The Role of the Cyanobacterial Type IV Pilus Machinery in Finding and Maintaining a Favourable Environment
Abstract
1. The Type IV Pilus Machinery Conveys Twitching Motility to Cyanobacteria
2. Twitching Motility Enables Cyanobacteria to Seek out Favourable and Escape Unfavourable Environments
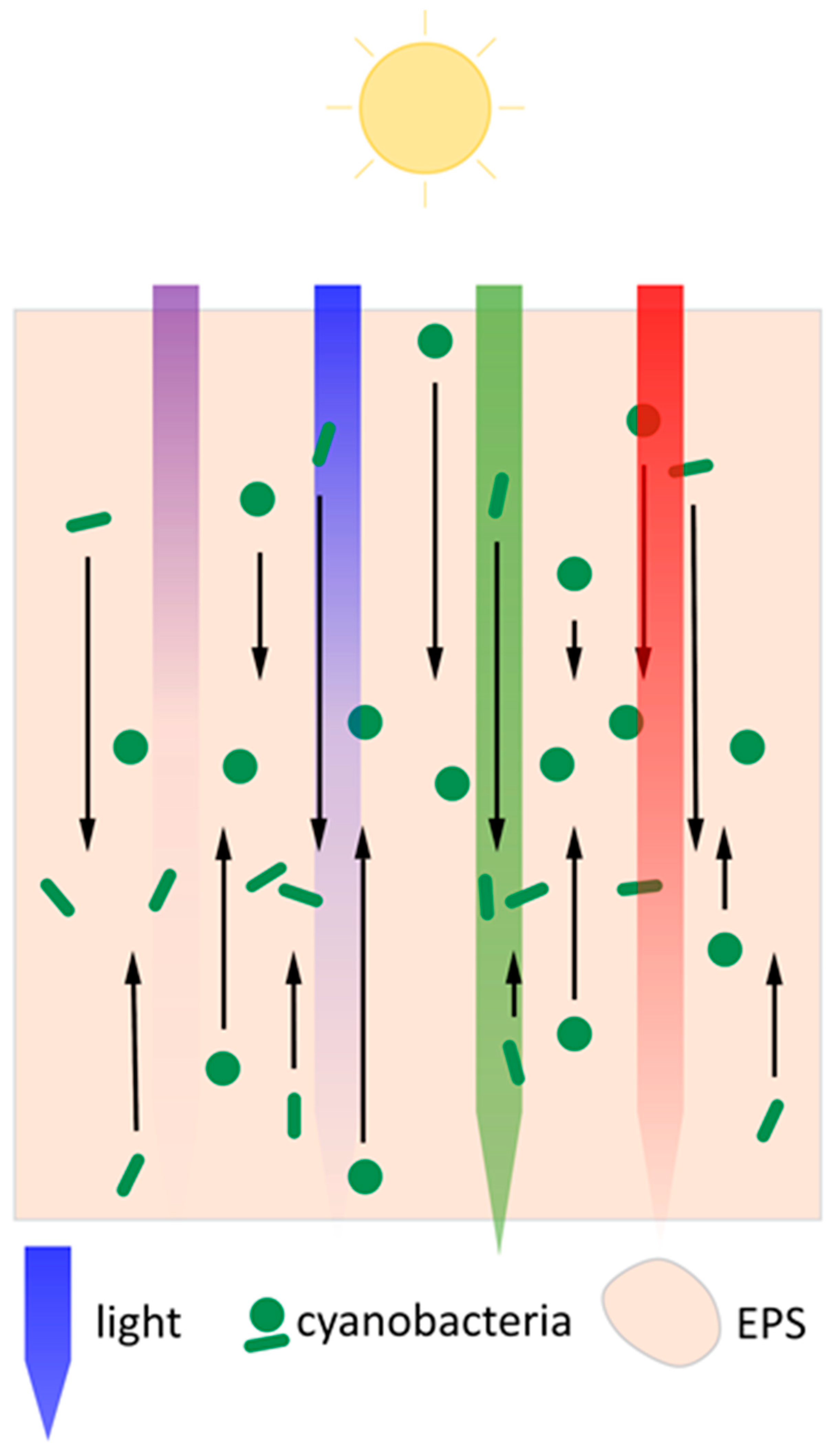
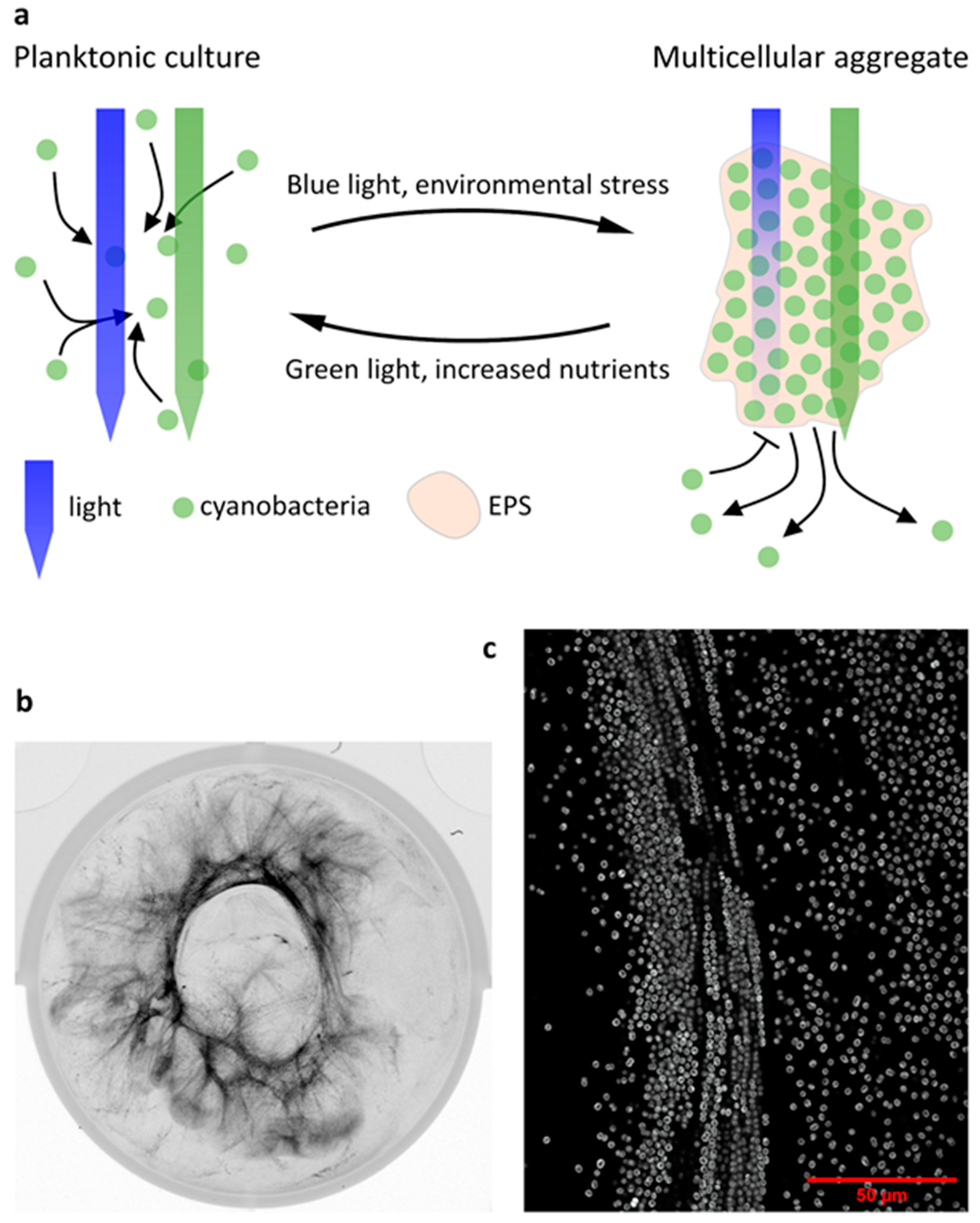
3. Many Species of Cyanobacteria Form Large-Scale Multicellular Assemblages
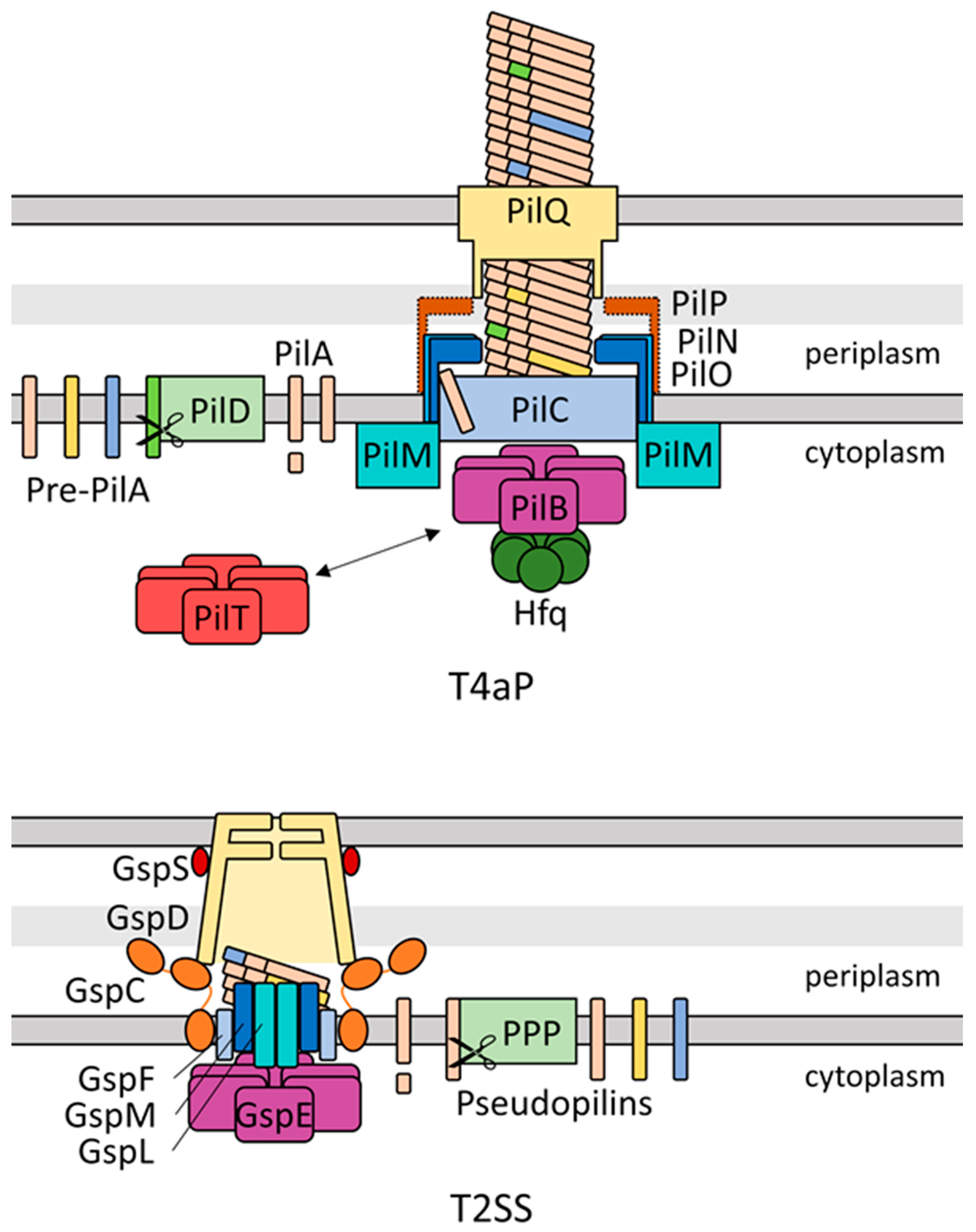
4. The T4P Apparatus Has Structural and Secretory Roles in Cyanobacterial Community Formation
5. Regulation of the T4P Machinery
6. Cyanobacterial Natural Competence Requires T4P
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Vigneron, A.; Cruaud, P.; Mohit, V.; Martineau, M.-J.; Culley, A.I.; Lovejoy, C.; Vincent, W.F. Multiple strategies for light-harvesting, photoprotection, and carbon flow in high latitude microbial mats. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chrismas, N.A.M.; Barker, G.; Anesio, A.M.; Sánchez-Baracaldo, P. Genomic mechanisms for cold tolerance and production of exopolysaccharides in the Arctic cyanobacterium Phormidesmis priestleyi BC1401. BMC Genom. 2016, 17, 533. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, M.; Henneberg, M.; Felde, V.J.M.N.L.; Berkowicz, S.M.; Raanan, H.; Pade, N.; Felix-Henningsen, P.; Kaplan, A. Cyanobacterial populations in biological soil crusts of the northwest Negev Desert, Israel-effects of local conditions and disturbance. FEMS Microbiol. Ecol. 2017, 93, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Flombaum, P.; Gallegos, J.L.; Gordillo, R.A.; Rincón, J.; Zabala, L.L.; Jiao, N.; Karl, D.M.; Li, W.K.W.; Lomas, M.W.; Veneziano, D.; et al. Present and future global distributions of the marine cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 9824–9829. [Google Scholar] [CrossRef]
- Yang, Y.; Lam, V.; Adomako, M.; Simkovsky, R.; Jakob, A.; Rockwell, N.C.; Cohen, S.E.; Taton, A.; Wang, J.; Lagarias, J.C.; et al. Phototaxis in a wild isolate of the cyanobacterium Synechococcus elongatus. Proc. Natl. Acad. Sci. USA 2018, 115, E12378–E12387. [Google Scholar] [CrossRef]
- Cho, Y.W.; Gonzales, A.; Harwood, T.V.; Huynh, J.; Hwang, Y.; Park, J.S.; Trieu, A.Q.; Italia, P.; Pallipuram, V.K.; Risser, D.D. Dynamic localization of HmpF regulates type IV pilus activity and directional motility in the filamentous cyanobacterium Nostoc punctiforme. Mol. Microbiol. 2017, 106, 252–265. [Google Scholar] [CrossRef]
- Schuergers, N.; Wilde, A. Appendages of the Cyanobacterial Cell. Life 2015, 5, 700–715. [Google Scholar] [CrossRef]
- Bhaya, D.; Bianco, N.R.; Bryant, D. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol. Microbiol. 2000, 37, 941–951. [Google Scholar] [CrossRef]
- Yoshihara, S.; Geng, X.X.; Okamoto, S.; Yura, K.; Murata, T.; Go, M.; Ohmori, M.; Ikeuchi, M. Mutational analysis of genes involved in pilus structure, motility and transformation competency in the unicellular motile cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2001, 42, 63–73. [Google Scholar] [CrossRef]
- Roux, N.; Spagnolo, J.; De Bentzmann, S. Neglected but amazingly diverse type IVb pili. Res. Microbiol. 2012, 163, 659–673. [Google Scholar] [CrossRef]
- Linhartová, M.; Bučinská, L.; Halada, P.; Ječmen, T.; Šetlík, J.; Komenda, J.; Sobotka, R. Accumulation of the Type IV prepilin triggers degradation of SecY and YidC and inhibits synthesis of Photosystem II proteins in the cyanobacterium Synechocystis PCC 6803. Mol. Microbiol. 2014, 93, 1207–1223. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Rettberg, L.A.; Treuner-Lange, A.; Iwasa, J.; Søgaard-Andersen, L.; Jensen, G.J. Architecture of the type IVa pilus machine. Science (80-) 2016, 351, aad2001. [Google Scholar] [CrossRef]
- Korotkov, K.V.; Sandkvist, M.; Hol, W.G.J. The type II secretion system: Biogenesis, molecular architecture and mechanism. Nat. Rev. Microbiol. 2012, 10, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Chernyatina, A.A.; Low, H.H. Core architecture of a bacterial type II secretion system. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Hay, I.D.; Belousoff, M.J.; Dunstan, R.A.; Bamert, R.S.; Lithgow, T. Structure and membrane topography of the vibrio-type secretin complex from the type 2 secretion system of enteropathogenic Escherichia coli. J. Bacteriol. 2018, 200, 1–15. [Google Scholar] [CrossRef]
- Taton, A.; Erikson, C.; Yang, Y.; Rubin, B.E.; Rifkin, S.A.; Golden, J.W.; Golden, S.S. The circadian clock and darkness control natural competence in cyanobacteria. Nat. Commun. 2020, 11, 1688. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.; Pique, M.E.; Tainer, J.A. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2004, 2, 363–378. [Google Scholar] [CrossRef]
- Neuhaus, A.; Selvaraj, M.; Salzer, R.; Langer, J.D.; Kruse, K.; Kirchner, L.; Sanders, K.; Daum, B.; Averhoff, B.; Gold, V.A.M. Cryo-electron microscopy reveals two distinct type IV pili assembled by the same bacterium. Nat. Commun. 2020, 11, 2231. [Google Scholar] [CrossRef]
- Duggan, P.S.; Gottardello, P.; Adams, D.G. Molecular analysis of genes in Nostoc punctiforme involved in pilus biogenesis and plant infection. J. Bacteriol. 2007, 189, 4547–4551. [Google Scholar] [CrossRef]
- Sukmana, A.; Yang, Z. The type IV pilus assembly motor PilB is a robust hexameric ATPase with complex kinetics. Biochem. J. 2018, 475, 1979–1993. [Google Scholar] [CrossRef]
- McCallum, M.; Tammam, S.; Khan, A.; Burrows, L.L.; Lynne Howell, P. The molecular mechanism of the type IVa pilus motors. Nat. Commun. 2017, 8, 15091. [Google Scholar] [CrossRef] [PubMed]
- del Mar Aguilo-Ferretjans, M.; Bosch, R.; Puxty, R.; Latva, M.; Zadjelovic, V.; Chhun, A.; Sousoni, D.; Polin, M.; Scanlan, D.; Christie-Oleza, J. Pili allow dominant marine cyanobacteria to avoid sinking and evade predation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Khayatan, B.; Meeks, J.C.; Risser, D.D. Evidence that a modified type IV pilus-like system powers gliding motility and polysaccharide secretion in filamentous cyanobacteria. Mol. Microbiol. 2015, 98, 1021–1036. [Google Scholar] [CrossRef]
- Rehman, A.U.; Cser, K.; Sass, L.; Vass, I. Characterization of singlet oxygen production and its involvement in photodamage of Photosystem II in the cyanobacterium Synechocystis PCC 6803 by histidine-mediated chemical trapping. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.L.; Meeks, J.C. Characteristics of hormogonia formation by symbiotic Nostoc spp. in response to the presence of Anthoceros punctatus or its extracellular products. Appl. Environ. Microbiol. 1989, 55, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Malin, G.; Walsby, A.E. Chemotaxis of a cyanobacterium on concentration gradients of carbon dioxide, bicarbonate and oxygen. Microbiology 1985, 131, 2643–2652. [Google Scholar] [CrossRef][Green Version]
- Choi, J.; Chungl, Y.; Moon, Y.; Kimtl, C.; Watanabe, M.; Song, P.; Joe, C.-O.; Bogorad, L.; Park, Y.M. Photomovement of the gliding cyanobacterium Synechocystis sp. PCC 6803. Photochem. Photobiol. 1999, 70, 95–102. [Google Scholar] [CrossRef]
- Wilde, A.; Mullineaux, C.W. Light-controlled motility in prokaryotes and the problem of directional light perception. FEMS Microbiol. Rev. 2017, 41, 900–922. [Google Scholar] [CrossRef]
- Gabai, V.L. A one-instant mechanism of phototaxis in the cyanobacterium Phormidium uncinatum. FEMS Microbiol. Lett. 1985, 30, 125–129. [Google Scholar] [CrossRef]
- Nultsch, W.; Wenderoth, K. Partial Irradiation Experiments with Anabaena variabilis (Kütz). Z. für Pflanzenphysiol. 1983, 111, 1–7. [Google Scholar] [CrossRef]
- Halfen, L.N.; Castenholz, R.W. Gliding motility in the blue-green alga Oscillatoria princeps. J. Phycol. 1971, 7, 133–145. [Google Scholar] [CrossRef]
- Nultsch, W.; Schuchart, H.; Höhl, M. Investigations on the phototactic orientation of Anabaena variabilis. Arch. Microbiol. 1979, 122, 85–91. [Google Scholar] [CrossRef]
- Hoiczyk, E.; Baumeister, W. The junctional pore complex, a prokaryotic secretion organelle, is the molecular motor underlying gliding motility in cyanobacteria. Curr. Biol. 1998, 8, 1161–1168. [Google Scholar] [CrossRef]
- Varuni, P.; Menon, S.N.; Menon, G.I. Phototaxis as a collective phenomenon in cyanobacterial colonies. Sci. Rep. 2017, 7, 17799. [Google Scholar] [CrossRef]
- Menon, S.N.; Varuni, P.; Menon, G.I. Information integration and collective motility in phototactic cyanobacteria. PLoS Comput. Biol. 2020, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wilde, A.; Mullineaux, C.W. Motility in cyanobacteria: Polysaccharide tracks and Type IV pilus motors. Mol. Microbiol. 2015, 98, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Schuergers, N.; Lenn, T.; Kampmann, R.; Meissner, M.V.; Esteves, T.; Temerinac-Ott, M.; Korvink, J.G.; Lowe, A.R.; Mullineaux, C.W.; Wilde, A. Cyanobacteria use micro-optics to sense light direction. eLife 2016, 5, e12620. [Google Scholar] [CrossRef]
- Nakane, D.; Nishizaka, T. Asymmetric distribution of type IV pili triggered by directional light in unicellular cyanobacteria. Proc. Natl. Acad. Sci. USA 2017, 114, 6593–6598. [Google Scholar] [CrossRef] [PubMed]
- Schuergers, N.; Nürnberg, D.J.; Wallner, T.; Mullineaux, C.W.; Wilde, A. PilB localization correlates with the direction of twitching motility in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology (United Kingdom) 2015, 161, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Luimstra, V.M.; Schuurmans, J.M.; Verschoor, A.M.; Hellingwerf, K.J.; Huisman, J.; Matthijs, H.C.P. Blue light reduces photosynthetic efficiency of cyanobacteria through an imbalance between photosystems I and II. Photosynth. Res. 2018, 138, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Vass, I. Molecular mechanisms of photodamage in the Photosystem II complex. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 209–217. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Y.; Häder, D.P. Reactive oxygen species and UV-B: Effect on cyanobacteria. Photochem. Photobiol. Sci. 2002, 1, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Scherer, S. UV protection in cyanobacteria. Eur. J. Phycol. 1999, 34, 329–338. [Google Scholar] [CrossRef]
- Wilde, A.; Fiedler, B.; Börner, T. The cyanobacterial phytochrome Cph2 inhibits phototaxis towards blue light. Mol. Microbiol. 2002, 44, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Cho, H.S.; Cho, J.-I.; Jeon, J.S.; Lagarias, J.C.; Park, Y. Il Near-UV cyanobacteriochrome signaling system elicits negative phototaxis in the cyanobacterium Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. USA 2011, 108, 10780–10785. [Google Scholar] [CrossRef] [PubMed]
- Angerer, V.; Schwenk, P.; Wallner, T.; Kaever, V.; Hiltbrunner, A.; Wilde, A. The protein Slr1143 is an active diguanylate cyclase in Synechocystis sp. PCC 6803 and interacts with the photoreceptor Cph2. Microbiology 2017, 163, 920–930. [Google Scholar] [CrossRef]
- Ng, W.O.; Grossman, A.R.; Bhaya, D. Multiple light inputs control phototaxis in Synechocystis sp. Strain PCC6803. J. Bacteriol. 2003, 185, 1599–1607. [Google Scholar] [CrossRef]
- Fushimi, K.; Narikawa, R. Cyanobacteriochromes: Photoreceptors covering the entire UV-to-visible spectrum. Curr. Opin. Struct. Biol. 2019, 57, 39–46. [Google Scholar] [CrossRef]
- Pudasaini, A.; El-Arab, K.K.; Zoltowski, B.D. LOV-based optogenetic devices: Light-driven modules to impart photoregulated control of cellular signaling. Front. Mol. Biosci. 2015, 2, 1–15. [Google Scholar] [CrossRef]
- Tanaka, K.; Nakasone, Y.; Okajima, K.; Ikeuchi, M.; Tokutomi, S.; Terazima, M. Light-induced conformational change and transient dissociation reaction of the BLUF photoreceptor Synechocystis PixD (Slr1694). J. Mol. Biol. 2011, 409, 773–785. [Google Scholar] [CrossRef]
- Yoshihara, S.; Ikeuchi, M. Phototactic motility in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Photochem. Photobiol. Sci. 2004, 3, 512–518. [Google Scholar] [CrossRef]
- Bhaya, D.; Takahashi, A.; Grossman, A.R. Light regulation of type IV pilus-dependent motility by chemosensor-like elements in Synechocystis PCC6803. Proc. Natl. Acad. Sci. USA 2001, 98, 7540–7545. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Koonin, E.V.; Haselkorn, R.; Galperin, M.Y. Cyanobacterial response regulator PatA contains a conserved N-terminal domain (PATAN) with an alpha-helical insertion. Bioinformatics 2006, 22, 1297–1301. [Google Scholar] [CrossRef]
- Campbell, E.L.; Hagen, K.D.; Chen, R.; Risser, D.D.; Ferreira, D.P.; Meeks, J.C. Genetic analysis reveals the identity of the photoreceptor for phototaxis in hormogonium filaments of Nostoc punctiforme. J. Bacteriol. 2015, 197, 782–791. [Google Scholar] [CrossRef]
- Ishizuka, T.; Shimada, T.; Okajima, K.; Yoshihara, S.; Ochiai, Y.; Katayama, M.; Ikeuchi, M. Characterization of cyanobacteriochrome TePixJ from a thermophilic cyanobacterium Thermosynechococcus elongatus strain BP-1. Plant Cell Physiol. 2006, 47, 1251–1261. [Google Scholar] [CrossRef]
- Narikawa, R.; Fukushima, Y.; Ishizuka, T.; Itoh, S.; Ikeuchi, M. A novel photoactive GAF domain of cyanobacteriochrome AnPixJ that shows reversible green/red photoconversion. J. Mol. Biol. 2008, 380, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, N.C.; Martin, S.S.; Lagarias, J.C. Red/green cyanobacteriochromes: Sensors of color and power. Biochemistry 2012, 51, 9667–9677. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Ono, T.A. Biochemical characterization of the major adenylyl cyclase, Cya1, in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 2004, 577, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Nakasone, Y.; Okajima, K.; Ikeuchi, M.; Tokutomi, S.; Terazima, M. Time-resolved tracking of interprotein signal transduction: Synechocystis PixD-PixE complex as a sensor of light intensity. J. Am. Chem. Soc. 2012, 134, 8336–8339. [Google Scholar] [CrossRef]
- Ren, S.; Sato, R.; Hasegawa, K.; Ohta, H.; Masuda, S. A predicted structure for the PixD-PixE complex determined by homology modeling, docking simulations, and a mutagenesis study. Biochemistry 2013, 52, 1272–1279. [Google Scholar] [CrossRef]
- Jakob, A.; Nakamura, H.; Kobayashi, A.; Sugimoto, Y.; Wilde, A.; Masuda, S. The (PATAN)-CheY-like response regulator PixE interacts with the motor ATPase PilB1 to control negative phototaxis in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2019, 0, 1–12. [Google Scholar] [CrossRef]
- Yoshihara, S.; Geng, X.; Ikeuchi, M. pilG gene cluster and split pilL genes involved in pilus biogenesis, motility and genetic transformation in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2002, 43, 513–521. [Google Scholar] [CrossRef]
- Savakis, P.; De Causmaecker, S.; Angerer, V.; Ruppert, U.; Anders, K.; Essen, L.O.; Wilde, A. Light-induced alteration of c-di-GMP level controls motility of Synechocystis sp. PCC 6803. Mol. Microbiol. 2012, 85, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The First 25 Years of a Universal Bacterial Second Messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef]
- Wallner, T.; Pedroza, L.; Voigt, K.; Kaever, V.; Wilde, A. The cyanobacterial phytochrome 2 regulates the expression of motility-related genes through the second messenger cyclic di-GMP. Photochem. Photobiol. Sci. 2020, 631–643. [Google Scholar] [CrossRef]
- Cao, Z.; Livoti, E.; Losi, A.; Gärtner, W. A blue light-inducible phosphodiesterase activity in the cyanobacterium Synechococcus elongatus. Photochem. Photobiol. 2010, 86, 606–611. [Google Scholar] [CrossRef]
- Lichtenberg, M.; Cartaxana, P.; Kühl, M. Vertical migration optimizes photosynthetic efficiency of motile cyanobacteria in a coastal mnicrobial mat. Front. Mar. Sci. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Nakasugi, K.; Neilan, B.A. Identification of pilus-like structures and genes in Microcystis aeruginosa PCC7806. Appl. Environ. Microbiol. 2005, 71, 7621–7625. [Google Scholar] [CrossRef]
- Ma, J.; Brookes, J.D.; Qin, B.; Paerl, H.W.; Gao, G.; Wu, P.; Zhang, W.; Deng, J.; Zhu, G.; Zhang, Y.; et al. Environmental factors controlling colony formation in blooms of the cyanobacteria Microcystis spp. in Lake Taihu, China. Harmful Algae 2014, 31, 136–142. [Google Scholar] [CrossRef]
- Liu, J.; Prindle, A.; Humphries, J.; Gabalda-Sagarra, M.; Asally, M.; Lee, D.Y.D.; Ly, S.; Garcia-Ojalvo, J.; Süel, G.M. Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature 2015, 523, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Conradi, F.D.; Zhou, R.; Oeser, S.; Schuergers, N.; Wilde, A.; Mullineaux, C.W. Factors Controlling Floc Formation and Structure in the Cyanobacterium Synechocystis sp. Strain PCC 6803. J. Bacteriol. 2019, 201, e00344-19. [Google Scholar] [CrossRef]
- Kühl, M.; Fenchel, T. Bio-optical Characteristics and the Vertical Distribution of Photosynthetic Pigments and Photosynthesis in an Artificial Cyanobacterial Mat. Microb. Ecol. 2000, 40, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.; Rittmann, B.E.; Curtiss, R. Axenic biofilm formation and aggregation by Synechocystis sp. strain PCC 6803 are induced by changes in nutrient concentration and require cell surface structures. Appl. Environ. Microbiol. 2019, 85, e02192-18. [Google Scholar] [CrossRef] [PubMed]
- Walters III, M.C.; Roe, F.; Bugnicourt, A.; Franklin, M.J.; Stewart, P.S. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 2003, 47, 317–323. [Google Scholar] [CrossRef]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef]
- Grujić, S.; Vasić, S.; Čomić, L.; Ostojić, A.; Radojević, I. Heavy metal tolerance and removal potential in mixed-species biofilm. Water Sci. Technol. 2017, 76, 806–812. [Google Scholar] [CrossRef]
- Giner-Lamia, J.; Pereira, S.B.; Bovea-Marco, M.; Futschik, M.E.; Tamagnini, P.; Oliveira, P. Extracellular proteins: Novel key components of metal resistance in cyanobacteria? Front. Microbiol. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Jittawuttipoka, T.; Planchon, M.; Spalla, O.; Benzerara, K.; Guyot, F.; Cassier-Chauvat, C.; Chauvat, F. Multidisciplinary evidences that Synechocystis PCC6803 exopolysaccharides operate in cell sedimentation and protection against salt and metal stresses. PLoS ONE 2013, 8, e55564. [Google Scholar] [CrossRef]
- Testa, S.; Berger, S.; Piccardi, P.; Oechslin, F.; Resch, G.; Mitri, S. Spatial structure affects phage efficacy in infecting dual-strain biofilms of Pseudomonas aeruginosa. Commun. Biol. 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Seiler, C.; van Velzen, E.; Neu, T.R.; Gaedke, U.; Berendonk, T.U.; Weitere, M. Grazing resistance of bacterial biofilms: A matter of predators’ feeding trait. FEMS Microbiol. Ecol. 2017, 93, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Graupner, S.; Weger, N.; Sohni, M.; Wackernagel, W. Requirement of novel competence genes pilT and pilU of Pseudomonas stutzeri for natural transformation and suppression of pilT deficiency by a hexahistidine tag on the type IV pilus protein PilAI. J. Bacteriol. 2001, 183, 4694–4701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bradley, D.E.; Pitt, T.L. Pilus-dependence of four Pseudomonas aeruginosa bacteriophages with non-contractile tails. J. Gen. Virol. 1974, 24, 1–15. [Google Scholar] [CrossRef]
- Romantschuk, M.; Bamford, D.H. Function of Pili in bacteriophage ∅6 penetration. J. Gen. Virol. 1985, 66, 2461–2469. [Google Scholar] [CrossRef]
- Agostoni, M.; Waters, C.M.; Montgomery, B.L. Regulation of biofilm formation and cellular buoyancy through modulating intracellular cyclic di-GMP levels in engineered cyanobacteria. Biotechnol. Bioeng. 2016, 113, 311–319. [Google Scholar] [CrossRef]
- Enomoto, G.; Nomura, R.; Shimada, T.; Narikawa, R.; Ikeuchi, M. Cyanobacteriochrome SesA is a diguanylate cyclase that induces cell aggregation in Thermosynechococcus. J. Biol. Chem. 2014, 289, 24801–24809. [Google Scholar] [CrossRef]
- Enomoto, G.; Win, N.; Narikawa, R.; Ikeuchi, M. Three cyanobacteriochromes work together to form a light color-sensitive input system for c-di-GMP signaling of cell aggregation. Proc. Natl. Acad. Sci. USA 2015, 112, 8082–8087. [Google Scholar] [CrossRef]
- Balskus, E.P.; Case, R.J.; Walsh, C.T. The biosynthesis of cyanobacterial sunscreen scytonemin in intertidal microbial mat communities. FEMS Microbiol. Ecol. 2011, 77, 322–332. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Wu, Q. Protective effects of mycosporine-like amino acids of Synechocystis sp. PCC 6803 and their partial characterization. J. Photochem. Photobiol. B Biol. 2007, 86, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, G.; Ikeuchi, M. Blue-/Green-light-responsive cyanobacteriochromes are cell shade sensors in red-light replete niches. iScience 2020, 23, 100936. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pichel, F.; Mechling, M.; Castenholtz, R.W. Diel migrations of microorganisms within a benthic, hypersaline mat community. Appl. Environ. Microbiol. 1994, 60, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Bebout, B.M.; Garcia-Pichel, F. UV B-induced vertical migrations of cyanobacteria in a microbial mat. Appl. Environ. Microbiol. 1995, 61, 4215–4222. [Google Scholar] [CrossRef] [PubMed]
- Schatz, D.; Nagar, E.; Sendersky, E.; Parnasa, R.; Zilberman, S.; Carmeli, S.; Mastai, Y.; Shimoni, E.; Klein, E.; Yeger, O.; et al. Self-suppression of biofilm formation in the cyanobacterium Synechococcus elongatus. Environ. Microbiol. 2013, 15, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.; Aaes-Jørgensen, A.; Molin, S.; Tolker-Nielsen, T. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 2003, 50, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.; Heydorn, A.; Ragas, P.; Lambertsen, L.; Aaes-Jørgensen, A.; Molin, S.; Tolker-Nielsen, T. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 2003, 48, 1511–1524. [Google Scholar] [CrossRef] [PubMed]
- Persat, A.; Inclan, Y.F.; Engel, J.N.; Stone, H.A.; Gitai, Z. Type IV pili mechanochemically regulate virulence factors in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2015, 112, 7563–7568. [Google Scholar] [CrossRef]
- Ellison, C.K.; Kan, J.; Dillard, R.S.; Kysela, D.T.; Ducret, A.; Berne, C.; Hampton, C.M.; Ke, Z.; Wright, E.R.; Biais, N.; et al. Obstruction of pilus retraction stimulates bacterial surface sensing. Science (80-) 2017, 358, 535–538. [Google Scholar] [CrossRef]
- Giltner, C.L.; Habash, M.; Burrows, L.L. Pseudomonas aeruginosa minor pilins are incorporated into type IV Pili. J. Mol. Biol. 2010, 398, 444–461. [Google Scholar] [CrossRef]
- Del Medico, L.; Cerletti, D.; Schächle, P.; Christen, M.; Christen, B. The type IV pilin PilA couples surface attachment and cell-cycle initiation in Caulobacter crescentus. Proc. Natl. Acad. Sci. USA 2020, 117, 9546–9553. [Google Scholar] [CrossRef]
- Nagar, E.; Zilberman, S.; Sendersky, E.; Simkovsky, R.; Shimoni, E.; Gershtein, D.; Herzberg, M.; Golden, S.S.; Schwarz, R. Type 4 pili are dispensable for biofilm development in the cyanobacterium Synechococcus elongatus. Environ. Microbiol. 2017, 19, 2862–2872. [Google Scholar] [CrossRef]
- Parnasa, R.; Nagar, E.; Sendersky, E.; Reich, Z.; Simkovsky, R.; Golden, S.; Schwarz, R. Small secreted proteins enable biofilm development in the cyanobacterium Synechococcus elongatus. Sci. Rep. 2016, 6, 32209. [Google Scholar] [CrossRef]
- Parsek, M.R.; Greenberg, E.P. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol. 2005, 13, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sharif, D.I.; Gallon, J.; Smith, C.J.; Dudley, E. Quorum sensing in Cyanobacteria: N-octanoyl-homoserine lactone release and response, by the epilithic colonial cyanobacterium Gloeothece PCC6909. ISME J. 2008, 2, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Abby, S.S.; Cury, J.; Guglielmini, J.; Néron, B.; Touchon, M.; Rocha, E.P.C. Identification of protein secretion systems in bacterial genomes. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Denise, R.; Abby, S.S.; Rocha, E.P.C. Diversification of the type IV filament superfamily into machines for adhesion, protein secretion, DNA uptake, and motility. PLoS Biol. 2019, 17, e3000390. [Google Scholar] [CrossRef]
- Russo, D.A.; Zedler, J.A.Z.; Wittmann, D.N.; Möllers, B.; Singh, R.K.; Batth, T.S.; Van Oort, B.; Olsen, J.V.; Bjerrum, M.J.; Jensen, P.E. Expression and secretion of a lytic polysaccharide monooxygenase by a fast-growing cyanobacterium. Biotechnol. Biofuels 2019, 12, 74. [Google Scholar] [CrossRef]
- Russo, D.A.; Zedler, J.A.Z. Genomic insights into cyanobacterial protein translocation systems. Biol. Chem. 2020. [Google Scholar] [CrossRef]
- Zuniga, E.G.; Boateng, K.K.A.; Bui, N.U.; Kurnfuli, S.; Muthana, S.M.; Risser, D.D. Identification of a hormogonium polysaccharide-specific gene set conserved in filamentous cyanobacteria. Mol. Microbiol. 2020, 1–12. [Google Scholar] [CrossRef]
- Fisher, M.L.; Allen, R.; Luo, Y.; Curtiss, R. Export of extracellular polysaccharides modulates adherence of the Cyanobacterium Synechocystis. PLoS ONE 2013, 8, e74514. [Google Scholar] [CrossRef]
- Schuergers, N.; Ruppert, U.; Watanabe, S.; Nürnberg, D.J.; Lochnit, G.; Dienst, D.; Mullineaux, C.W.; Wilde, A. Binding of the RNA chaperone Hfq to the type IV pilus base is crucial for its function in Synechocystis sp. PCC 6803. Mol. Microbiol. 2014, 92, 840–852. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chin, K.H.; Tu, Z.L.; He, J.; Jones, C.J.; Sanchez, D.Z.; Yildiz, F.H.; Galperin, M.Y.; Chou, S.H. Nucleotide binding by the widespread high-affinity cyclic di-GMP receptor MshEN domain. Nat. Commun. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Terauchi, K.; Ohmori, M. Blue light stimulates cyanobacterial motility via a cAMP signal transduction system. Mol. Microbiol. 2004, 52, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, K.; Ohmori, M. An adenylate cyclase, Cya1, regulates cell motility in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 1999, 40, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Ono, T.A. Adenylyl cyclase activity of Cya1 from the cyanobacterium Synechocystis sp. strain PCC 6803 is inhibited by bicarbonate. J. Bacteriol. 2005, 187, 5032–5035. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhaya, D.; Nakasugi, K.; Fazeli, F.; Burriesci, M.S. Phototaxis and impaired motility in adenylyl cyclase and cyclase receptor protein mutants of Synechocystis sp. strain PCC 6803. J. Bacteriol. 2006, 188, 7306–7310. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Yoshihara, S.; Okamoto, S.; Ikeuchi, M.; Ohmori, M. A cAMP receptor protein, SYCRP1, is responsible for the cell motility of Synechocystis sp. PCC 6803. Plant Cell Physiol. 2002, 43, 460–463. [Google Scholar] [CrossRef]
- Song, W.-Y.; Zang, S.-S.; Li, Z.-K.; Dai, G.-Z.; Liu, K.; Chen, M.; Qiu, B.-S. Sycrp2 is essential for twitching motility in the cyanobacterium Synechocystis sp. Strain PCC 6803. J. Bacteriol. 2018, 200, 1–13. [Google Scholar] [CrossRef]
- Hu, J.; Zhan, J.; Chen, H.; He, C.; Cang, H.; Wang, Q. The small regulatory antisense RNA PilR affects pilus formation and cell motility by negatively regulating pilA11 in Synechocystis sp. PCC 6803. Front. Microbiol. 2018, 9, 786. [Google Scholar] [CrossRef]
- Higo, A.; Ikeuchi, M.; Ohmori, M. cAMP regulates respiration and oxidative stress during rehydration in Anabaena sp. PCC 7120. FEBS Lett. 2008, 582, 1883–1888. [Google Scholar] [CrossRef]
- Ohmori, K.; Hirose, M.; Ohmori, M. Function of cAMP as a mat-forming factor in the cyanobacterium Spirulina platensis. Plant Cell Physiol. 1992, 33, 21–25. [Google Scholar] [CrossRef]
- Ohmori, K.; Hirose, M.; Ohmori, M. An increase in the intracellular concentration of cAMP triggers formation of an algal mat by the cyanobacterium Spirulina platensis. Plant Cell Physiol. 1993, 34, 169–171. [Google Scholar] [CrossRef]
- Imamura, S.; Asayama, M. Sigma factors for cyanobacterial transcription. Gene Regul. Syst. Biol. 2009, 3, GRSB-S2090. [Google Scholar] [CrossRef] [PubMed]
- Asayama, M.; Imamura, S. Stringent promoter recognition and autoregulation by the group 3 σ-factor SigF in the cyanobacterium Synechocystis sp. strain PCC 6803. Nucleic Acids Res. 2008, 36, 5297–5305. [Google Scholar] [CrossRef] [PubMed]
- Bhaya, D.; Watanabe, N.; Ogawa, T.; Grossman, A.R. The role of an alternative sigma factor in motility and pilus formation in the cyanobacterium Synechocystis sp. strain PCC6803. Proc. Natl. Acad. Sci. USA 1999, 96, 3188–3193. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.; Santos, M.; Pereira, S.B.; Mota, R.; Rossi, F.; De Philippis, R.; Couto, N.; Karunakaran, E.; Wright, P.C.; Oliveira, P.; et al. The alternative sigma factor SigF is a key player in the control of secretion mechanisms in Synechocystis sp. PCC 6803. Environ. Microbiol. 2019, 21, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Miranda, H.; Immerzeel, P.; Gerber, L.; Hörnaeus, K.; Lind, S.B.; Pattanaik, B.; Lindberg, P.; Mamedov, F.; Lindblad, P. Sll1783, a monooxygenase associated with polysaccharide processing in the unicellular cyanobacterium Synechocystis PCC 6803. Physiol. Plant. 2017, 161, 182–195. [Google Scholar] [CrossRef]
- Gonzalez, A.; Riley, K.W.; Harwood, T.V.; Zuniga, E.G.; Risser, D.D. A tripartite, hierarchical sigma factor cascade promotes hormogonium development in the filamentous cyanobacterium Nostoc punctiforme. mSphere 2019, 4, e00231-19. [Google Scholar] [CrossRef] [PubMed]
- Ellison, C.K.; Dalia, T.N.; Vidal Ceballos, A.; Wang, J.C.Y.; Biais, N.; Brun, Y.V.; Dalia, A.B. Retraction of DNA-bound type IV competence pili initiates DNA uptake during natural transformation in Vibrio cholerae. Nat. Microbiol. 2018, 3, 773–780. [Google Scholar] [CrossRef]
- Van Schaik, E.J.; Giltner, C.L.; Audette, G.F.; Keizer, D.W.; Slupsky, C.M.; Irvin, R.T. DNA binding: A novel function of Pseudomonas aeruginosa type IV pili. J. Bacteriol. 2005, 187, 1455–1464. [Google Scholar] [CrossRef]
- Salleh, M.Z.; Karuppiah, V.; Snee, M.; Thistlethwaite, A.; Levy, C.W.; Knight, D.; Derrick, J.P. Structure and properties of a natural competence-associated pilin suggest a unique pilus Tip-associated DNA receptor. MBio 2019, 10, e00614-19. [Google Scholar] [CrossRef]
- Nakasugi, K.; Svenson, C.J.; Neilan, B.A. The competence gene, comF, from Synechocystis sp. strain PCC 6803 is involved in natural transformation, phototactic motility and piliation. Microbiology 2006, 152, 3623–3631. [Google Scholar] [CrossRef]
- Yu, J.; Liberton, M.; Cliften, P.F.; Head, R.D.; Jacobs, J.M.; Smith, R.D.; Koppenaal, D.W.; Brand, J.J.; Pakrasi, H.B. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Sci. Rep. 2015, 5, 8132. [Google Scholar] [CrossRef] [PubMed]
- Nakasugi, K.; Alexova, R.; Svenson, C.J.; Neilan, B.A. Functional Analysis of PilT from the toxic cyanobacterium Microcystis aeruginosa PCC 7806. J. Bacteriol. 2007, 189, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Nies, F.; Mielke, M.; Pochert, J.; Tilman, L. Natural transformation of the filamentous cyanobacterium Phormidium lacuna. PLoS ONE 2020, 15, e0234440. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Katoh, H.; Katayama, M.; Ikeuchi, M. Improved genetic transformation of the thermophilic cyanobacterium, Thermosynechococcus elongatus BP-1. Plant Cell Physiol. 2004, 45, 171–175. [Google Scholar] [CrossRef]
- Cheng, Y.I.; Chou, L.; Chiu, Y.F.; Hsueh, H.T.; Kuo, C.H.; Chu, H.A. Comparative genomic analysis of a novel strain of Taiwan hot-spring cyanobacterium Thermosynechococcus sp. CL-1. Front. Microbiol. 2020, 11, 82. [Google Scholar] [CrossRef]
- Kawano, Y.; Saotome, T.; Ochiai, Y.; Katayama, M.; Narikawa, R.; Ikeuchi, M. Cellulose accumulation and a cellulose synthase gene are responsible for cell aggregation in the cyanobacterium Thermosynechococcus vulcanus RKN. Plant Cell Physiol. 2011, 52, 957–966. [Google Scholar] [CrossRef]
- Trautmann, D.; Voß, B.; Wilde, A.; Al-Babili, S.; Hess, W.R. Microevolution in cyanobacteria: Re-sequencing a motile substrain of Synechocystis sp. PCC 6803. DNA Res. 2012, 19, 435–448. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conradi, F.D.; Mullineaux, C.W.; Wilde, A. The Role of the Cyanobacterial Type IV Pilus Machinery in Finding and Maintaining a Favourable Environment. Life 2020, 10, 252. https://doi.org/10.3390/life10110252
Conradi FD, Mullineaux CW, Wilde A. The Role of the Cyanobacterial Type IV Pilus Machinery in Finding and Maintaining a Favourable Environment. Life. 2020; 10(11):252. https://doi.org/10.3390/life10110252
Chicago/Turabian StyleConradi, Fabian D., Conrad W. Mullineaux, and Annegret Wilde. 2020. "The Role of the Cyanobacterial Type IV Pilus Machinery in Finding and Maintaining a Favourable Environment" Life 10, no. 11: 252. https://doi.org/10.3390/life10110252
APA StyleConradi, F. D., Mullineaux, C. W., & Wilde, A. (2020). The Role of the Cyanobacterial Type IV Pilus Machinery in Finding and Maintaining a Favourable Environment. Life, 10(11), 252. https://doi.org/10.3390/life10110252



