The Development of a Gracilis and Quadriceps Tendons Calibration Device for Uniaxial Tensile Tests
Abstract
:1. Introduction
- Structures with more or less parallel fibres in the form of strips or ribbons—tendons and ligaments;
- Structures in the form of membranes, wherein the fibres create a two-dimensional network of different interrelationships of these fibres;
- Three-dimensional connective structures in which collagen fibres create a spatial network.
2. Materials and Methods
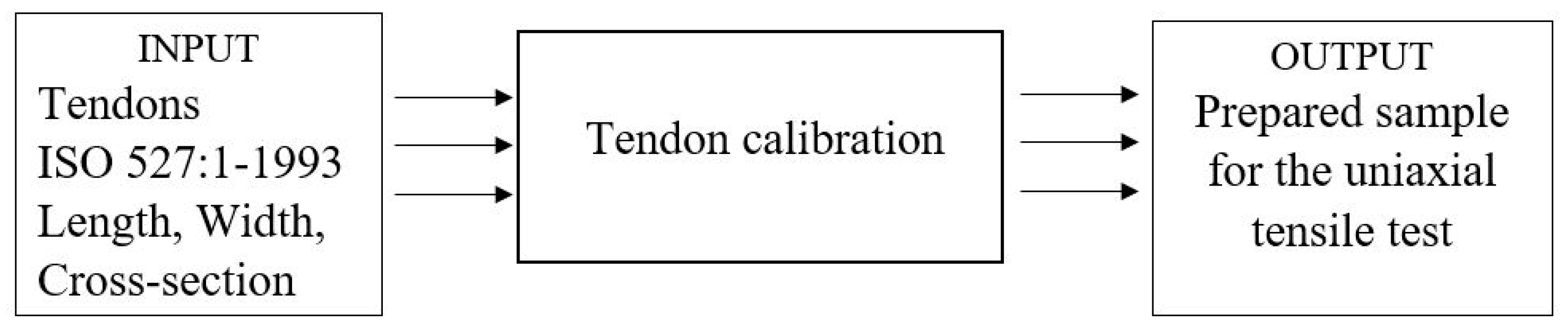
2.1. The Functional Structure
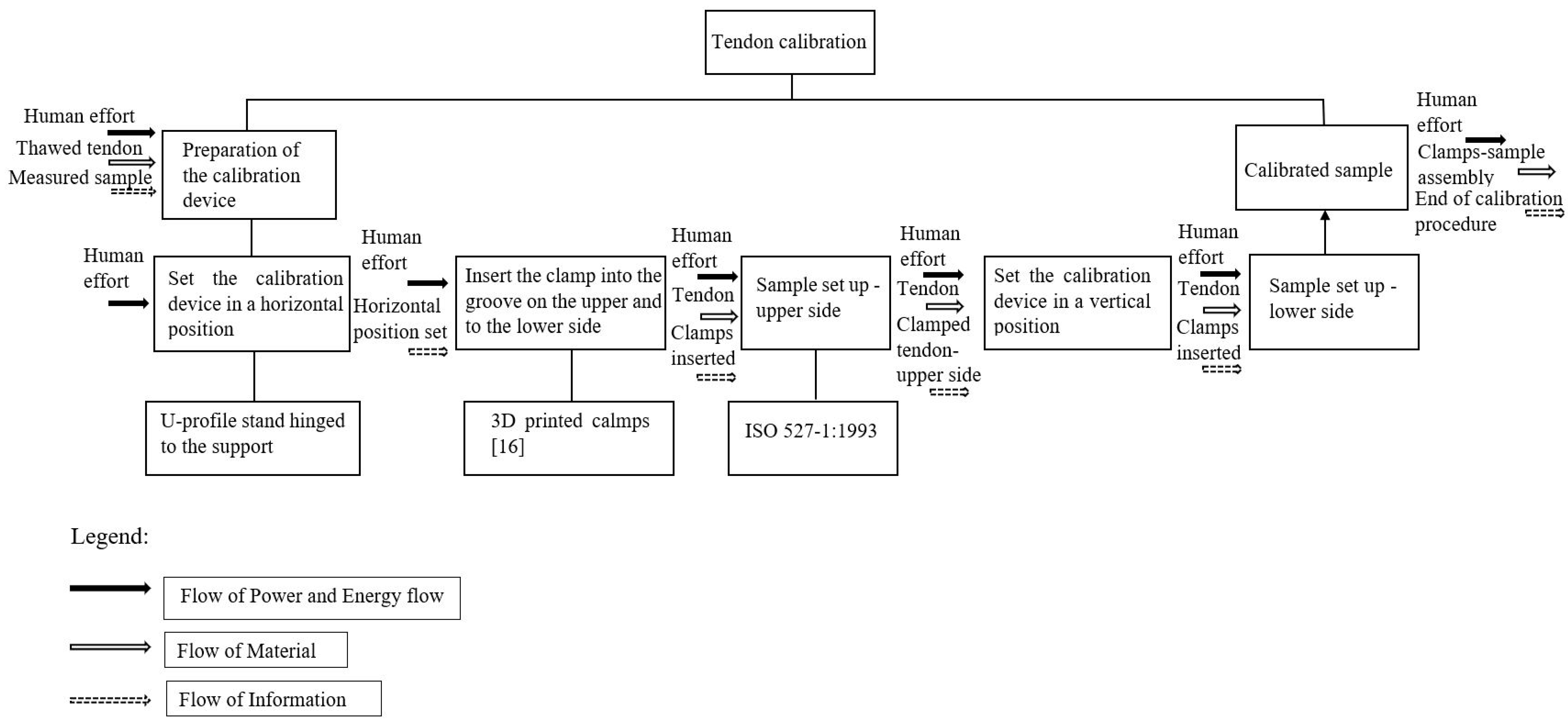
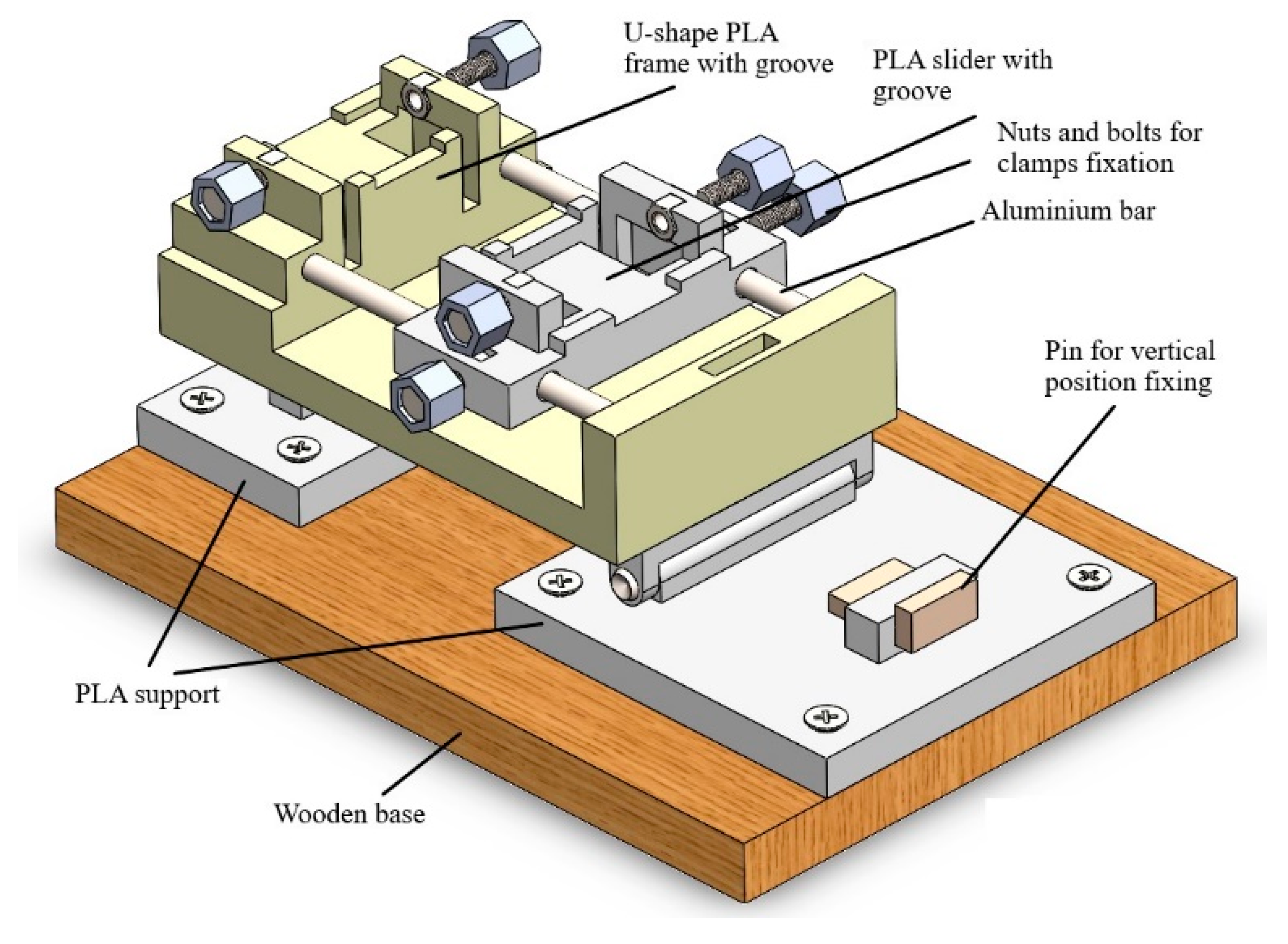
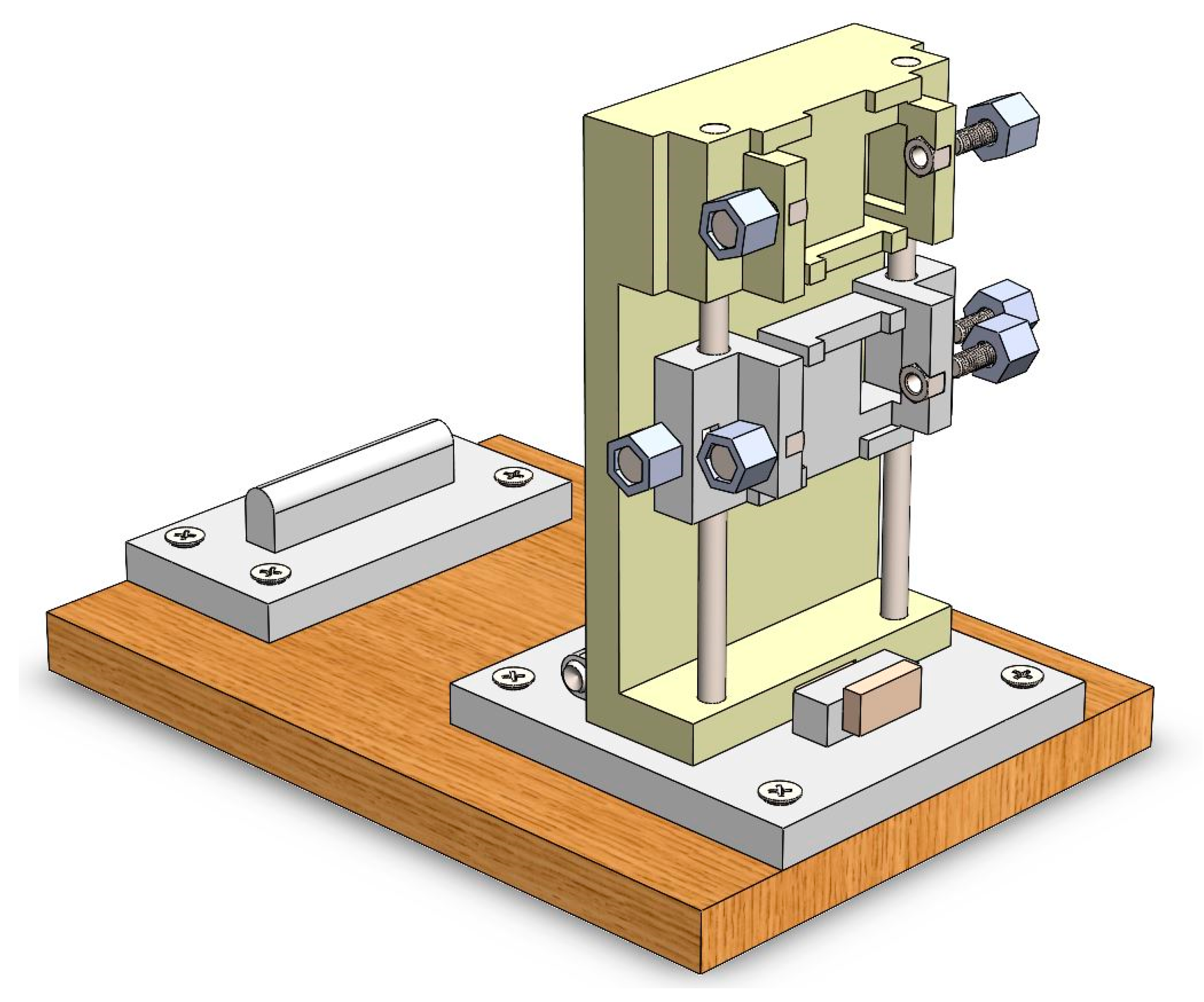
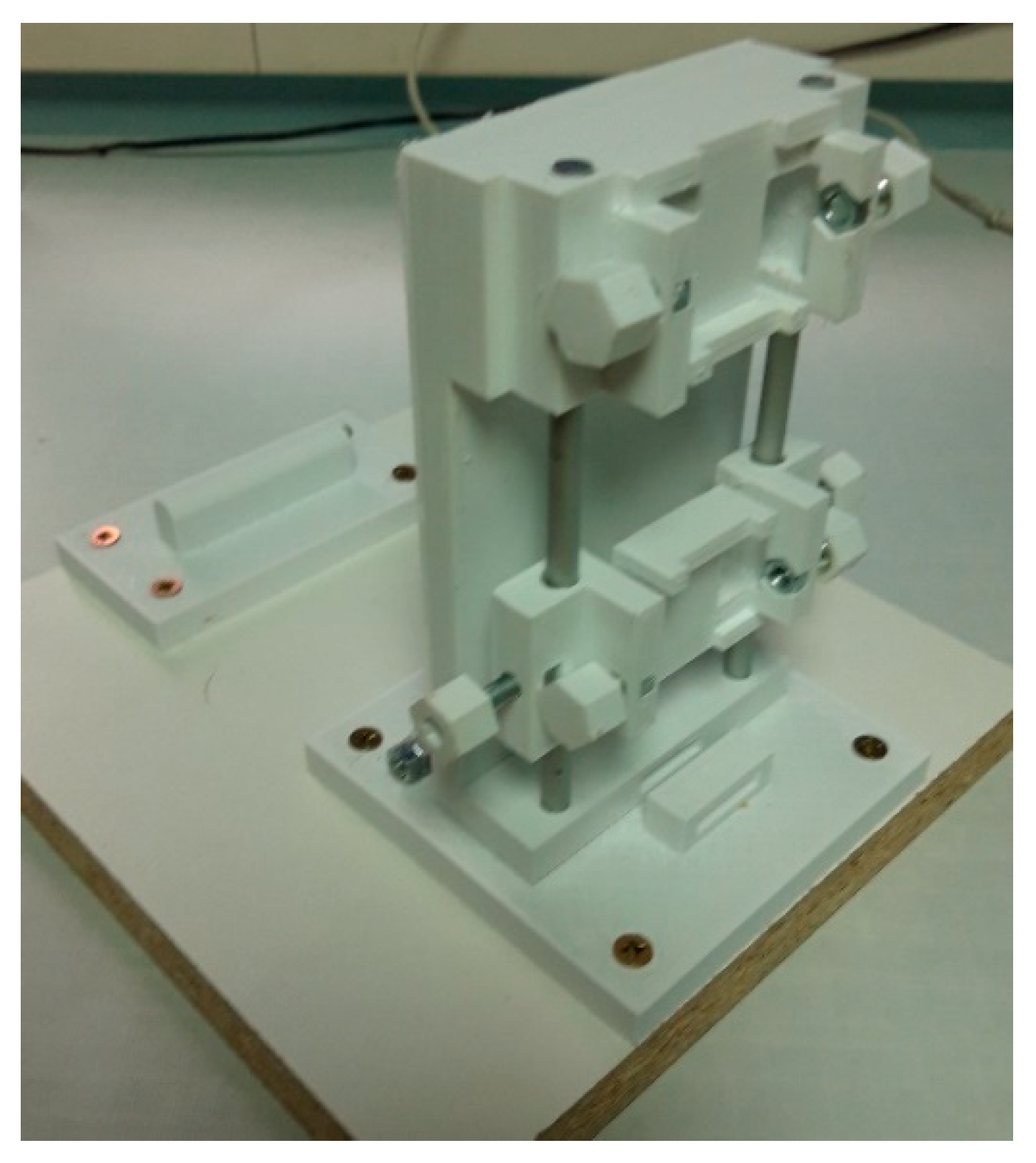
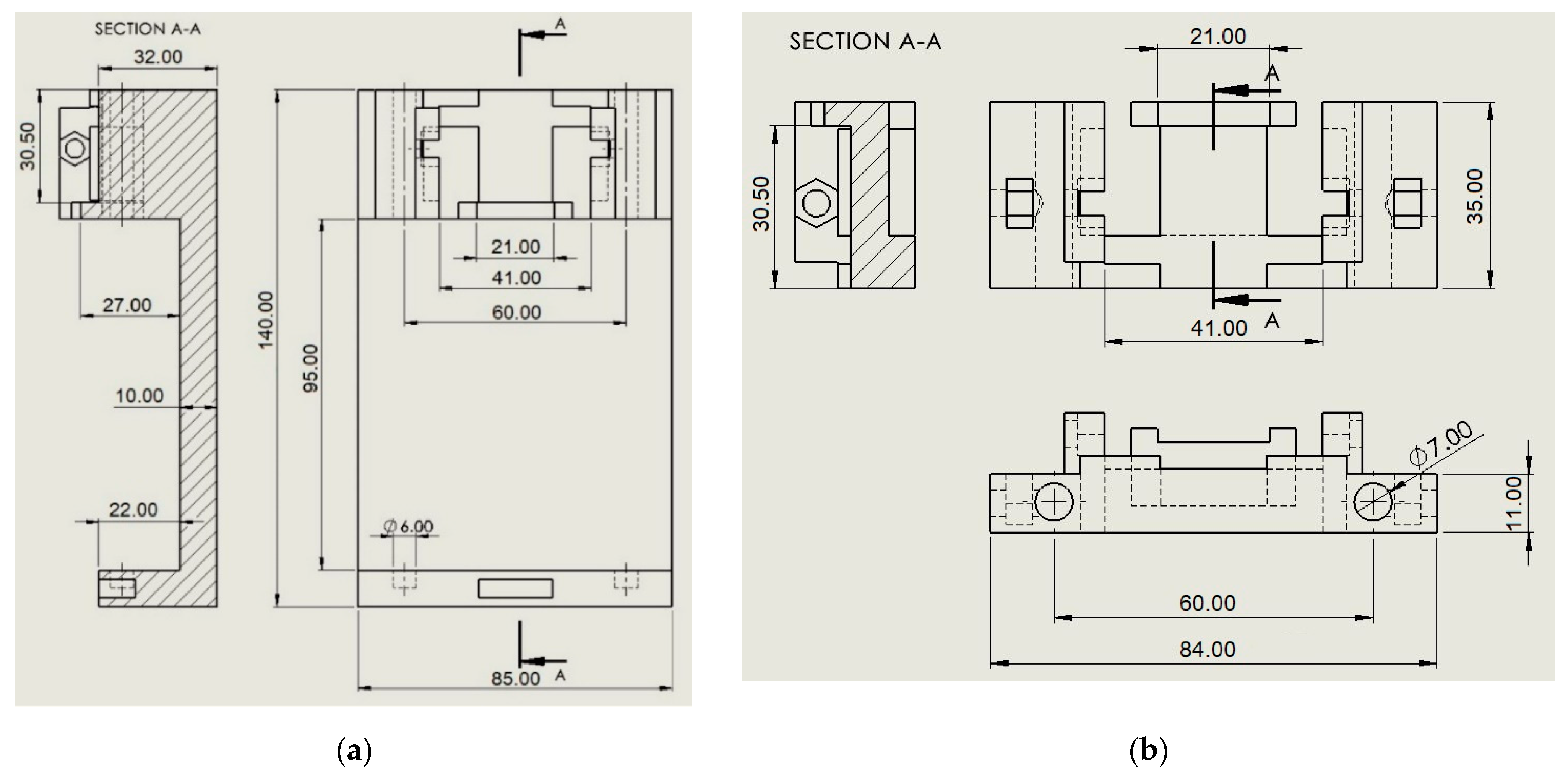
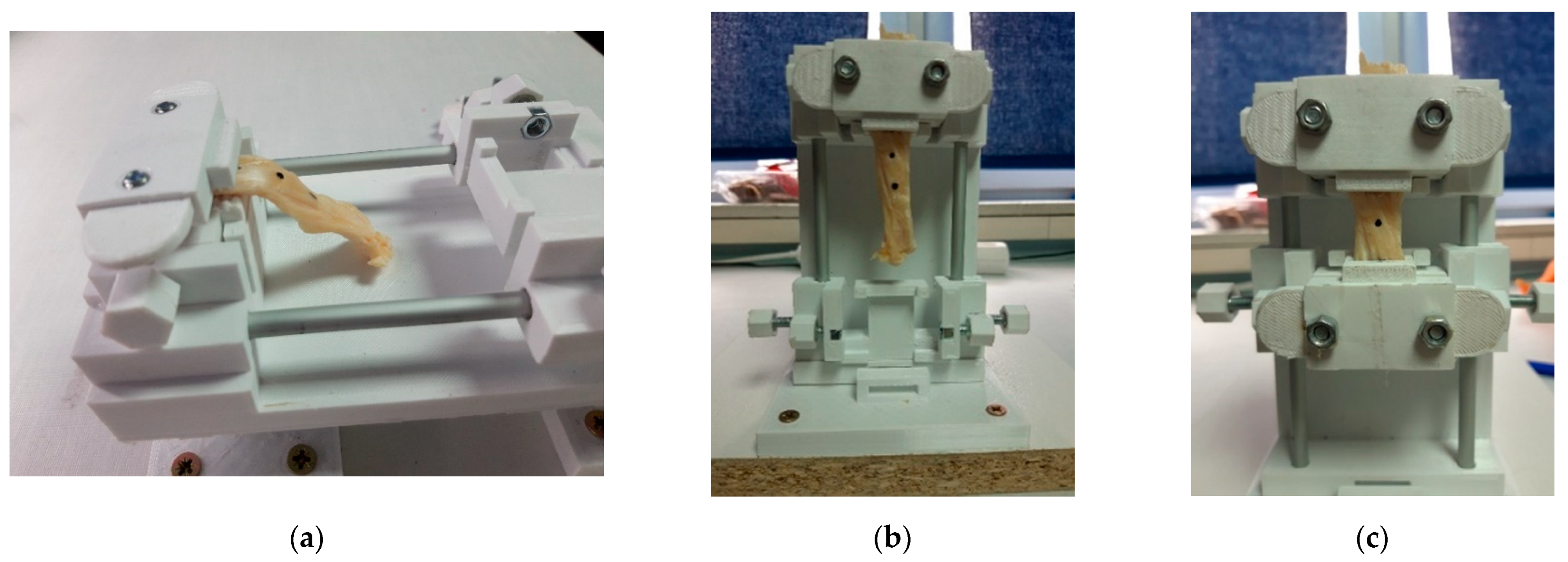
2.2. Design of the Calibrator
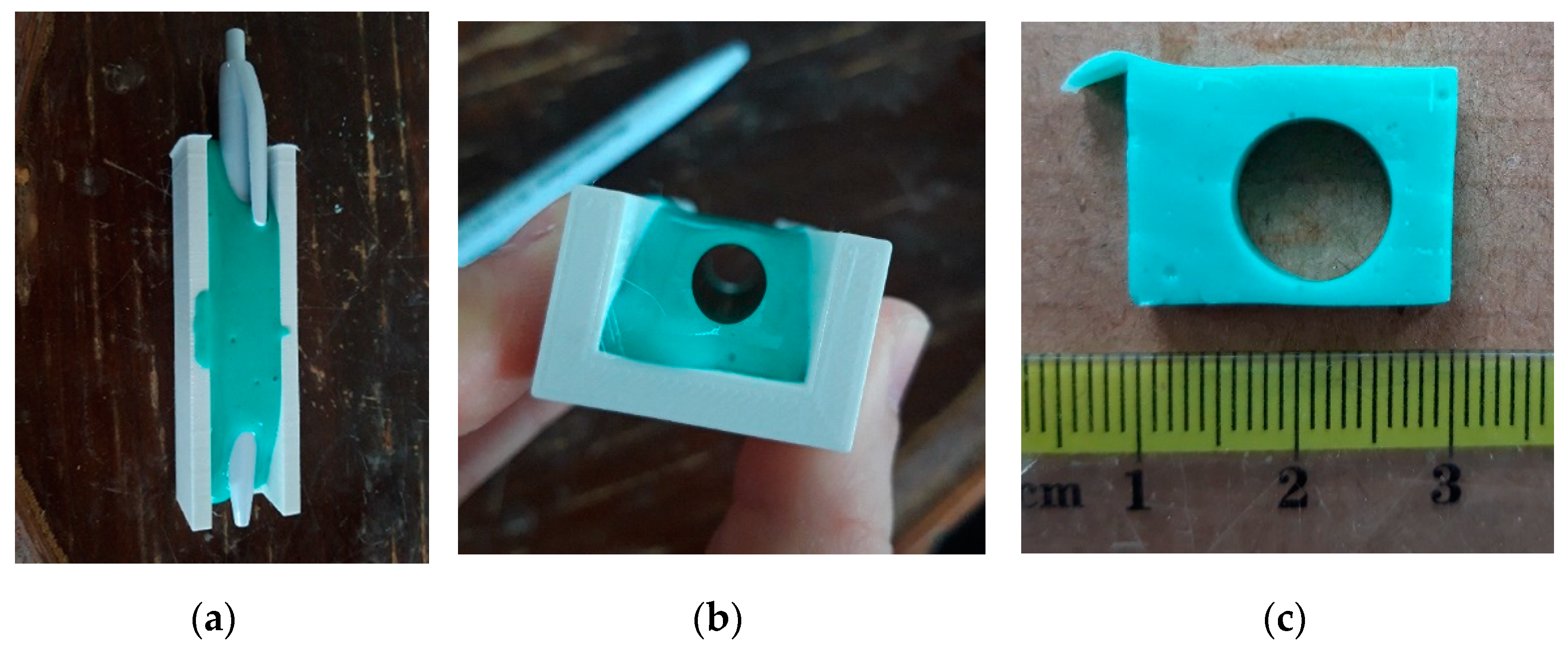
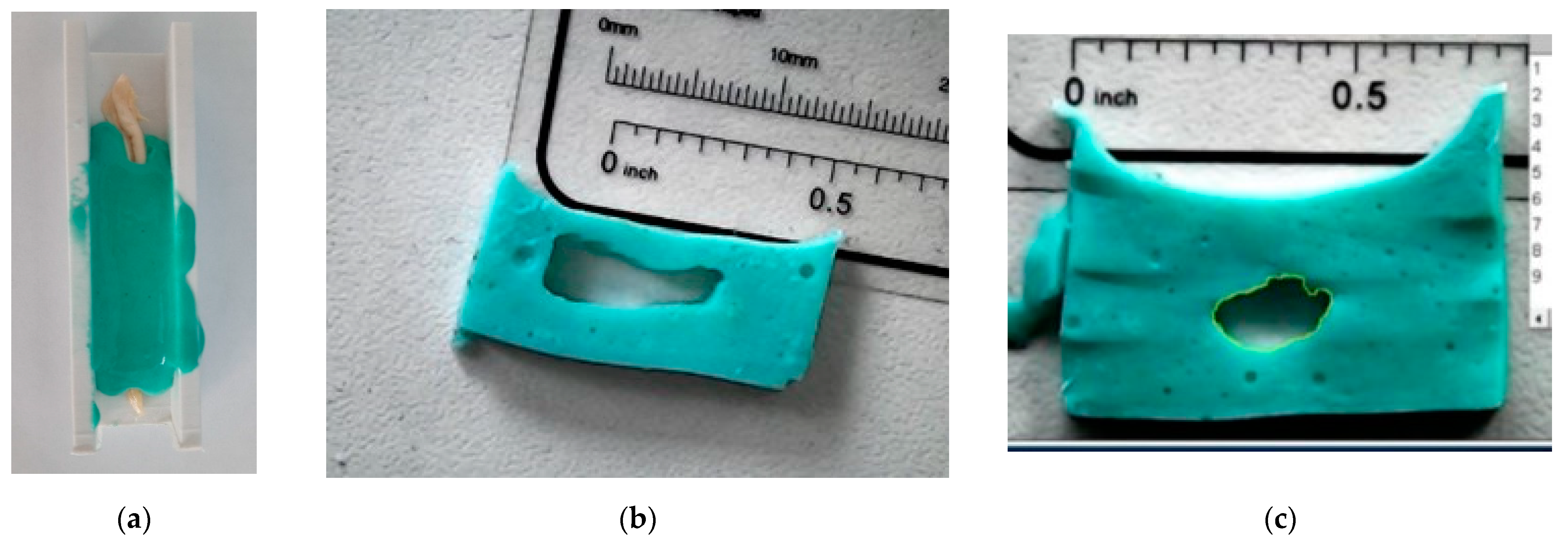
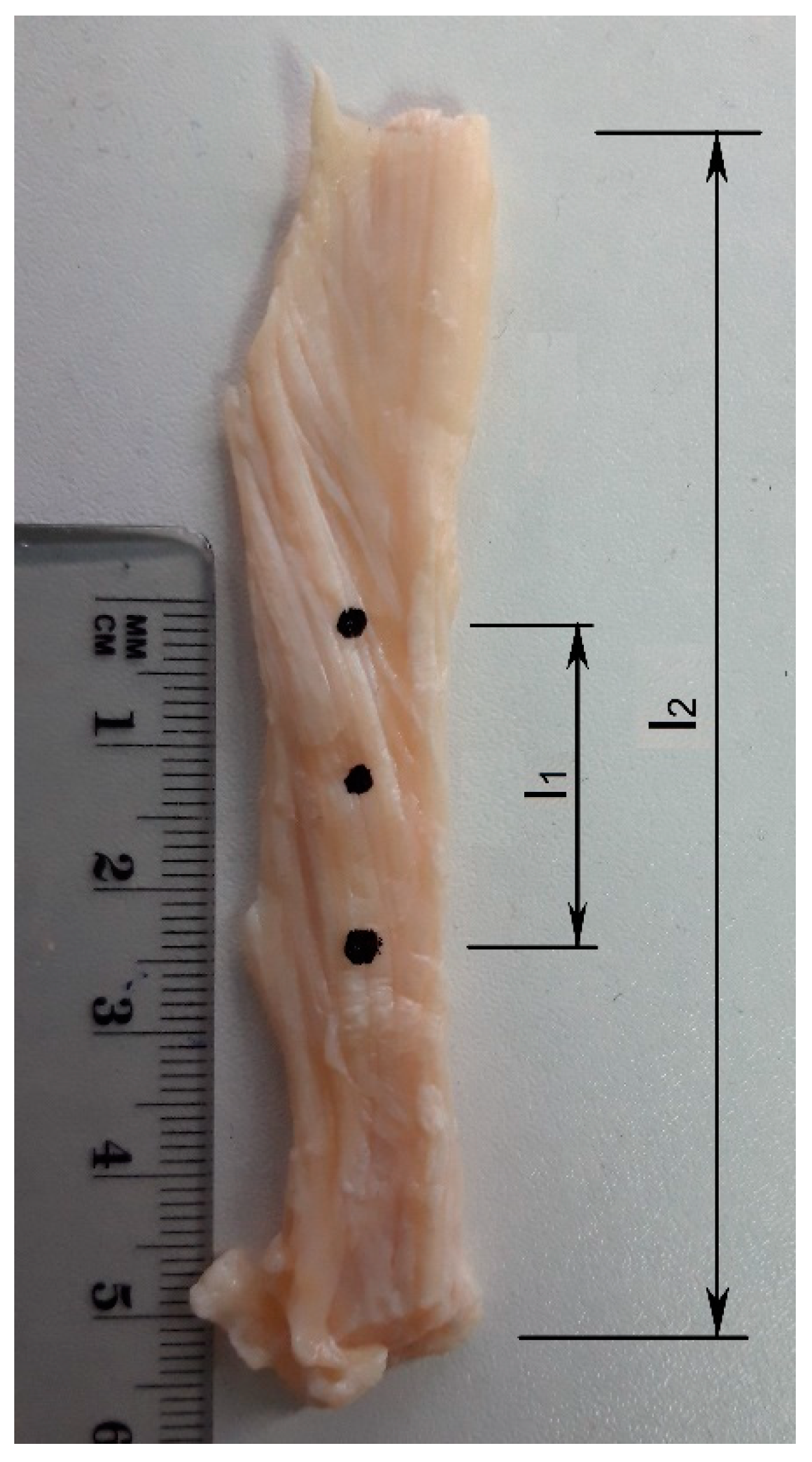
2.3. Specimen Preparation
2.4. Utilisation of ISO 527-1:1993
2.5. Functionality Test
3. Results and Discussion
4. Conclusions
- Functional device for tendons calibration and preparation for the tensile tests;
- Utilisation of ISO 527-1:1993 standard to test tendons in their natural shape;
- Preparation in horizontal and vertical positions.
- Future research will seek to improve the calibrator in terms of the following features:
- Immersing the tendon in liquid during calibration to avoid dehydration of the tissue and spraying procedures;
- Heating the liquid to a temperature of 33 °C for the tensile test procedure to warm the tendon tissue as much as possible in advance;
- Improving the design to be less robust.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Özkaya, N.; Nordin, M.; Goldsheyder, D.; Leger, D. Fundamentals of Biomechanics: Equilibrium, Motion and Deformation; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. Anisotropic Materials. In Analytical Methods in Anisotropic Elasticity; Birkhäuser: Boston, MA, USA, 2005. [Google Scholar] [CrossRef]
- Chanda, A.; Callaway, C. Tissue Anisotropy Modeling Using Soft Composite Materials. Appl. Bionics Biomech. 2018, 2018, 4838157. [Google Scholar] [CrossRef] [Green Version]
- Thorpe, C.T.; Screen, H.R.C. Tendon Structure and Composition. In Metabolic Influences on Risk for Tendon Disorders; Ackermann, P., Hart, D., Eds.; Springer: Cham, Switzerland, 2016; Volume 920. [Google Scholar] [CrossRef]
- Nikolić, V.; Hudec, M.B. Principi Biomehnaike; Ljevak: Zagreb, Croatia, 2011; Available online: https://www.bib.irb.hr/611801 (accessed on 20 November 2021).
- Wan Abas, W.A. Biaxial tension test of human skin in vivo. Biomed. Mater. Eng. 1994, 4, 473–486. [Google Scholar]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef]
- Smeets, K.; Bellemans, J.; Scheys, L.; Eijnde, B.O.; Slane, J.; Claes, S. Mechanical Analysis of Extra-Articular Knee Ligaments. Part two: Tendon grafts used for knee ligament reconstruction. Knee 2017, 24, 957–964. [Google Scholar] [CrossRef]
- Criscenti, G.; De Maria, C.; Sebastiani, E.; Tei, M.; Placella, G.; Speziali, A.; Vozzi, G.; Cerulli, G. Material and structural tensile properties of the human medial patello-femoral ligament. J. Mech. Behav. Biomed. Mater. 2016, 54, 141–148. [Google Scholar] [CrossRef]
- Criscenti, G.; De Maria, C.; Sebastiani, E.; Tei, M.; Placella, G.; Speziali, A.; Vozzi, G.; Cerulli, G. Quasi-linear viscoelastic properties of the human medial patello-femoral ligament. J. Biomech. 2015, 48, 4297–4302. [Google Scholar] [CrossRef]
- Woo, L.-Y.; Orlando, C.A.; Gomez, M.A.; Frank, C.B.; Akeson, W.H. Tensile properties of the medial collateral ligament as a function of age. J. Orthop. Res. 1986, 4, 133–141. [Google Scholar] [CrossRef]
- Abramowitch, S.D.; Zhang, X.; Curran, M.; Kilger, R. A comparison of the quasi-static mechanical and non-linear viscoelastic properties of the human semitendinosus and gracilis tendons. Clin. Biomech. 2010, 25, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Mlyniec, A.; Dabrowska, S.; Heljak, M.; Weglarz, E.P.; Wojcik, K.; Ekiert-Radecka, M.; Obuchowicz, R.; Swieszkowski, M. The dispersion of viscoelastic properties of fascicle bundles within the tendon results from the presence of interfascicular matrix and flow of body fluids. Mater. Sci. Eng. C 2021, 130, 112435. [Google Scholar] [CrossRef]
- Duenwald, S.E.; Vanderby, R.; Lakes, R.S. Viscoelastic Relaxation and Recovery of Tendon. Ann. Biomed. Eng. 2009, 37, 1131–1140. [Google Scholar] [CrossRef]
- Hayes, A.; Easton, K.; Devanaboyina, P.T.; Wu, J.-P.; Kirk, T.B.; Lloyd, D. A review of methods to measure tendon dimensions. J. Orthop. Surg. Res. 2019, 14, 18. [Google Scholar] [CrossRef]
- Woo, S.L.-Y.; Akeson, W.H.; Jemmott, G.F. Measurements of nonhomogeneous, directional mechanical properties of articular cartilage in tension. J. Biomech. 1976, 9, 785–791. [Google Scholar] [CrossRef]
- Lionello, G.; Sirieix, C.; Baleani, M. An effective procedure to create a speckle pattern on biological soft tissue for digital image correlation measurements. J. Mech. Behav. Biomed. Mater. 2014, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.K.; Vanderby, R., Jr.; Ulm, M.J.; Rogalski, R.P.; Thielke, R.J. Effect of preconditioning on the viscoelastic response of primate patellar tendon. Arthrosc.: J. Arthrosc. Relat. Surgery. 1994, 10, 90–96. [Google Scholar] [CrossRef]
- Duenwald, S.E.; Vanderby, R., Jr.; Lakes, R.S. Stress relaxation and recovery in tendon and ligament: Experiment and modeling. Biorheology 2010, 47, 1–14. [Google Scholar] [CrossRef]
- Scholze, M.; Singh, A.; Lozano, P.F.; Ondruschka, B.; Ramezani, M.; Werner, M.; Hammer, N. Utilization of 3D printing technology to facilitate and standardize soft tissue testing. Sci. Rep. 2018, 8, 11340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholze, M.; Safavi, S.; Li, K.C.; Ondruschka, B.; Werner, M.; Zwirner, J.; Hammer, N. Standardized tensile testing of soft tissue using a 3D printed clamping system. HardwareX 2020, 8, e00159. [Google Scholar] [CrossRef]
- Grgić, I.; Wertheimer, V.; Karakašić, M.; Ivandić, Ž. 3D Printed Clamps for In Vitro Tensile Tests of Human Gracilis and the Superficial Third of Quadriceps Tendons. Appl. Sci. 2021, 11, 2563. [Google Scholar] [CrossRef]
- Grgić, I.; Wertheimer, V.; Karakašić, M.; Ivandić, Ž. Development of a 3D Printed Double-Acting Linear Pneumatic Actuator for the Tendon Gripping. Polymers 2021, 13, 2528. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, V.; Grgić, I.; Zelić, Z.; Ivandić, Ž.; Koprivčić, I.; Zelenić, M.; Karakašić, M. Biomechanical Analysis of the Gracilis and Superficial Third of the Quadriceps Tendons Concerning MPFL Biomechanics. Tech. Gaz. 2021, 28, 1575–1581. [Google Scholar] [CrossRef]
- Husain, K.N.; Stojković, M.; Vitković, N.; Milovanović, J.; Trajanović, M.; Rashid, M.; Milovanović, A. Procedure for Creating Personalized Geometrical Models of the Human Mandible and Corresponding Implants. Tech. Gaz. 2019, 26, 1044–1051. [Google Scholar] [CrossRef]
- Alghrairi, M.K.; Sulaiman, N.B.; Sidek, R.B.M.; Mutashar, S. Simple and Efficient Transcutaneous Inductive Micro-System Device Based on ASK Modulation at 6.78 MHz ISM Band. Tech. Gaz. 2020, 27, 1478–1485. [Google Scholar] [CrossRef]
- Zuccon, G.; Bottin, M.; Ceccarelli, M.; Rosati, G. Design and Performance of an Elbow Assisting Mechanism. Machines 2020, 8, 68. [Google Scholar] [CrossRef]
- Russo, M.; Ceccarelli, M. Analysis of a Wearable Robotic System for Ankle Rehabilitation. Machines 2020, 8, 48. [Google Scholar] [CrossRef]
- Goodship, A.E.; Birch, H.L. Cross sectional area measurement of tendon and ligament in vitro: A simple, rapid, non-destructive technique. J. Biomech. 2005, 38, 605–608. [Google Scholar] [CrossRef]
- Race, A.; Amis, A.A. Cross-sectional area measurement of soft tissue. A new casting method. J. Biomech. 1996, 29, 1207–1212. [Google Scholar] [CrossRef]
- Smith, R.K.; Jones, R.; Webbon, P.M. The cross-sectional areas of normal equine digital flexor tendons determined ultrasonographically. Equine Vet. J. 1994, 26, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.-J.; Zhang, L.; Xiang, G.; Hu, Y.-C.; Lun, D.-X. Cross-Sectional Area Measurement Techniques of Soft Tissue: A Literature Review. Orthop. Surg. 2020, 12, 1547–1566. [Google Scholar] [CrossRef] [PubMed]
- Hongpaisan, J.; Roomans, G.M. Retaining ionic concentrations during in vitro storage of tissue for microanalytical studies. J. Microsc. 1999, 193, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Noyes, F.R.; Butler, D.L.; Grood, E.S.; Zernicke, R.F.; Hefzy, M.S. Biomechanical analysis of human ligament grafts used in knee ligament repairs and reconstructions. J. Bone Jt. Surg. 1984, 66, 344–352. [Google Scholar] [CrossRef]
- Handl, M.; Drzik, M.; Cerulli, G.; Povysil, C.; Chlpik, J.; Varga, F. Reconstruction of the anterior cruciate ligament: Dynamic strain evaluation of the graft. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.L.; Grood, E.S.; Noyes, F.R.; Zernicke, R.F.; Brackett, K. Effects of structure and strain measurement technique on the material properties of young human tendons and fascia. J. Biomech. 1984, 17, 579–596. [Google Scholar] [CrossRef]
- Mabe, I.; Hunter, S. Quadriceps tendon allografts as an alternative to Achilles tendon allografts: A biomechanical comparison. Cell Tissue Bank 2014, 15, 523–529. [Google Scholar] [CrossRef]
- Staubli, H.U.; Schatzmann, L.; Brunner, P.; Rincon, L.; Nolte, L.-P. Mechanical Tensile Properties of the Quadriceps Tendon and Patellar Ligament in Young Adults. Am. J. Sports Med. 1999, 27, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Shani, R.; Umpierez, E.; Nasert, M.; Hiza, E.; Xerogeanes, J. Biomechanical Comparison of Quadriceps and Patellar Tendon Grafts in Anterior Cruciate Ligament Reconstruction. J. Arthrosc. Relat. Surg. 2015, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Value |
|---|---|
| Layer height | 0.01 mm |
| Shell thickness | 0.4 mm |
| Overlap percentage | 40% |
| Infill density | 100% |
| Printing temperature | 200 °C |
| Infill speed | 20 mm/s |
| Wall speed | 10 mm/s |
| Build plate temperature | 70 °C |
| Mean (SD) | ||
|---|---|---|
| Gracilis | Quadriceps | |
| CSA, mm2 | 10.65 (1.4) | 19.36 (3.2) |
| Length, mm | 90.12 (7.6) | 85.97 (4.1) |
| Width, mm | 5 (0.5) | 9.91 (0.8) |
| Thickness, mm | 2.73 (0.4) | 2.46 (0.3) |
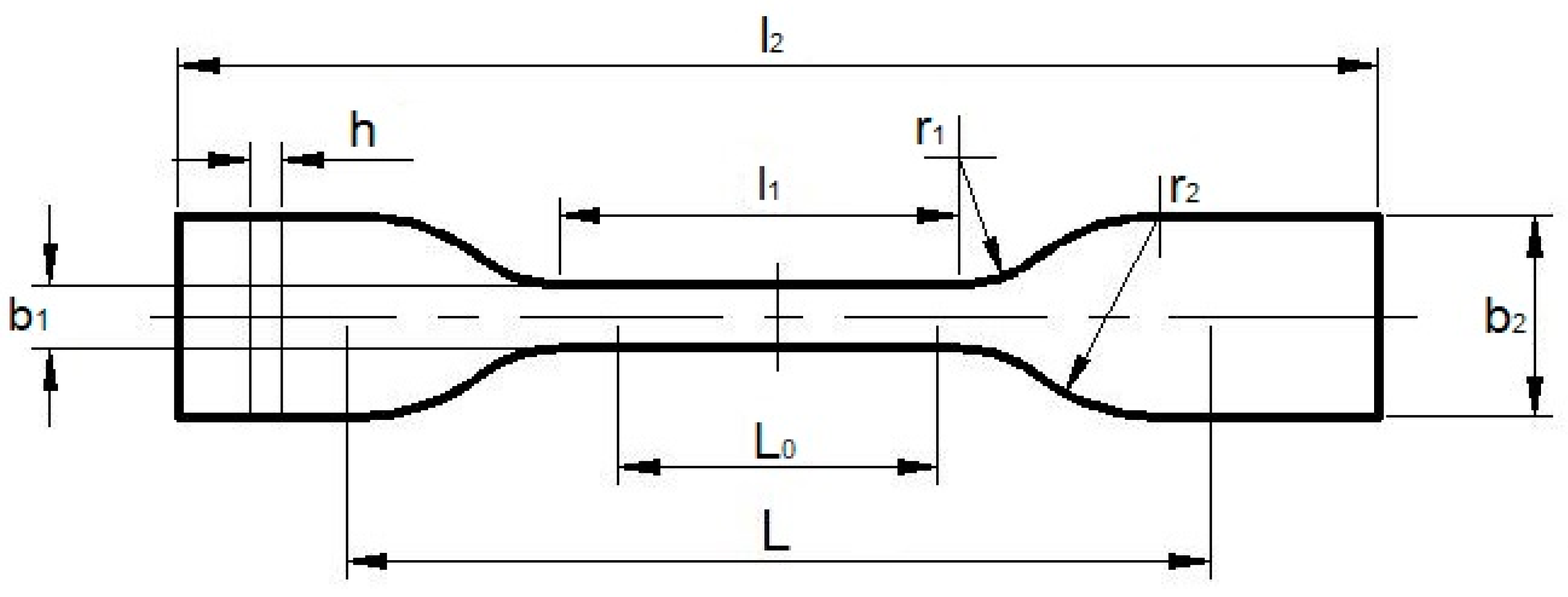
| Sample Type | Dimensions, mm | |
|---|---|---|
| 5A | 5B | |
| l2 | ≥75 | ≥35 |
| b2 | 12.5 ± 1 | 6 ± 0.5 |
| l1 | 25 ± 1 | 12 ± 0.5 |
| b1 | 4 ± 0.1 | 2 ± 0.1 |
| r1 | 8 ± 0.5 | 3 ± 0.1 |
| r2 | 12.5 ± 1 | 3 ± 0.1 |
| L | 50 ± 2 | 20 ± 2 |
| L0 | 20 ± 0.5 | 10 ± 0.2 |
| h | ≥2 | ≥1 |
| Mean (SD) | 95% CI | |||||
|---|---|---|---|---|---|---|
| Gracilis | Quadriceps | Divergence | From | to | p-Value (Student t-Test) | |
| Maximum force, N | 563.9 (119.6) | 788.3 (155.3) | −224.3 | −373 | −75.7 | 0.006 |
| Extension, mm | 2.45 (0.25) | 2.9 (0.4) | −0.43 | −0.77 | −0.11 | 0.01 |
| Tensile strength, MPa | 55.9 (20.5) | 36 (4.6) | 19.9 | 2.68 | 37.2 | 0.03 |
| Elongation, % | 12.2 (1.2) | 14.4 (1.8) | −2.2 | −3.83 | −0.54 | 0.01 |
| Stiffness, N/mm | 66.6 (23.0) | 78.4 (17.5) | −11.9 | −33.8 | 10.1 | 0.27 |
| Elastic modulus, MPa | 559.9 (226.6) | 303.2 (35.1) | 252.8 | 62.8 | 442.8 | 0.008 |
| Mean (SD) | ||||
|---|---|---|---|---|
| n | Elastic Modulus, MPa | Tensile Strength, MPa | Elongation, % | |
| GRACILIS | ||||
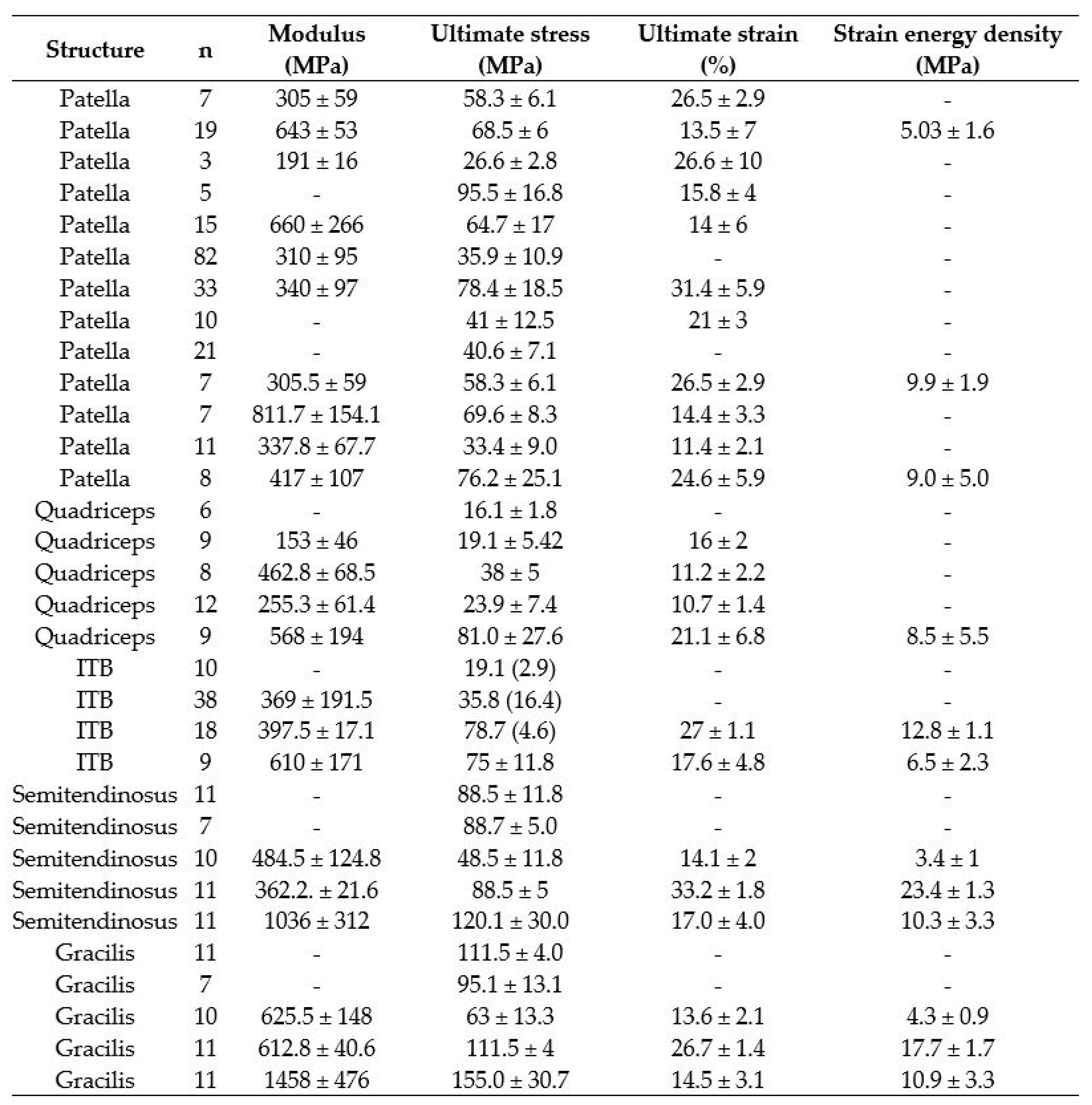
| Noyes et al. [34] | 11 | - | 115.5 (4) | - |
| Handl et al. [35] | 7 | - | 95.1 (13.1) | - |
| Abramowitch et al. [12] | 10 | 625.5 (148) | 63 (13.3) | 13.6 (2.1) |
| Butler et al. [36] | 11 | 612.8 (40.6) | 111.5 (4) | 26.7 (1.4) |
| Smeets et al. [8] | 11 | 1458 (476) | 155 (30.7) | 14.5 (3.1) |
| This study | 8 | 555.9 (226.6) | 55.9 (20.5) | 12.1 (1.2) |
| QUADRICEPS | ||||
| Noyes et al. [34] | 6 | - | 16.1 (1.8) | - |
| Mabe [37] | 9 | 153 (46) | 19.1 (5.42) | 16 (2) |
| Staubli et al. [38] | 8 | 462.8 (68.5) | 38 (5) | 11.2 (2.2) |
| Shani et al. [39] | 12 | 255.3 (61.4) | 23.9 (7.4) | 10.7 (1.4) |
| Smeets et al. [8] | 9 | 568 (194) | 81 (27.6) | 21.1 (6.8) |
| This study | 8 | 303.2 (35.1) | 36 (4.6) | 14.4 (1.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grgić, I.; Karakašić, M.; Ivandić, Ž.; Jurčević Lulić, T. The Development of a Gracilis and Quadriceps Tendons Calibration Device for Uniaxial Tensile Tests. Machines 2021, 9, 364. https://doi.org/10.3390/machines9120364
Grgić I, Karakašić M, Ivandić Ž, Jurčević Lulić T. The Development of a Gracilis and Quadriceps Tendons Calibration Device for Uniaxial Tensile Tests. Machines. 2021; 9(12):364. https://doi.org/10.3390/machines9120364
Chicago/Turabian StyleGrgić, Ivan, Mirko Karakašić, Željko Ivandić, and Tanja Jurčević Lulić. 2021. "The Development of a Gracilis and Quadriceps Tendons Calibration Device for Uniaxial Tensile Tests" Machines 9, no. 12: 364. https://doi.org/10.3390/machines9120364
APA StyleGrgić, I., Karakašić, M., Ivandić, Ž., & Jurčević Lulić, T. (2021). The Development of a Gracilis and Quadriceps Tendons Calibration Device for Uniaxial Tensile Tests. Machines, 9(12), 364. https://doi.org/10.3390/machines9120364







