Fractional Order Mathematical Modelling of HFMD Transmission via Caputo Derivative
Abstract
1. Introduction
2. Model Formulation
3. Preliminaries
4. Positivity and Boundedness
5. Existence and Uniqueness
6. Model Analysis
6.1. Equilibrium Points
6.2. Basic Reproduction Number (BRN)
6.3. Local Stability
- , , , , ,
- , , , , ,
- , , , , ,
- , .
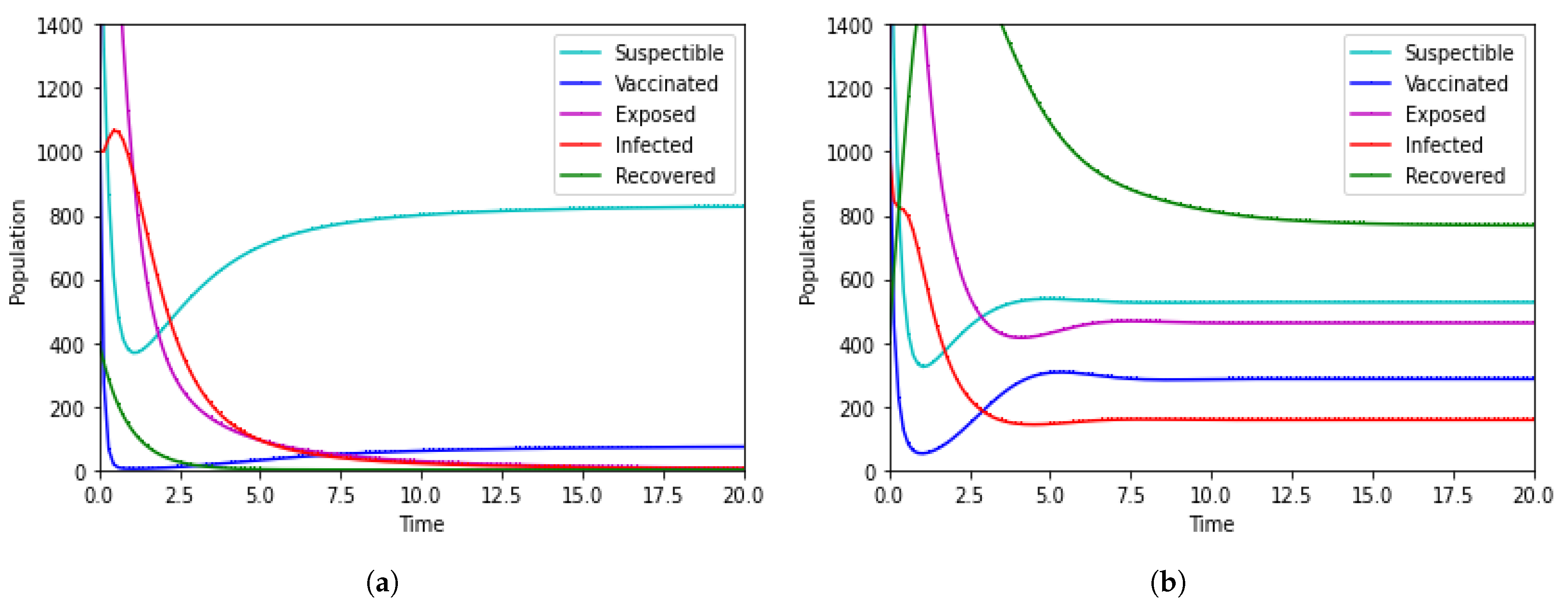
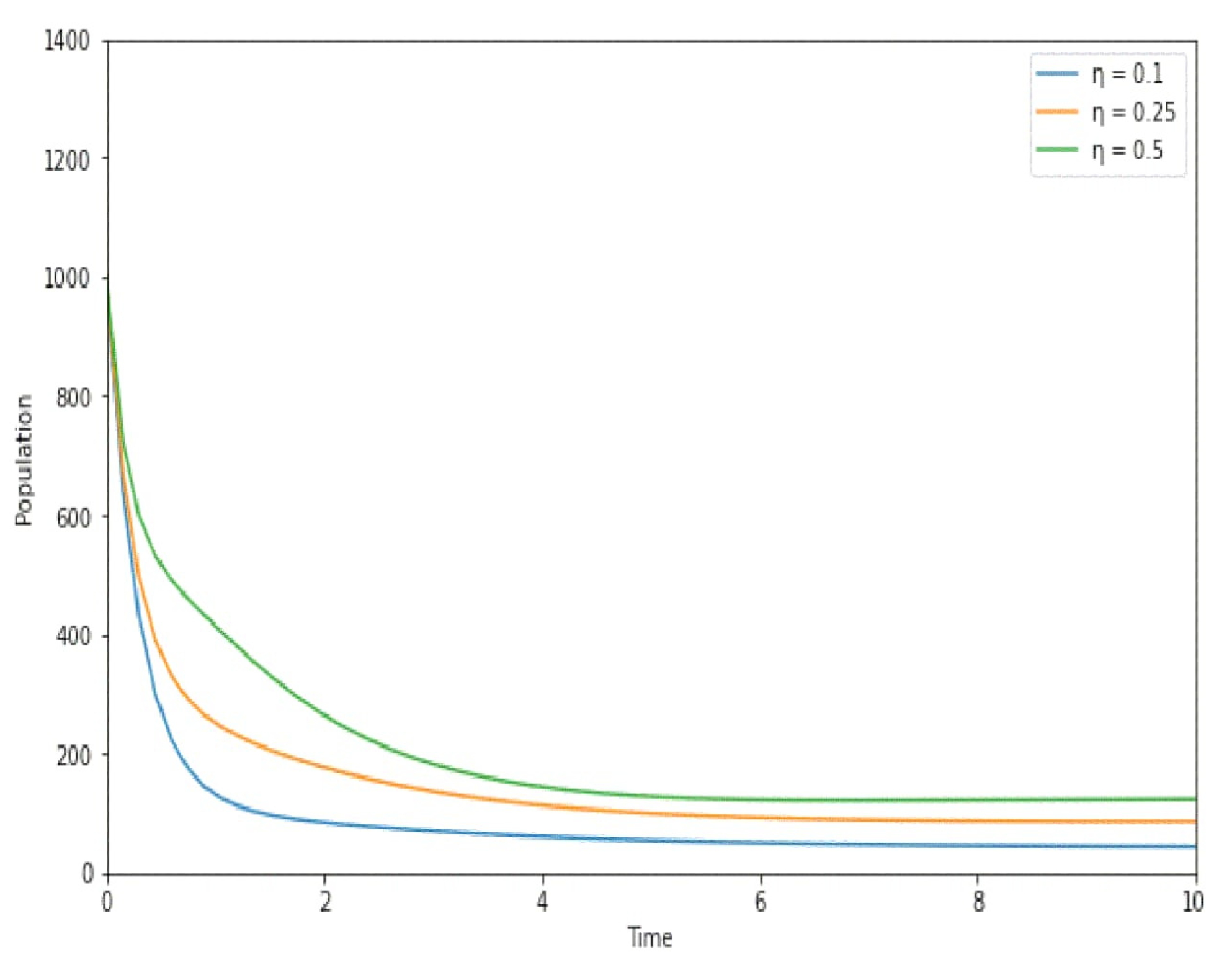
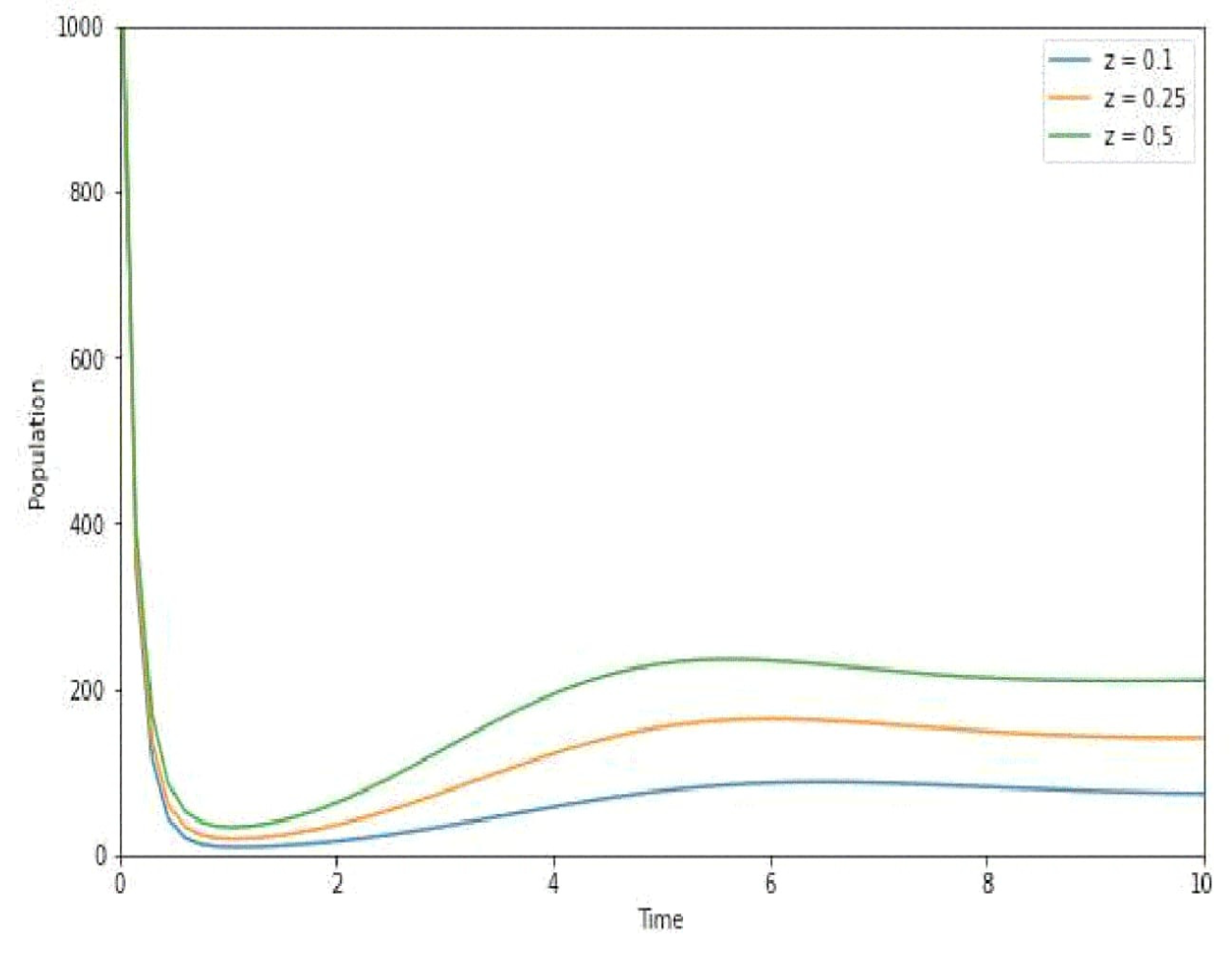
7. Numerical Simulation
8. Discussion
- Improved Understanding of Transmission Dynamics: The proposed fractional order mathematical model enhanced the understanding of the transmission dynamics of HFMD. By utilizing non-integer derivatives, the model may capture more accurately the complex and memory-dependent aspects of disease spread. This improved understanding is fundamental for designing targeted control strategies;
- Predictive Capabilities for Outbreaks: This model contributes to the development of more accurate predictive tools for HFMD outbreaks. By considering non-local interactions and historical data more effectively, the model could provide better forecasts, enabling authorities to prepare for and respond to potential outbreaks more proactively;
- Assessing the Impact of Control Measures: The model’s ability to capture the intricacies of HFMD transmission can be valuable in assessing the impact of implemented control measures. This feedback loop is crucial for evaluating the effectiveness of interventions and refining strategies as needed to enhance overall disease control efforts;
- Informing Vaccination Policies: Fractional order modeling may assist in informing vaccination policies by providing a more nuanced understanding of how vaccination efforts impact disease transmission. This could aid in optimizing vaccination coverage, determining the most effective age groups for vaccination, and adapting strategies in response to changing epidemiological trends;
- Resource Allocation and Public Health Planning: A more accurate representation of HFMD transmission dynamics can contribute to better resource allocation and planning. Health agencies can use the model to identify high-risk areas or populations, allocate resources strategically, and plan interventions in a way that maximizes their impact on disease prevention.
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santhosh Kumar, G.; Gunasundari, C. Turing instability of a Diffusive Predator-Prey Model along with an Allee Effect on a Predator. Commun. Math. Biol. Neurosci. 2022, 40, 1–15. [Google Scholar]
- Sharmila, N.B.; Gunasundari, C. Travelling Wave Solutions for a Diffusive Prey-Predator Model with One Predator and Two Preys. Int. J. Appl. Math. 2022, 35, 661. [Google Scholar] [CrossRef]
- Ahmadini, A.H.A.; Hassan, A.S.; Zaky, A.N.; Alshqaq, S.S. Bayesian inference of dynamic cumulative residual entropy from Pareto II distribution with application to COVID-19. AIMS Math. 2020, 6, 2196–2216. [Google Scholar] [CrossRef]
- Aakash, M.; Gunasundari, C.; Al-Mdallal, Q.M. Mathematical modeling and simulation of SEIR model for COVID-19 outbreak: A case study of Trivandrum. Front. Appl. Math. Stat. 2023, 9, 1124897. [Google Scholar]
- Aakash, M.; Gunasundari, C. Effect of Partially and Fully Vaccinated Individuals in some Regions of India: A Mathematical Study on COVID-19 Outbreak. Commun. Math. Biol. Neurosci. 2023, 2023, 25. [Google Scholar]
- Sivakumar, S.; Moni, S.I.A.; Aamena, J.; Mohammed, E.E.; Duaa, A.; Gassem, G.; Bassem, O.; Abdulla, M.F.; Ahmed, A.J.; Mahdi, M.A.; et al. Advancements in Vaccine Adjuvants: The Journey from Alum to Nano Formulations. Vaccines 2023, 11, 1704. [Google Scholar]
- Abdulaziz, A.; Famara, S.; Mouna, L.; Magloire, P.N.; Francis, B.B.; Ilka, E.; Enagnon, K.A.; Didier, H. Enteroviruses and Type 1 Diabetes Mellitus: An Overlooked Relationship in Some Regions. Microorganisms 2020, 8, 1458. [Google Scholar]
- Syed, Z.S.; Basit, J.; Muhammad, U.M.; Muhammad, W.; Shahkaar, A.; Sobia, A.H.; Amjad, A.; Shazia, R.; Muhammad, I.; Asaad, K.; et al. An Immunoinformatics Approach to Design a Potent Multi-Epitope Vaccine against Asia-1 Genotype of Crimean-Congo Haemorrhagic Fever Virus Using the Structural Glycoproteins as a Target. Vaccines 2023, 11, 61. [Google Scholar]
- Wongvanich, N.; Tang, I.-M.; Dubois, M.-A.; Pongsumpun, P. Mathematical Modeling and Optimal Control of the Hand Foot Mouth Disease Affected by Regional Residency in Thailand. Mathematics 2021, 9, 2863. [Google Scholar] [CrossRef]
- Sun, J.; Wu, S.; Yan, Z.; Li, Y.; Yan, C.; Zhang, F.; Liu, R.; Du, Z. Using geographically weighted regression to study the seasonal influence of potential risk factors on the incidence of HFMD on the Chinese mainland. ISPRS Int. J. Geo-Inf. 2021, 10, 448. [Google Scholar] [CrossRef]
- Shi, R.; Lu, T. Dynamic analysis and optimal control of a fractional order model for hand-foot-mouth disease. J. Appl. Math. Comput. 2020, 64, 565–590. [Google Scholar] [CrossRef]
- Gashirai, T.B.; Hove-Musekwa, S.D.; Mushayabasa, S. Dynamical analysis of a fractional-order foot-and-mouth disease model. Math. Sci. 2021, 15, 65–82. [Google Scholar] [CrossRef]
- Gashirai, T.B.; Hov-Musekwa, S.D.; Mushayabasa, S. Optimal control applied to a fractional-order foot-and-mouth disease model. Int. J. Appl. Comput. Math. 2021, 7, 73. [Google Scholar] [CrossRef]
- Rashid, J.; Boulaaras, S.; Alyobi, S.; Jawad, M. Transmission dynamics of Hand–Foot–Mouth Disease with partial immunity through non-integer derivative. Int. J. Biomath. 2023, 16, 2250115. [Google Scholar]
- Rao, S.N.; Alesemi, M. On a coupled system of fractional differential equations with nonlocal non-separated boundary conditions. Adv. Differ. Equ. 2019, 2019, 97. [Google Scholar] [CrossRef]
- Li, X.P.; DarAssi, M.H.; Khan, M.A.; Chukwu, C.W.; Mohammad, Y.A.; Mesfer, A.S.; Muhammad, B.R. Assessing the potential impact of COVID-19 Omicron variant: Insight through a fractional piecewise model. Results Phys. 2022, 38, 105652. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.R.; Haloi, R.; Bahuguna, D.; Dwijendra, N.P. Existence of solutions to a new class of abstract non-instantaneous impulsive fractional integro-differential equations. Nonlinear Dyn. Syst. Theory. 2016, 16, 73–85. [Google Scholar]
- Atangana, A.; Baleanu, D. New fractional derivatives with nonlocal and non-singular kernel: Theory and application to heat transfer model. Therm Sci. 2016, 20, 763–769. [Google Scholar] [CrossRef]
- Fatmawati; Shaiful, E.M.; Utoyo, M.I. A fractional-order model for HIV dynamics in a two-sex population. Int. J. Math. Math. Sci. 2018, 2018, 763–769. [Google Scholar]
- Bonyah, E.; Zarin, R.; Fatmawati, E.M. Mathematical modeling of cancer and hepatitis co-dynamics with non-local and non-singular kernel. Commun. Math. Biol. Neurosci. 2020.
- Tarasova, V.V.; Tarasov, V.E. Comments on the article long and short memory in economics: Fractional-order difference and differentiation. Probl. Mod. Sci. Educ. 2017, 31, 26–28. [Google Scholar] [CrossRef]
- Wang, W.; Khan, M.A.; Fatmawati; Kumam, P.; Thounthong, P. A Comparison study of bank data in fractional calculus. Chaos Solitons Fractals 2019, 126, 369–384. [Google Scholar] [CrossRef]
- Khan, F.S.; Khalid, M.; Al-moneef, A.; Ali, A.H.; Bazighifan, O. Freelance Model with Atangana–Baleanu Caputo Fractional Derivative. Symmetry 2022, 14, 2424. [Google Scholar] [CrossRef]
- Haq, I.U.; Yavuz, M.; Ali, N.; Akgül, A. A SARS-CoV-2 Fractional-Order Mathematical Model via the Modified Euler Method. Math. Comput. Appl. 2022, 27, 82. [Google Scholar] [CrossRef]
- Sweilam, N.H.; AL-Mekhlafi, S.M.; Hassan, S.M.; Alsenaideh, N.R.; Radwan, A.E. New Coronavirus (2019-nCov) Mathematical Model Using Piecewise Hybrid Fractional Order Derivatives; Numerical Treatments. Mathematics 2022, 10, 4579. [Google Scholar] [CrossRef]
- Sharmila, N.B.; Gunasundari, C. Stability Analysis of a Fractional Order Prey-Predator Model with Disease in Preys. Math. Appl. 2022, 50, 287–302. [Google Scholar]
- Nortey, S.N.; Juga, M.; Bonyah, E. Fractional order modelling of Anthrax-Listeriosis coinfection with nonsingular Mittag Leffler law. Sci. Afr. 2022, 16, 287–302. [Google Scholar] [CrossRef]
- Aakash, M.; Gunasundari, C.; Rashid, J. Modelling and Analysis of Vaccination Effects on Hand, Foot, and Mouth Disease Transmission Dynamics. Math. Model. Eng. Probl. 2023, 6, 1937–1949. [Google Scholar]
- Ramesh, P.; Sambath, M.; Mohd Hafiz, M.; Krishnan, B. Stability analysis of the fractional-order prey-predator model with infection. Int. J. Model. Simul. 2020, 41, 434–450. [Google Scholar] [CrossRef]
- Belgaid, Y.; Helal, M.; Lakmeche, A.; Venturino, E. Mathematical Study of a Coronavirus Model with the Caputo Fractional-Order Derivative. Fractal Fract. 2021, 5, 87. [Google Scholar] [CrossRef]
- Choi, S.K.; Kang, B.; Koo, N. Stability for Caputo fractional differential systems. Abstr. Appl. Anal. 2014, 2014, 631419. [Google Scholar] [CrossRef]
- Wei, Z.; Li, Q.; Che, J. Initial value problems for fractional differential equations involving Riemann-Liouville sequential fractional derivative. J. Math. Anal. Appl. 2010, 367, 260–272. [Google Scholar] [CrossRef]
- Ahmad, S.; Ullah, A.; Al-Mdallal, Q.; Khan, H.; Shah, K.; Khan, A. Fractional order mathematical modeling of COVID-19 transmission. Chaos Solitons Fractals 2020, 139, 110256. [Google Scholar] [CrossRef]
- Arshad, S.; Siddique, I.; Nawaz, F.; Shaheen, A.; Khurshid, H. Dynamics of a fractional order mathematical model for COVID-19 epidemic transmission. Phys. A Stat. Mech. Its Appl. 2023, 609, 128383. [Google Scholar] [CrossRef]
- Askar, S.S.; Ghosh, D.; Santra, P.K.; Elsadany, A.A.; Mahapatra, G.S. A fractional order SITR mathematical model for forecasting of transmission of COVID-19 of India with lockdown effect. Results Phys. 2021, 24, 104067. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Description |
|---|---|
| A | Rate of recruitment. |
| Rate of transmission from I to S. | |
| Rate of transmission from E to S. | |
| Rate of transmission from infected to vaccinated. | |
| Rate of transmission from exposed to vaccinated. | |
| Natural death rate. | |
| Transmission from recovered to vaccinated. | |
| Rate of progression from exposed to infected. | |
| Rate of recovery. | |
| Death due to HFMD disease. | |
| Rate from recovered to susceptible. | |
| z | Rate of vaccination [28]. |
| Eigenvalues | Sign | Conditions | Stability |
|---|---|---|---|
| − | , , and , and hence | Stable | |
| − | , , and , and hence | Stable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohandoss, A.; Chandrasekar, G.; Meetei, M.Z.; Msmali, A.H. Fractional Order Mathematical Modelling of HFMD Transmission via Caputo Derivative. Axioms 2024, 13, 213. https://doi.org/10.3390/axioms13040213
Mohandoss A, Chandrasekar G, Meetei MZ, Msmali AH. Fractional Order Mathematical Modelling of HFMD Transmission via Caputo Derivative. Axioms. 2024; 13(4):213. https://doi.org/10.3390/axioms13040213
Chicago/Turabian StyleMohandoss, Aakash, Gunasundari Chandrasekar, Mutum Zico Meetei, and Ahmed H. Msmali. 2024. "Fractional Order Mathematical Modelling of HFMD Transmission via Caputo Derivative" Axioms 13, no. 4: 213. https://doi.org/10.3390/axioms13040213
APA StyleMohandoss, A., Chandrasekar, G., Meetei, M. Z., & Msmali, A. H. (2024). Fractional Order Mathematical Modelling of HFMD Transmission via Caputo Derivative. Axioms, 13(4), 213. https://doi.org/10.3390/axioms13040213






