Anti-Inflammatory Effects of Fermented Lotus Root and Linoleic Acid in Lipopolysaccharide-Induced RAW 264.7 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of FLR and LA
2.2. LA Detection by Gas Chromatography with Flame Ionization Detector (GC-FID)
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Nitric Oxide (NO) Production
2.6. RNA Isolation and cDNA Synthesis
2.7. Immune Gene Expression Analysis by Quantitative Reverse Transcription PCR (qRT-PCR)
2.8. Luciferase Assay
2.9. Western Blot Analysis
2.10. Immunofluorescence Staining
2.11. Statistical Analysis
3. Results
3.1. Effects of FLR on Cell Viability
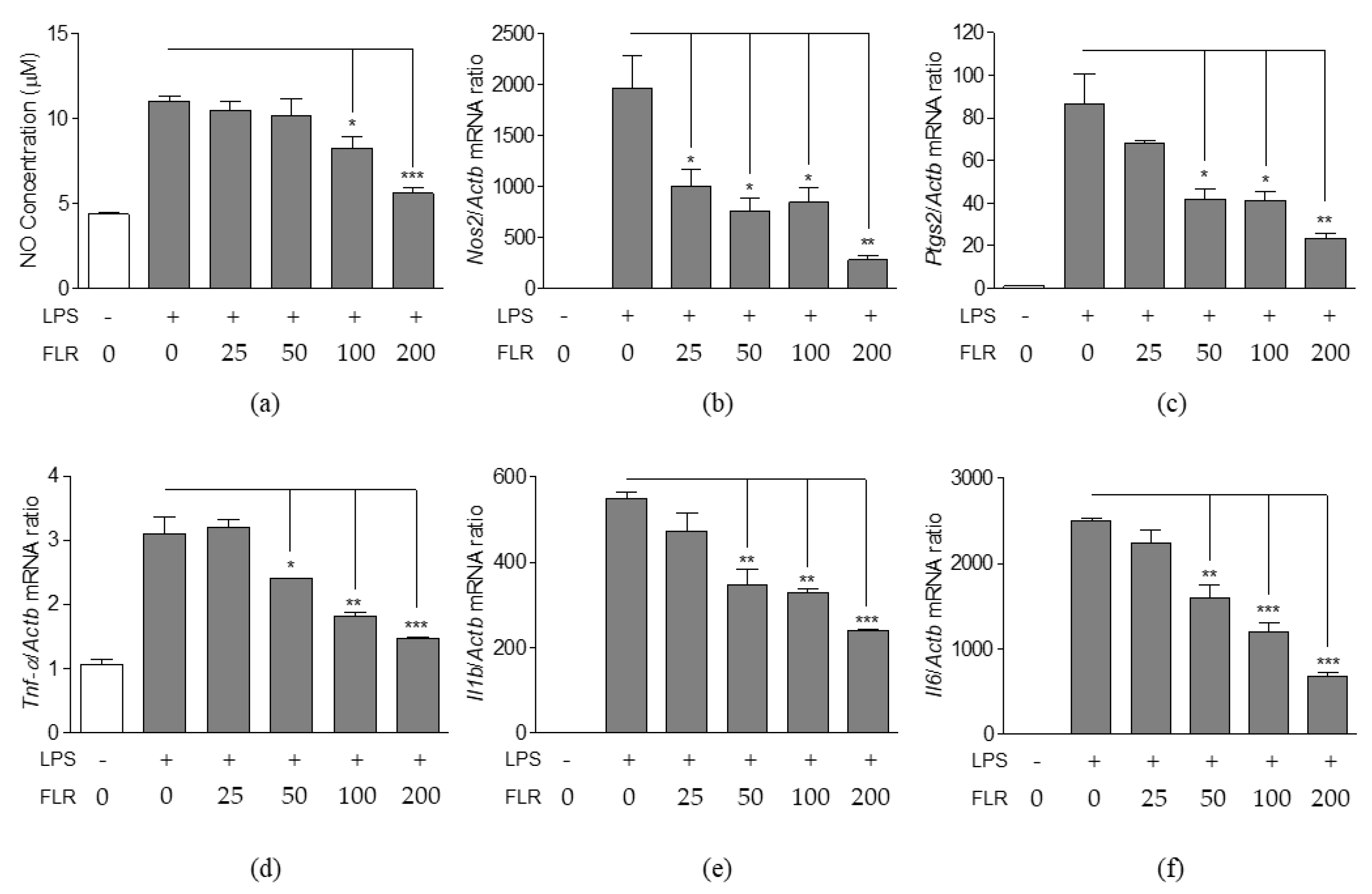
3.2. Effects of FLR on NO Production and Expression of Pro-Inflammatory Mediators
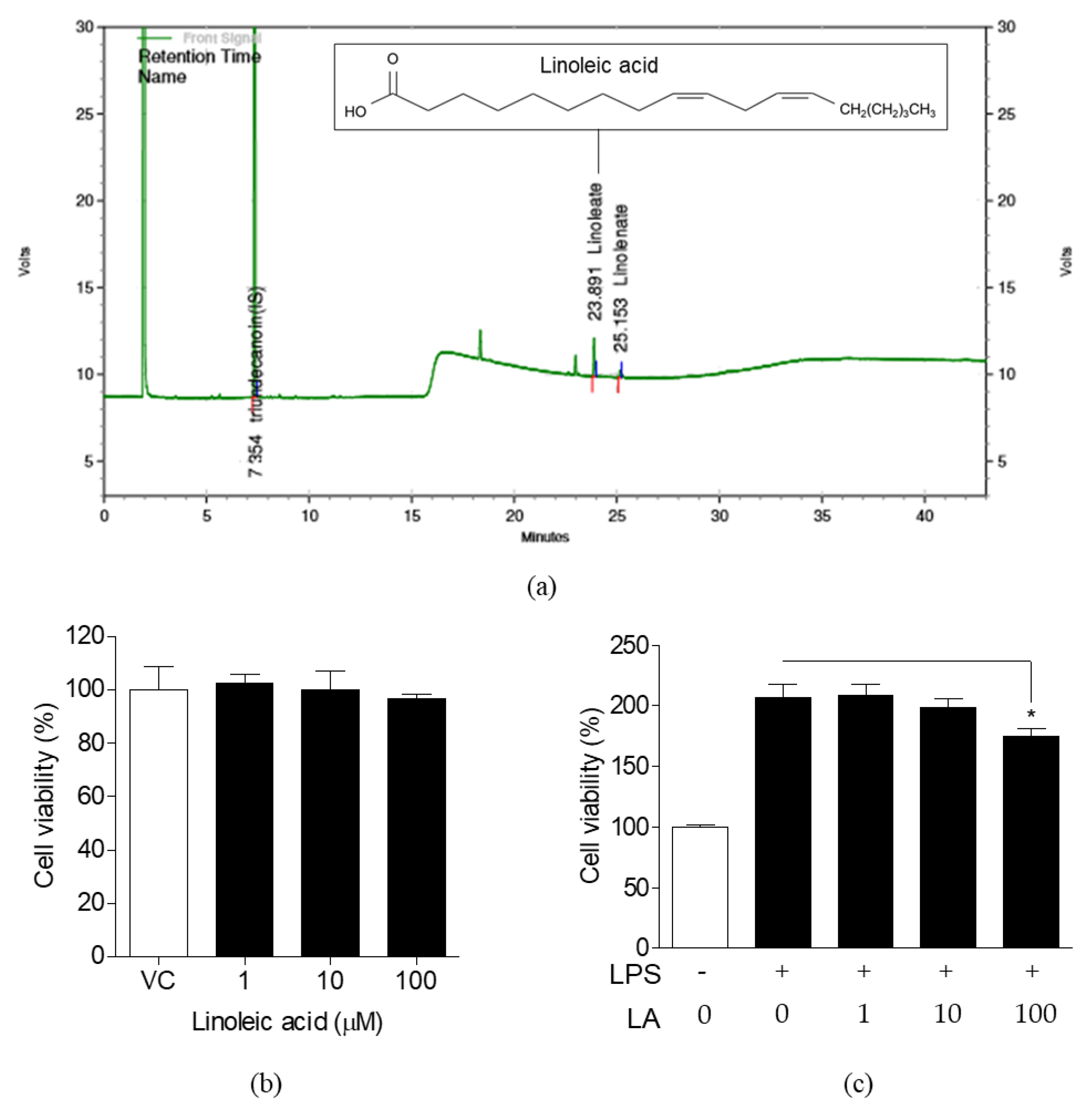
3.3. Detection of LA in FLR and Effects of LA on Cell Viability
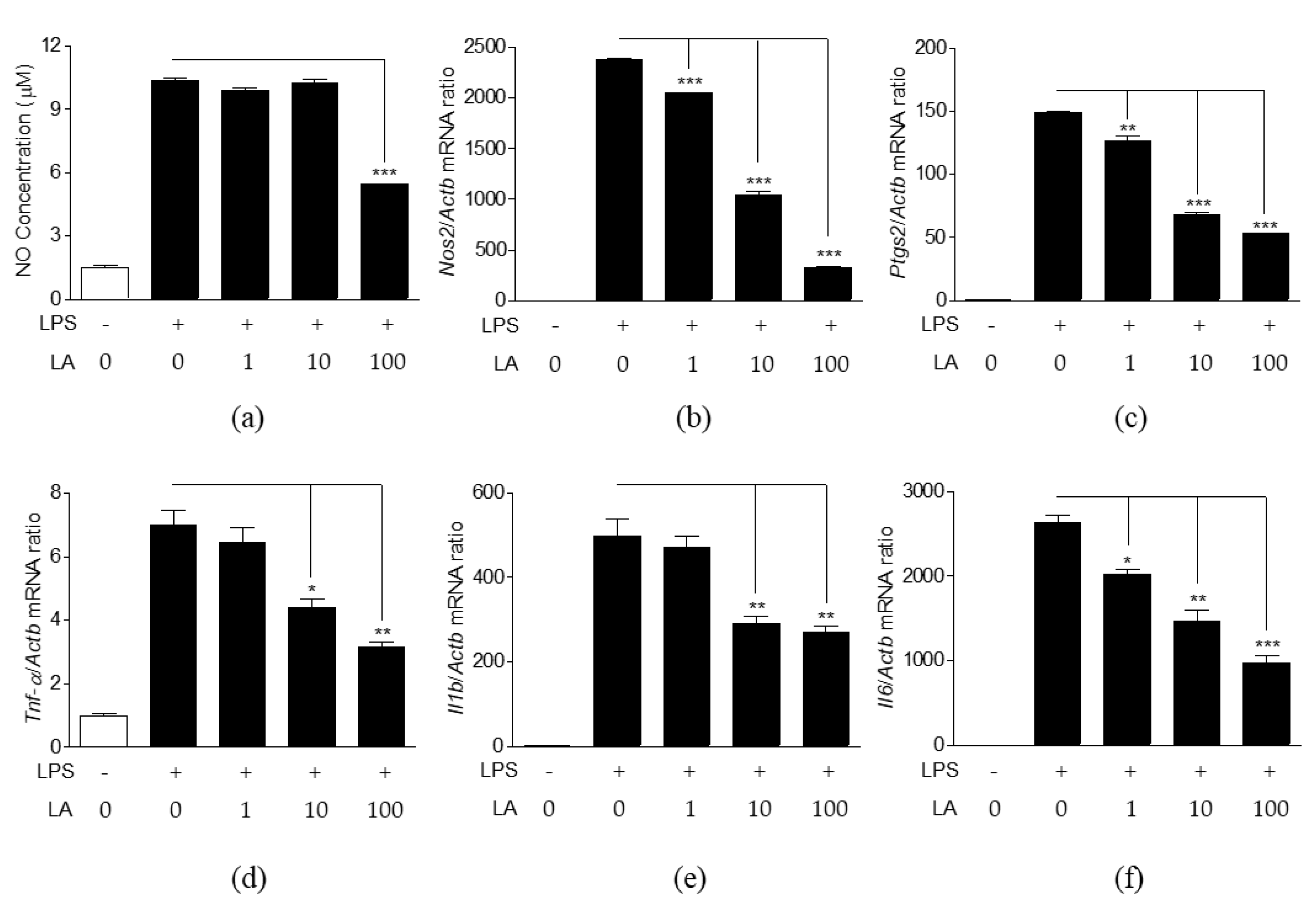
3.4. Effects of LA on NO Production and Expression of Pro-Inflammatory Mediators
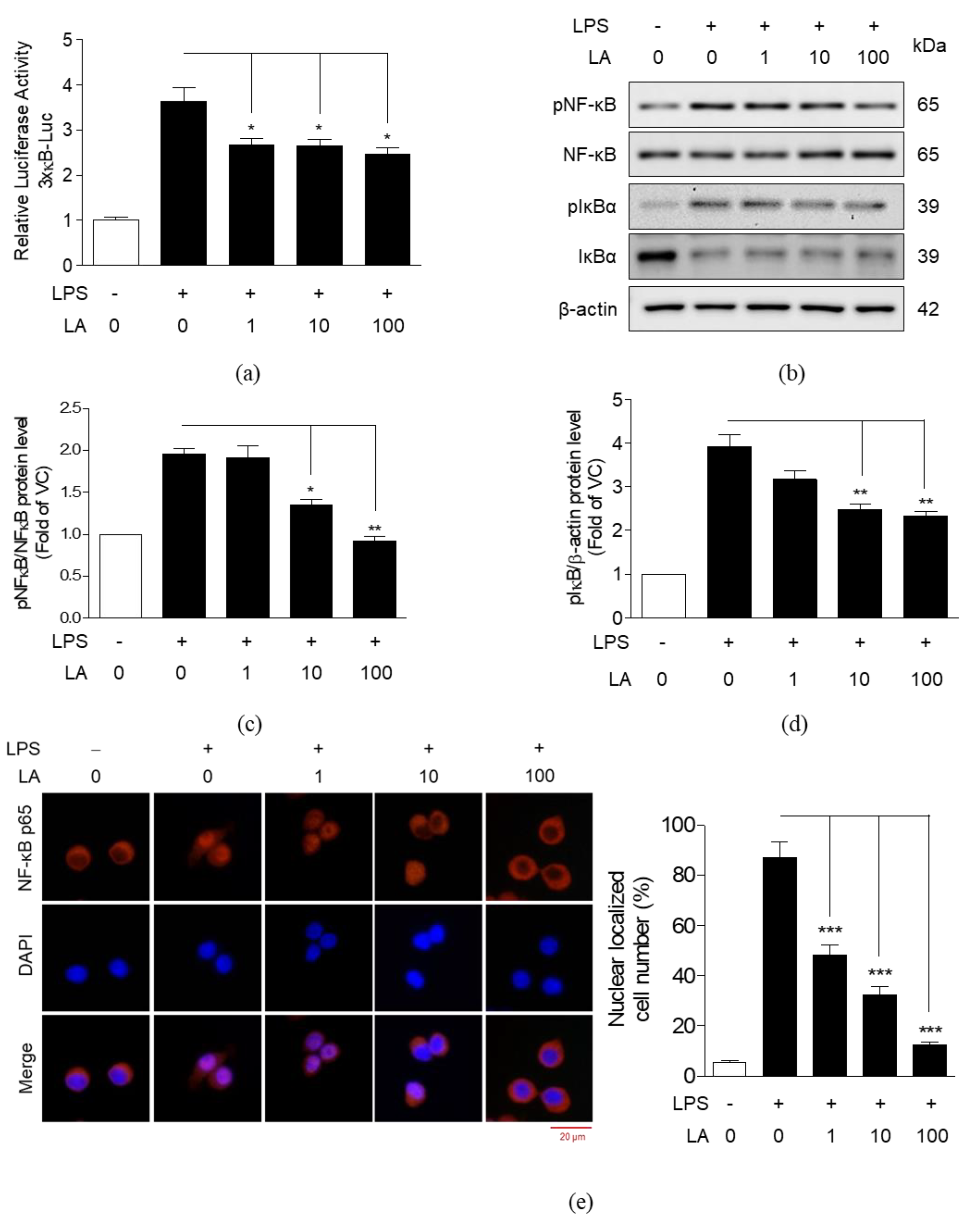
3.5. Effects of LA on the Activation and Translocation of NF-κB p65
3.6. Effects of LA on MAPK Activation
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FLR | Fermented lotus root |
| LA | Linoleic acid |
| LPS | Lipopolysaccharide |
| TLR4 | Toll-like receptor 4 |
| NF-κB | Nuclear factor-kappa B |
| IκBα | Inhibitor of kappa B |
| MAPK | Mitogen-activated protein kinase |
| NO | Nitric oxide |
| iNOS | Inducible nitric oxide synthase |
| COX-2 | Cyclooxygenase-2 |
| TNF-α | Tumor necrosis factor-alpha |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| Nos2 | Nitric oxide synthase 2 |
| Ptgs2 | Prostaglandin-endoperoxide synthase 2 |
| ERK | Extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| GC-FID | Gas chromatography with flame ionization detector |
| VC | Vehicle control |
| CCK-8 | Cell counting kit-8 |
| DAPI | 4′,6-Diamidino-2-phenylindole dihydrochloride |
| ANOVA | Analysis of variance |
| SEM | Standard error of the mean |
References
- De Lavor, E.M.; Fernandes, A.W.C.; de Andrade Teles, R.B.; Leal, A.; de Oliveira Junior, R.G.; Gama, E.S.M.; de Oliveira, A.P.; Silva, J.C.; de Moura Fontes Araujo, M.T.; Coutinho, H.D.M.; et al. Essential Oils and Their Major Compounds in the Treatment of Chronic Inflammation: A Review of Antioxidant Potential in Preclinical Studies and Molecular Mechanisms. Oxid. Med. Cell. Longev. 2018, 2018, 6468593. [Google Scholar] [CrossRef]
- Freigang, S. The regulation of inflammation by oxidized phospholipids. Eur. J. Immunol. 2016, 46, 1818–1825. [Google Scholar] [CrossRef]
- Ara, T.; Nakatani, S.; Kobata, K.; Sogawa, N.; Sogawa, C. The Biological Efficacy of Natural Products against Acute and Chronic Inflammatory Diseases in the Oral Region. Medicines 2018, 5. [Google Scholar] [CrossRef]
- Zerem, E. Treatment of severe acute pancreatitis and its complications. World J. Gastroenterol. 2014, 20, 13879–13892. [Google Scholar] [CrossRef]
- Lu, S.; Wu, D.; Sun, G.; Geng, F.; Shen, Y.; Tan, J.; Sun, X.; Luo, Y. Gastroprotective effects of Kangfuxin against water-immersion and restraint stress-induced gastric ulcer in rats: Roles of antioxidation, anti-inflammation, and pro-survival. Pharm. Biol. 2019, 57, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Han, H.I.; Skvarca, L.B.; Espiritu, E.B.; Davidson, A.J.; Hukriede, N.A. The role of macrophages during acute kidney injury: Destruction and repair. Pediatr. Nephrol. 2019, 34, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.U.; Lee, J.H.; Shehzad, A.; Ahn, E.M.; Lee, Y.M.; Lee, Y.S. Decursinol Angelate Inhibits LPS-Induced Macrophage Polarization through Modulation of the NFkappaB and MAPK Signaling Pathways. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Cui, X.; Lee, D.S.; Sohn, J.H.; Yim, J.H.; Kim, Y.C.; Oh, H. Anti-inflammatory effect of neoechinulin a from the marine fungus Eurotium sp. SF-5989 through the suppression of NF-small ka, CyrillicB and p38 MAPK Pathways in lipopolysaccharide-stimulated RAW264.7 macrophages. Molecules 2013, 18, 13245–13259. [Google Scholar] [CrossRef]
- Duan, Y.; An, W.; Wu, H.; Wu, Y. Salvianolic Acid C Attenuates LPS-Induced Inflammation and Apoptosis in Human Periodontal Ligament Stem Cells via Toll-Like Receptors 4 (TLR4)/Nuclear Factor kappa B (NF-kappaB) Pathway. Med. Sci. Monit. 2019, 25, 9499–9508. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W.; Yang, M.; Hou, C. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4/NF-kappaB pathway activation. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- Yuan, F.; Chen, J.; Sun, P.P.; Guan, S.; Xu, J. Wedelolactone inhibits LPS-induced pro-inflammation via NF-kappaB pathway in RAW 264.7 cells. J. Biomed. Sci. 2013, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, J.; Cui, L.; Wang, Y.; Lin, J.; Qu, Y.; Wang, H. Cortisol modulates inflammatory responses in LPS-stimulated RAW264.7 cells via the NF-kappaB and MAPK pathways. BMC Vet. Res. 2018, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappaB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Kim, D.H.; Chung, J.H.; Yoon, J.S.; Ha, Y.M.; Bae, S.; Lee, E.K.; Jung, K.J.; Kim, M.S.; Kim, Y.J.; Kim, M.K.; et al. Ginsenoside Rd inhibits the expressions of iNOS and COX-2 by suppressing NF-kappaB in LPS-stimulated RAW264.7 cells and mouse liver. J. Ginseng Res. 2013, 37, 54–63. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.; Karin, M. Regulation and Function of NF-kappa B Transcription Factors in the Immune System. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Kim, D.H.; Cho, W.Y.; Yeon, S.J.; Choi, S.H.; Lee, C.H. Effects of Lotus (Nelumbo nucifera) Leaf on Quality and Antioxidant Activity of Yogurt during Refrigerated Storage. Food Sci. Anim. Resour. 2019, 39, 792–803. [Google Scholar] [CrossRef]
- Tsuruta, Y.; Nagao, K.; Shirouchi, B.; Nomura, S.; Tsuge, K.; Koganemaru, K.; Yanagita, T. Effects of lotus root (the edible rhizome of Nelumbo nucifera) on the deveolopment of non-alcoholic fatty liver disease in obese diabetic db/db mice. Biosci. Biotechnol. Biochem. 2012, 76, 462–466. [Google Scholar] [CrossRef]
- Jiang, X.L.; Wang, L.; Wang, E.J.; Zhang, G.L.; Chen, B.; Wang, M.K.; Li, F. Flavonoid glycosides and alkaloids from the embryos of Nelumbo nucifera seeds and their antioxidant activity. Fitoterapia 2018, 125, 184–190. [Google Scholar] [CrossRef]
- Yang, M.Y.; Hung, T.W.; Wang, C.J.; Tseng, T.H. Inhibitory Effect of Nelumbo nucifera Leaf Extract on 2-Acetylaminofluorene-induced Hepatocarcinogenesis Through Enhancing Antioxidative Potential and Alleviating Inflammation in Rats. Antioxidants 2019, 8. [Google Scholar] [CrossRef]
- Knipping, K.; Garssen, J.; van’t Land, B. An evaluation of the inhibitory effects against rotavirus infection of edible plant extracts. Virol. J. 2012, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Hattori, E.; Fukaya, Y.; Imai, S.; Ohizumi, Y. Anti-obesity effect of Nelumbo nucifera leaves extract in mice and rats. J. Ethnopharmacol. 2006, 106, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Poornima, P.; Weng, C.F.; Padma, V.V. Neferine, an alkaloid from lotus seed embryo, inhibits human lung cancer cell growth by MAPK activation and cell cycle arrest. Biofactors 2014, 40, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Wahile, A.; Mukherjee, K.; Saha, B.P.; Mukherjee, P.K. Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. J. Ethnopharmacol. 2006, 104, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Nishimura, K.; Itoh, A.; Tanahashi, T.; Nakajima, H.; Oshiro, H.; Sun, S.; Toda, T.; Yamada, J. Serotonergic mechanisms are involved in antidepressant-like effects of bisbenzylisoquinolines liensinine and its analogs isolated from the embryo of Nelumbo nucifera Gaertner seeds in mice. J. Pharm. Pharmacol. 2015, 67, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- You, J.S.; Lee, Y.J.; Kim, K.S.; Kim, S.H.; Chang, K.J. Ethanol extract of lotus (Nelumbo nucifera) root exhibits an anti-adipogenic effect in human pre-adipocytes and anti-obesity and anti-oxidant effects in rats fed a high-fat diet. Nutr. Res. 2014, 34, 258–267. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Saha, K.; Pal, M.; Saha, B.P. Effect of Nelumbo nucifera rhizome extract on blood sugar level in rats. J. Ethnopharmacol. 1997, 58, 207–213. [Google Scholar] [CrossRef]
- Tsuruta, Y.; Nagao, K.; Kai, S.; Tsuge, K.; Yoshimura, T.; Koganemaru, K.; Yanagita, T. Polyphenolic extract of lotus root (edible rhizome of Nelumbo nucifera) alleviates hepatic steatosis in obese diabetic db/db mice. Lipids Health Dis. 2011, 10, 202. [Google Scholar] [CrossRef]
- Mukherjee, D.; Khatua, T.N.; Venkatesh, P.; Saha, B.P.; Mukherjee, P.K. Immunomodulatory potential of rhizome and seed extracts of Nelumbo nucifera Gaertn. J. Ethnopharmacol. 2010, 128, 490–494. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Saha, K.; Das, J.; Pal, M.; Saha, B.P. Studies on the anti-inflammatory activity of rhizomes of Nelumbo nucifera. Planta Med. 1997, 63, 367–369. [Google Scholar] [CrossRef]
- Yong, C.C.; Yoon, Y.; Yoo, H.S.; Oh, S. Effect of Lactobacillus Fermentation on the Anti-Inflammatory Potential of Turmeric. J. Microbiol. Biotechnol. 2019, 29, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Villavicencio, M.L.; Vinqvist-Tymchuk, M.; Kalt, W.; Matar, C.; Alarcon Aguilar, F.J.; Escobar Villanueva, M.D.; Haddad, P.S. Fermented blueberry juice extract and its specific fractions have an anti-adipogenic effect in 3 T3-L1 cells. BMC Complement. Altern. Med. 2017, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, H.; Liu, H.; Ma, R.; Ma, J.; Fang, H. Fermentation by Multiple Bacterial Strains Improves the Production of Bioactive Compounds and Antioxidant Activity of Goji Juice. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Park, E.J.; Kim, S.H.; Lee, H.J. Gastroprotective Effects of Fermented Lotus Root against Ethanol/HCl-Induced Gastric Mucosal Acute Toxicity in Rats. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef] [PubMed]
- Monmai, C.; Go, S.H.; Shin, I.S.; You, S.G.; Lee, H.; Kang, S.B.; Park, W.J. Immune-Enhancement and Anti-Inflammatory Activities of Fatty Acids Extracted from Halocynthia aurantium Tunic in RAW264.7 Cells. Mar. Drugs 2018, 16. [Google Scholar] [CrossRef]
- Park, E.-J.; Lee, Y.-S.; Kim, S.M.; Jung, A.J.; Yoo, J.-H.; Lee, S.-H.; Jeong, H.C.; Lee, H.-J. Immune-Enhancing Effects of Red Platycodon grandiflorus Root Extract via p38 MAPK-Mediated NF-κB Activation. Appl. Sci. 2020, 10. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Zhang, H.; Ren, Q.C.; Ren, Y.; Zhao, L.; Yang, F.; Zhang, Y.; Zhao, W.J.; Tan, Y.Z.; Shen, X.F. Ajudecumin A from Ajuga ovalifolia var. calantha exhibits anti-inflammatory activity in lipopolysaccharide-activated RAW264.7 murine macrophages and animal models of acute inflammation. Pharm. Biol. 2018, 56, 649–657. [Google Scholar] [CrossRef]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Kimbrough, C.W.; Lakshmanan, J.; Matheson, P.J.; Woeste, M.; Gentile, A.; Benns, M.V.; Zhang, B.; Smith, J.W.; Harbrecht, B.G. Resveratrol decreases nitric oxide production by hepatocytes during inflammation. Surgery 2015, 158, 1095–1101; discussion 1101. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Li, W.M.; Zeb, M.; Yaqoob, A.; Tackaberry, L.E.; Massicotte, H.B.; Egger, K.N.; Cheung, P.C.K.; Payne, G.W.; Lee, C.H. Anti-Inflammatory Activity of the Wild Mushroom, Echinodontium tinctorium, in RAW264.7 Macrophage Cells and Mouse Microcirculation. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Davis, R.J. TNF and MAP kinase signalling pathways. Semin. Immunol. 2014, 26, 237–245. [Google Scholar] [CrossRef]
- Koistinaho, M.; Koistinaho, J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia 2002, 40, 175–183. [Google Scholar] [CrossRef]
- Saha, R.N.; Jana, M.; Pahan, K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J. Immunol. 2007, 179, 7101–7109. [Google Scholar] [CrossRef]
- Grynberg, K.; Ma, F.Y.; Nikolic-Paterson, D.J. The JNK Signaling Pathway in Renal Fibrosis. Front. Physiol. 2017, 8, 829. [Google Scholar] [CrossRef]
- Ruan, J.; Qi, Z.; Shen, L.; Jiang, Y.; Xu, Y.; Lan, L.; Luo, L.; Yin, Z. Crosstalk between JNK and NF-kappaB signaling pathways via HSP27 phosphorylation in HepG2 cells. Biochem. Biophys. Res. Commun. 2015, 456, 122–128. [Google Scholar] [CrossRef]
- Wan, F.; Lenardo, M.J. Specification of DNA binding activity of NF-kappaB proteins. Cold Spring Harb. Perspect. Biol. 2009, 1, a000067. [Google Scholar] [CrossRef]
- Hinz, M.; Scheidereit, C. The IkappaB kinase complex in NF-kappaB regulation and beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Li, Y.; Chen, Q.; Liu, F.; Zhu, X.; Mei, L.; Song, X.; Liu, X.; Song, Z.; et al. Anti-Inflammatory Effects of a Mytilus coruscus alpha-d-Glucan (MP-A) in Activated Macrophage Cells via TLR4/NF-kappaB/MAPK Pathway Inhibition. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, H.; Jantan, I.; Haque, M.A.; Kumolosasi, E. Anti-inflammatory effects of Phyllanthus amarus Schum. & Thonn. through inhibition of NF-kappaB, MAPK, and PI3K-Akt signaling pathways in LPS-induced human macrophages. BMC Complement. Altern. Med. 2018, 18, 224. [Google Scholar] [CrossRef]
- Akanda, M.R.; Kim, I.S.; Ahn, D.; Tae, H.J.; Nam, H.H.; Choo, B.K.; Kim, K.; Park, B.Y. Anti-Inflammatory and Gastroprotective Roles of Rabdosia inflexa through Downregulation of Pro-Inflammatory Cytokines and MAPK/NF-kappaB Signaling Pathways. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.H.; Kim, H.; Yang, S.; Kim, J.K.; Kim, J.G. Linoleic acid rescues microglia inflammation triggered by saturated fatty acid. Biochem. Biophys. Res. Commun. 2019, 513, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, H.G.; Vinolo, M.A.; Magdalon, J.; Vitzel, K.; Nachbar, R.T.; Pessoa, A.F.; dos Santos, M.F.; Hatanaka, E.; Calder, P.C.; Curi, R. Oral administration of oleic or linoleic acid accelerates the inflammatory phase of wound healing. J. Invest. Dermatol. 2012, 132, 208–215. [Google Scholar] [CrossRef]
- Marchix, J.; Choque, B.; Kouba, M.; Fautrel, A.; Catheline, D.; Legrand, P. Excessive dietary linoleic acid induces proinflammatory markers in rats. J. Nutr. Biochem. 2015, 26, 1434–1441. [Google Scholar] [CrossRef]
- Investigators, I.B.D.i.E.S.; Tjonneland, A.; Overvad, K.; Bergmann, M.M.; Nagel, G.; Linseisen, J.; Hallmans, G.; Palmqvist, R.; Sjodin, H.; Hagglund, G.; et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: A nested case-control study within a European prospective cohort study. Gut 2009, 58, 1606–1611. [Google Scholar] [CrossRef]
- Jandacek, R.J. Linoleic Acid: A Nutritional Quandary. Healthcare 2017, 5. [Google Scholar] [CrossRef]
- Fritsche, K.L. Linoleic acid, vegetable oils & inflammation. Mo. Med. 2014, 111, 41–43. [Google Scholar]
- Vaughan, R.A.; Garrison, R.L.; Stamatikos, A.D.; Kang, M.; Cooper, J.A.; Paton, C.M. A High Linoleic Acid Diet does not Induce Inflammation in Mouse Liver or Adipose Tissue. Lipids 2015, 50, 1115–1122. [Google Scholar] [CrossRef]
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Rett, B.S.; Whelan, J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: A systematic review. Nutr. Metab. 2011, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Saiki, P.; Kawano, Y.; Van Griensven, L.; Miyazaki, K. The anti-inflammatory effect of Agaricus brasiliensis is partly due to its linoleic acid content. Food Funct. 2017, 8, 4150–4158. [Google Scholar] [CrossRef] [PubMed]



| Genes | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Product Size (bp) |
|---|---|---|---|
| Actb | CCACAGCTGAGAGGAAATC | AAGGAAGGCTGGAAAAGAGC | 193 |
| Nos2 | TTCCAGAATCCCTGGACAAG | TGGTCAAACTCTTGGGGTTC | 180 |
| Ptgs2 | AGAAGGAAATGGCTGCAGAA | GCTCGGCTTCCAGTATTGAG | 194 |
| Tnf-α | ATGAGCACAGAAAGCATGATC | TACAGGCTTGTCACTCGAATT | 276 |
| Il1b | GGGCCTCAAAGGAAAGAA | TACCAGTTGGGGAACTCTGC | 183 |
| Il6 | AGTTGCCTTCTTGGGACTGA | CAGAATTGCCATTGCACAAC | 191 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.M.; Park, E.-J.; Kim, J.-Y.; Choi, J.; Lee, H.-J. Anti-Inflammatory Effects of Fermented Lotus Root and Linoleic Acid in Lipopolysaccharide-Induced RAW 264.7 Cells. Life 2020, 10, 293. https://doi.org/10.3390/life10110293
Kim SM, Park E-J, Kim J-Y, Choi J, Lee H-J. Anti-Inflammatory Effects of Fermented Lotus Root and Linoleic Acid in Lipopolysaccharide-Induced RAW 264.7 Cells. Life. 2020; 10(11):293. https://doi.org/10.3390/life10110293
Chicago/Turabian StyleKim, Sung Min, Eun-Jung Park, Jong-Yeon Kim, Jihee Choi, and Hae-Jeung Lee. 2020. "Anti-Inflammatory Effects of Fermented Lotus Root and Linoleic Acid in Lipopolysaccharide-Induced RAW 264.7 Cells" Life 10, no. 11: 293. https://doi.org/10.3390/life10110293
APA StyleKim, S. M., Park, E.-J., Kim, J.-Y., Choi, J., & Lee, H.-J. (2020). Anti-Inflammatory Effects of Fermented Lotus Root and Linoleic Acid in Lipopolysaccharide-Induced RAW 264.7 Cells. Life, 10(11), 293. https://doi.org/10.3390/life10110293






