Mineralogical Imaging for Characterization of the Per Geijer Apatite Iron Ores in the Kiruna District, Northern Sweden: A Comparative Study of Mineral Liberation Analysis and Raman Imaging
Abstract
:1. Introduction
2. Geology of the Per Geijer Deposits
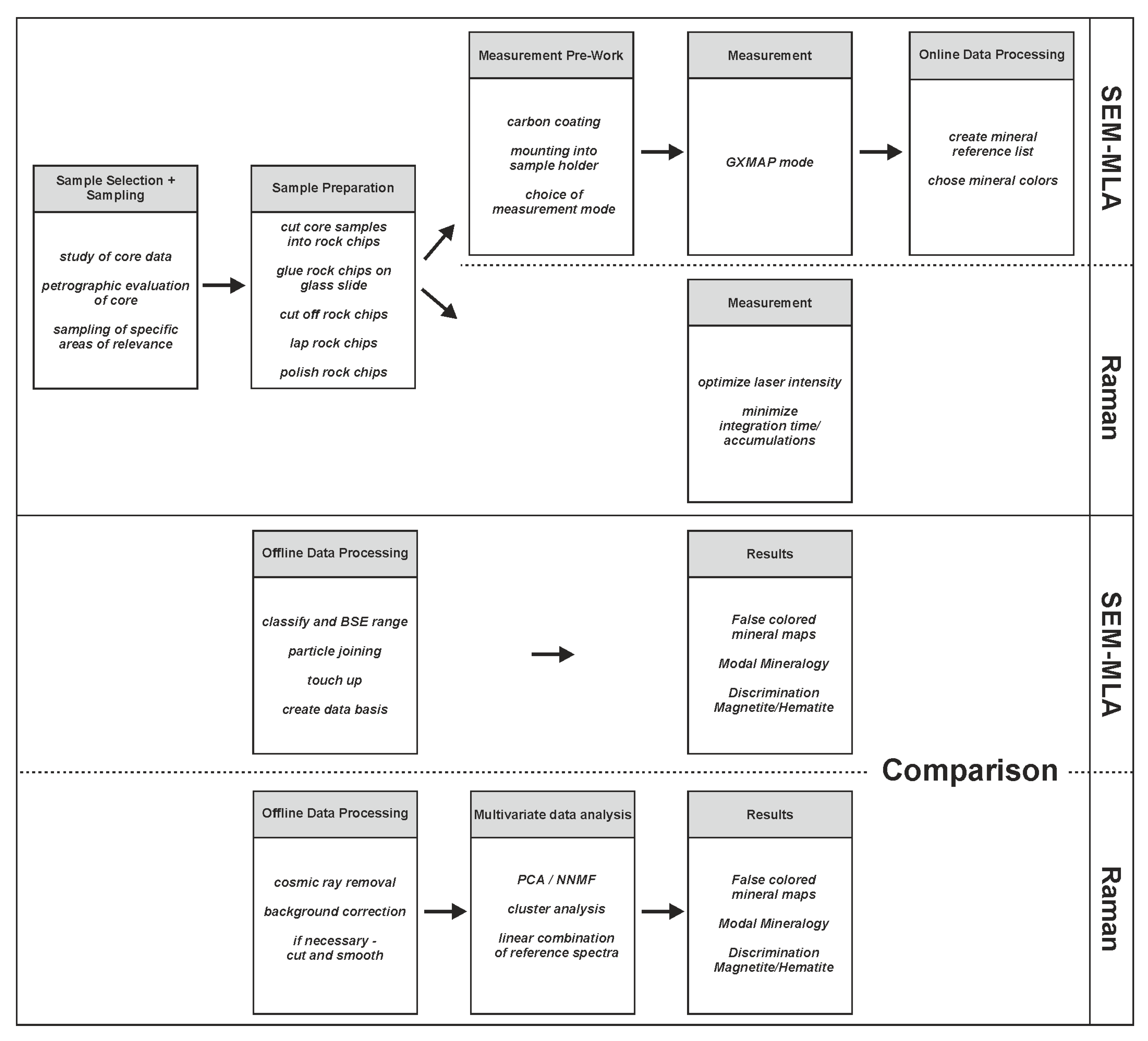
3. Materials and Methods
3.1. Samples and Preparation
3.2. Scanning Electron Microscope (SEM) and Mineral Liberation Analyzer (MLA)
3.2.1. Measurement Mode and Setup
3.2.2. Mineral Reference List
3.2.3. Online Data Processing
3.2.4. Offline Data Processing
3.3. Raman Spectroscopy and Imaging
3.3.1. Measurement Mode and Setup
3.3.2. Mineral Reference List
3.3.3. Offline Data Processing-Data Preprocessing
3.3.4. Offline Data Processing-Multivariate Analysis
4. Results
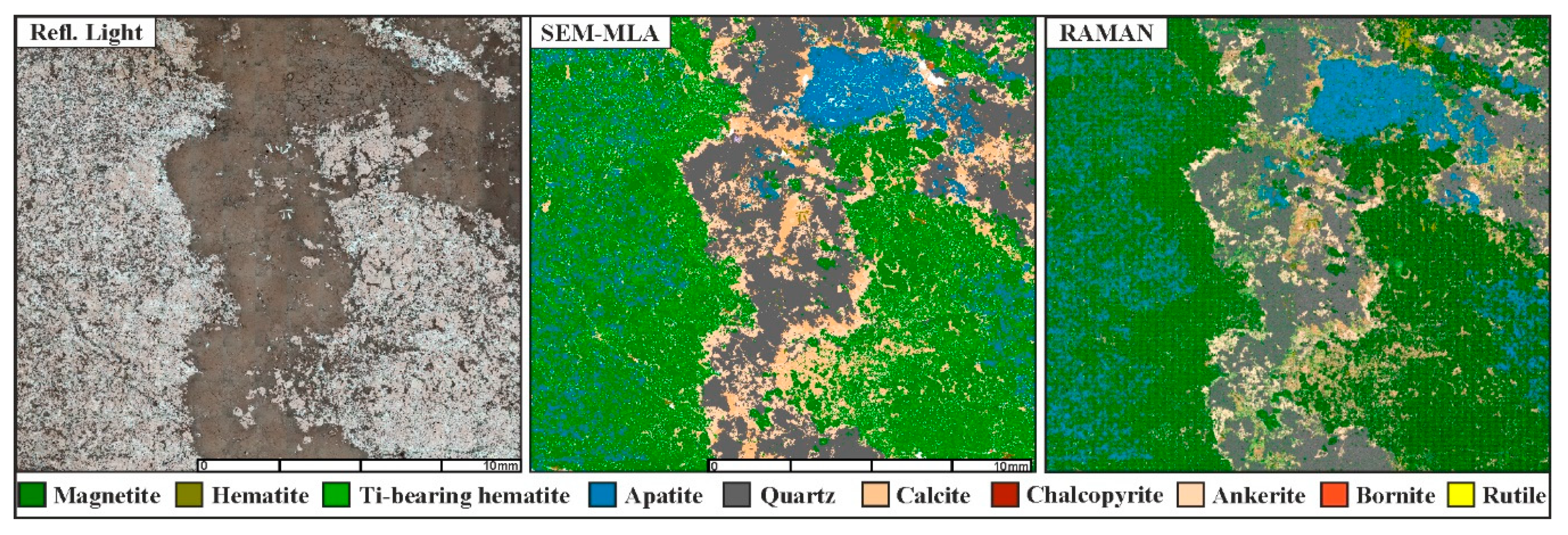
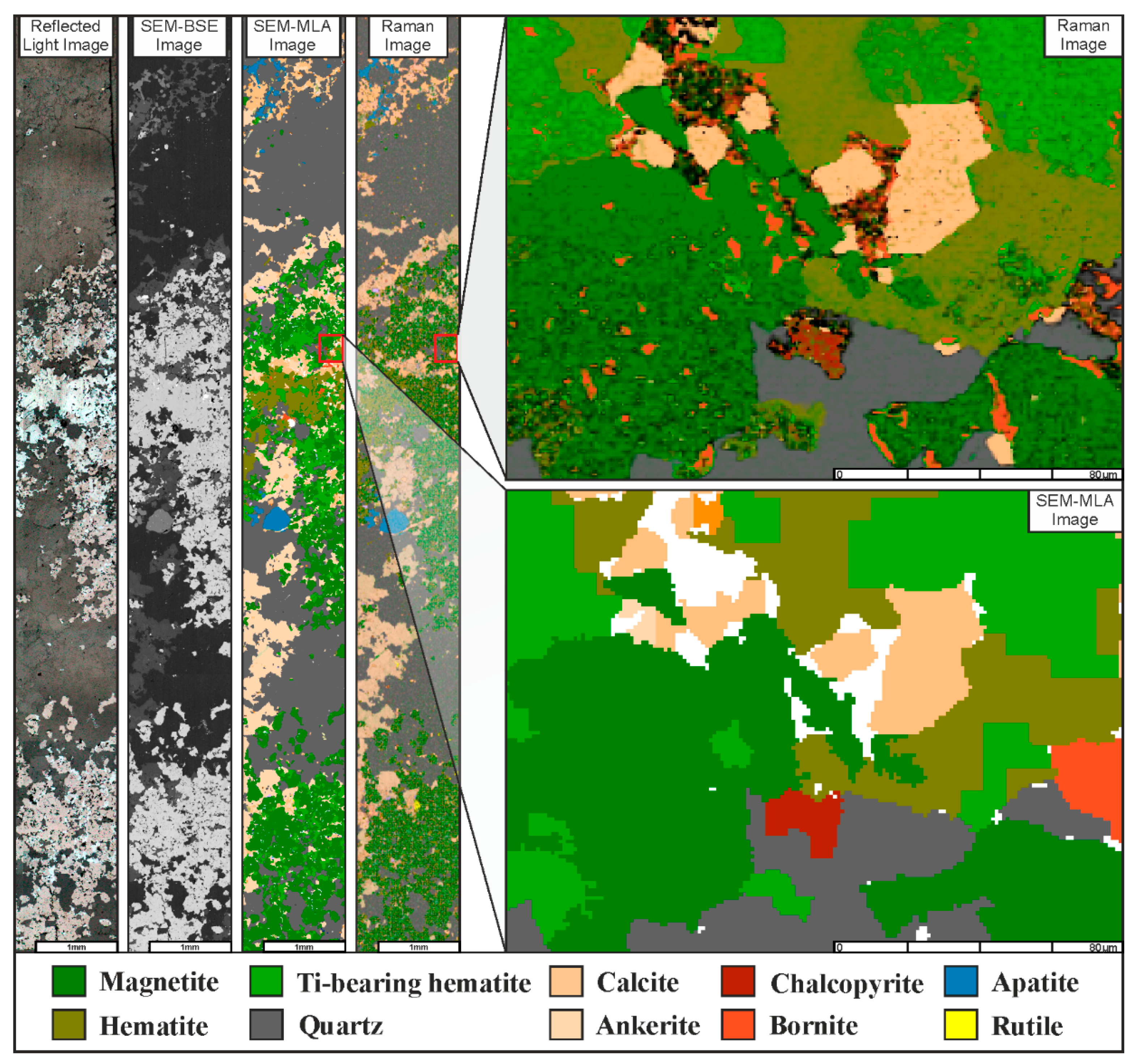
4.1. Classification and Imaging
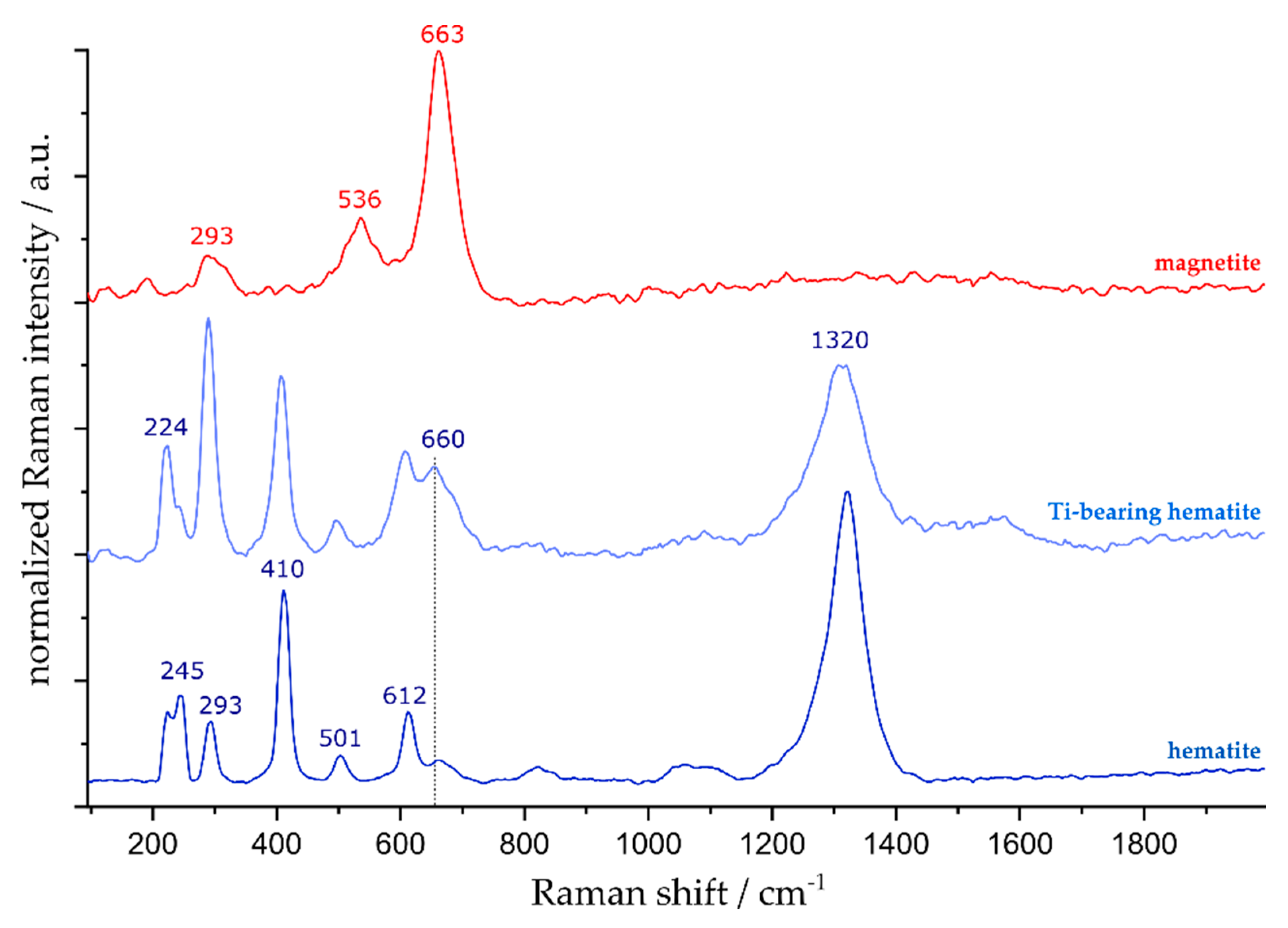
4.2. Discrimination of Magnetite and Hematite
4.3. Modal Mineralogy
4.4. Time Constraints
4.4.1. Measurement Time
4.4.2. Data Processing Time
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andersen, J.C.Ø.; Rollinson, G.K.; Snook, B.; Herrington, R.; Fairhurst, R.J. Use of QEMSCAN® for the characterization of Ni-rich and Ni-poor goethite in laterite ores. Miner. Eng. 2009, 22, 1119–1129. [Google Scholar] [CrossRef]
- Rollinson, G.K.; Andersen, J.C.Ø.; Stickland, R.J.; Boni, M.; Fairhurst, R. Characterisation of non-sulphide zinc deposits using QEMSCAN®. Miner. Eng. 2011, 24, 778–787. [Google Scholar] [CrossRef]
- Hunt, J.; Berry, R.; Bradshaw, D. Characterising chalcopyrite liberation and flotation potential. Examples from an IOCG deposit. Miner. Eng. 2011, 24, 1271–1276. [Google Scholar] [CrossRef]
- Sandmann, D.; Haser, S.; Gutzmer, J. Characterisation of graphite by automated mineral liberation analysis. Miner. Process. Extr. Metall. 2014, 123, 184–189. [Google Scholar] [CrossRef]
- Lund, C.; Lamberg, P.; Lindberg, T. Development of a geometallurgical framework to quantify mineral textures for process prediction. Miner. Eng. 2015, 82, 61–77. [Google Scholar] [CrossRef]
- Niiranen, K. Characterization of the Kiirunavaara Iron Ore Deposit for Mineral Processing with a Focus on the High Silica Ore Type B2. Ph.D. Thesis, Montanuniversität Leoben, Leoben, Austria, 2015. [Google Scholar]
- Lund, C. Mineralogical, Chemical and Textural Characterisation of the Malmberget Iron Ore Deposit for a Geometallurgical Model. Ph.D. Thesis, Luleå tekniska universitet, Luleå, Sweden, 2013. [Google Scholar]
- Gilligan, M.; Costanzo, A.; Feely, M.; Rollinson, G.K.; Timmins, E.; Henry, T.; Morrison, L. Mapping arsenopyrite alteration in a quartz vein-hosted gold deposit using microbeam analytical techniques. Mineral. Mag. 2016, 80, 739–748. [Google Scholar] [CrossRef]
- Escolme, A.; Cooke, D.; Hunt, J.; Berry, R.; Fisher, L.; Potma, W. Ore characterisation and geometallurgy modelling. Productora Cu-Au-Mo deposit, Chile. In Proceedings of the Second International Seminar on Geometallurgy, Santiago, Chile, 1–3 December 2014; p. 1. [Google Scholar]
- Kern, M.; Möckel, R.; Krause, J.; Teichmann, J.; Gutzmer, J. Calculating the deportment of a fine-grained and compositionally complex Sn skarn with a modified approach for automated mineralogy. Miner. Eng. 2018, 116, 213–225. [Google Scholar] [CrossRef]
- Warlo, M.; Wanhainen, C.; Bark, G.; Butcher, A.R.; McElroy, I.; Brising, D.; Rollinson, G.K. Automated Quantitative Mineralogy Optimized for Simultaneous Detection of (Precious/Critical) Rare Metals and Base Metals in A Production-Focused Environment. Minerals 2019, 9, 440. [Google Scholar] [CrossRef]
- Fandrich, R.; Gu, Y.; Burrows, D.; Moeller, K. Modern SEM-based mineral liberation analysis. Int. J. Miner. Process. 2007, 84, 310–320. [Google Scholar] [CrossRef]
- Figueroa, G.; Moeller, K.; Buhot, M.; Gloy, G.; Haberla, D. Advanced discrimination of hematite and magnetite by automated mineralogy. In Proceedings of the 10th International Congress for Applied Mineralogy (ICAM), Trondheim, Norway, 1–5 August 2012; Springer: Berlin, Germany; pp. 197–204. [Google Scholar]
- Weihed, P.; Bergman, J.; Bergström, U. Metallogeny and tectonic evolution of the Early Proterozoic Skellefte district, northern Sweden. Precambrian Res. 1992, 58, 143–167. [Google Scholar] [CrossRef]
- Lahtinen, R.; Nironen, M.; Korja, A. Palaeoproterozoic orogenic evolution of the Fennoscandian Shield at 1.92–1.77 Ga with notes on the metallogeny of FeOx–Cu–Au, VMS, and orogenic gold deposits. In Proceedings of the Seventh Biennial SGA Conference “Mineral Exploration and Sustainable Development”, Athens, Greece, 24–28 August 2003; pp. 1057–1060. [Google Scholar]
- Lahtinen, R.; Korja, A.; Nironen, M. Paleoproterozoic tectonic evolution. In Developments in Precambrian Geology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 14, pp. 481–531. [Google Scholar]
- Lahtinen, R.; Hölttä, P.; Kontinen, A.; Niiranen, T.; Nironen, M.; Saalmann, K.; Sorjonen-Ward, P. Tectonic and metallogenic evolution of the Fennoscandian Shield. Key questions with emphasis on Finland. Geol. Surv. Finl. Spec. Pap. 2011, 49, 23–33. [Google Scholar]
- Martinsson, O. Genesis of the Per Geijer apatite iron ores, Kiruna area, northern Sweden. In Proceedings of the Abstract Volume, SGA Biennal Meeting, Nancy, France, 24–27 August 2015; pp. 23–27. [Google Scholar]
- Martinsson, O. Geology and Metallogeny of the Northern Norrbotten Fe-Cu-Au Province. In Svecofennian Ore-Forming Environments Field Trip Volcanic-Associated Zn-Cu-Au-Ag and Magnetite-Apatite, Sediment-Hosted Pb-Zn, and Intrusion-Associated Cu-Au Deposits in Northern Sweden; Rodney, A., Olof, M., Pär, W., Eds.; Society of Economic Geologists Guidebook Series; Society of Economic Geologists: Littleton, CO, USA, 2004; Volume 33, pp. 131–148. [Google Scholar]
- Krolop, P.; Niiranen, K.; Gilbricht, S.; Seifert, T. Ore type characterization of the Per Geijer iron ore deposits in Kiruna, Northern Sweden. In Proceedings of the Iron Ore 2019 Conference, Perth, Australia, 22–24 July 2019; The Australasian Institute of Mining and Metallurgy: Perth, Australia, 2019; pp. 343–353. [Google Scholar]
- FEI Company. MLA System User Training Course. 2011; 380 p, unpublished work. [Google Scholar]
- Sandmann, D. Method Development in Automated Mineralogy. Ph.D. Thesis, TU Bergakademie Freiberg, Freiberg, Germany, 2015. [Google Scholar]
- Dobbe, R.; Gottlieb, P.; Gu, Y.; Butcher, A.R.; Fandrich, R.; Lemmens, H. Scanning Electron Beambased Automated Mineralogy-Outline of Technology and Selected Applications in the Natural Resources Industry. In European Workshop on Modern developments and Applications in Microbeam Analysis: The Book of Tutorials and abstracts of EMAS 2009 11th European Workshop on Modern Developments and Applications in Microbeam Analysis, Gdynia, Poland, 10–14 May 2009; Gdynia/Rumia: Gdansk, Poland, 2009; Volume 10, pp. 169–189. [Google Scholar]
- Gu, Y. Automated scanning electron microscope based mineral liberation analysis an introduction to JKMRC/FEI mineral liberation analyser. J. Miner. Mater. Charact. Eng. 2003, 2, 33. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venâncio, S.S.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P.; Montenero, A. Micro-Raman investigation of iron oxide films and powders produced by sol–gel syntheses. J. Raman Spectrosc. 1999, 30, 355–360. [Google Scholar] [CrossRef]
- Chamritski, I.; Burns, G. Infrared-and Raman-active phonons of magnetite, maghemite, and hematite. A computer simulation and spectroscopic study. J. Phys. Chem. 2005, 109, 4965–4968. [Google Scholar] [CrossRef] [PubMed]
- Ramanaidou, E.; Wells, M.; Lau, I.; Laukamp, C. Characterization of iron ore by visible and infrared reflectance and, Raman spectroscopies. In Iron ore; Woodhead Publishing: Cambridge, UK, 2015; pp. 191–228. [Google Scholar]
- Colomban, P. Potential and drawbacks of Raman (micro)spectrometry for the understanding of iron and steel corrosion. In New Trends and Developments in Automotive System Engineering; Chiaberge, M., Ed.; InTech: London, UK, 2011; pp. 567–584. [Google Scholar]
- Shebanova, O.N.; Lazor, P. Raman spectroscopic study of magnetite (FeFe2O4): A new assignment for the vibrational spectrum. J. Solid State Chem. 2003, 174, 424–430. [Google Scholar] [CrossRef]
- Zoppi, A.; Lofrumento, C.; Castellucci, E.; Dejoie, C.; Sciau, P. Micro-Raman study of aluminium-bearing hematite from the slip of Gaul sigillata wares. J. Raman Spectrosc. 2006, 37, 1131–1138. [Google Scholar] [CrossRef]
- Zoppi, A.; Lofrumento, C.; Castellucci, E.; Sciau, P. Al-for-Fe substitution in hematite: The effect of low Al concentrations in the Raman spectrum of Fe2O3. J. Raman Spectrosc. 2008, 39, 40–46. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Zou, X.; Qing, C.; Frost, R. Effect of Al content on the structure of Al-substituted goethite: A micro-Raman spectroscopic study. J. Raman Spectrosc. 2013, 44, 1609–1614. [Google Scholar] [CrossRef]
- Varnshey, D.; Yogi, A. Structural and Electrical conductivity of Mn doped Hematite (α-Fe2O3) phase. J. Mol. Struct. 2011, 995, 157–162. [Google Scholar]
- Varnshey, D.; Yogi, A. Influence of Cr and Mn substitution on the structural and spectroscopic properties of doped haematite: α-Fe2-xMxO3 (0.0 ≤ x <0.50). J. Mol. Struct. 2013, 1052, 105–111. [Google Scholar]
- Varshney, D.; Yogi, A. Structural, vibrational and magnetic properties of Ti substituted bulk hematite. A-Fe2− xTixO3. J. Adv. Ceram. 2014, 3, 269–277. [Google Scholar] [CrossRef]
- Rividi, N.; van Zuilen, M.; Philippot, P.; Ménez, B.; Godard, G.; Poidatz, E. Calibration of Carbonate Composition Using Micro-Raman Analysis: Application to Planetary Surface Exploration. Astrobiology 2010, 3, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, W.; Sharma, S.; Mackenzie, F. Carbonate ion disorder in synthetic and biogenic magnesian calcites: A Raman spectral study. Am. Mineral. 1985, 70, 581–589. [Google Scholar]
- Perrin, J.; Vielzeuf, D.; Laporte, D.; Ricolleau, A.; Rossman, G.R.; Floquet, N. Raman characterization of synthetic magnesian calcites. Am. Mineral. 2016, 101, 2525–2538. [Google Scholar] [CrossRef]
- Borromeo, L.; Zimmermann, U.; Andò, S.; Coletti, G.; Bersani, D.; Basso, D.; Gentile, P.; Schulz, B.; Garzanti, E. Raman spectroscopy as a tool for magnesium estimation in Mg-calcite. J. Raman Spectrosc. 2017, 48, 983–992. [Google Scholar] [CrossRef]
- Schmid, T.; Schäfer, N.; Levcenko, S.; Rissom, T.; Abou-Ras, D. Orientation-distribution mapping of polycrystalline materials by Raman microspectroscopy. Sci. Rep. 2015, 5, 18410. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T.; Schäfer, N.; Abou-Ras, D. Raman microspectroscopy provides access to compositional and microstructural details of polycrystalline materials. Spectrosc. Eur. 2016, 28, 16–20. [Google Scholar]
- Ramanaidou, E.R.; Wells, M.A. Raman I Do–Raman spectroscopy for the mineralogical characterisation of banded iron formation and iron ore. In Proceedings of the Iron Ore 2011 Conference, The Australasian Institute of Mining and Metallurgy, Perth, Australia, 11–13 July 2011; pp. 11–13. [Google Scholar]
| Device | MLA GXMAP (f) | MLA GXMAP (s) | Raman |
|---|---|---|---|
| Sample type Excitation energy | ca. 20 mm × 40 mm ts 25 kV | 20 mm × 40 mm ts 25 kV | 20 mm × 40 mm ts 532 nm, 5–20 mW laser power |
| Step size | 36 µm | 12 µm | 30 µm |
| Acquisition time | 7 ms | 7 ms | 275 ms/200 ms |
| Measurement time | 0.75 h/cm2 | 1.5 h/cm2 | 9 h/cm2 (30 µm Renishaw) 45 h/cm2 (12 µm WITEC) 42 h/mm2 (1 µm WITEC) |
| Mineral | MLA GXMAP(s) | MLA GXMAP(f) | Raman |
|---|---|---|---|
| Magnetite | 35.54 | 33.62 | 34.9 |
| Hematite_Ti | 14.62 | 15.59 | 11.6 |
| Quartz | 22.02 | 21.60 | 19.5 |
| Apatite | 9.78 | 9.38 | 9.0 |
| Ankerite | 8.61 | 8.85 | 7.4 |
| Calcite | 7.68 | 7.70 | 7.6 |
| Hematite | 0.72 | 2.21 | 0.7 |
| MLA | Raman Imaging |
|---|---|
|
|
|
|
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krolop, P.; Jantschke, A.; Gilbricht, S.; Niiranen, K.; Seifert, T. Mineralogical Imaging for Characterization of the Per Geijer Apatite Iron Ores in the Kiruna District, Northern Sweden: A Comparative Study of Mineral Liberation Analysis and Raman Imaging. Minerals 2019, 9, 544. https://doi.org/10.3390/min9090544
Krolop P, Jantschke A, Gilbricht S, Niiranen K, Seifert T. Mineralogical Imaging for Characterization of the Per Geijer Apatite Iron Ores in the Kiruna District, Northern Sweden: A Comparative Study of Mineral Liberation Analysis and Raman Imaging. Minerals. 2019; 9(9):544. https://doi.org/10.3390/min9090544
Chicago/Turabian StyleKrolop, Patrick, Anne Jantschke, Sabine Gilbricht, Kari Niiranen, and Thomas Seifert. 2019. "Mineralogical Imaging for Characterization of the Per Geijer Apatite Iron Ores in the Kiruna District, Northern Sweden: A Comparative Study of Mineral Liberation Analysis and Raman Imaging" Minerals 9, no. 9: 544. https://doi.org/10.3390/min9090544
APA StyleKrolop, P., Jantschke, A., Gilbricht, S., Niiranen, K., & Seifert, T. (2019). Mineralogical Imaging for Characterization of the Per Geijer Apatite Iron Ores in the Kiruna District, Northern Sweden: A Comparative Study of Mineral Liberation Analysis and Raman Imaging. Minerals, 9(9), 544. https://doi.org/10.3390/min9090544





