Abstract
The ability to microscopically image diseased or damaged tissue throughout a longitudinal study in living mice would provide more insight into disease progression than having just a couple of time points to study. In vivo disease development and monitoring provides more insight than in vitro studies as well. In this study, we developed permanent 3D-printed, surgically implantable abdominal imaging windows (AIWs) to allow for longitudinal imaging of deep-lying tissues or organs in the abdominal cavity of living mice. They are designed to prevent organ movement while allowing the animal to behave normally throughout longitudinal studies. The AIW also acts as its own mounting bracket for attaching them to a custom 3D printed microscope mount that attaches to the stage of a microscope and houses the animal inside. During the imaging of the living animal, cellular and macroscopic changes over time in one location can be observed because markers can be used to find the same spot in each imaging session. We were able to deliver cancer cells to the pancreas and use the AIW to image the disease progression. The design of the AIWs can be expanded to include secondary features, such as delivery and manipulation ports and guides, and to make windows for imaging the brain, subcutaneous implants, and mammary tissue. In all, these 3D-printed AIWs and their microscope mount provide a system for enhancing the ability to image and study cellular and disease progression of deep-lying abdominal tissues of living animals during longitudinal studies.
1. Introduction
Many diseases, such as cancers, occur internally and can develop over an extended period of time, making them difficult to interrogate in vivo. It is important to study these diseases longitudinally to fully understand how they change and develop over time and to develop effective treatments for them. This is especially difficult for deep-seated organs in the abdomen, including the pancreas, liver, kidney, and others. It can be expensive to use traditional imaging modalities, such as computed tomography (CT) and magnetic resonance imaging (MRI), and they are not able to tell the story on a cellular level. Intravital microscopy gives researchers the ability to see how living cells behave in their native environments [1,2,3]. Highly focused light energy can be used to stimulate emission shifts of specific fluorescently labeled cellular components, allowing us to not only visualize the cellular landscape of a tumor but even the sub-cellular features and markers of individual cells (allowing for robust differentiation of each cell and monitoring of its individual responses). Most knowledge of cellular processes and pathways comes from static and biologically isolated conditions, but intravital microscopy facilitates the viewing of cell-to-cell interactions, real-time responses to signaling and stimuli, and other questions that are unable to be answered through cell cultures or fixed tissues. A problem with this powerful technology is the inability to physically present the tissue of a live animal to the microscope objective and light source in a manner that is stable enough to perform deep tissue scans for minutes or hours [4,5]. There is also a problem with imaging artifacts from movement during imaging that needs to be accounted for or prevented [6]. There have been efforts made to develop techniques to improve the effectiveness of intravital imaging [7,8,9,10]. Being able to manipulate the changes in the tissue microenvironment can be important for a complete understanding of cellular changes under certain conditions, but this is difficult to achieve. Efforts have been made to develop ways to manipulate the microenvironments of tissues to be used with intravital imaging [11]. Developing an effective, easy-to-use, and cost-efficient in vivo method for solving these issues would greatly benefit the ability to image, study, and understand cellular interactions and stimuli responses in their native environments. This, in turn, would increase the knowledge of disease progression in internal organs at the cellular level and eventually could lead to better treatments and outcomes.
Abdominal imaging windows (AIWs) have thus been developed to visualize distinct biological processes in internal organs. The dorsal skin fold chamber is the most frequently used to visualize subcutaneous tumor formation and angiogenesis and is commercially available [12,13,14]. Some other types of AIWs have also been fabricated and implemented to intravitally image pancreatic islets [15], mammary glands [16,17,18], the brain [19,20], ovaries [21], and liver [22], etc. Typically, these devices consist of a glass coverslip inserted into a metal (usually titanium) frame, which is sutured or glued to the skin [23]. There are several limitations with these devices. First, metal materials are heavy compared to lighter plastics, and the use of metal-based AIWs may cause changes in animal behavior if they are too heavy. Second, the current devices are usually expensive due to the complicated manufacturing steps, including design, mold fabrication and modification, and device assembly. Third, these devices lack capacities to incorporate other functions, and each modification will require a new mold fabrication, which is time- and resource-consuming. Fourth, effort has been made to lighten AIWs by using lighter and cheaper materials [24], but molds are still required for their production and are difficult to change if required.
To address these issues, we 3D printed permanent, surgically implanted AIWs that can be used with multiphoton microscopy to observe cellular behaviors in longitudinal disease progression in internal tissues and organs of the abdomen of living mice. These abdominal imaging windows are tolerated by mice (tested out to three months in vivo) and allow for repeated imaging of tissues of interest in the abdomen throughout disease progression. These windows are 3D printed from plastic, and this manufacturing method allows the AIWs to be inexpensively built in the lab for each use and lets them be lighter in weight. Since each use might be different, they can be customized for location and size. We developed additional functionalities into the AIWs, such as channels for delivery of materials to the tissue of interest, a method for locking the window into a fixed position for imaging the same location over time, and a method for mounting them to imaging devices. We developed a mount design specifically to mount the AIWs to microscopes, and this mount can be easily customized to different devices. The design of the AIWs can also be expanded to fit other areas of the body that would need to be imaged, such as the brain, subcutaneous implants, or mammary tissue. These 3D-printable abdominal imaging windows are engineered to be versatile, multifunctional, inexpensive, light, and effective devices that allow for longitudinal in vivo imaging of disease progression in tissues in the abdominal cavity.
2. Materials and Methods
2.1. Three-Dimensional Printing of Abdominal Imaging Windows
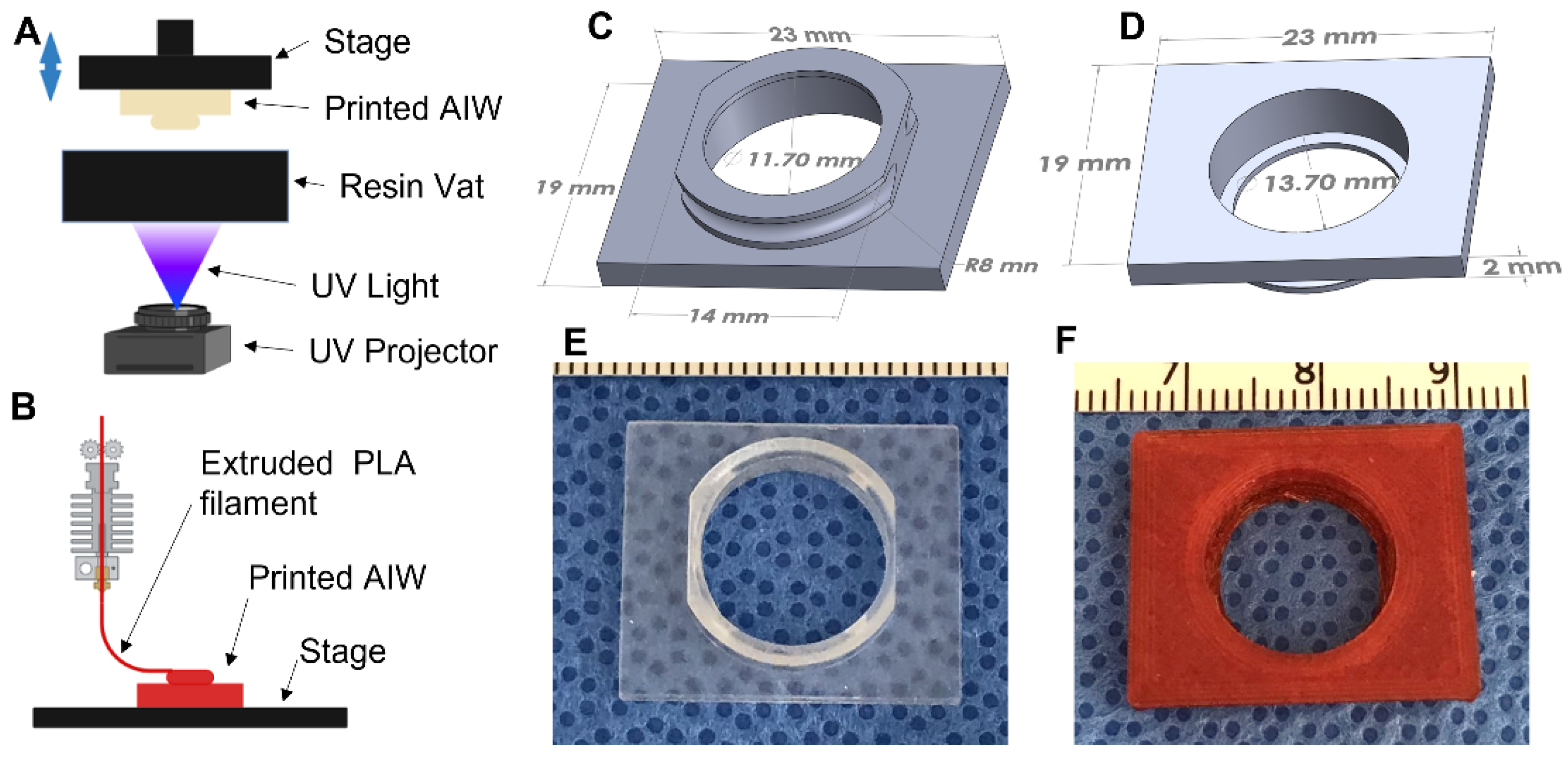
The AIWs were designed and converted to STL files for printing using Solidworks. They were printed using a Vida (EnvisionTEC, Gladbeck, Germany) 3D printer, which is a digital light processing (DLP) 3D printer (Figure 1A). The material used for printing was a methacrylic/acrylic resin called Clear Guide (EnvisionTEC, Gladbeck, Germany). They were also able to be printed using poly(lactic) acid (PLA, Ultimaker, Utrecht, The Netherlands) in an Ultimaker fused deposition modeling (FDM) 3D printer (Figure 1B). After printing, the AIWs were washed in a 70% isopropanol (Fisher Scientific, Waltham, MA, USA) bath until all uncrosslinked resin was washed away. They were then dried and post-cured in a UV curing box (EnvisionTEC, Gladbeck, Germany) for 10 min. After curing, the rings of the windows were coated in polycaprolactone (PCL, 80,000 MW, Sigma-Aldrich, St. Louis, MO, USA) and dissolved in Trifluoroethanol (TFE, Acros Organics, Geel, Belgium) (10% wt/vol) to help with glue attachment during the surgical implantation. The windows were then fully dried in order to remove all of the TFE before use.
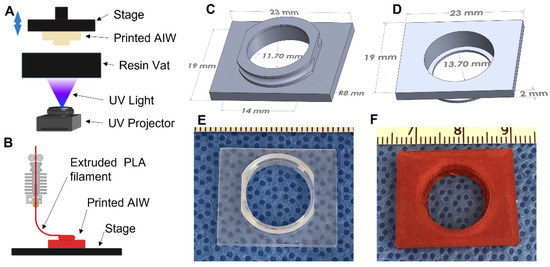
Figure 1.
AIW 3D CAD design and printed AIWs. Schematics of (A) FDM and (B) DLP printing of the AIWs (created with BioRender.com). (C,D) The 3D CAD design of basic AIW; (E) DLP 3D-printed AIW made of a methacrylic/acrylic resin; (F) FDM 3D-printed AIW made of PLA.
2.2. Surgical Implantation of Abdominal Imaging Windows
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Nebraska Medical Center (UNMC). All surgeries were performed using aseptic techniques. C57BL/6 mice (male and female, 12 weeks old, weighing 18–20 g, Charles River Laboratories, Wilmington, MA, USA) were used. The mice were anesthetized using isoflurane and were given Buprenorphine for pain relief. Prior to surgery, hair was removed by a commercially available depilatory cream (Nair, Ewing, NJ, USA). The printed window was disinfected by soaking it in glutaraldehyde, Betadine, Chlorohexidine, or another suitable disinfectant. Immediately before use, the window was rinsed with 70% ethanol. A sterile coverslip was glued into the inside of the circular portion of the window using a veterinary grade 2-octyl cyanoacrylate (super glue). To implant the AIW, the animal was anesthetized, and a 0.5 cm incision was made in both the skin and abdominal wall above the tissue of interest. In this case, we were interested in the pancreas, so the spleen was removed to prevent visual obstruction. The internal component was implanted two to four mm inferior to the ribs and six to ten mm superior to the pelvis. The splenic vasculature was pressure cauterized with a chromic gut 4–0 suture. The tissue of interest was then moved into place against the coverslip and held in place by super glue. The abdominal muscle wall was then glued to the outside of the round portion of the AIW to create a biological seal. Alternatively, this can also be achieved by using a purse-string suture (Ethilon Black Monofilament, Bridgewater, NJ, USA) to suture the abdominal wall into the indented portion of the outer ring. The skin was then sutured into the concave indention portion of the outer ring using a purse-string suture. Buprenorphine was administered every 12 h for up to 72 h after surgery. After surgery, all animals were monitored daily to check for any infection, health issues, or discomfort and were treated accordingly.
2.3. Microscope Mount 3D Printing
The mount for the microscope was designed and converted to an STL file using Solidworks. It was 3D printed using PLA and an Ultimaker FDM 3D printer (Ultimaker, Utrecht, The Netherlands). It was printed as seven separate pieces (4 sides, back, top, and bottom) that were later assembled after some sanding to complete the device.
2.4. In Vivo Imaging
Human pancreatic cancer cells (S2-013) were injected into the pancreas of mice during implantation of the AIW. The tumor cells were labeled with green fluorescent protein (GFP) to fluoresce green, and type IV collagen innately fluoresces through second harmonic resonance and fluoresces blue. Mice with implanted AIWs were lightly anesthetized with Ketamine-Xylazine and attached by the AIW to the microscope mount, which was attached to the microscope. The window fits into the slot of the mount to hold the mouse in place during imaging. Visualization of vital signs of the animals, including respiratory rate and heart rate, was performed throughout the imaging process. Longitudinal images of tumor cell congregation were captured through an AIW using a two-photon microscope (Olympus FVMPE-RS, Olympus, Tokyo, Japan) at 24 and 48 h after injection to view the tumor cells and collagen changes in the same location. A 60× water immersion Olympus objective was used. The imaging took about 10 min per mouse. Myelin-directed NP41 labeled neurons, with purple fluorescence, from a penetrating mass of pancreatic cancer cells were also imaged using an AIW and the same two-photon microscope.
3. Results
We were able to engineer 3D printable AIWs that have the ability to mount directly onto a customizable microscope stage mount. They are designed to be inexpensive, convenient, light, and effective. They are single-use, implantable AIWs that can be used on a variety of different tissues and with different imaging modalities. By using these, we were able to longitudinally image tumor-bearing mice on an upright multiphoton microscope with minimal respiratory artifacts.
3.1. Design of 3D Printed Abdominal Imaging Windows
The basic design (Figure 1C,D) has a 16 mm outer diameter circular immersed well with truncated sides so that it ergonomically fits small animals without inhibiting the ambulatory movement, especially in the hips and ribs. The outer wall of the well contains a small concave, semicircular recess that allows for a tight and permanent placement of a surgical purse-string suture of the dermis to hold everything in place. The inner diameter of the well is customizable for the microscopic imaging needs. This AIW has a 13.7 mm inner diameter with a 1 mm wide lip on the inside to allow for a circular coverslip to be securely glued in place to create a tight seal with no leakage. The customizable 4 mm well depth allows for imaging-objective positioning within microns of the tissue of interest and maximization of the tissue imaging focal length, all while providing a deep reservoir for superficial imaging.
To image the tissue with the least amount of imaging artifacts from movement and respiration, we designed the AIW to be able to be mounted to a microscope. On the top side of the well, there is a 19 × 23 mm rectangular mounting bracket that is designed to fit into a stage mount insert. The whole AIW slides into the mount and is held tightly in place to reduce any movement during the imaging process. Gravity helps to suspend the animal slightly to reduce artifacts secondary to respiration and motility.
3.2. Three-Dimensional Printing of Abdominal Imaging Windows
The AIWs were designed in 3D CAD software (Solidworks) and were 3D printed using a DLP-based 3D printer (Figure 1A) and a biocompatible methacrylic/acrylic resin [25,26] (Figure 1E) or an FDM (Figure 1B) 3D printer and a PLA material (Figure 1F), which makes them convenient and inexpensive to make. The materials are also biocompatible and will not cause any adverse effects. The 3D-printed AIWs have light weights (0.67 ± 0.01 g and 0.83 ± 0.01 g for DLP-printed and FDM-printed AIWs, respectively, n = 3–4) and will not significantly affect the daily behaviors of the mice. The 3D printing allows for them to be printed right before use and gives the option of customizing the size, if needed. Since they are plastic and inexpensive, they are single-use and do not need to be harvested and cleaned to be reused, which reduces the potential risks of contamination.
3.3. Abdominal Window Implantation
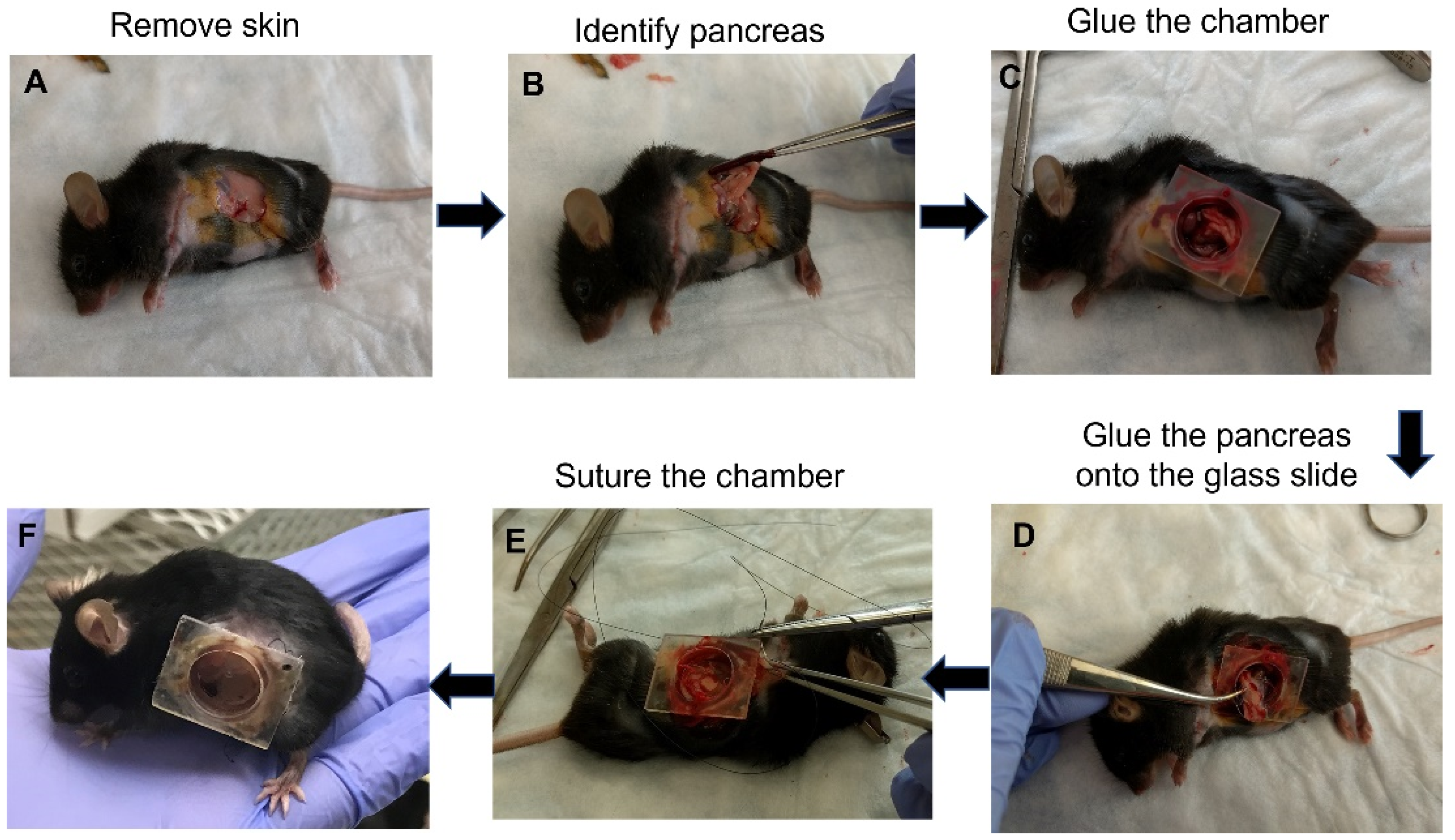
The surgery for implanting the AIW into mice is quick and simple (Figure 2A–F). The AIW is designed to make the surgery easy and consistent. Before the surgery, the AIW is sterilized, and a sterile glass coverslip is glued into place in the well. Briefly, to implant the AIW, a 0.5 cm incision is made in the skin and abdominal wall above the tissue of interest (Figure 2A). In this case, it was above the pancreas. The spleen was removed to prevent visual obstruction, and the pancreas was identified (Figure 2B), moved into position, and glued to the coverslip to prevent movement (Figure 2C,D). Then the abdominal muscle wall was glued to the outside of the well of the AIW to create a biological seal. The skin was then fit into the groove of the AIW and held in place using a purse-string suture (Figure 2E). The groove in the ring of the AIW allows for consistent, easy placement of the abdominal wall and skin to create a seal and prevent infection. During the surgery, the tissue of interest (pancreas in this study) can be manipulated and have anything added to it that is needed, and then it can be sealed for sterility while being able to keep visually monitoring it (Figure 2F). The mice tolerated the AIWs well (for at least three months), and they did not inhibit the movement or health of the mice that had them implanted (Supplementary Video S1).
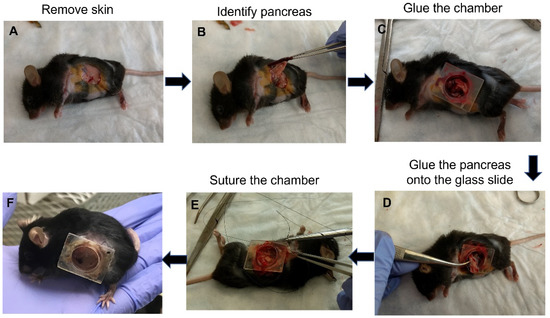
Figure 2.
Procedure for implanting the AIW into a mouse for pancreatic imaging: (A) Skin opening to expose internal organs; (B) Identification of the pancreas; (C) Gluing of the AIW into the skin; (D) Gluing of the pancreas on the glass slide and insertion of the glass slide into the AIW; (E) Suturing of the AIW into the skin; (F) Mouse with attached AIW after surgery.
3.4. Microscope Mount Design and 3D Printing
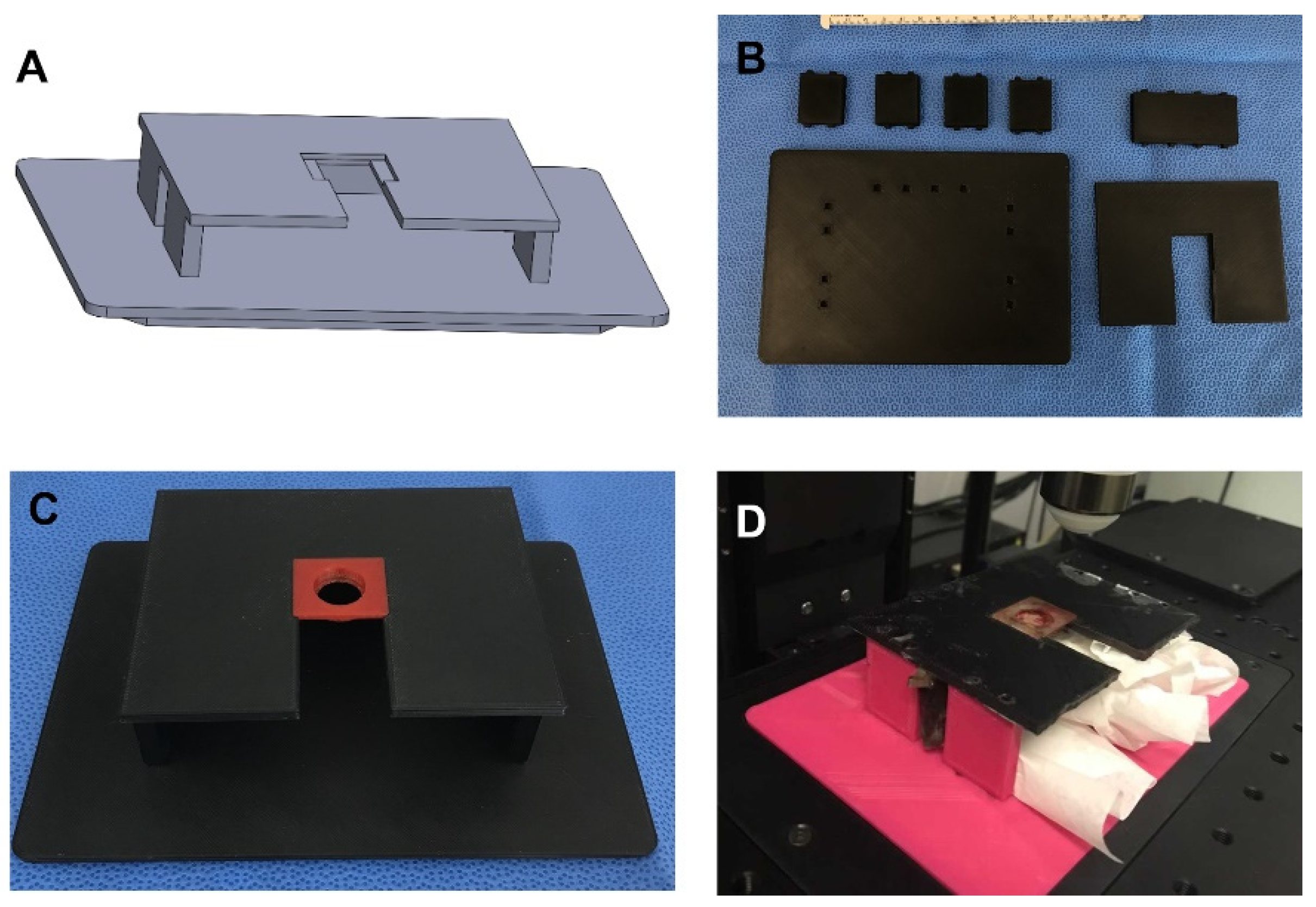
The customizable microscope mount is an important tool for facilitating the imaging with a low number of artifacts. It was 3D printed using PLA, so it is inexpensive and can be easily customized to fit various needs (Figure 3). It can also be designed to accommodate animals of various sizes, tissue presentation, and microscope stages (Figure 3A). The microscope mount is made up of a base, five side brackets (4 sides and 1 back), and the top mount (Figure 3B). The base is designed to fit into the stage without any movement. We fit it to the stage of an Olympus FVMPE-RS two-photon microscope. It has slots to fit all five of the side brackets. The vertical side brackets are designed to fit with openings in order to facilitate continuous monitoring of the animal as well as support the use of devices, such as oxygen, isoflurane, and a heating apparatus. The side brackets are tall enough to comfortably fit animals of various sizes. The top piece is designed as the mount. It has slots on the underside that fit onto the tops of the side brackets. There is a slot to allow for the well and skin of the AIWs to slide through it. This slot leads to an indention that is made to fit the AIW tightly and hold it steady (Figure 3C). The indention is centered under the objective. This solid plastic top mount is also able to protect the animal from rogue light/laser sources and mounting media. The gap between the top mount and the base is designed to be large enough to comfortably fit animals of multiple sizes and also allow for some slight tension from gravity on the skin and AIW to prevent unwanted movement from the animal (Figure 3D).
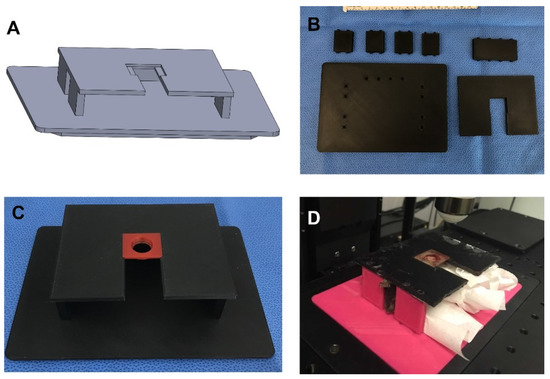
Figure 3.
Microscope mount for AIWs: (A) 3D CAD design of the microscope mount for AIWs; (B) all microscope mount parts before assembling; (C) assembled microscope mount with AIW mounted; (D) full AIW and mount system with the mouse on the microscope.
3.5. In Vivo Imaging
The design of the AIW allows for repeated in vivo imaging of abdominal tissues of interest of a living animal. The AIW also allows for imaging of the same location on the tissue of interest longitudinally, so researchers can compare changes in a particular area over time.
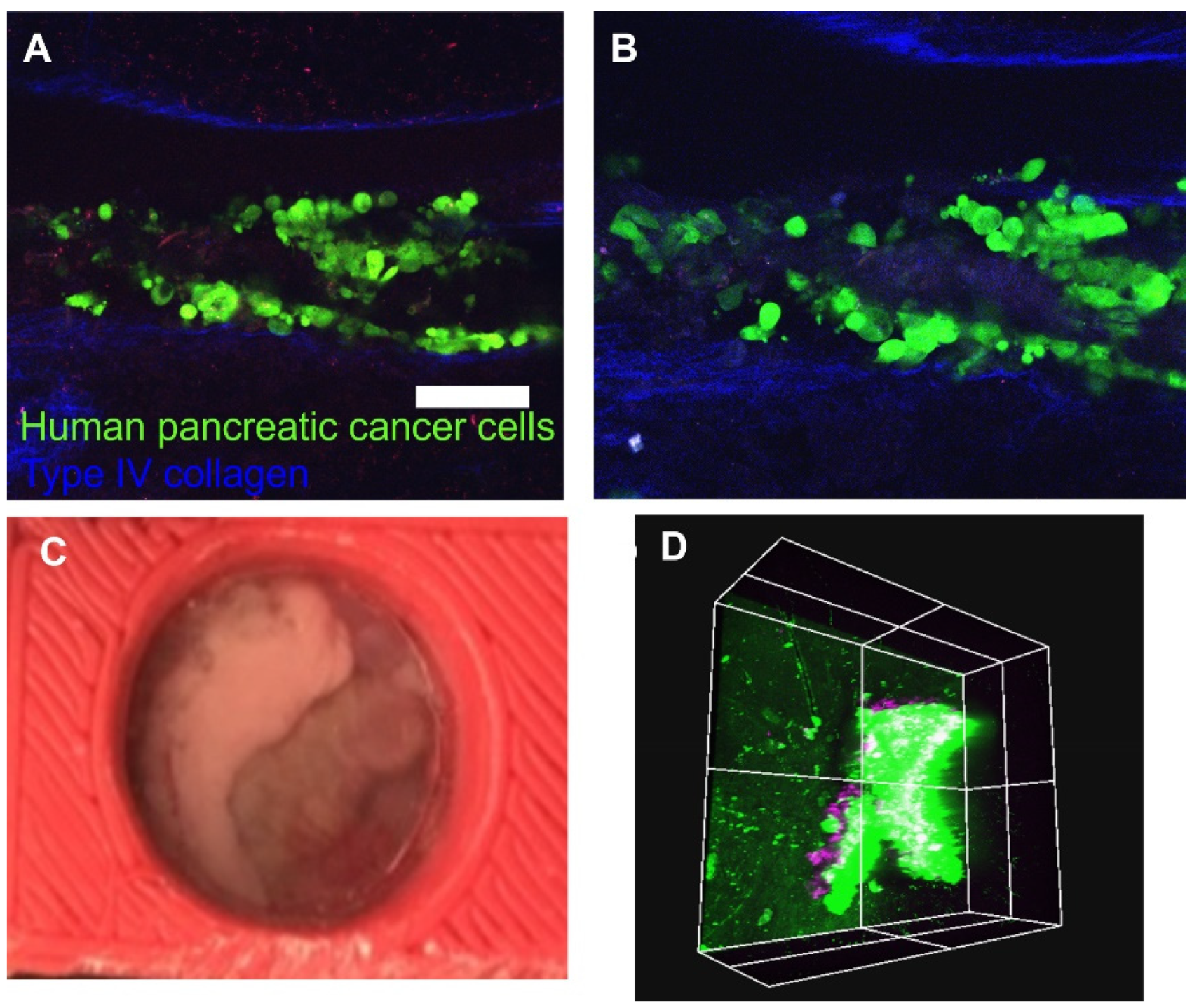
In the current study, we delivered human pancreatic cancer cells to the pancreas to image changes in the tissue over time (Figure 4A,B). After the AIW was implanted, features of the organ were macroscopically visible through the coverslip (Figure 4C). It would be feasible to observe vascular formation, macroscopic metastasis, cyst formation, and other features macroscopically or with the aid of a dissecting microscope.
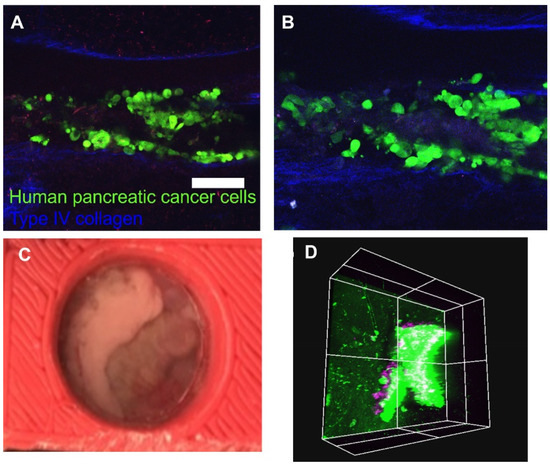
Figure 4.
In vivo imaging. (A,B) Longitudinal images captured from a windowed specimen that depict tumor cell congregation (green) between deep groove collagen structures (blue), (A) 24 h post-injection, (B) 48 h post-injection. The green color represents human pancreatic cancer cells (S2-013), and the blue color represents Type IV collagen; (C) macroscopic view of the pancreas attached to the AIW; (D) in vivo image of myelin-directed NP41 labeled neuron (purple) penetrating a mass of orthotopically implanted GFP expressing pancreatic cancer cells, acquired via AIW. Scale bar: 100 μm.
After the subject with the AIW is mounted, it is able to be imaged by any epifluorescent light or excitation source. We were able to lightly sedate the animal during imaging, but they could also be heavily sedated if needed. The same tissue location can be imaged over the course of hours, days, or months through different mapping techniques. In vivo images of the same location on the pancreas 24 h (Figure 4A) and 48 h (Figure 4B) after cancer cell injection were taken with the two-photon microscope through the AIW to observe the changes. GFP labeled pancreatic cancer cells are shown in green, and deep groove type IV collagen, which is innately fluorescent, structures are shown in blue. Myelin-directed NP41 labeled neurons (shown in purple) from a penetrating mass of orthotopically implanted GFP expressing pancreatic cancer cells were imaged through the AIW and two-photon microscope (Figure 4D). Large features of the tissue, such as large vasculature and collagen branches, can be used as fiducial markers to guide the imaging back to a certain location of interest. The imaging can also be guided by marks made on the coverslip itself, if needed. Endogenously fluorescent proteins or exogenous labeling, such as injected antibodies or nanoparticles, could also be used as landmarks depending on how the labels react and changes in the tissue.
3.6. Extension Designs and Applications
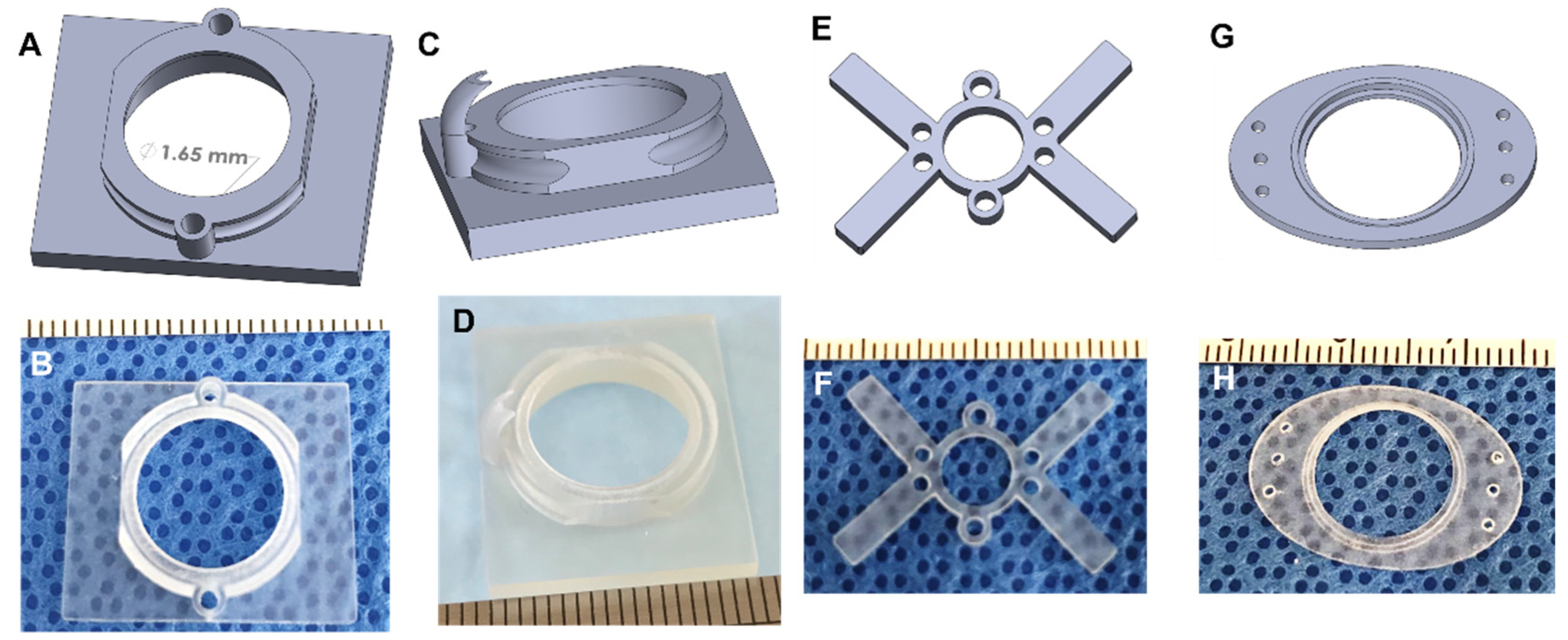
Apart from the basic design, several different types of AIWs were designed to enable some extra functionalities. One design has a port (or two) that allows for access to the tissue while the AIW is implanted and can be used to deliver drugs, cells, or other materials to the tissue of interest and for blunt probe manipulation (Figure 5A,B). These ports are a convenient way to modify the tissue and maintain the imaging location without another surgery and while maintaining sterility. These ports can be plugged or covered with repeat-puncture-safe rubber to maintain sterility. Another design uses the delivery port, but it has a guide added to the underside of the AIW (Figure 5C,D). This guide can be used to hold a small tube in position for the delivery of various materials, or it can be used to hold a specific piece of tissue in position for manipulation multiple times.
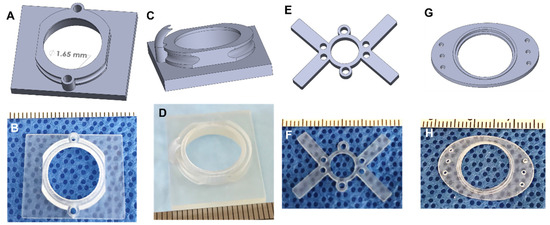
Figure 5.
Extended designs of the imaging windows. (A) The 3D CAD design of 2-port AIW; (B) the 3D printed 2-port AIW; (C) the 3D CAD design of 1-guide AIW; (D) the 3D-printed 1-guide AIW; (E) the 3D CAD design of brain imaging window with delivery ports; (F) the 3D-printed brain imaging window with delivery ports; (G) the 3D CAD design of the subcutaneous or mammary imaging window; (H) the 3D-printed subcutaneous or mammary imaging window.
While using the AIWs for abdominal tissue imaging, we realized that their features would be useful for imaging other areas of the body. We engineered 3D printable plastic imaging windows with the same coverslip, port, and mounting features for brain imaging (Figure 5E,F) and coverslip features for subcutaneous or mammary imaging (Figure 5G,H). Methods for using a similar brain imaging window have been established, but our brain imaging windows add extra features for expanded use. They are also made of a light plastic, so they are significantly lighter than the current metal ones. The brain imaging window fits into the same mount as the AIWs, but it is made to be lighter and less bulky, so that it does not hinder the head movement or feeding of the animal. Similar to the AIWs, the ports serve the function of delivering materials to the brain without another surgery while maintaining sterility. The brain windows allow for longitudinal imaging, and the well depth can be customized to the imaging modality. They are implanted into the animal in a similar manner to the AIWs as well, so they are easy to use.
We designed a 3D printable plastic subcutaneous or mammary imaging window that is a slimmed down and unobtrusive way to monitor and image materials that are implanted under the skin or for mammary tissue. The device is implanted into the skin layer above the implanted material, which can be attached to the window itself, as with the tissue in the AIW. It can be secured using a purse string suture as well as through suture holes on the ends of the device. A mounting bracket can be added to the device for use in fluorescent imaging. This subcutaneous and mammary imaging window can be customized to fit different sized animals and for different mounting purposes. Ports can also be used with this design to deliver substances to the implanted material.
4. Discussion
Advances in two-/multi-photon microscopy allow for deep tissue cellular imaging, but this is limited by the ability to image the tissue at a single time point after an animal has been sacrificed. Having the ability to longitudinally study biological cellular processes and disease progression of living animals in vivo would increase the knowledge of those cellular processes and diseases and lead to progress in treatments. We have engineered permanent, 3D printable, surgically implanted AIWs for the intravital imaging of internal organs of living animals.
Two-/Multi-photon imaging works by sending highly focused light energy to stimulate the fluorescence of labeled cellular components. This allows the visualization of the cellular landscape of tissues and tumors, but the sub-cellular features and individual cellular markers can also be seen. A limitation of this form of microscopy is that skin absorbs this energy, which prevents the visceral tissue from being excited. Parting the skin to facilitate photon penetrance causes surgically induced changes to the tumor progression upon closure (a cascade of inflammatory regulators, clotting factors, and immune cells all flood the area and incidentally alter tumor progression). These pathological responses can be mitigated by allowing for recovery time after the surgical placement of a device followed then by orthotopic challenge. Being able to image deep tissue through the skin without pathological responses altering the tissue of interest is an important hurdle to overcome.
We engineered permanent 3D-printable, surgically implantable AIWs that solve issues of intravital imaging while providing several other features that ensure accurate imaging and tissue manipulation. The AIWs and their mounting systems are engineered to facilitate longitudinal imaging with little artifacts from the movement of the animal from respiration or other movements. They were 3D printed using biocompatible materials, so there are limited adverse effects from implantation. By suturing the AIW into the abdominal wall and skin and gluing a cover-slip into the bottom of the center well, it provides a biological seal to prevent infection while maintaining a line of sight with the tissue of interest. The tissue of interest is attached to the glass coverslip. This means that it stays in place, which allows for landmarks in the tissue, such as vasculature, collagen branches, endogenous fluorescent proteins, and exogenous fluorescent markers, as well as markers on the glass, to be used to image the same location of the tissue multiple times over various time points. The center well is made to be able to accommodate several different types of microscope objectives.
We developed AIWs with extra features for tissue manipulation after AIW implantation. One version of the AIW has a port near the side of the center well. These ports allow access to the tissue while the AIWs are implanted. They can be used for the delivery of materials to the tissue of interest through needles, tubes, or other devices, as well as for the manipulation of the tissue using a blunt probe. The ports can be plugged or covered with a repeat-puncture safe rubber to maintain sterility. Another version of the AIW has the same ports, but it has an added guide on the underside of the port. This guide can be used to hold a tube or other device in place to deliver materials to the tissue over time, or it could be used to hold a piece of the tissue in place for ease of access.
To make the AIW completely functional with microscopy, we created a 3D printable mounting system that attaches to the stage of a microscope and steadily holds the animal with the implanted window in place under the objective. The mount is customizable to the size of the animals, the microscope stage, and the size of the AIW mounting bracket used. The whole AIW and microscope mount system are able to work together to facilitate the imaging of visceral abdominal tissues over time with minimal artifacts.
Various organ features, including changes to vascular formation, macroscopic metastasis, cyst formation, and other features, can be observed macroscopically or with the aid of a dissecting microscope. Variable surgical placement allows for visualization of various regions of interest, including the pancreatic body, fluid suspension of tumor cells, major pancreatic vasculature, and liver lobes. Our AIW captured a view of the pancreas head and body, duodenum, and portal duct. Once mounted, the windowed subject’s tissues are imageable via any epifluorescent light or excitation source. The user can easily image the same tissue location over the course of hours, days, or months through various mapping techniques. As an example, we longitudinally imaged the same point on the pancreas at 24 and 48 h after cancer cell injection through the AIWs and a two-photon microscope. This allows for the viewing of the cellular and structural changes of the environment. Large vasculature and collagen branches can be used as fiduciary markers to guide the user to their region of interest. The same can be achieved by using markings on the coverslip or using gridded coverslips, especially for multiple regions of interest. Endogenously expressing fluorescent proteins can also make unique landmarks, although it is worthwhile to consider that any number of conditions can affect repeated use of exogenous labels, such as injected labeled antibodies or nanoparticles. Animals can be heavily or lightly sedated during imaging.
In the future, the AIWs should be tested on larger animal types and be used to collect time-lapse imaging data of cancer progression. They would work under the same idea but would have to be expanded to be larger. More types of microscopes should be used to test for any adjustments that would need to be made to the AIWs and their mounts so that they can accommodate all types. The AIWs could be further improved by adding more secondary features to extend their functionality, such as adding a removable mounting bracket or location markings on the windows themselves. Using the windows for monitoring more types of cells and more types of tissue would prove their functionality and allow for more knowledge of cellular and disease progression over time.
5. Conclusions
We were able to engineer a 3D printable, surgically implantable abdominal imaging window that allows for imaging of visceral tissues and cells of living animals for longitudinal studies. The system of using the mountable AIW with a 3D printable, customizable microscope mount allows for imaging with multiple devices and with minimal artifacts from animal movement. We also developed AIWs with features that allow for the delivery of materials to the tissue of interest along with the ability to manipulate it, all while maintaining sterility. We were able to longitudinally image pancreatic tumor cells, matrix, and neurons in a pancreas using a two-photon microscope. We also designed some imaging windows that can be used for brain, subcutaneous, and mammary imaging that have features for enhancing longitudinal imaging. This AIW with a microscope mount system is an effective method for facilitating imaging of cellular changes and disease progression in the same location of visceral abdominal tissues of living animals on multiple occasions over an extended period of time.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/machines10080697/s1, Video S1: Mice moving freely with implanted AIWs.
Author Contributions
Conceptualization, M.A.H. and B.D.; methodology, A.J.C. and M.K.; validation, O.A.A.; writing—original draft preparation, A.J.C. and M.K.; writing—review and editing, O.A.A., M.A.H., and B.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the Mary and Dick Holland Regenerative Medicine Program start-up grant, the Mary and Dick Holland Regenerative Medicine Program pilot project grant, Nebraska Research Initiative funding, and the University of Nebraska Collaboration Initiative Seed Grant to B.D. The authors would like to thank the McGoogan Health Sciences Library at the University of Nebraska Medical Center for the use of the Jim and Karen Linder Maker Studio. The authors acknowledge the Multiphoton Intravital and Tissue Imaging Core (MITI), which receives support through the Nebraska Center for Nanomedicine and the Cognitive Neuroscience of Development and Aging Centers for Biomedical Research Excellence (NIH NIGMS P30 GM127200, P20 GM130447), the Fred and Pamela Buffett Cancer Center support grant (NIH NCI P30CA036727), state funds from the Nebraska Research Initiative, and institutionally through the UNMC Office of the Vice Chancellor for Research. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center (protocol code 16-124-12-FC, approval date 1 October 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Turk, M.; Naumenko, V.; Mahoney, D.J.; Jenne, C.N. Tracking Cell Recruitment and Behavior within the Tumor Microenvironment Using Advanced Intravital Imaging Approaches. Cells 2018, 7, 69. [Google Scholar] [CrossRef]
- Dawson, C.A.; Mueller, S.N.; Lindeman, G.J.; Rios, A.C.; Visvader, J.E. Intravital microscopy of dynamic single-cell behavior in mouse mammary tissue. Nat. Protoc. 2021, 16, 1907–1935. [Google Scholar] [CrossRef]
- Entenberg, D.; Pastoriza, J.M.; Oktay, M.H.; Voiculescu, S.; Wang, Y.; Sosa, M.S.; Aguirre-Ghiso, J.; Condeelis, J. Time-lapsed, large-volume, high-resolution intravital imaging for tissue-wide analysis of single cell dynamics. Methods 2017, 128, 65–77. [Google Scholar] [CrossRef]
- Cao, L.; Kobayakawa, S.; Yoshiki, A.; Abe, K. High Resolution Intravital Imaging of Subcellular Structures of Mouse Abdominal Organs Using a Microstage Device. PLoS ONE 2012, 7, e33876. [Google Scholar] [CrossRef]
- Deng, D.; Dai, B.; Wei, J.; Yuan, X.; Yang, X.; Qi, S.; Zhang, Z. A drawer-type abdominal window with an acrylic/resin coverslip enables long-term intravital fluorescence/photoacoustic imaging of the liver. Nanophotonics 2021, 10, 3369–3381. [Google Scholar] [CrossRef]
- Lee, S.; Vinegoni, C.; Sebas, M.; Weissleder, R. Automated motion artifact removal for intravital microscopy, without a priori information. Sci. Rep. 2014, 4, 4507. [Google Scholar] [CrossRef]
- Andresen, V.; Pollok, K.; Rinnenthal, J.-L.; Oehme, L.; Günther, R.; Spiecker, H.; Radbruch, H.; Gerhard, J.; Sporbert, A.; Cseresnyes, Z.; et al. High-Resolution Intravital Microscopy. PLoS ONE 2012, 7, e50915. [Google Scholar] [CrossRef]
- Hato, T.; Winfree, S.; Dagher, P.C. Intravital imaging of the kidney. Methods 2017, 128, 33–39. [Google Scholar] [CrossRef]
- Warren, S.C.; Nobis, M.; Magenau, A.; Mohammed, Y.H.; Herrmann, D.; Moran, I.; Vennin, C.; Conway, J.R.; Mélénec, P.; Cox, T.R.; et al. Removing physiological motion from intravital and clinical functional imaging data. eLife 2018, 7, e35800. [Google Scholar] [CrossRef]
- Burkel, B.M.; Inman, D.R.; Virumbrales-Muñoz, M.; Hoffmann, E.J.; Ponik, S.M. A label-free segmentation approach for intravital imaging of mammary tumor microenvironment. J. Vis. Exp. 2022, 183, e63413. [Google Scholar] [CrossRef]
- Williams, J.K.; Entenberg, D.; Wang, Y.; Avivar-Valderas, A.; Padgen, M.; Clark, A.; Aguirre-Ghiso, J.A.; Castracane, J.; Condeelis, J.S. Validation of a device for the active manipulation of the tumor microenvironment during intravital imaging. IntraVital 2016, 5, e1182271. [Google Scholar] [CrossRef][Green Version]
- Sorg, H.; Krueger, C.; Vollmar, B. Intravital insights in skin wound healing using the mouse dorsal skin fold chamber. J. Anat. 2007, 211, 810–818. [Google Scholar] [CrossRef]
- Koehl, G.E.; Gaumann, A.; Geissler, E.K. Intravital microscopy of tumor angiogenesis and regression in the dorsal skin fold chamber: Mechanistic insights and preclinical testing of therapeutic strategies. Clin. Exp. Metastasis 2009, 26, 329–344. [Google Scholar] [CrossRef]
- Rücker, M.; Laschke, M.W.; Junker, D.; Carvalho, C.; Schramm, A.; Mülhaupt, R.; Gellrich, N.-C.; Menger, M.D. Angiogenic and inflammatory response to biodegradable scaffolds in dorsal skinfold chambers of mice. Biomaterials 2006, 27, 5027–5038. [Google Scholar] [CrossRef]
- Ritsma, L.; Steller, E.J.A.; Ellenbroek, S.; Kranenburg, O.; Rinkes, I.H.M.B.; Van Rheenen, J. Surgical implantation of an abdominal imaging window for intravital microscopy. Nat. Protoc. 2013, 8, 583–594. [Google Scholar] [CrossRef]
- Lloyd-Lewis, B. Multidimensional Imaging of Mammary Gland Development: A Window Into Breast Form and Function. Front. Cell Dev. Biol. 2020, 8, 203. [Google Scholar] [CrossRef]
- Mourao, L.; Ciwinska, M.; van Rheenen, J.; Scheele, C.L.G.J. Longitudinal Intravital Microscopy using a Mammary Imaging Window with Replaceable Lid. J. Vis. Exp. 2022, 179, e63326. [Google Scholar] [CrossRef]
- Sobolik, T.; Su, Y.-J.; Ashby, W.; Schaffer, D.K.; Wells, S.; Wikswo, J.P.; Zijlstra, A.; Richmond, A. Development of novel murine mammary imaging windows to examine wound healing effects on leukocyte trafficking in mammary tumors with intravital imaging. IntraVital 2016, 5, e1125562. [Google Scholar] [CrossRef]
- Heo, C.; Park, H.; Kim, Y.-T.; Baeg, E.; Kim, Y.H.; Kim, S.-G.; Suh, M. A soft, transparent, freely accessible cranial window for chronic imaging and electrophysiology. Sci. Rep. 2016, 6, 27818. [Google Scholar] [CrossRef]
- Roome, C.J.; Kuhn, B. Chronic cranial window with access port for repeated cellular manipulations, drug application, and electrophysiology. Front. Cell. Neurosci. 2014, 8, 379. [Google Scholar] [CrossRef]
- Bochner, F.; Fellus-Alyagor, L.; Kalchenko, V.; Shinar, S.; Neeman, M. A Novel Intravital Imaging Window for Longitudinal Microscopy of the Mouse Ovary. Sci. Rep. 2015, 5, srep12446. [Google Scholar] [CrossRef] [PubMed]
- Ritsma, L.; Steller, E.J.A.; Beerling, E.; Loomans, C.J.M.; Zomer, A.; Gerlach, C.; Vrisekoop, N.; Seinstra, D.; van Gurp, L.; Schäfer, R.; et al. Intravital Microscopy Through an Abdominal Imaging Window Reveals a Pre-Micrometastasis Stage During Liver Metastasis. Sci. Transl. Med. 2012, 4, 158ra145. [Google Scholar] [CrossRef] [PubMed]
- Alieva, M.; Ritsma, L.; Giedt, R.J.; Weissleder, R.; van Rheenen, J. Imaging windows for long-term intravital imaging: General overview and technical insights. IntraVital 2014, 3, e29917. [Google Scholar] [CrossRef]
- Jacquemin, G.; Benavente-Diaz, M.; Djaber, S.; Bore, A.; Dangles-Marie, V.; Surdez, D.; Tajbakhsh, S.; Fre, S.; Lloyd-Lewis, B. Longitudinal high-resolution imaging through a flexible intravital imaging window. Sci. Adv. 2021, 7, eabg7663. [Google Scholar] [CrossRef]
- Shi, W.; Dong, P.; Kuss, M.A.; Gu, L.; Kievit, F.; Kim, H.J.; Duan, B. Design and Evaluation of an In Vitro Mild Traumatic Brain Injury Modeling System Using 3D Printed Mini Impact Device on the 3D Cultured Human iPSC Derived Neural Progenitor Cells. Adv. Health Mater. 2021, 10, 2100180. [Google Scholar] [CrossRef]
- Qi, D.; Wu, S.; Lin, H.; Kuss, M.A.; Lei, Y.; Krasnoslobodtsev, A.; Ahmed, S.; Zhang, C.; Kim, H.J.; Jiang, P.; et al. Establishment of a Human iPSC- and Nanofiber-Based Microphysiological Blood–Brain Barrier System. ACS Appl. Mater. Interfaces 2018, 10, 21825–21835. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).