 2014, 156, 413–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Zhang, X.; Tang, X.; Wang, P.; Wang, H.; Wang, Y. MiR-21 is continually elevated long-term in the brain after exposure to ionizing radiation. Radiat. Res. 2011, 177, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ding, N.; Pei, H.; Hu, W.; Wei, W.; Zhang, X.; Zhou, G.; Wang, J. MiR-21 is involved in radiation-induced bystander effects. RNA Biol. 2014, 11, 1161–1170. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Tian, W.; Wang, L.; Wang, J.; Zhang, S.; Cao, J.; Yang, H. Radiation quality-dependence of bystander effect in unirradiated fibroblasts is associated with TGF-β1-Smad2 pathway and miR-21 in irradiated keratinocytes. Sci. Rep. 2015, 5, 11373. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Wang, X.; Hu, S.; Ying, X.; Wang, P.; Zhang, X.; Wang, J.; Wang, H.; Wang, Y. miR-21-mediated Radioresistance Occurs via Promoting Repair of DNA Double Strand Breaks. J. Biol. Chem. 2017, 292, 3531–3540. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Chen, L.; Wang, G.; Xiao, Y.; Ju, L.; Wang, X. Identification of a three-miRNA signature as a novel potential prognostic biomarker in patients with clear cell renal cell carcinoma. J. Cell. Biochem. 2019, 120, 13751–13764. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, M.; Shan, X.; Zhou, X.; Wang, T.; Zhang, J.; Tao, J.; Cheng, W.; Chen, G.; Li, J.; et al. A panel of seven-miRNA signature in plasma as potential biomarker for colorectal cancer diagnosis. Gene 2018, 687, 246–254. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Q.; Ma, S.; Sun, Y.; Vadamootoo, A.S.; Jin, C. A four serum-miRNA panel serves as a potential diagnostic biomarker of osteosarcoma. Int. J. Clin. Oncol. 2019, 24, 976–982. [Google Scholar] [CrossRef]
- Li, Z.; Ye, L.; Wang, L.; Quan, R.; Zhou, Y.; Li, X. Identification of miRNA signatures in serum exosomes as a potential biomarker after radiotherapy treatment in glioma patients. Ann. Diagn. Pathol. 2020, 44, 151436. [Google Scholar] [CrossRef]
- Zhou, H.; Tang, K.; Xiao, H.; Zeng, J.; Guan, W.; Guo, X.; Xu, H.; Ye, Z. A panel of eight-miRNA signature as a potential biomarker for predicting survival in bladder cancer. J. Exp. Clin. Cancer Res. 2015, 34, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.-S.; Lin, H.; Chen, D.; Yi, Z.; Zeng, B.; Jiang, Y.; Ren, G. A four-miRNA signature as a novel biomarker for predicting survival in endometrial cancer. Gene 2019, 697, 86–93. [Google Scholar] [CrossRef] [PubMed]







| miRNA | Change Direction | Fold Change | Time Point | p-Value |
|---|---|---|---|---|
| hsa-miR-3197 | Up | 16.69 | 0.5 h | <0.001 |
| Up | 5.23 | 2 h | <0.001 | |
| hsa-miR-1246 | Up | 1.03 | 0.5 h | <0.001 |
| Up | 4.06 | 2 h | <0.001 | |
| hsa-miR-1307-3p | Up | 2.39 | 0.5 h | <0.001 |
| UP | 4.14 | 2 h | <0.001 | |
| hsa-miR-4267 | Up | 4.11 | 0.5 h | <0.001 |
| Down | 1.69 | 2 h | <0.001 | |
| hsa-miR-5096 | Up | 14.92 | 0.5 h | <0.001 |
| UP | 2.42 | 2 h | <0.001 | |
| hsa-miR-7641 | UP | 1.10 | 0.5 h | <0.001 |
| UP | 3.45 | 2 h | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Xie, D.; Gao, S.; Bai, C.-J.; Zhu, M.-X.; Guan, H.; Zhou, P.-K. A Biomarker Panel of Radiation-Upregulated miRNA as Signature for Ionizing Radiation Exposure. Life 2020, 10, 361. https://doi.org/10.3390/life10120361
Song M, Xie D, Gao S, Bai C-J, Zhu M-X, Guan H, Zhou P-K. A Biomarker Panel of Radiation-Upregulated miRNA as Signature for Ionizing Radiation Exposure. Life. 2020; 10(12):361. https://doi.org/10.3390/life10120361
Chicago/Turabian StyleSong, Man, Dafei Xie, Shanshan Gao, Chen-Jun Bai, Mao-Xiang Zhu, Hua Guan, and Ping-Kun Zhou. 2020. "A Biomarker Panel of Radiation-Upregulated miRNA as Signature for Ionizing Radiation Exposure" Life 10, no. 12: 361. https://doi.org/10.3390/life10120361






