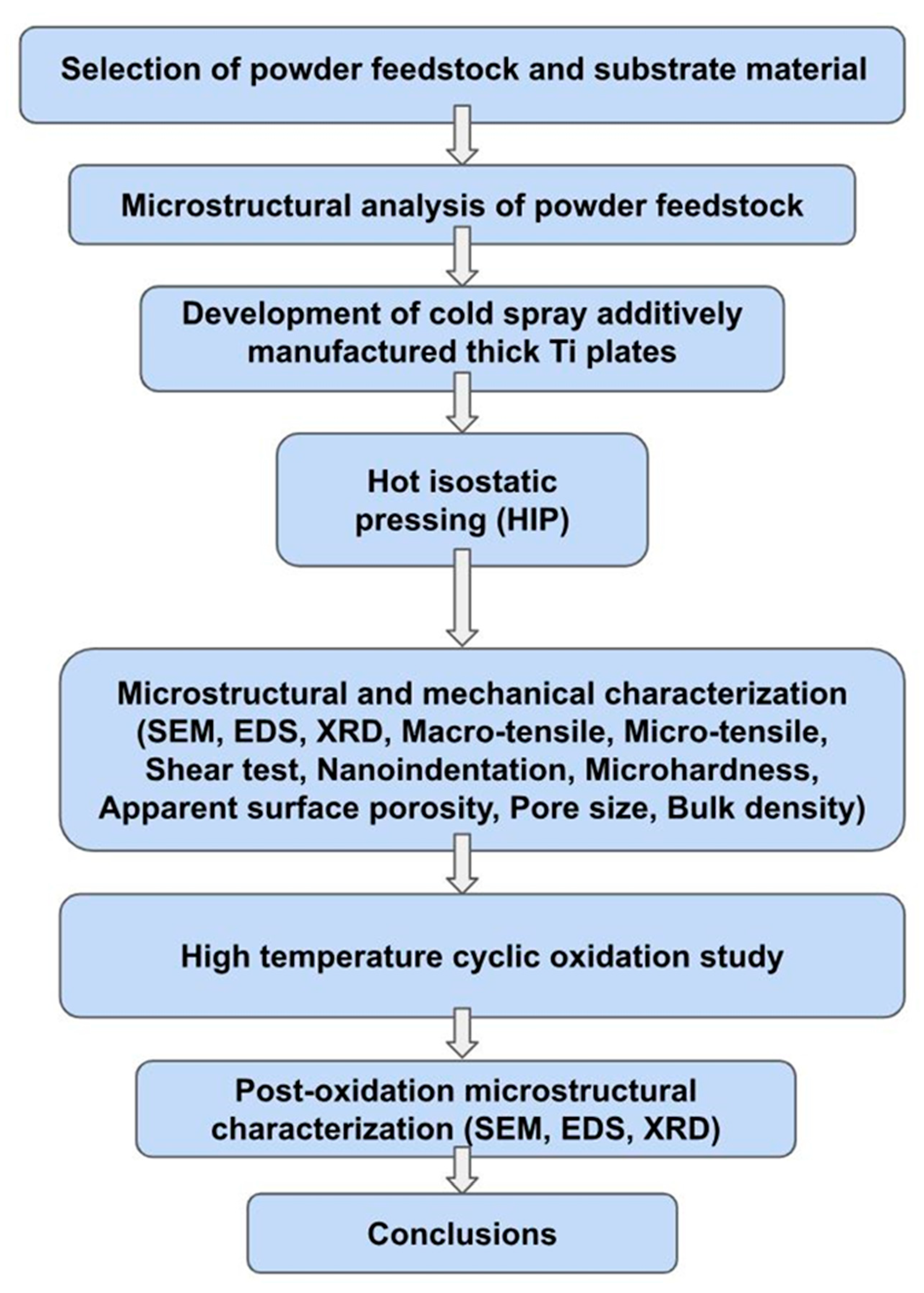
3.1. Microstructural Analyses
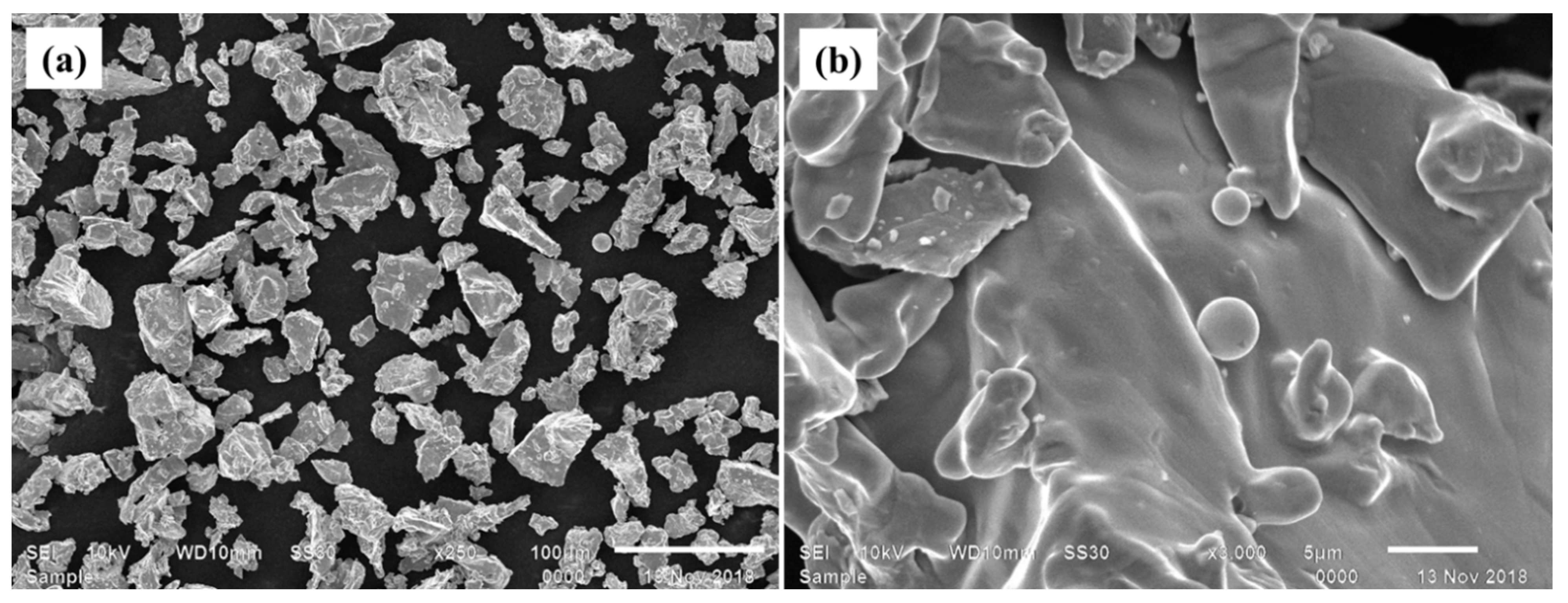
SEM images of the Ti feedstock powder revealed an irregular morphology with an angular particle shape, as shown in
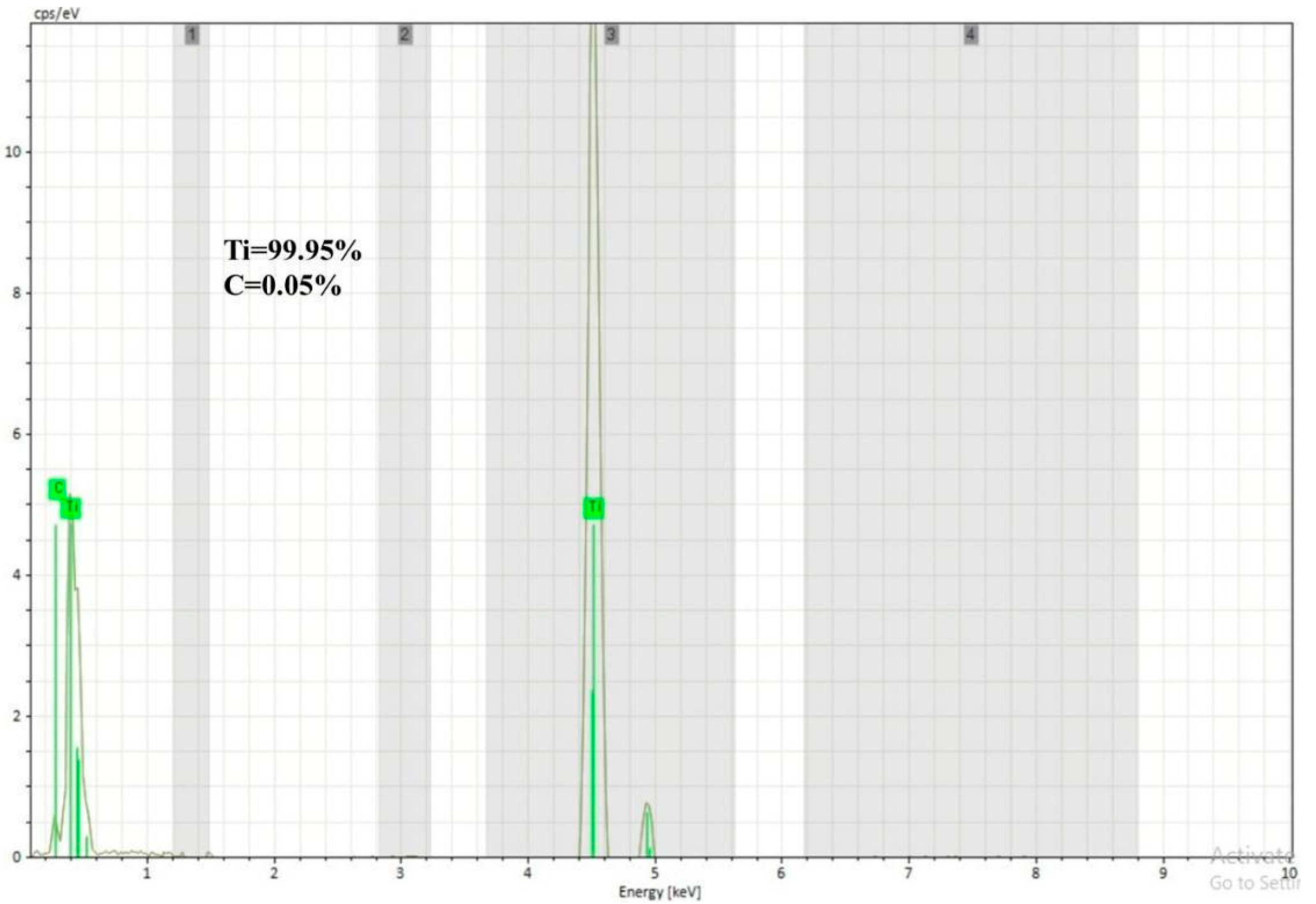
Figure 2. The EDS examination of the powder revealed the existence of only the Ti phase (99.95%), as displayed in
Figure 3, which was further confirmed via XRD investigation (
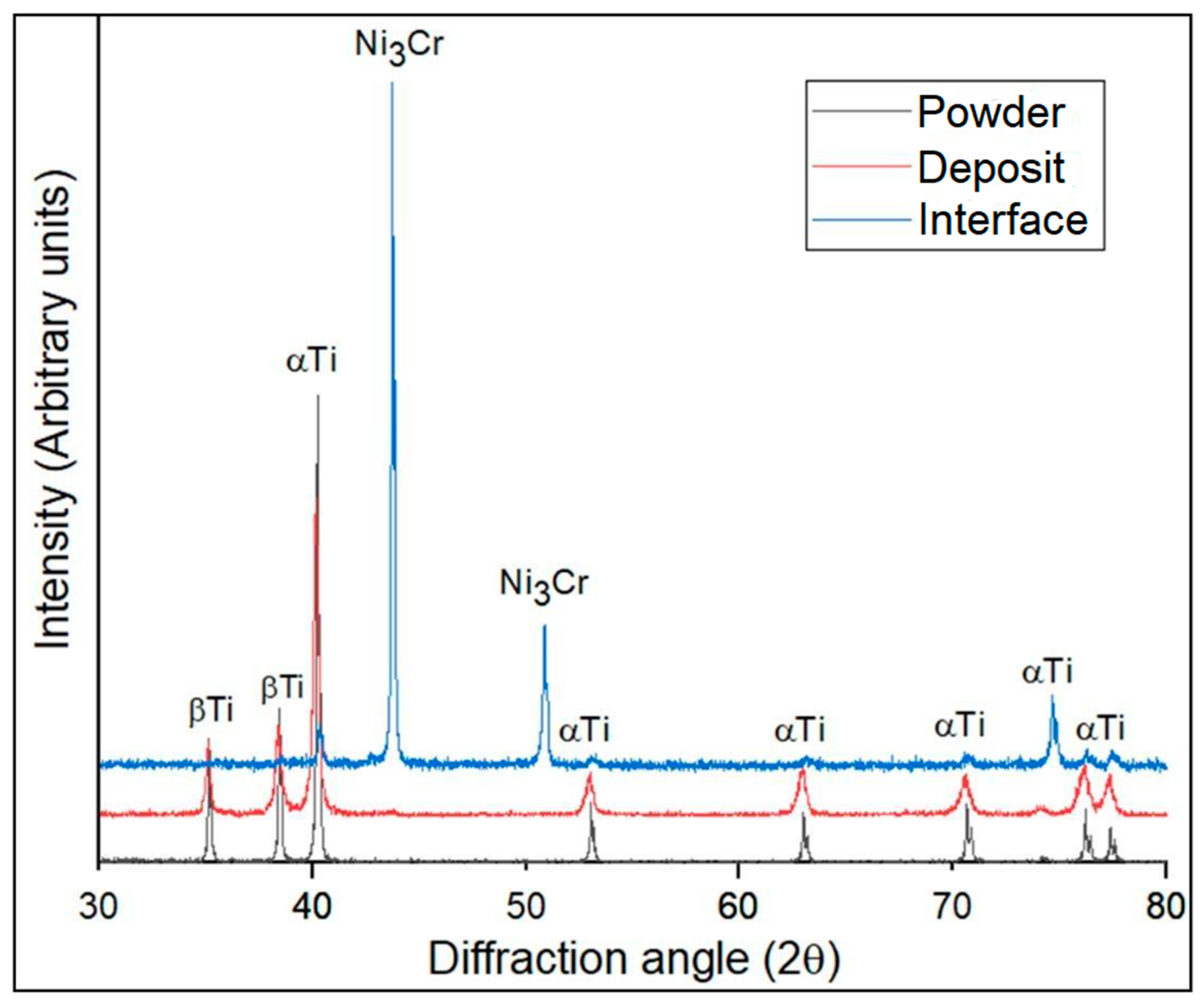
Figure 4). The carbon traces in the EDS data could be attributed to the carbon tape used during analysis to avoid the charging effect of electrons. The XRD analysis revealed that the deposit exhibited a more pronounced peak broadening compared to the powder and interface regions, as depicted in
Figure 4. This phenomenon could be attributed to the combined influence of strain and size effects in cold-sprayed HIP-treated pure titanium deposits. The strain and dislocation movements might vary the lattice spacing, resulting in peak broadening. The crystallite size decreased due to grain subdivision, dynamic recrystallization, and grain refinement. Smaller crystallites had a limited number of lattice planes contributing to the diffraction, resulting in broader diffraction peaks [
15].
The optical micrographs of the cold-sprayed HIP-treated pure titanium deposit are represented in
Figure 5. The deposit is found to have two different types of microstructures. A granular structure with spherical grains of (α + β phase of titanium) has been observed near the center of the deposit. However, a bunch of bush-like structures (Widmanstatten) has been observed near the interface, which was further identified (via XRD, SEM, and microhardness) as α-titanium.
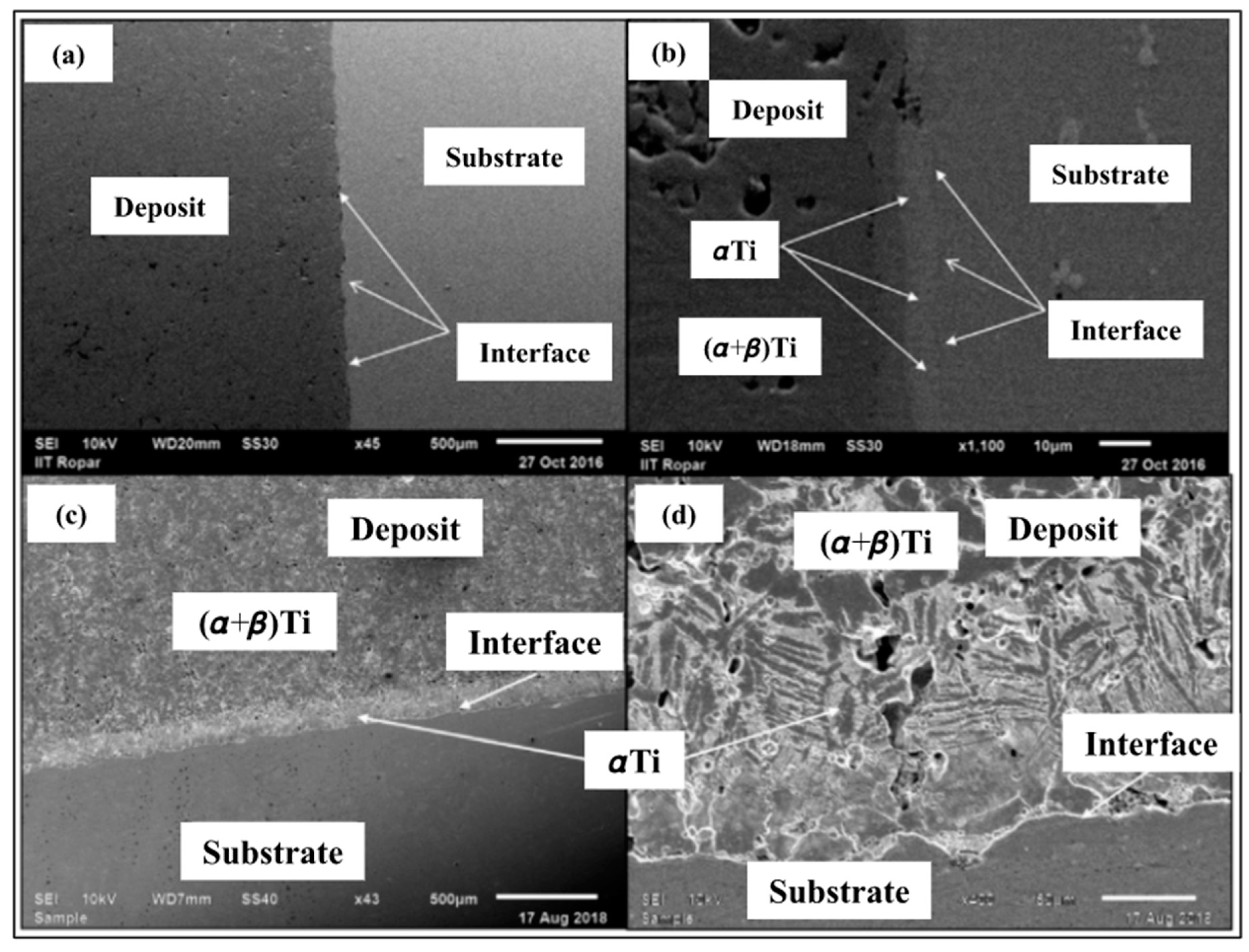
SEM images of the developed Ti deposit are presented in
Figure 6. It can be seen from
Figure 6a that there is no gap at the interface, which indicates appropriate adhesion between the substrate and the first layer of the deposit. This information can be useful if one is interested in developing Ti deposits on IN718. There were no cracks found in the Ti coating deposit. However, micron-sized inter-particulate pores, as well as weakly bonded inter-particulate boundaries, were found. These imperfections, cavities, and pores could be the result of inadequate plastic deformation of powder particles during deposition. It is worth mentioning as a side note that these features are very important in medical prostheses, such as orthopedic or dental implants, to act as nucleation sites for the cells to proliferate, aiding in osseointegration. Osseointegration helps in improving prosthetics for amputees.
A white layer at the interface was also observed. A high-magnification SEM image shown in
Figure 6d also shows a bunch of bush-like structures (Widmanstatten) near the interface, which were further characterized as α-titanium via XRD (
Figure 3) and microhardness analysis). The EDS mappings (
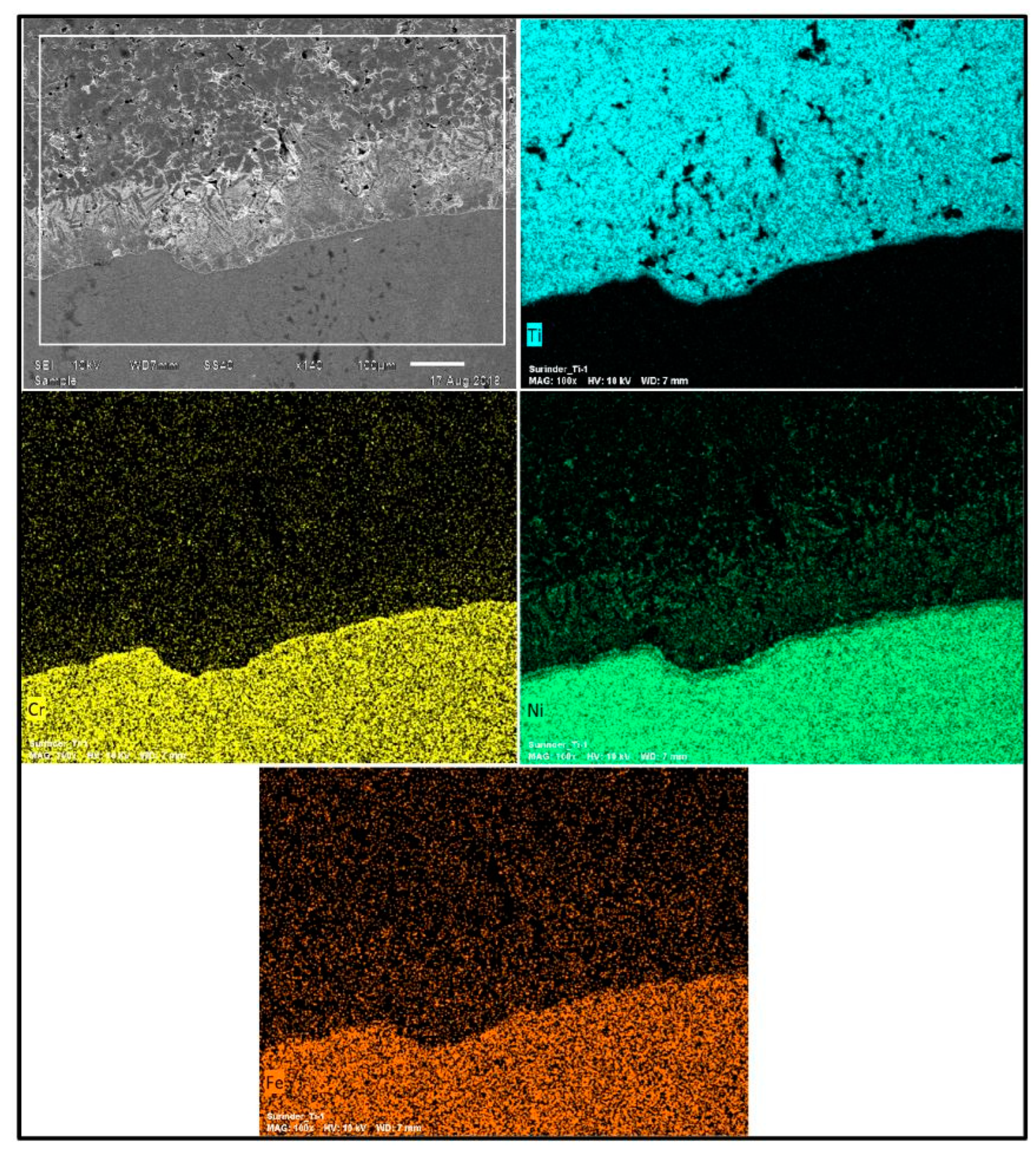
Figure 7) showed pure titanium on the deposit side and nickel, chromium, and iron on the substrate side. There was no oxide formation in the deposit during cold spraying and HIP processing, highlighting the capability of the used approach for the development of standalone pure titanium products. Moreover, no inter-diffusion across the Ti-layer and substrate material was observed. A microstructure comprising a variety of irregularly shaped particles and voids has been observed at the inter-particle boundaries. These voids were not noticeable in the un-etched sample (
Figure 6a,b), while the mass loss due to attack by the etchant (
Figure 6c,d) exposed the hidden pores. Therefore, it can be concluded that the un-etched surface can lead to the underestimation of porosity [
16].
Figure 4 shows the XRD analysis of the developed HIP-treated Ti deposit near the interfacial area and inside the deposit performed by placing the sample in line with the mark of the XRD fixture, which indicates the sight of the XRD scan. XRD analysis of both areas indicates that no oxide phases were formed. This observation was also supported by the EDS analysis. This is a positive attribute regarding the development of pure-Ti products using the cold spraying process. Additionally, the absence of intermetallic phases at the interface proved that cold spraying (CS) is superior to conventional high-temperature additive manufacturing (AM) technologies in inhibiting the synthesis of brittle intermetallic phases at the multi-materials interface. The XRD analysis of the cold-sprayed HIP-treated deposit and the used powder showed the presence of both α- and β-titanium, while at the interface, only α-titanium peaks were observed. The presence of both the phases (α and β) in the titanium powder, as illustrated in
Figure 4, was a result of the material’s unique phase diagram and the specific manufacturing processes used to produce the powder. At high temperatures (above the β transus temperature, typically around 882 °C or 1620°F), titanium exists in the β phase, which has a body-centered cubic (BCC) crystal structure. As the temperature decreases below the β transus, titanium undergoes a phase transformation to the α phase, which has a hexagonal close-packed (HCP) crystal structure. It is important to mention that the method used for making the feedstock titanium powder was atomization in this case. In this method, molten titanium is sprayed or atomized into fine droplets using high-pressure gas or other means. These droplets are rapidly cooled and solidified into powder particles. During the rapid cooling process, the material could become “frozen” in a metastable state, preserving both the α and β phases in the powder. This was because the cooling rate is so fast that the phase transformation from β to α may not have had time to complete before the particles solidified. The presence of only α-Ti peaks at the interface suggests that a transformation of β to α occurred in the titanium deposits. This transformation is often influenced by two main factors: rapid cooling from the β phase or α + β regions, which might have taken place after HIP, especially the interfacial region, which was closer to the substrate than the other part of the deposit, and plastic deformation during cold spraying, which induced this transformation. The fast cooling at the interface led to the formation of Widmanstatten-α laths. Moreover, the HIP treatment applied to the cold-sprayed Ti deposits resulted in the spheroidization of the colony microstructure within each β grain. This process produced a micro-duplex structure comprising primary α particles in a matrix of β grains/sub-grains. These β grains/sub-grains developed during the slow cooling of the deposit after HIP in contrast to the interfacial region. This is the reason for the α and β peaks in the case of the deposit, as depicted in
Figure 4 [
17,
18].
The density for the cold-sprayed HIP-treated pure titanium sample was observed at 4.14 g/cc when determined using the Archimedes method, respectively. However, the density of the pure bulk Ti (manufactured through special casting machines) was measured as being 4.50 g/cc when determined using the aforesaid method. It is very challenging to produce a pore-free deposit of titanium powder using cold spraying (solid-state deposition) alone due to the irregularly shaped morphology of powder particles. Furthermore, owing to its low density and hexagonal closed-pack (HCP) crystal structure, it is very difficult to densify Ti [
16]. Therefore, the HIP treatment of the thick Ti deposits has been performed to achieve a comparatively denser microstructure with a density approaching that of bulk Ti. An apparent surface porosity having a value of less than 1% has been achieved in a HIP-treated Ti deposit. However, pores with size variation from 3 to 10 µm have been observed in the deposit when measured using the Image-J analyzer. Relatively higher amounts of smaller pores (having a pore size ranging between 3 and 6 µm) have been observed as compared to that of larger pores (having a pore size range of 6–10 µm).
3.2. Mechanical Properties Evaluation
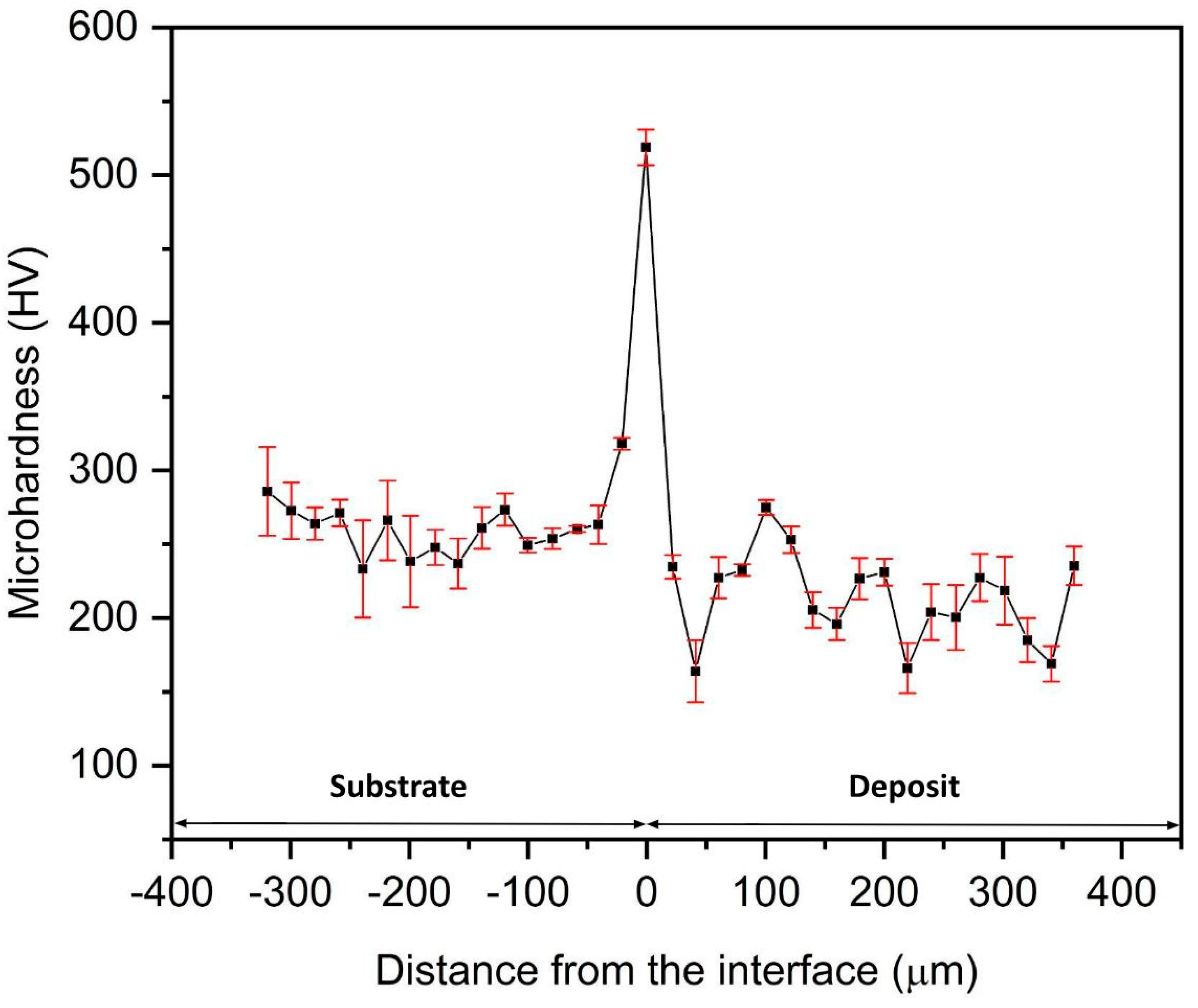
The microhardness plot (hardness v/s thickness) of the cold-sprayed HIP-treated pure titanium deposits is shown in
Figure 8. The average microhardness of the Ti-layers is 214 HV, which is slightly higher than that of the commercially available bulk titanium (202 HV) [
19]. The average microhardness value at the interface was observed as being 519 HV (at the white line observed in SEM images). This jump was observed because of the strain hardening of the substrate at the interfacial region due to the extremely high-velocity bombardment of the Ti particles. Additionally, it may be due to the presence of α phase, which has hexagonal close packing (HCP) compared to the β-phase possessing a body-centered cubic (BCC) crystal structure, leading to the greater hardness of the α phase compared to the β phase [
19]. The transition elements in the substrate might have stabilized the α phase of titanium by lowering the β–α phase transition temperature during HIP treatment at 900 °C [
20]. The XRD data (
Figure 4) also established the stabilization of the α phase because β phase peaks were not seen in the XRD analysis at the interface [
21].
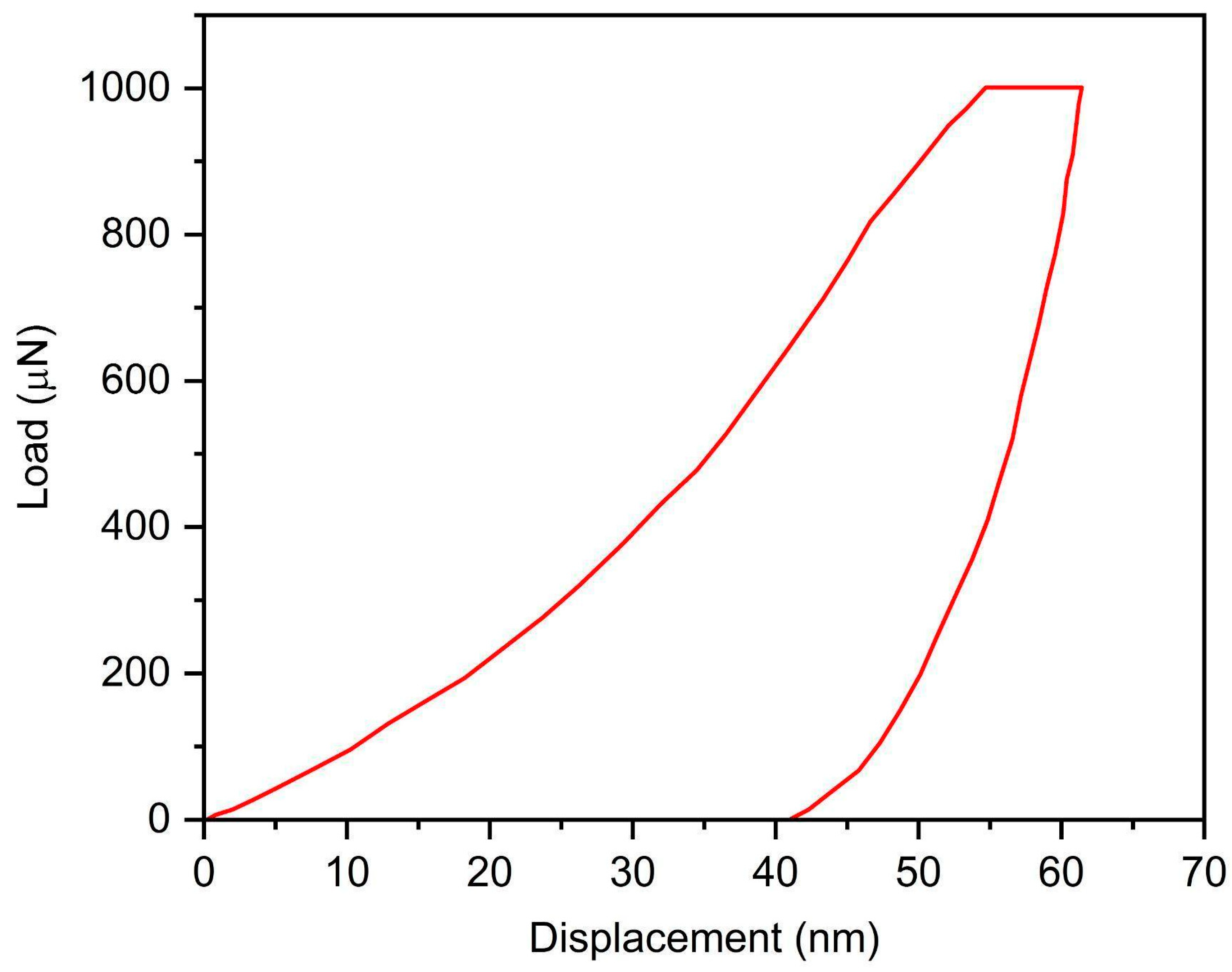
Localized mechanical properties of the deposited titanium without the substrate were measured via nanoindentation. The load–displacement curve is depicted in
Figure 9. The nanohardness was found to be 5.2 GPa. Young’s modulus of the sample was found to be 148 GPa in comparison to 105–115 GPa of the bulk titanium [
22]. The magnitude of Young’s modulus was found to be higher because the applied stress needs to first overcome the residual compressive stresses owing to cold spraying and HIP treatment before straining the material. It may also be due to individual particle sampling in nanoindentation, which offers less in terms of contribution from pores/oxides [
19].
The ultimate tensile strength (UTS) was found to be 384 MPa, with a total elongation of 6%, as represented in
Figure 10. The UTS achieved in the present study is more than that achieved by Hussain et al., which was 247 ± 15 MPa for titanium deposit sprayed with a commercially available CGT system using nitrogen at 800 °C at 4.0 MPa. Additionally, the achieved UTS was near to the UTS of bulk titanium (434 MPa) [
22]. This may be due to the HIP treatment of as-sprayed titanium, which led to lesser porosity and higher UTS. It is well known that the elongation can be augmented via the post-heat treatment routes available in the literature.
The shear strength between the first layer of the deposit and the substrate was found to be 26 MPa. This poor adhesion may be due to the formation of a hard face (α-titanium) near the interface. Additionally, the diffusion coefficient of HCP α-titanium is lower than the diffusion coefficient of BCC β-titanium due to the dense packing of atoms in HCP α-titanium [
22]. This decreased diffusion and formation of the hard phase of HCP α-titanium at the interface might have led to poor metallurgical bonding at the interface. It is pertinent to mention that this lower shear strength is beneficial with regard to the AM aspect of cold spraying, as one has to remove the substrate in the case that a standalone structure of Ti is required to be manufactured.
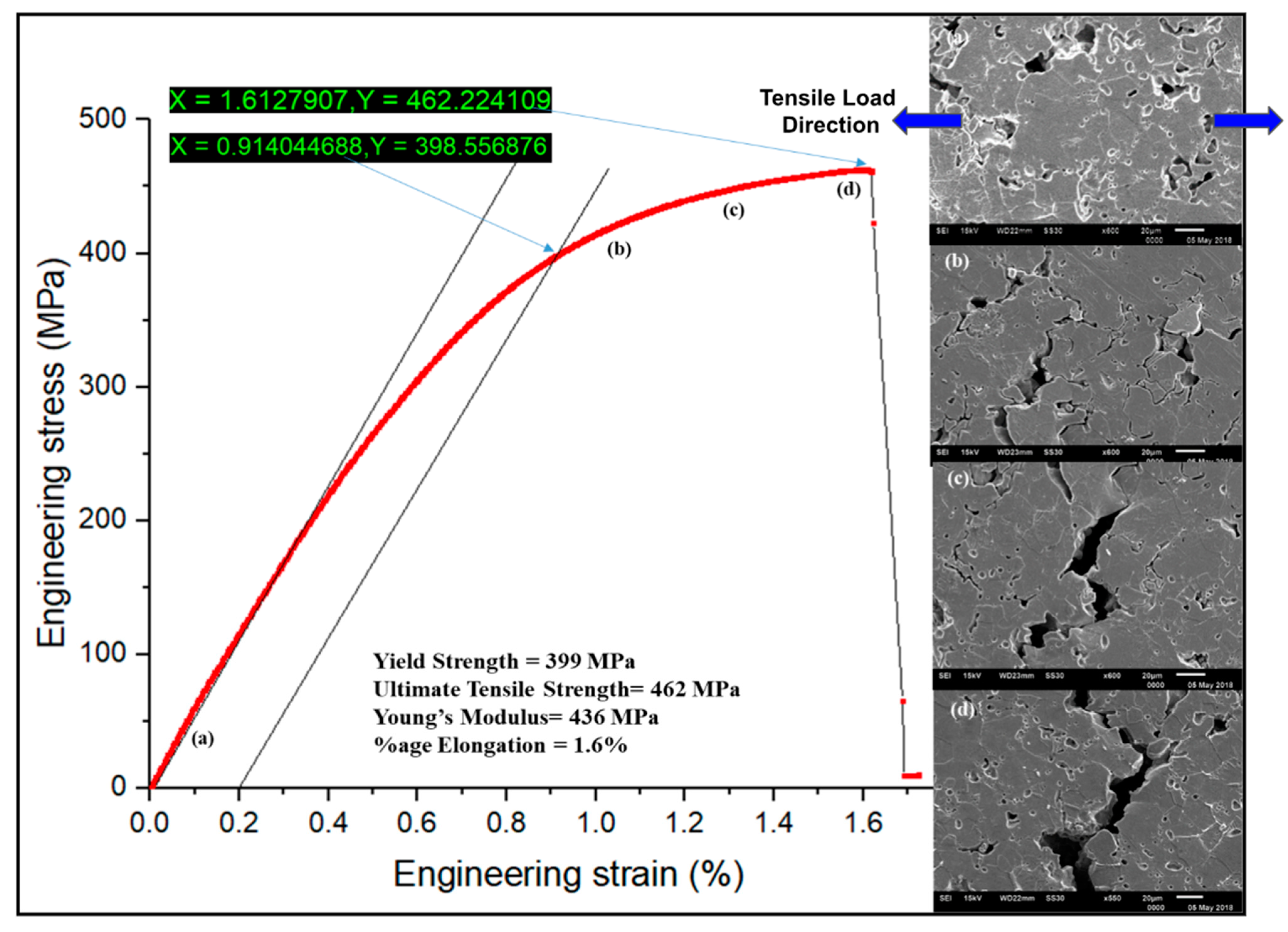
Micro-tensile plot showing engineering stress versus engineering strain, along with the corresponding SEM images at various stages, is shown in
Figure 11. A complete brittle nature of the fracture can be observed from the tensile curve. The ultimate tensile strength was found to be 462 MPa, with an elongation of 1.6% only. Moreover, micro-UTS was found to be higher than macro-UTS. It may be due to the polishing performed on the micro-tensile sample. A finely polished surface might have delayed the crack initiation, which led to higher yield strength and UTS. Young’s modulus achieved by micro-tensile (436 MPa) is higher than that achieved from a macro-tensile sample (281 MPa); however, it is very less than that observed during nanoindentation (143 GPa). The lower modulus in macro-tensile compared to micro-tensile analysis could be attributed to the polishing of samples required for micro-tensile for in situ analysis in SEM. It has been reported that the sprayed deposits have a significantly lower modulus often as compared to bulk material because of less compliance between the splats [
23]. Moreover, higher values achieved through nanoindentation may be due to individual particle sampling, which offers less contribution from pores/oxides.
It was also observed during the in situ tensile testing that no cracks were nucleated (
Figure 11a) till 399 MPa (Yield strength). The cracks started nucleating from the pore boundaries above the yield point, as represented in
Figure 11b. Further, straining of the material led to the propagation of cracks through the splat boundaries. The cracks passed from pore to pore through the splat and grain boundaries (
Figure 11c). The final fracture of the material showed a brittle nature (
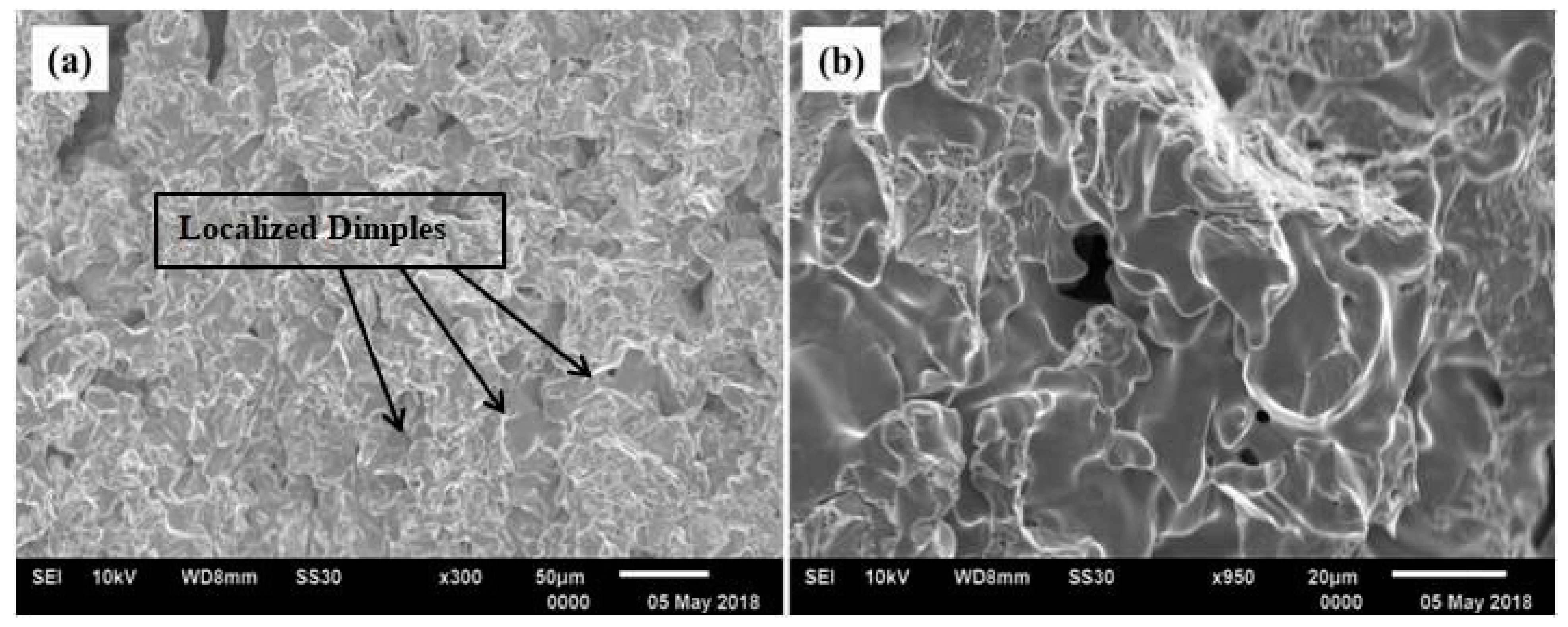
Figure 11d). An examination of the fractured surfaces of the deposited Ti sample was performed using SEM, which is shown in
Figure 12. Despite the fact that some localized dimples were also visible, the fractography study does not provide any indications of distinctive features like cup and cone. These fractured surfaces are significantly similar to those found in powder metallurgy [
12].
3.3. High Temperature Cyclic Oxidation Study
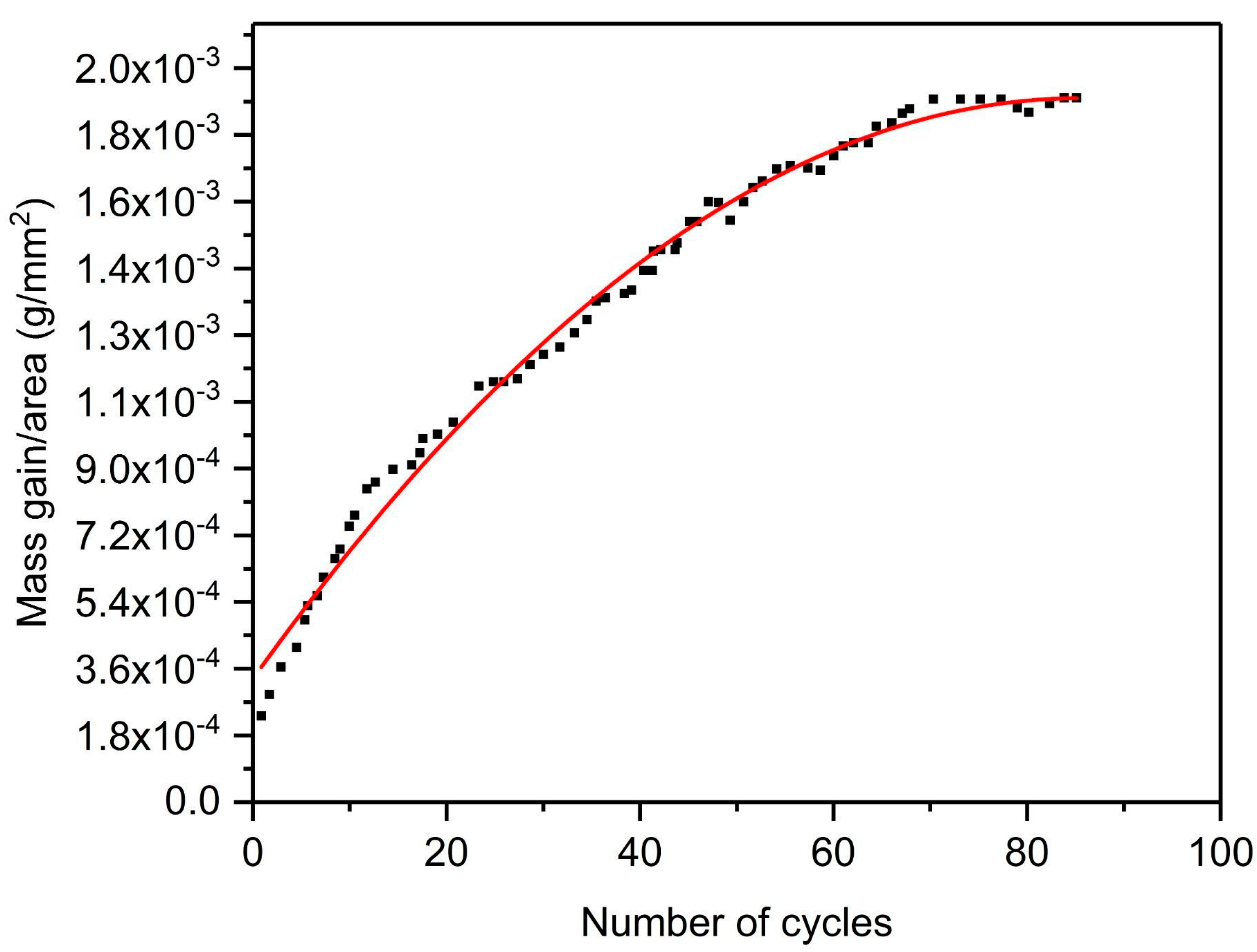
Cyclic oxidation was performed to evaluate the deposit’s sustainability under high-temperature conditions. The plot of the mass gain vs. number of cycles is represented in
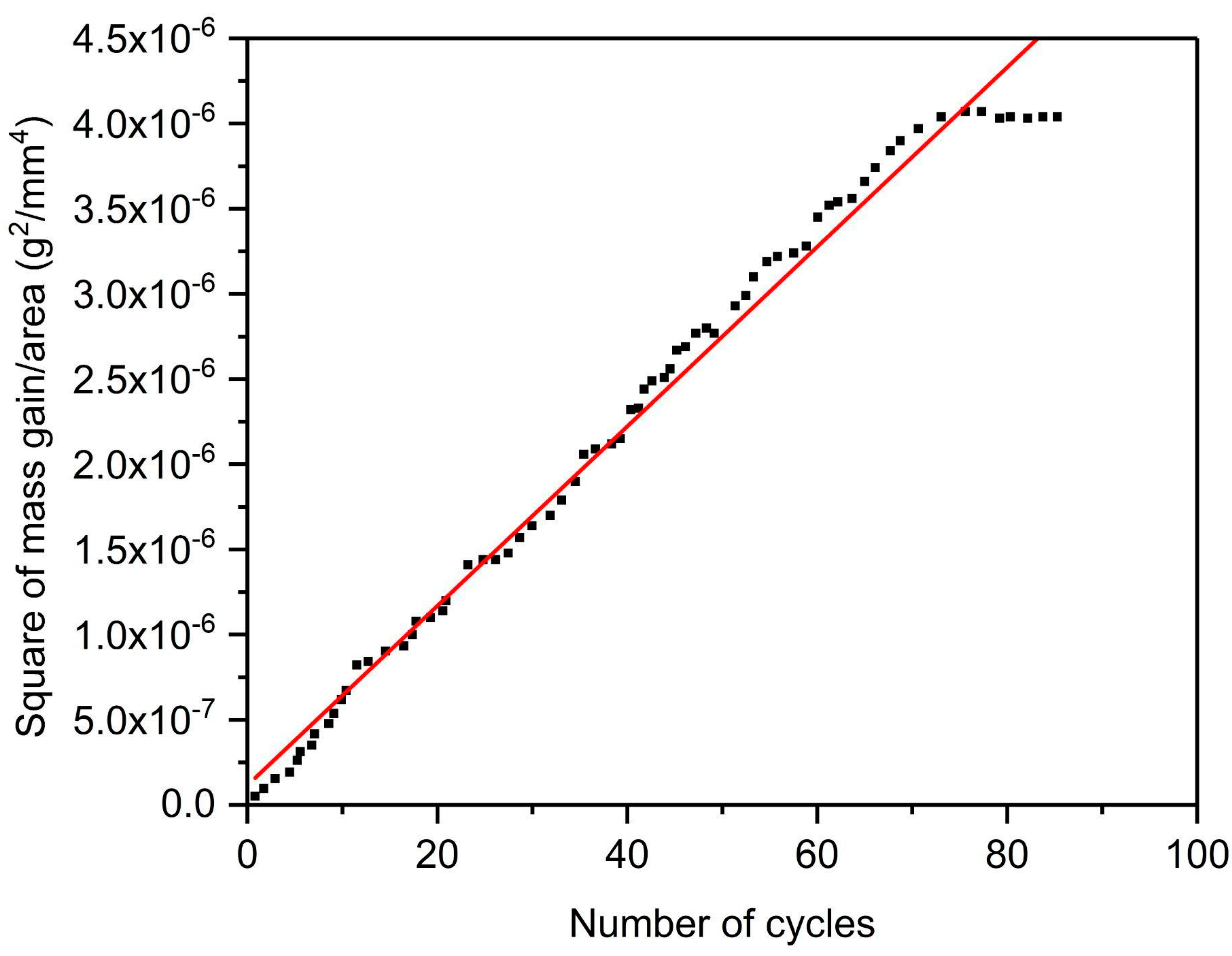
Figure 13. To ascertain the rate law for high-temperature cyclic oxidation of the sample, the square of mass gain vs. number of cycles plot is drawn as displayed in
Figure 14. A parabolic relationship was observed to approximate the data. The parabolic rate constant of deposited Ti can be calculated as follows [
24]:
where ∆W is the mass change per total surface area, K
p is the parabolic rate constant, and t is the oxidation time. The value of K
p for the cold-sprayed HIP-treated pure titanium sample is found to be 1.17 × 10
−11 g
2 mm
−4 s
−1, which describes the cyclic oxidation rate quantitatively. The magnitude of K
p was observed to be less than that of titanium specimens prepared via the powder metallurgy process assisted with inductive sintering (1.43 × 10
−11 g
2 mm
−4 s
−1) [
25]. The mass of the cold-sprayed HIP-treated pure titanium sample was found to increase during the first 67th cycles. However, the mass of the sample stabilized after the 68th cycle. This mass stabilization effect is evident from the parabolic nature of the curve, as shown in
Figure 13 [
26]. No spallation was observed during the whole oxidation study. The mass change trend shows that the material could have good oxidation resistance during longer usage. That may be due to the forming of a protective layer of titanium oxide. However, no stabilization in mass gain was observed in the studies available in the literature, even after 1500 cycles of oxidation in air at a 700 °C temperature for pure bulk titanium [
25]. This concretes that cold-sprayed HIP-treated pure titanium deposit is more advantageous for high-temperature applications.
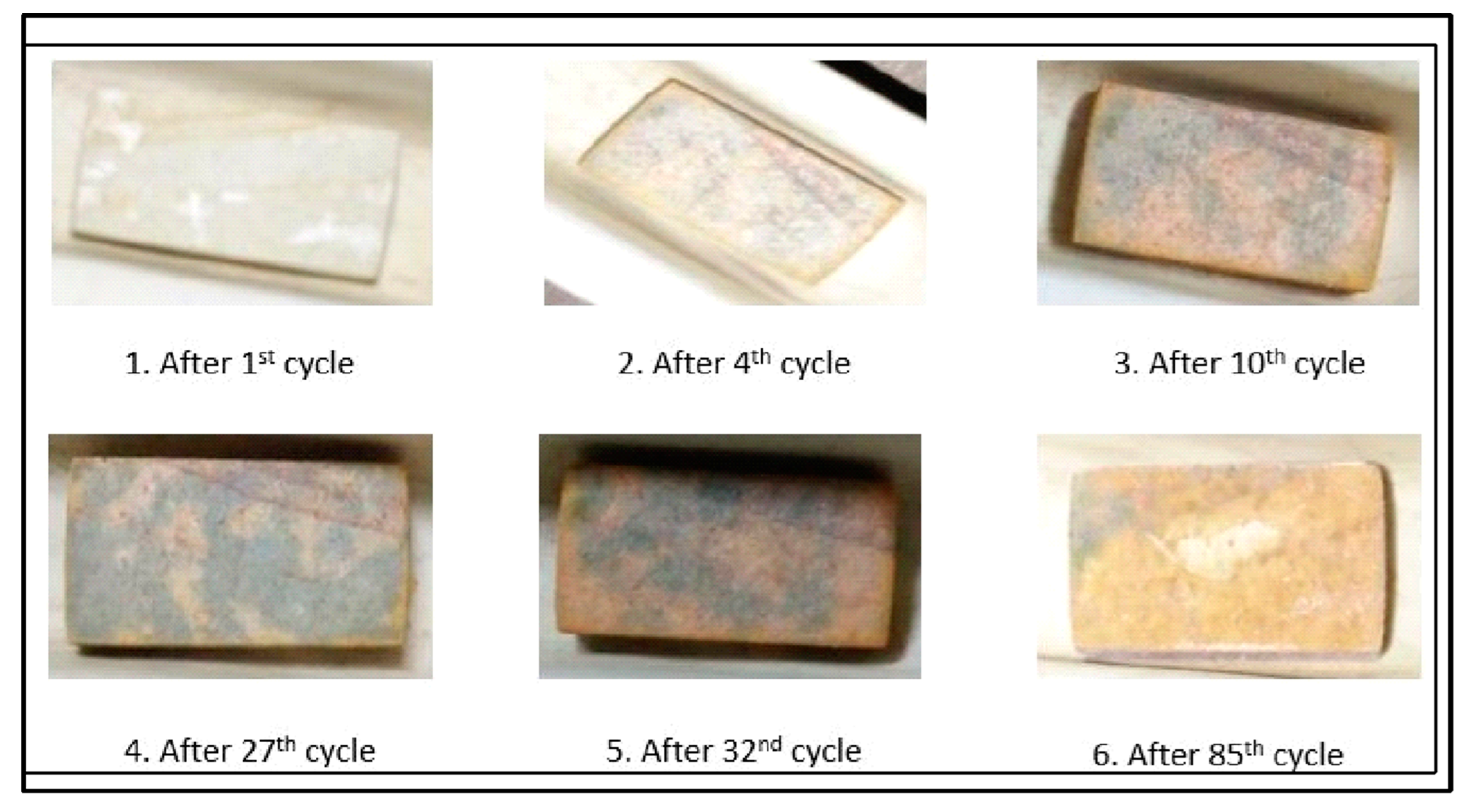
The macrographs of one of the samples subjected to cyclic oxidation at high temperatures are presented in
Figure 15. Overall, the scale was smooth and continuous without cracks, with no trend of scale spalling during the entire study. The sample at its initial stage was metallic in color with a shining surface due to fine polishing. After the first oxidation cycle, the whole surface became creamish in color, and gray spots started appearing after the 4th cycle with a cream color in the backdrop. These gray spots started increasing in size, and almost 80% of the surface became gray after the 27th cycle. Further, the gray shade became creamish, and the creamish color began changing to a light-yellow tone. Almost the whole surface of the sample turned light yellow towards the end of the high-temperature cyclic oxidation study carried out for 85 cycles. These color variations may be due to changes in the mineral form of titanium dioxide from anatase to rutile or vice versa during heating [
27,
28].
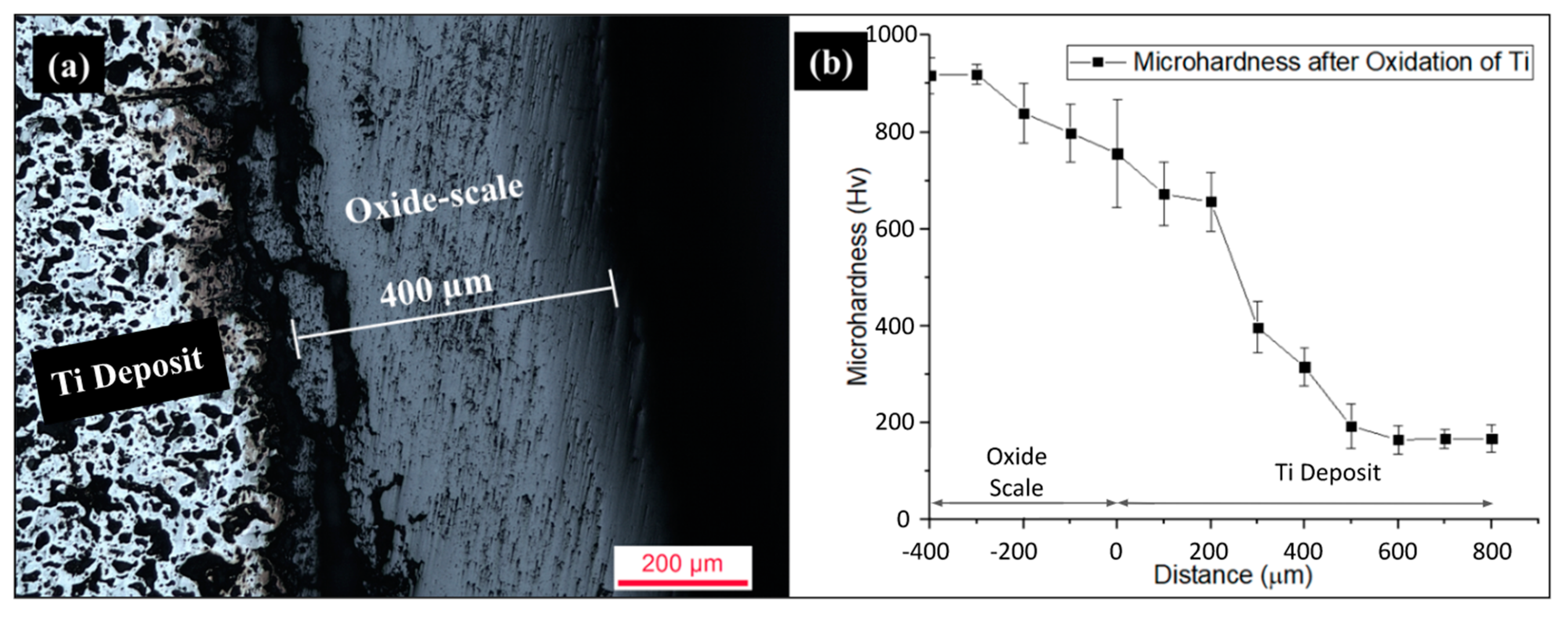
Cross-sectional optical images and microhardness analysis of the cold-sprayed HIP-treated pure titanium deposit subjected to high-temperature cyclic oxidation are represented in
Figure 16a,b, respectively. High-temperature cyclic oxidation induced the development of a layered oxide structure through a complex interplay of oxidation and reduction cycles. This cyclic process led to the continuous growth of the oxide scale with each cycle, resulting in distinct layers. Moreover, diffusion and segregation of elements at the oxide–metal interface significantly influenced the composition and structure of the layers. Additionally, phase changes of the oxide layers in response to varying conditions and the intricate influence of microstructural features, such as grain boundaries, precipitates, and defects, were pivotal factors shaping the layered oxide structure. Understanding these phenomena was crucial for improving the performance and durability of materials in high-temperature environments, particularly in gas turbine components, heat exchangers, and other applications subjected to repeated thermal cycling in oxidizing atmospheres [
29]. A cracked oxide scale of 400 µm thickness and an average microhardness of 818 HV was observed, which implies the critical scale thickness for spallation. The tremendous rise in the oxide scale microhardness is because of the mineral forms of titanium dioxide present in the oxide scale. Rutile and anatase phases were the mineral forms of titanium dioxide investigated through XRD analysis. These two mineral forms have body-centered tetragonal (BCT) crystalline structures, which have fewer active slip systems and hence high hardness and brittleness. The microhardness of the cold-sprayed HIP-treated Ti deposit was observed to be higher (775 HV) at the deposit-oxide scale interface (0 µm), which starts to decrease up to 500 µm deeper inside the deposit, attaining a microhardness of 202 HV. The microhardness value stabilized to an average value of 175 HV after 500 µm distance from the deposit–oxide scale interface. The variation in hardness may be due to comparatively higher oxygen concentration (enrichment of titanium with oxygen) in the outer layers than in the inner layers of the deposit. Therefore, from the obtained microhardness values and optical micrographs, it can be concluded that oxidation of titanium comprises both oxidation dissolution of the titanium near the interface and oxide scale formation.
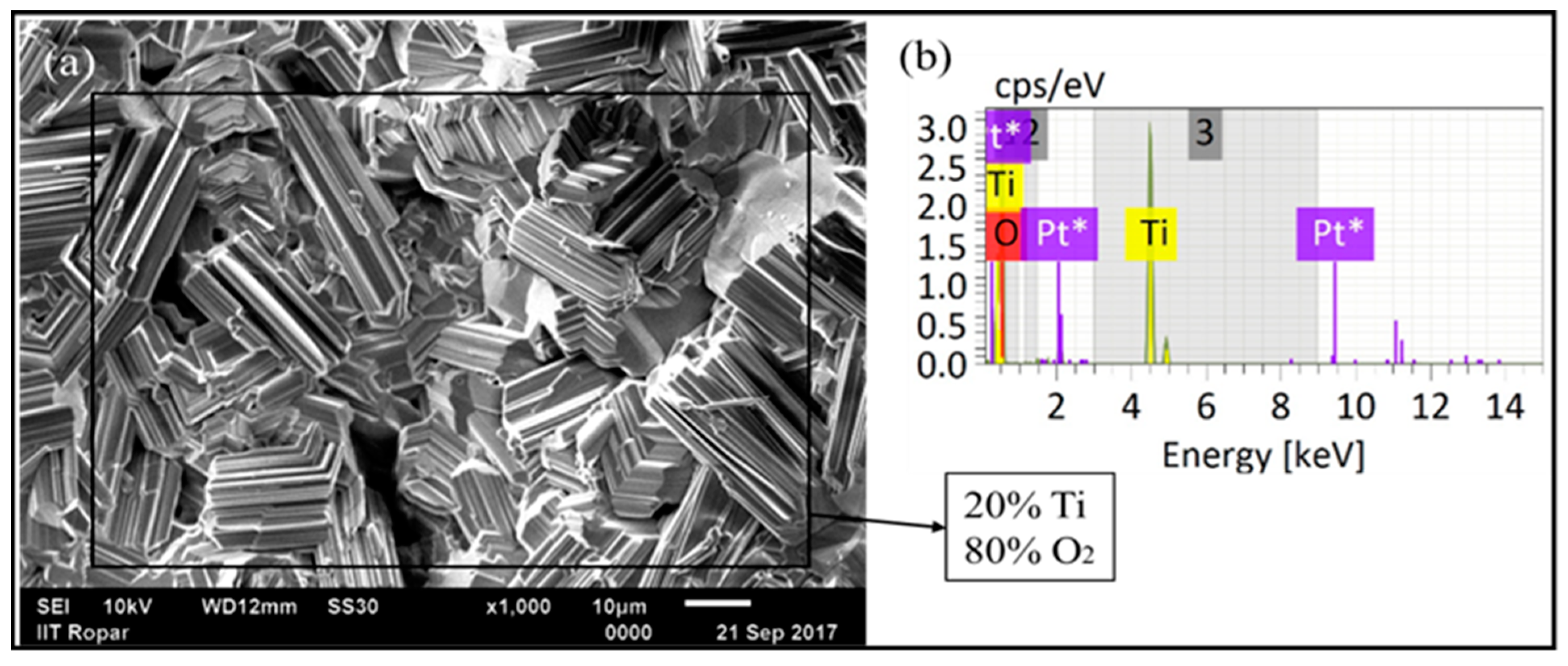
Surface SEM analysis of the oxidized deposit is provided in
Figure 17a. A crystalline structure can be seen, like a bunch of rods with variations in length. The development of a crystalline rod-like structure with varying lengths during high-temperature cyclic oxidation of titanium (Ti) arose from the influence of Ti’s hexagonal close-packed (HCP) crystal structure. The repeated oxidation–reduction cycles in an oxidizing environment facilitated the preferential growth of rutile titanium oxide (TiO
2) along specific crystallographic directions, resulting in the formation of rod-like morphologies. This phenomenon was affected by crucial parameters such as temperature, oxygen concentration, and the number of oxidation cycles, which contributed to the observed variations in rod lengths. Additionally, microstructural features, including grain boundaries and defects, played a significant role by serving as preferential sites for oxide growth, thereby enhancing the diversity in rod lengths. A comprehensive understanding of these factors was essential for tailoring and optimizing the performance of titanium-based materials under high-temperature conditions, thus enabling advancements in various industrial applications [
30]. EDS analysis with point spectrum and mapping showed the presence of titanium and oxides on the whole surface (
Figure 17b and
Figure 18, respectively). EDS square spectrum analysis (shown in
Figure 17b) showed an 80% concentration of oxide and a 20% concentration of titanium on the surface. The presence of platinum in the EDS spectrum is due to the sputtering performed before the SEM/EDS analysis to make the oxide layer conductive for obtaining better results in SEM/EDS.
The X-ray diffractogram for the cold-sprayed HIP-treated pure titanium deposit subjected to high-temperature cyclic oxidation is displayed in
Figure 19. It is evident from the XRD analysis that the cold-sprayed HIP-treated pure titanium sample developed a layer of titanium oxide TiO
2 after high-temperature cyclic oxidation. Diffraction peaks observed were of TiO
2 having a crystalline structure in the form of rutile and anatase, which are the two mineral forms of the crystalline structure of titanium dioxide [
28]. Most of the peaks are rutile, and very few have an anatase structure. The formation and transformation of rutile and anatase phases during high-temperature cyclic oxidation were governed by a complex interplay of temperature-dependent thermodynamics, phase transition kinetics, and microstructural influences. Initially, rapid oxidation resulted in the thermodynamically favored rutile phase formation. Subsequent heating and cooling cycles induced fluctuations in temperature and oxygen concentration, impacting microstructure and stability. Lower temperatures promoted the kinetics-favorable transformation to metastable anatase, while higher temperatures reverted anatase to rutile due to equilibrium thermodynamics. Microstructural features, such as grain boundaries and defects, influenced phase nucleation and growth. Additionally, oxygen diffusion into the oxide scale during oxidation–reduction cycles significantly affected phase transformation kinetics, contributing to the observed phase changes [
31]. Therefore, it may be concluded that initially (after 1st cycle) rutile structure of titanium dioxide was formed, which probably appeared creamish in color. After further heating cycles, it converted into anatase and appeared blackish after the 27th cycle. Further heating cycles may lead to the conversion of anatase to a rutile crystal structure of titanium dioxide. The rutile crystal is the most stable polymorph of TiO
2 at all temperatures compared to metastable anatase. The color variation is due to a change in crystal structure having a difference in refractive index. Rutile has a higher refractive index (RI) when compared to anatase. Therefore, the scattering of light is relatively higher by the rutile phase, leading to creamish/yellow color, whereas anatase has comparatively lesser RI, scatters less light, and appears grayish. The rutile type of pigments is generally preferred over anatase because they scatter light more efficiently, are more stable, and are less likely to catalyze photodegradation [
32]. Additionally, rutile can be converted into a titanium sponge through the Kroll process.
Summarizing the above discussion on oxidation behavior, it is concluded that the manufactured titanium deposit has a high oxidation resistance.