Synergistic Recovery of Copper, Antimony, and Silver Refractory Sulfide Minerals Using an ADD/Z-200 Mixed Collector System
Abstract
1. Introduction
2. Materials and Methods
2.1. Minerals
2.2. Reagents
2.3. Flotation Tests
2.4. Scanning Electron Microscopy and Energy Dispersive X-Ray Spectroscopy (SEM-EDS) Analysis
2.5. Particle Size Measurement
3. Results and Discussion
3.1. Effect of Grinding Fineness on Flotation
3.2. Effect of Collector on Flotation
3.3. Effect of Sodium Silicate on Flotation
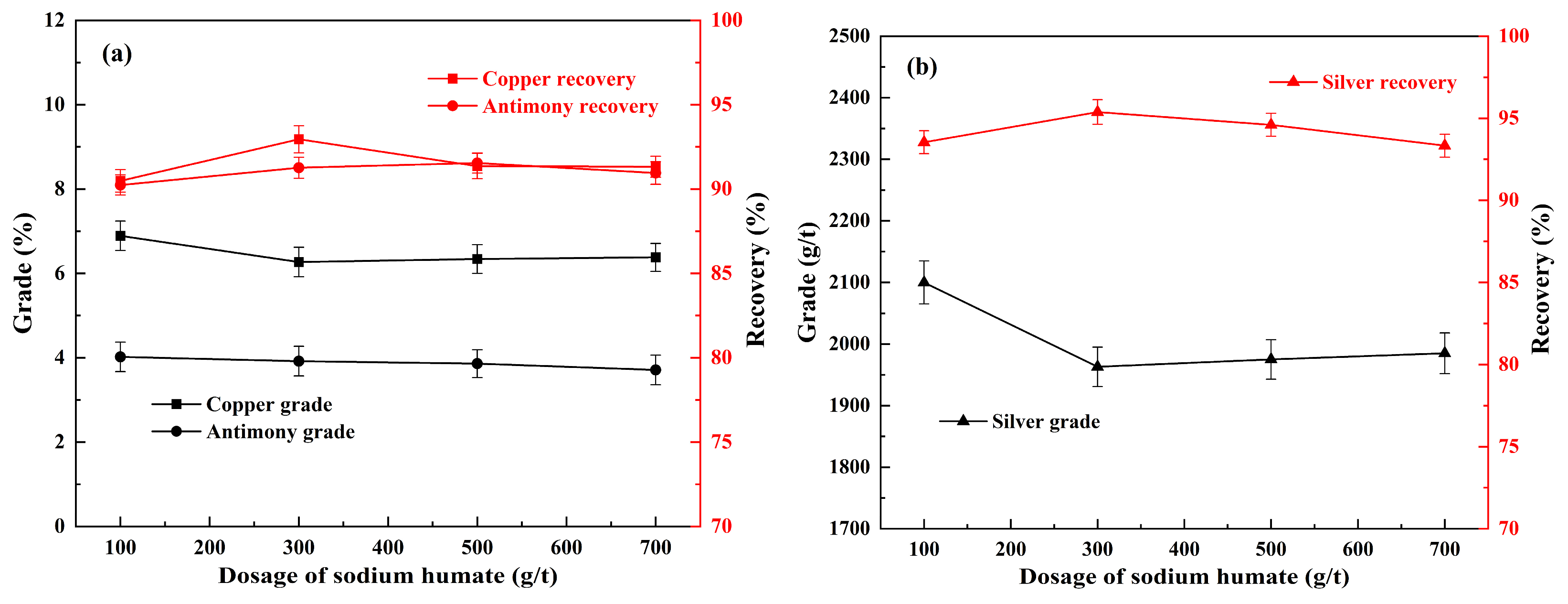
3.4. Effect of Sodium Humate on Flotation
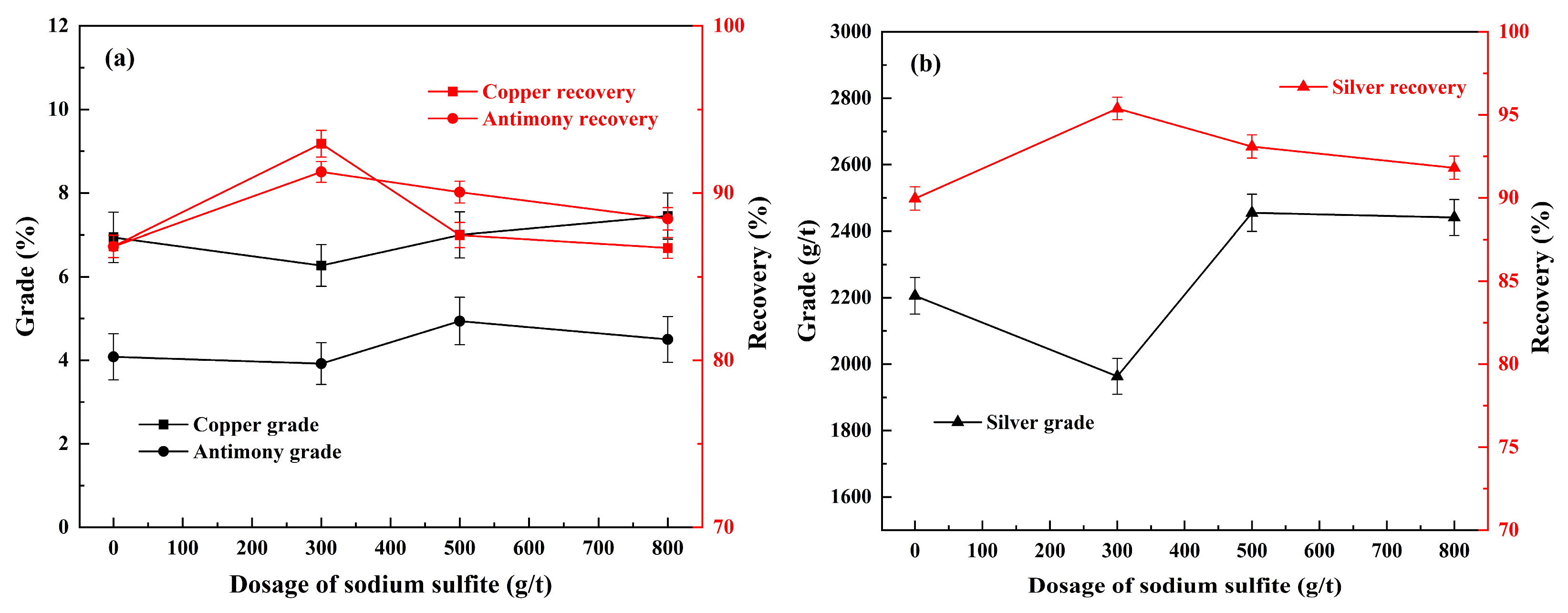
3.5. Effect of Sodium Sulfite Dosage on Flotation
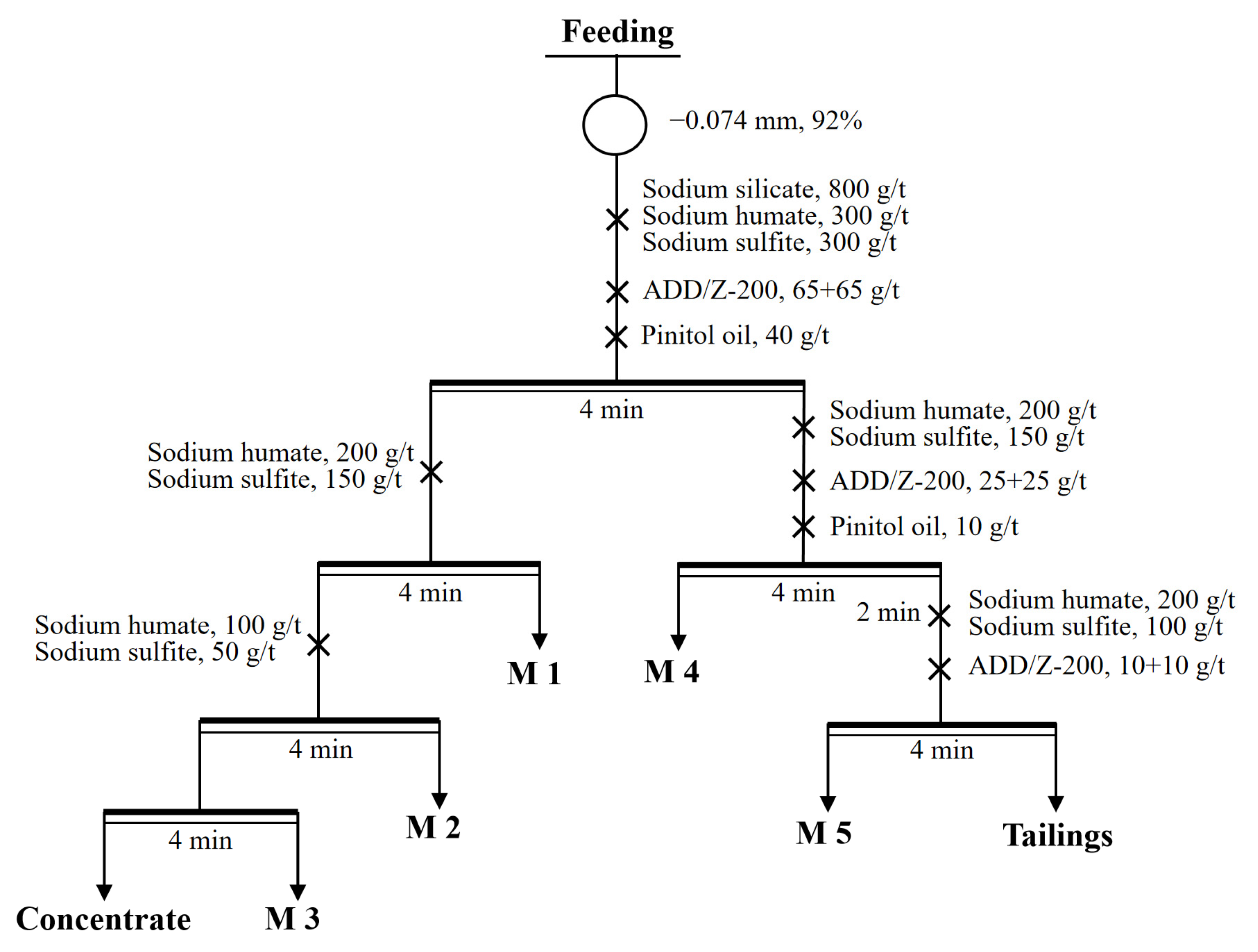
3.6. Open-Circle Flotation
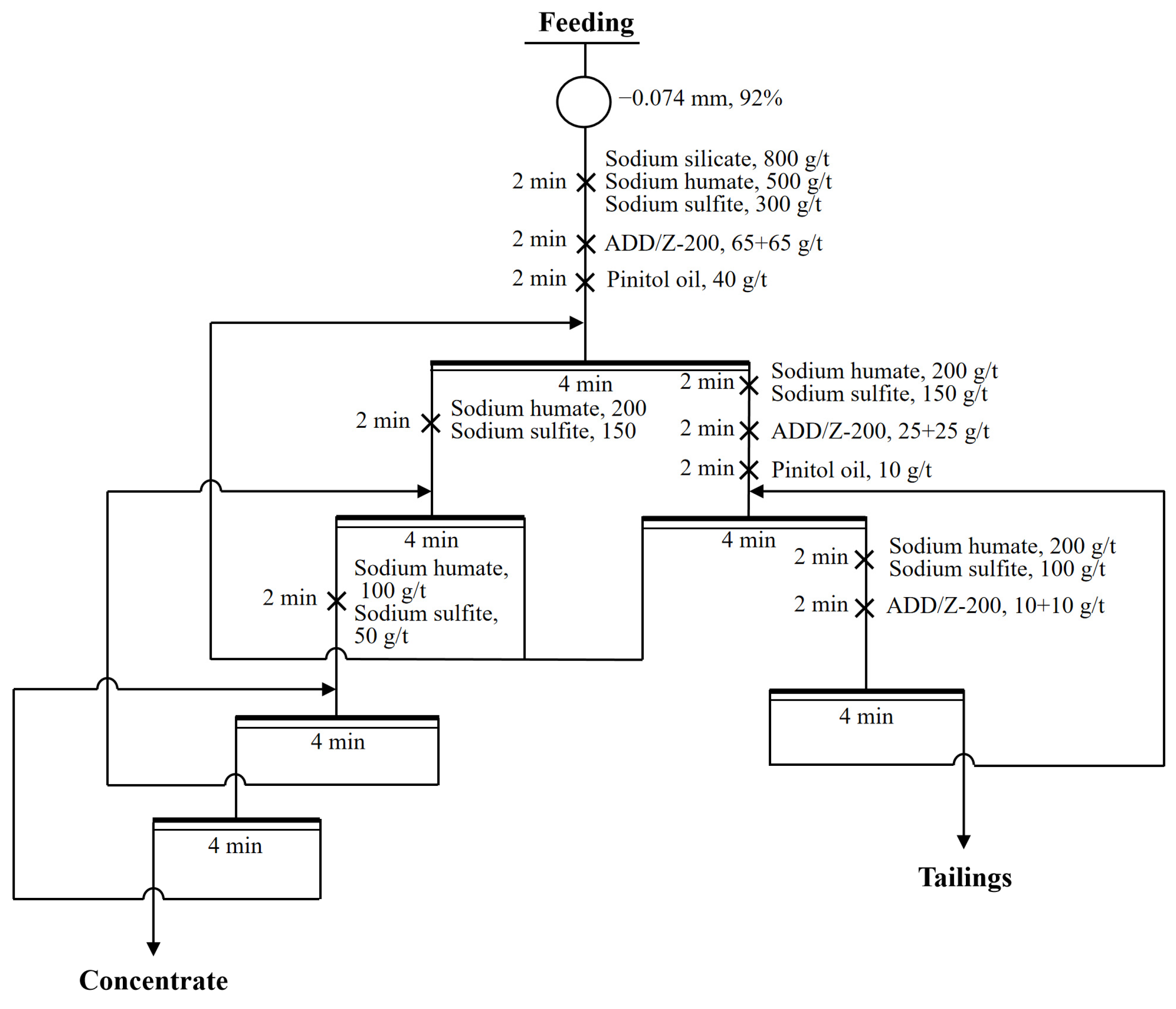
3.7. Closed-Circuit Flotation Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.X.; Wen, S.M.; Zhang, J.H.; Wang, C.L.; Liu, J.; Shen, H.Y. Comprehensive recovery of valuable metals from copper polymetallic sulphide ore. Adv. Mater. Res. 2012, 524–527, 957–964. [Google Scholar] [CrossRef]
- Li, G.; Zou, X.; Cheng, H.; Geng, S.; Xiong, X.; Xu, Q.; Zhou, Z.; Lu, X. A novel ammonium chloride roasting approach for high-efficiency co-sulfation of nickel, cobalt, and copper in polymetallic sulfide minerals. Metall. Mater. Trans. B 2020, 51, 2769–2784. [Google Scholar] [CrossRef]
- Qin, S.; Ren, H.; Hu, Y.; Ma, S.; Ding, J.; Lai, F.; Zhang, C.; Shen, C.; Zhao, H. Efficient recovery of Cu, Co, and Au from polymetallic magnetite: Insights into mineral liberation mechanisms and interfacial behavior. J. Environ. Chem. Eng. 2025, 13, 115940. [Google Scholar] [CrossRef]
- Zou, K.; Zhang, Y.; Shen, L.; Shang, H.; Chen, B.; Wen, J.; Zhao, H. Highly efficient resource recovery from polymetallic sulfide ores under neutral–alkaline conditions: Chemical oxidation and coordination leaching. Chem. Eng. J. 2025, 503, 158198. [Google Scholar] [CrossRef]
- Shengo, M.L.; Kime, M.-B.; Mambwe, M.P.; Nyembo, T.K. A review of the beneficiation of copper–cobalt-bearing minerals in the Democratic Republic of Congo. J. Sustain. Min. 2019, 18, 226–246. [Google Scholar] [CrossRef]
- Cui, W.; Chen, J. Insight into mineral flotation fundamentals through the DFT method. Int. J. Min. Sci. Technol. 2021, 31, 983–994. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, J.; Liu, Z.; Chen, J. Selective enhancement of jamesonite flotation using Aerophine 3418A/DDTC mixture. Miner. Eng. 2023, 191, 107934. [Google Scholar] [CrossRef]
- Wills, B.A.; Napier-Munn, T. Wills’ Mineral Processing Technology; Elsevier: Oxford, UK, 2006. [Google Scholar]
- Tolley, W.; Kotlyar, D.; Van Wagoner, R. Fundamental electrochemical studies of sulfide mineral flotation. Miner. Eng. 1996, 9, 603–637. [Google Scholar] [CrossRef]
- Pana, R.J.; William, T.; Junji, K.; Kazutoshi, H.; Yasushi, T.; Atsushi, S. Effect of flotation reagents for upgrading and recovery of Cu and Mo from mine tailing by flotation. Resour. Process. 2011, 58, 14–21. [Google Scholar] [CrossRef]
- Sun, Z.M.; Sun, C.B.; Wang, J.Z.; Yin, W.Z. Optimization and mechanism of gold-bearing sulfide flotation. Rare Met. 2014, 33, 363–368. [Google Scholar] [CrossRef]
- Ndoro, T.O.; Witika, L.K. A review of the flotation of copper minerals. Int. J. Sci. Basic Appl. Res. 2017, 34, 145–165. [Google Scholar]
- Feng, Q.; Yang, W.; Chang, M.; Wen, S.; Liu, D.; Han, G. Advances in depressants for flotation separation of Cu–Fe sulfide minerals at low alkalinity: A critical review. Int. J. Miner. Metall. Mater. 2024, 31, 1–17. [Google Scholar] [CrossRef]
- Plaksin, I.N.; Glembotskii, V.A.; Okolovich, A.M. Simultaneous use of several collector reagents for the intensification of the flotation of pyrite. Izv. Akad. Nauk SSSR Otd. Tekh. Nauk 1952, 51, 405–415. [Google Scholar]
- Buckley, A.N.; Hope, G.A.; Parker, G.K.; Steyn, J.; Woods, R. Mechanism of mixed dithiophosphate and mercaptobenzothiazole collectors for Cu sulfide ore minerals. Miner. Eng. 2017, 109, 80–97. [Google Scholar] [CrossRef]
- Lee, K.; Archibald, D.; McLean, J.; Reuter, M.A. Flotation of mixed copper oxide and sulphide minerals with xanthate and hydroxamate collectors. Miner. Eng. 2009, 22, 395–401. [Google Scholar] [CrossRef]
- Roy, S.; Datta, A.; Rehani, S. Flotation of copper sulphide from copper smelter slag using multiple collectors and their mixtures. Int. J. Miner. Process. 2015, 143, 43–49. [Google Scholar] [CrossRef]
- Dhar, P.; Thornhill, M.; Kota, H.R. Comparison of single and mixed reagent systems for flotation of copper sulphides from Nussir ore. Miner. Eng. 2019, 142, 105930. [Google Scholar] [CrossRef]
- Guner, M.K.; Lode, S.; Malafeevskiy, N.; Aasly, K.; Kowalczuk, P. Mixed thiol collector system in the flotation of copper ore using the REFLUX Flotation Cell (RFC). Miner. Eng. 2024, 216, 108843. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Li, Y.; Chen, J. Co-adsorption mechanism of selective Aerophine 3418A/DTP collectors and their industrial application for chalcopyrite flotation. Miner. Eng. 2025, 228, 109304. [Google Scholar] [CrossRef]
- Pavlova, G.G.; Borisenko, A.S. The age of Ag–Sb deposits of Central Asia and their correlation with other types of ore systems and magmatism. Ore Geol. Rev. 2009, 35, 164–185. [Google Scholar] [CrossRef]
- Nyamdelger, S.; Burmaa, G.; Narangarav, T.; Ariunaa, G. Dissolution behaviour of freibergite–tetrahedrite concentrate in acidic dichromate solution. Mong. J. Chem. 2014, 14, 36–40. [Google Scholar] [CrossRef]
- Avirmed, D. Mineral resources of Mongolia as a driving force of the country. Mong. J. Int. Aff. 2021, 22, 61–66. [Google Scholar] [CrossRef]
- Miettinen, T.; Ralston, J.; Fornasiero, D. The limits of fine particle flotation. Miner. Eng. 2010, 23, 420–437. [Google Scholar] [CrossRef]
- Leistner, T.; Peuker, U.A.; Rudolph, M. How gangue particle size can affect the recovery of ultrafine and fine particles during froth flotation. Miner. Eng. 2017, 109, 1–9. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Li, Y.; Liu, M.; Liu, Y.; Zhao, C.; Cui, W. Effects of surface spatial structures and electronic properties of chalcopyrite and pyrite on Z-200 selectivity. Miner. Eng. 2025, 163, 106803. [Google Scholar] [CrossRef]
- Tussupbayev, N.K.; Rulyov, N.N.; Kravtchenco, O.V. Microbubble augmented flotation of ultrafine chalcopyrite from quartz mixtures. Trans. Inst. Min. 2016, 125, 5–9. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Li, W.; Zhu, Y. A fundamental study of chalcopyrite flotation in sea water using sodium silicate. Miner. Eng. 2019, 139, 10586. [Google Scholar] [CrossRef]
- Xia, L.; Hart, B.; Sidorkiewicz, V.; Shaw, D. The role of soluble sodium silicate for enhancing flotation selectivity of sulphides: Example from a Cu-sulphide ore. In Proceedings of the First Global Conference on Extractive Metallurgy, Denver, CO, USA, 24–27 February 2019; pp. 2901–2913. [Google Scholar]
- Dong, Z.; Zhi, H.; Li, W.; Man, X.; Yang, X.; Fu, Y.; Liu, J. Study on inhibition effect and mechanism of sodium humate in hematite reverse flotation. Miner. Eng. 2022, 189, 107883. [Google Scholar] [CrossRef]
- Sun, D.; Li, M.; Fu, Y.; Pan, Z.; Cui, R.; Wang, D.; Zhang, M.; Yao, W. Selective separation of chalcopyrite from pyrite using sodium humate: Flotation behavior and adsorption mechanism. ACS Omega 2023, 47, 45129–45136. [Google Scholar] [CrossRef]
- Yuan, Z.; Du, Y.; Meng, Q.; Li, Y. Selective dispersion and separation mechanisms of sodium humate in ilmenite flotation. Sep. Purif. Technol. 2025, 376, 134081. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Miki, H.; Ochi, D.; Aoki, Y.; Ura, K.; Berdakh, D.; Ulmaszoda, A.; Dwitama, E.P.; Sasaki, K.; Hirajima, T. Sodium metabisulfite as a copper depressant in the selective flotation of copper-molybdenum concentrate using seawater. Adv. Powder Technol. 2023, 34, 104258. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, F.; Cui, Y.; Lin, X.; Qin, W.; Wei, Q. Application and electrochemical depression mechanism of sodium sulfite on the flotation separation of galena–pyrite mixed concentrate in Yiliang typical high–sulfur lead–zinc deposit. Geochemistry 2025, 85, 126219. [Google Scholar] [CrossRef]
- Yang, X.; Lai, H.; Wei, X.; Shen, P.; Wang, Y.; Li, M.; Cai, J.; Liu, D. Adsorption mechanism of Na2S2O3 and FeSO4 as a combined depressant for galena in chalcopyrite–galena flotation separation. Colloids Surf. A Physicochem. Eng. Asp. 2025, 706, 135799. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Sun, W.; Li, W.; Dong, Y.; Wang, C. Electrochemical mechanism and flotation of chalcopyrite and galena in the presence of water glass and sodium sulfite. Trans. Nonferrous Met. Soc. China 2020, 30, 1091–1101. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K.; Kuroiwa, S.; Aoki, Y. Effect of Na2SO3 on the floatability of chalcopyrite and enargite. Miner. Eng. 2021, 173, 107222. [Google Scholar] [CrossRef]
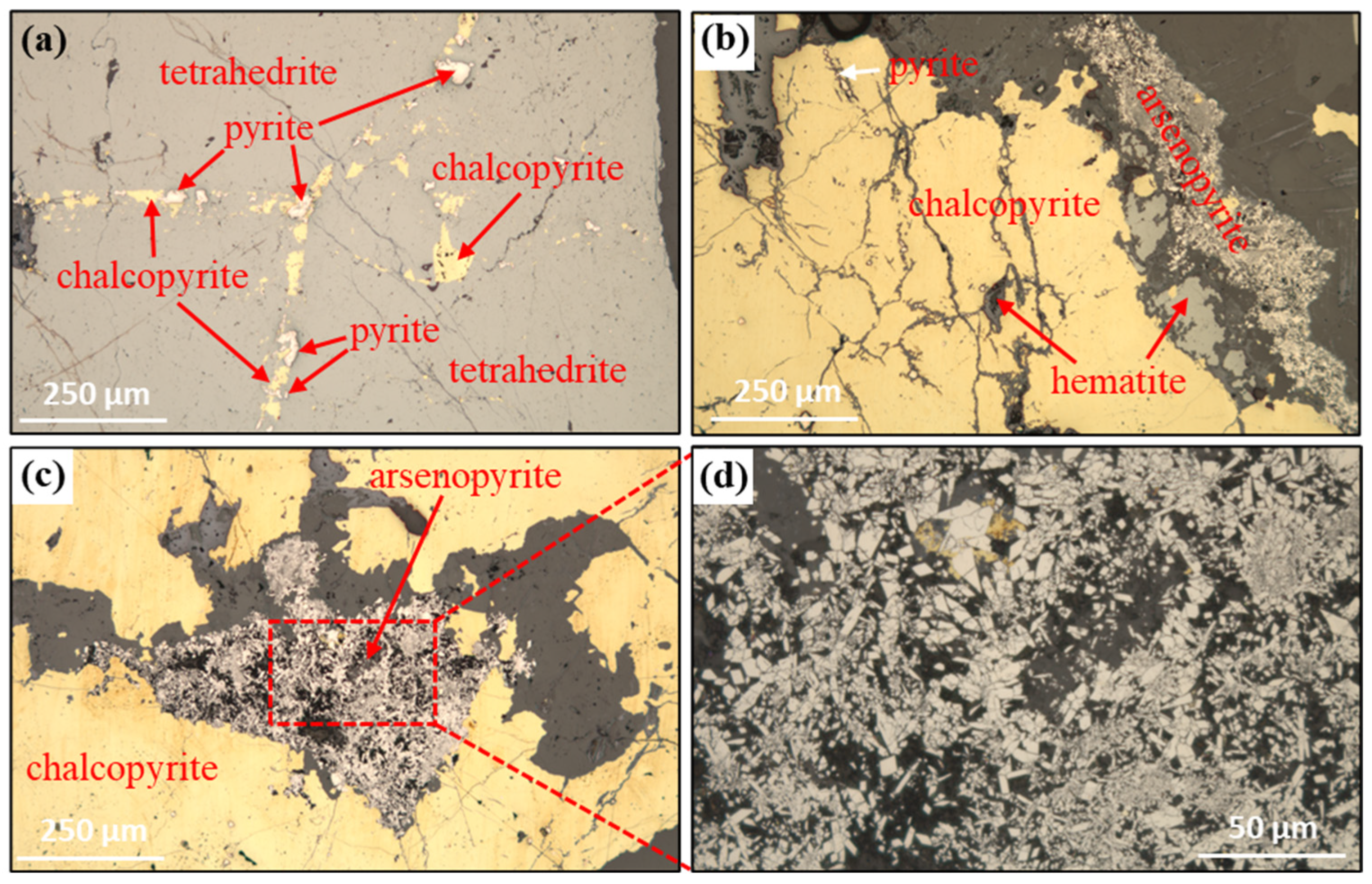
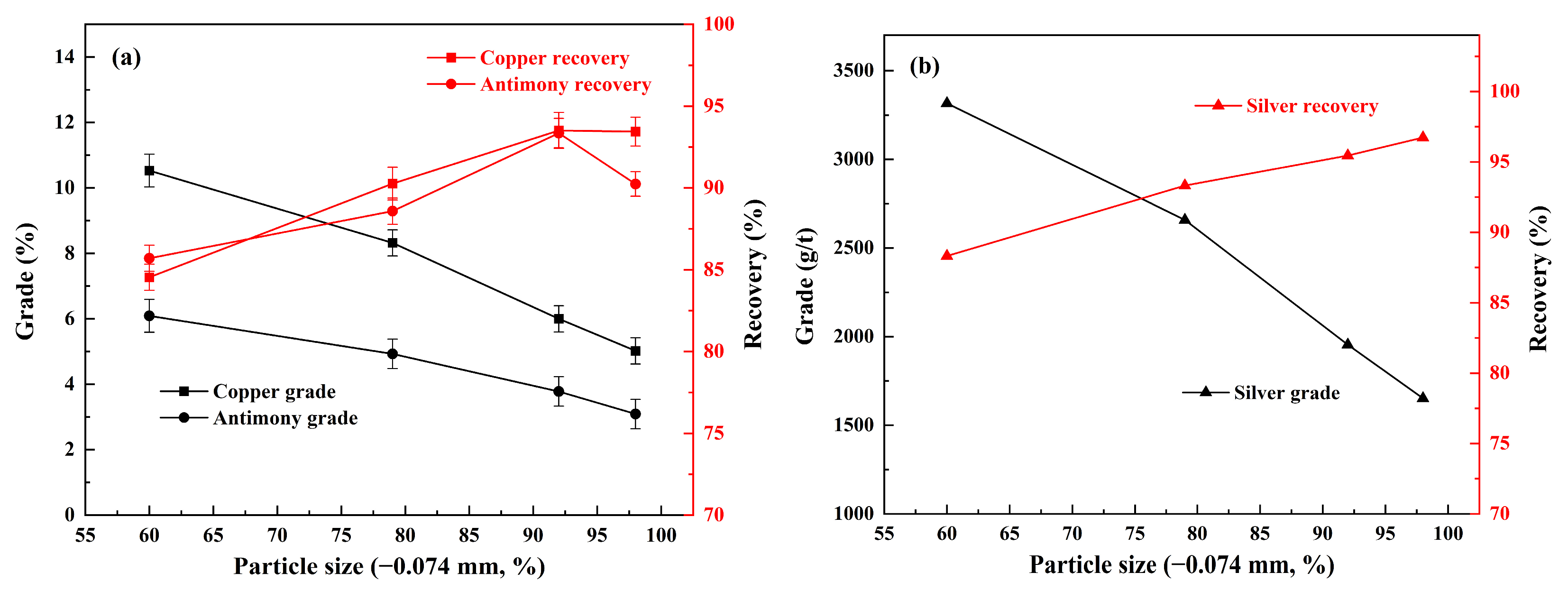
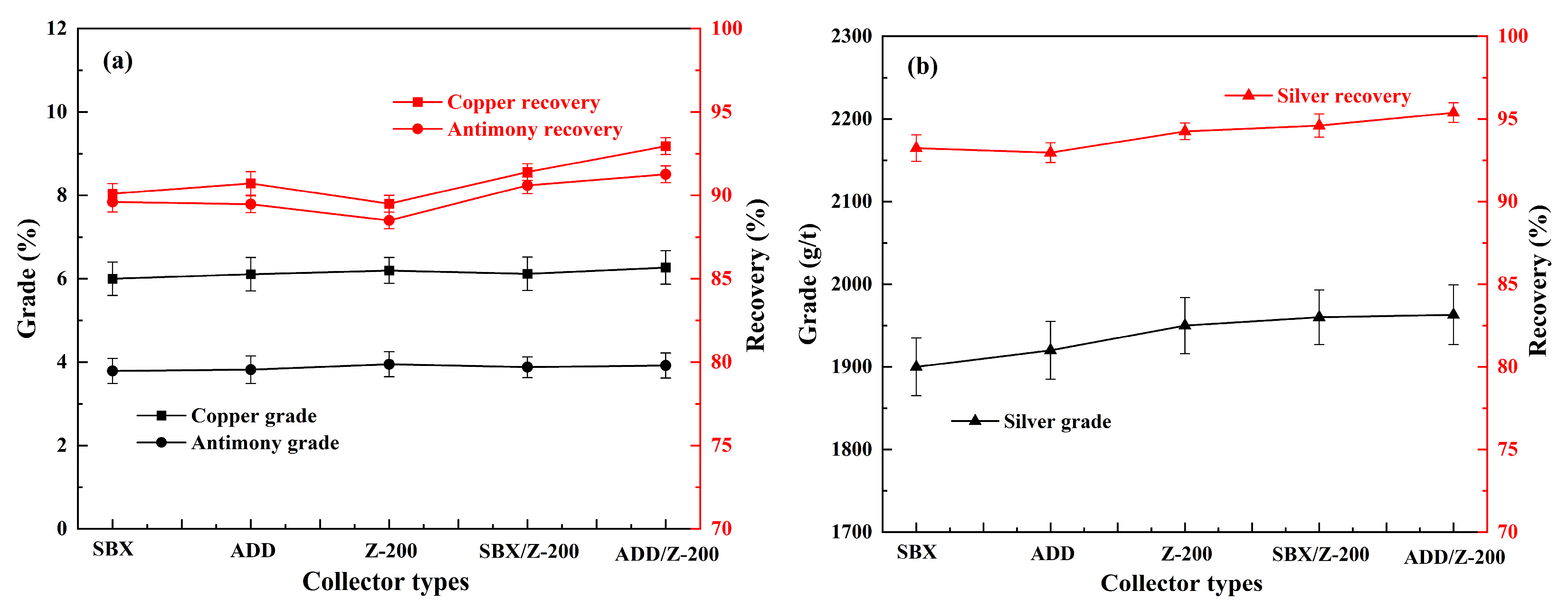
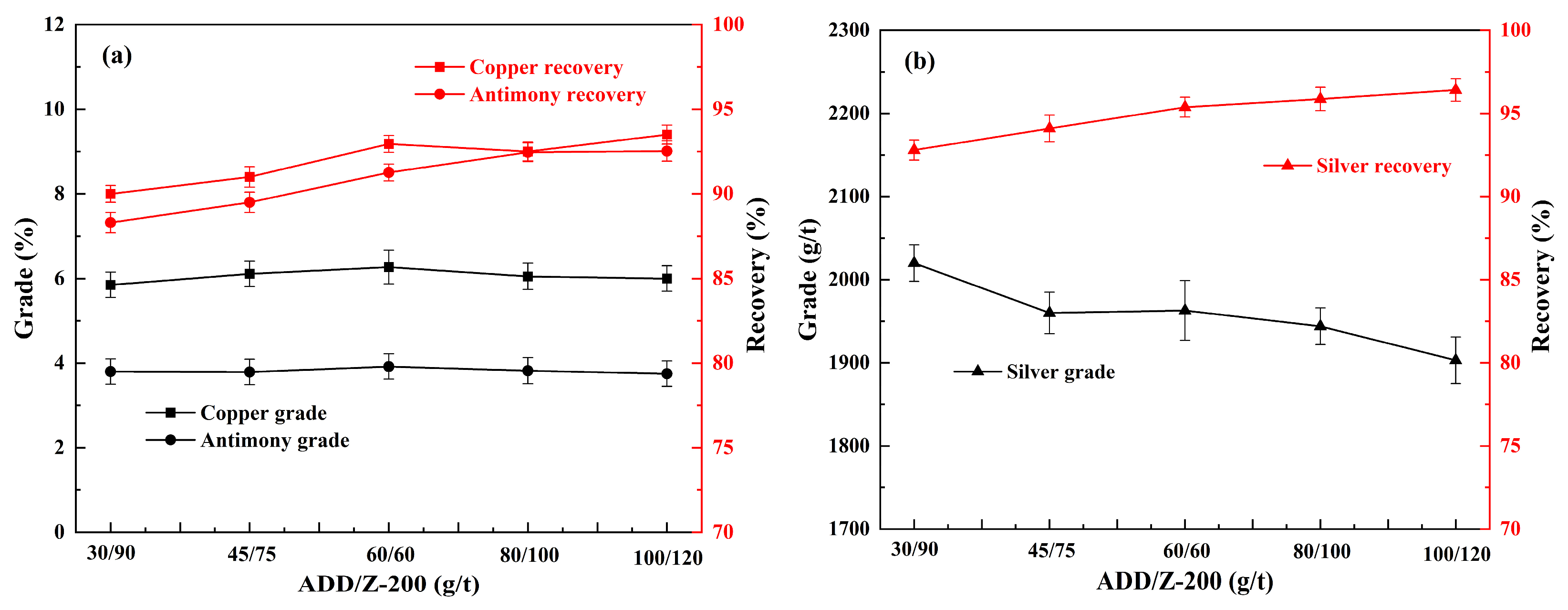


| Elements | Fe | Ti | Si | Cu | Mn | Sb | Mg | Al | As | Ag * |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt. %) | 36.70 | 6.60 | 5.15 | 1.93 | 1.09 | 1.22 | 1.09 | 0.72 | 0.23 | 592 |
| Product | Yield (%) | Grade (%) | Recovery (%) | ||||
|---|---|---|---|---|---|---|---|
| Copper | Antimony | Silver * | Copper | Antimony | Silver | ||
| Concentrate | 3.4 | 33.45 | 18.25 | 10,968 | 58.7 | 51.3 | 62.7 |
| Middings 1 | 14.7 | 0.49 | 0.96 | 113 | 3.7 | 11.7 | 2.8 |
| Middings 2 | 2.3 | 3.76 | 1.65 | 1563 | 4.5 | 3.2 | 6.1 |
| Middings 3 | 2.1 | 22.60 | 10.28 | 6540 | 24.9 | 18.1 | 23.5 |
| Middings 4 | 7.4 | 0.53 | 0.65 | 140 | 2.0 | 4.0 | 1.8 |
| Middings 5 | 5.4 | 0.38 | 0.34 | 90 | 1.1 | 1.5 | 0.8 |
| Tailings | 64.6 | 0.15 | 0.19 | 21 | 5.0 | 10.2 | 2.3 |
| Feeding | 100.0 | 1.93 | 1.20 | 591 | 100.0 | 100.0 | 100.0 |
| Prod | Product | Yield (%) | Grade (%) | Recovery (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Copper | Antimony | Silver * | Copper | Antimony | Silver | |||
| ADD/Z-200 | Concentrate | 5.9 | 30.30 | 19.20 | 9538 | 92.7 | 93.0 | 95.1 |
| Tailings | 94.1 | 0.15 | 0.09 | 31 | 7.3 | 7.0 | 4.9 | |
| Feeding | 100.0 | 1.93 | 1.22 | 592 | 100.0 | 100.0 | 100.0 | |
| Z-200 | Concentrate | 5.0 | 32.73 | 21.73 | 10,270 | 84.6 | 87.6 | 86.3 |
| Tailings | 95.0 | 0.31 | 0.16 | 85 | 15.4 | 12.4 | 13.7 | |
| Feeding | 100.0 | 1.92 | 1.23 | 590 | 100.0 | 100.0 | 100.0 | |
| Reagent | Consumption (g/t) | Unit Price (CNY/t) | Total (CNY) |
|---|---|---|---|
| ADD | 100 | 15,000.0 | 8.4 |
| Z-200 | 100 | 26,000.0 | |
| Pinitol oil | 50 | 8000.0 | |
| Sodium silicate | 1100 | 900.0 | |
| Sodium humate | 1200 | 1500.0 | |
| Sodium sulfite | 750 | 1500.0 | |
| Z-200 | 205 | 26,000.0 | 9.7 |
| Pinitol oil | 50 | 8000.0 | |
| Sodium silicate | 1100 | 900.0 | |
| Sodium humate | 1200 | 1500.0 | |
| Sodium sulfite | 750 | 1500.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, B.; Liu, Y.; Jia, X.; Enkhtur, O.; Tumendelger, A.; Bian, Z. Synergistic Recovery of Copper, Antimony, and Silver Refractory Sulfide Minerals Using an ADD/Z-200 Mixed Collector System. Minerals 2025, 15, 1219. https://doi.org/10.3390/min15111219
Yan B, Liu Y, Jia X, Enkhtur O, Tumendelger A, Bian Z. Synergistic Recovery of Copper, Antimony, and Silver Refractory Sulfide Minerals Using an ADD/Z-200 Mixed Collector System. Minerals. 2025; 15(11):1219. https://doi.org/10.3390/min15111219
Chicago/Turabian StyleYan, Baobao, Yongmao Liu, Xianbing Jia, Otgonjargal Enkhtur, Azzaya Tumendelger, and Zhiwei Bian. 2025. "Synergistic Recovery of Copper, Antimony, and Silver Refractory Sulfide Minerals Using an ADD/Z-200 Mixed Collector System" Minerals 15, no. 11: 1219. https://doi.org/10.3390/min15111219
APA StyleYan, B., Liu, Y., Jia, X., Enkhtur, O., Tumendelger, A., & Bian, Z. (2025). Synergistic Recovery of Copper, Antimony, and Silver Refractory Sulfide Minerals Using an ADD/Z-200 Mixed Collector System. Minerals, 15(11), 1219. https://doi.org/10.3390/min15111219







