Crystal Chemistry and Properties of Elpidite and Its Ag-Exchanged Forms
Abstract
:1. Introduction
2. Materials and Methods
3. Results
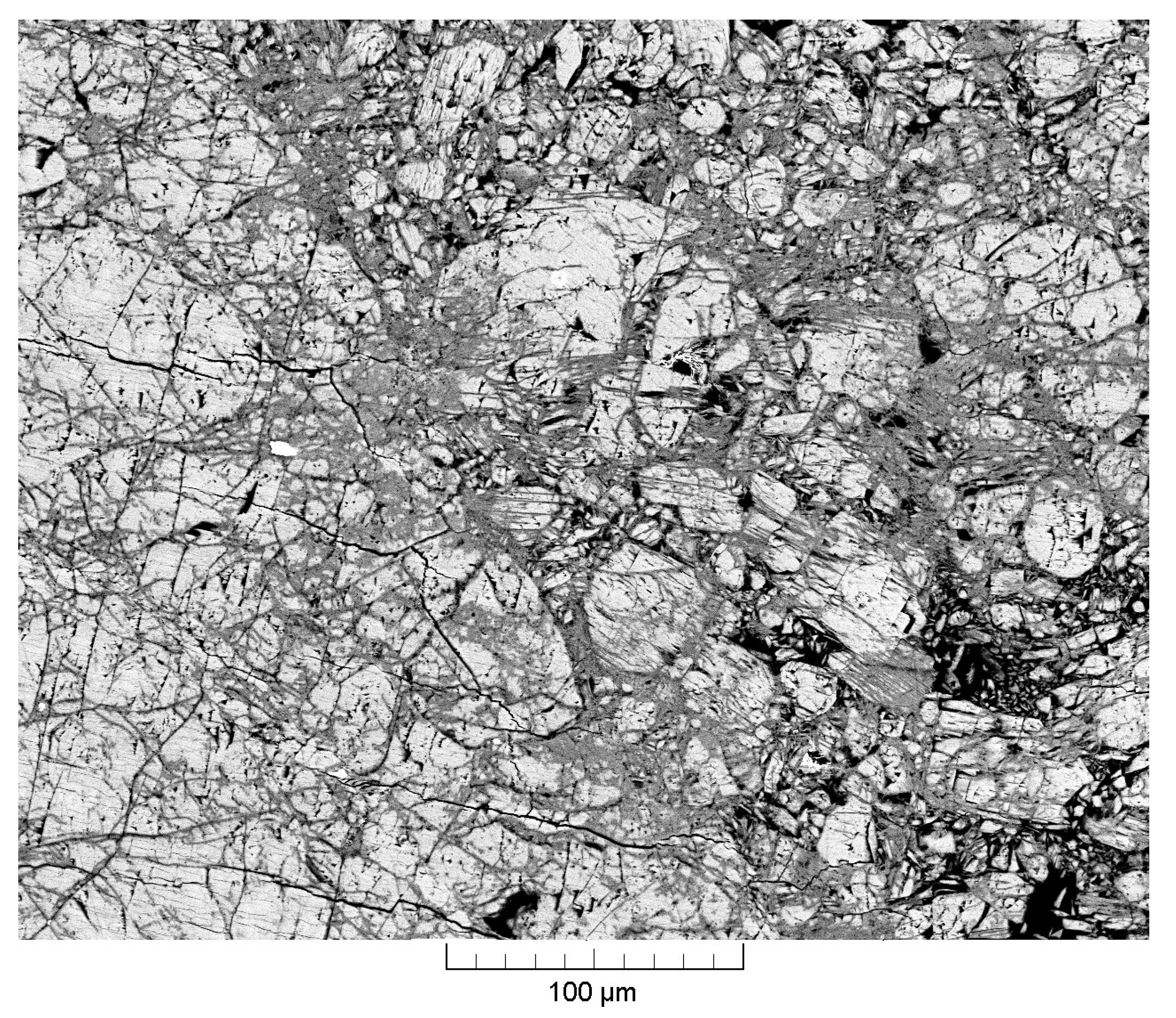
3.1. Chemical Composition
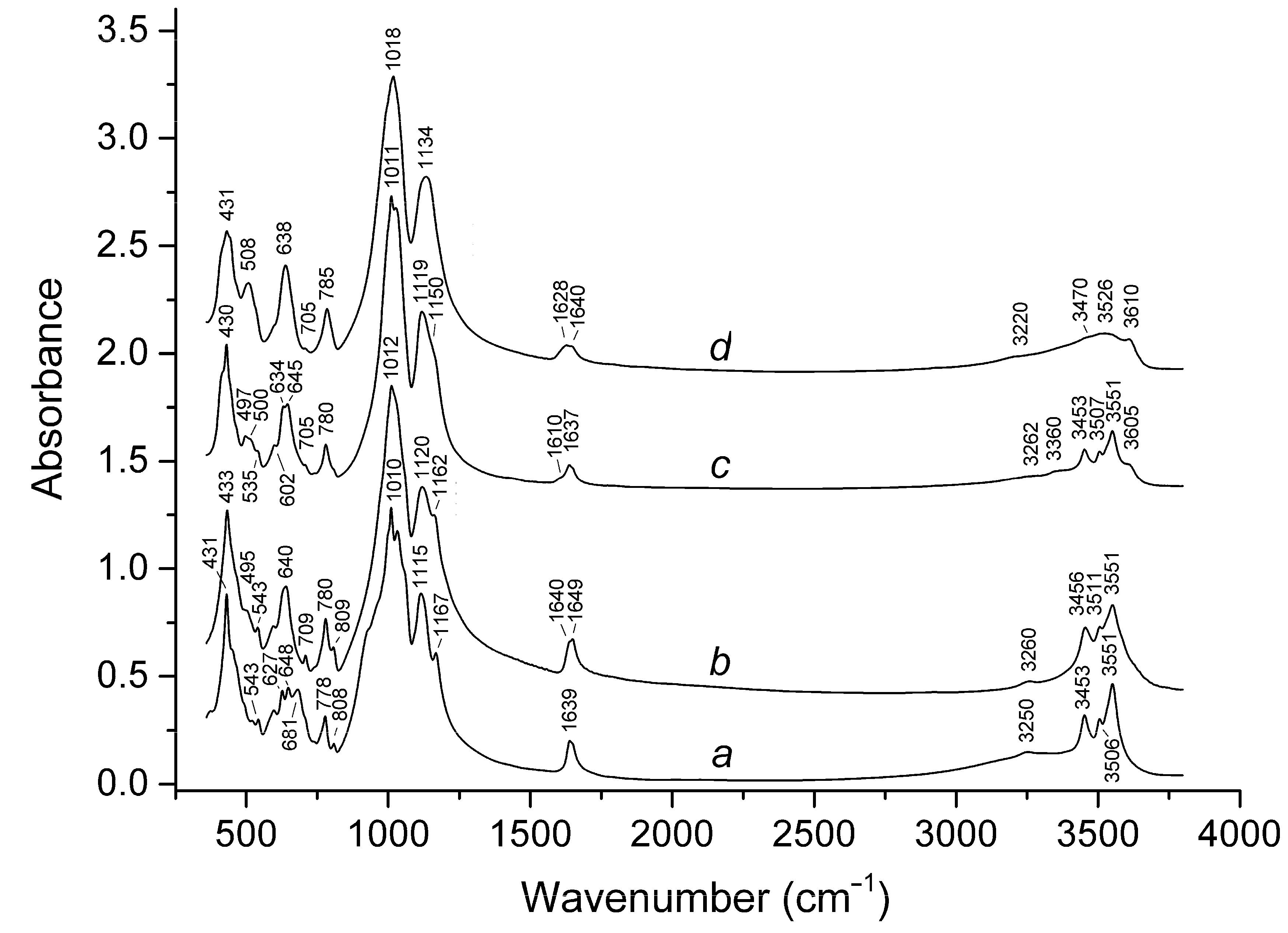
3.2. Infrared Specroscopy
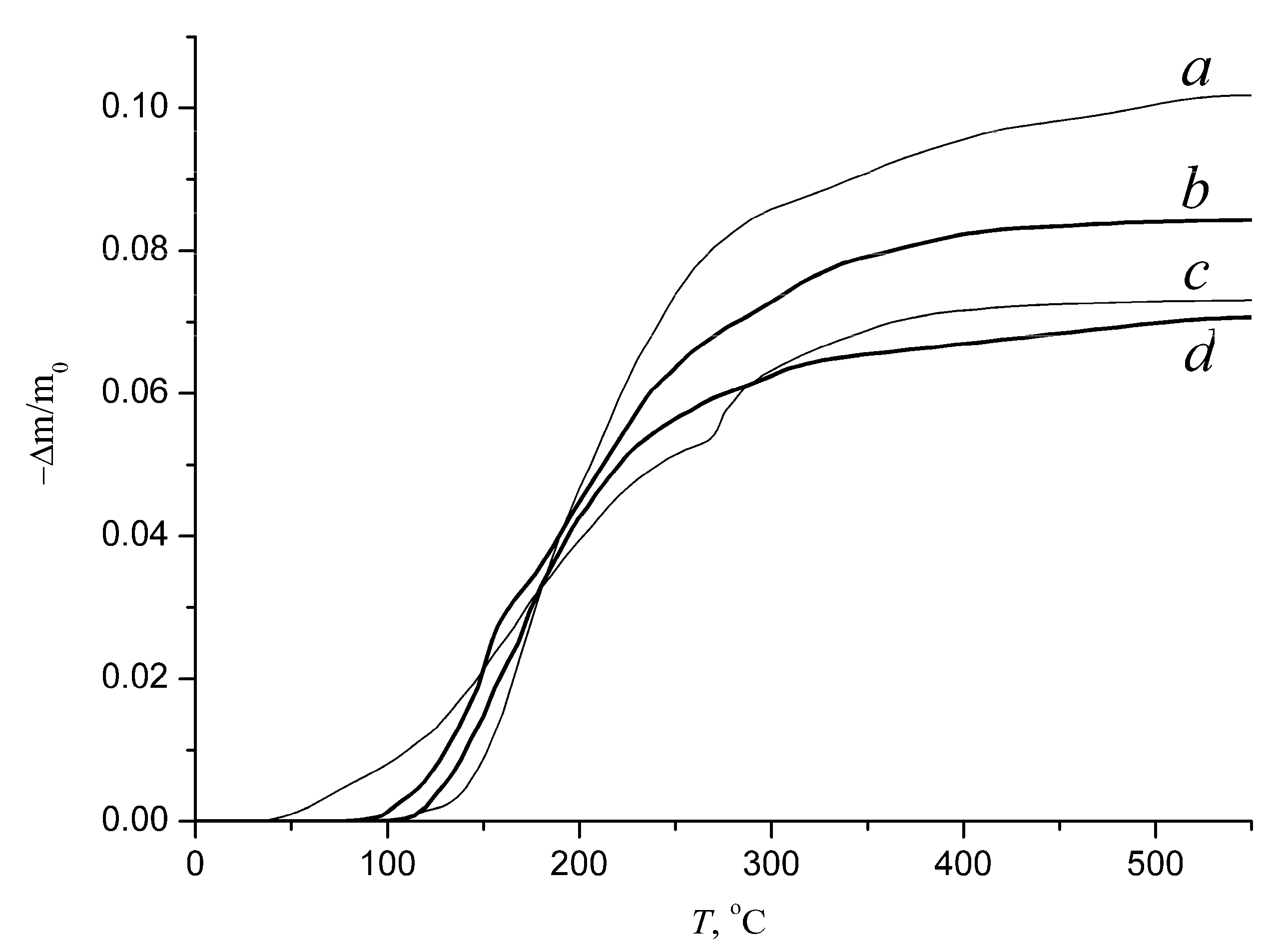
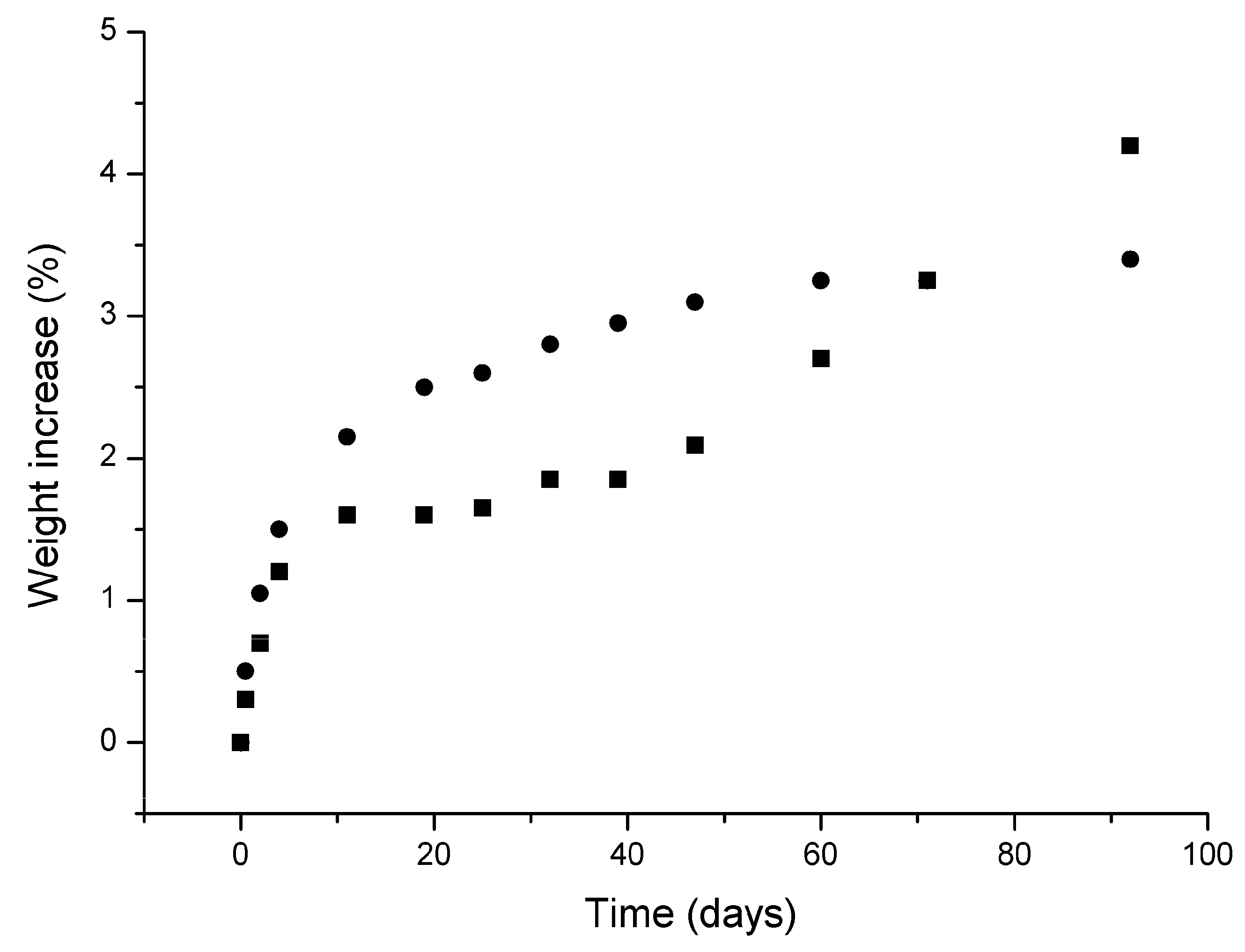
3.3. Dehydration and Rehydration Kinetics
3.4. X-Ray Diffraction Data and Crystal Structures
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Chukanov, N.V.; Pekov, I.V.; Rastsvetaeva, R.K. Crystal chemistry, properties and synthesis of microporous silicates containing transition elements. Russ. Chem. Rev. 2004, 73, 227–246. [Google Scholar] [CrossRef]
- Turchkova, A.G.; Pekov, I.V.; Bryzgalov, I.A. Cation-exchange properties of natural zeolite-like sodium zirconosilicates: An experimental study in aqueous solutions at 80–90 °C and 1 atm. In Proceedings of the 19th General Meet of IMA, Kobe, Japan, 23–28 July 2006. [Google Scholar]
- Grigor’eva, A.A.; Zubkova, N.V.; Pekov, I.V.; Kolitsch, U.; Pushcharovsky, D.Y.; Vigasina, M.F.; Giester, G.; Ðorðevic, T.; Tillmanns, E.; Chukanov, N.V. Crystal chemistry of elpidite from Khan Bogdo (Mongolia) and its K-and Rb-exchanged forms. Crystallogr. Rep. 2011, 56, 832–841. [Google Scholar] [CrossRef]
- Salvi, S.; Williams-Jones, A.E. Zirconosilicate phase relations in the Strange Lake (Lac Brisson) Pluton, Quebec-Labrador, Canada. Amer. Mineral. 2001, 13, 355–363. [Google Scholar] [CrossRef]
- Neronova, N.N.; Belov, N.V. Crystal structure of elpidite Na2ZrSi6O15·(H2O)3. Dimorphism of the dimetasilicate radical Si6O15. Dokl. Akad. Nauk SSSR 1963, 150, 642–645. (In Russian) [Google Scholar]
- Neronova, N.N.; Belov, N.V. Crystal structure of elpidite, Na2ZrSi6O15·(H2O)3. Sov. Phys. Crystallogr. 1964, 9, 700–705. [Google Scholar]
- Cannillo, E.; Rossi, G.; Ungaretti, L. The crystal structure of elpidite. Am. Mineral. 1973, 58, 106–109. [Google Scholar]
- Sapozhnikov, A.N.; Kashaev, A.A. Features of the crystal structure of calcium-containing elpidite. Sov. Phys. Crystallogr. 1978, 23, 24–27. [Google Scholar]
- Sapozhnikov, A.N.; Kashaev, A.A. The crystal structure of calcined Ca-containing elpidite. Sov. Phys. Crystallogr. 1980, 25, 357–359. [Google Scholar]
- Zubkova, N.V.; Ksenofontov, D.A.; Kabalov, Y.K.; Chukanov, N.V.; Nedel’ko, V.V. Dehydration-induced structural transformations of the microporous zirconosilicate elpidite. Inorg. Mat. 2011, 47, 506–512. [Google Scholar] [CrossRef]
- Cametti, G.; Armbruster, T.; Nagashima, M. Dehydration and thermal stability of elpidite: An in-situ single crystal X-ray diffraction study. Microporous Mesoporous Mat. 2016, 227, 81–87. [Google Scholar] [CrossRef]
- Nedel’ko, V.V.; Chukanov, N.V.; Pekov, I.V. Dehydration kinetics of the microporous zirconosilicate elpidite. Inorg. Mat. 2011, 47, 502–505. [Google Scholar] [CrossRef]
- Agakhanov, A.A.; Pautov, L.A.; Karpenko, V.Y.; Sokolova, E.; Abdu, Y.A.; Hawthorne, F.C.; Pekov, I.V.; Siidra, O.I. Yusupovite, Na2Zr(Si6O15)(H2O)3, a new mineral species from the Darai-Pioz alkaline massif and its implications as a new microporous filter for large ions. Am. Mineral. 2015, 100, 1502–1508. [Google Scholar] [CrossRef]
- Pekov, I.V. Lovozero Massif: History, Pegmatites, Minerals; OP: Moscow, Russia, 2000; p. 480. [Google Scholar]
- Agilent Technologies, CrysAlisPro Software System, Version 1.171.35.21; Agilent Technologies: Santa Clara, CA, USA, 2012.
- Rigaku Oxford Diffraction, CrysAlisPro Software System, Version 1.171.39.46; Rigaku Oxford Diffraction: Oxford, UK, 2018.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Libowitzky, E. Correlation of O–H stretching frequencies and O–H···O hydrogen bond lengths in minerals. Mon. für Chem. 1999, 130, 1047–1059. [Google Scholar]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Cryst. 2015, 71, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]

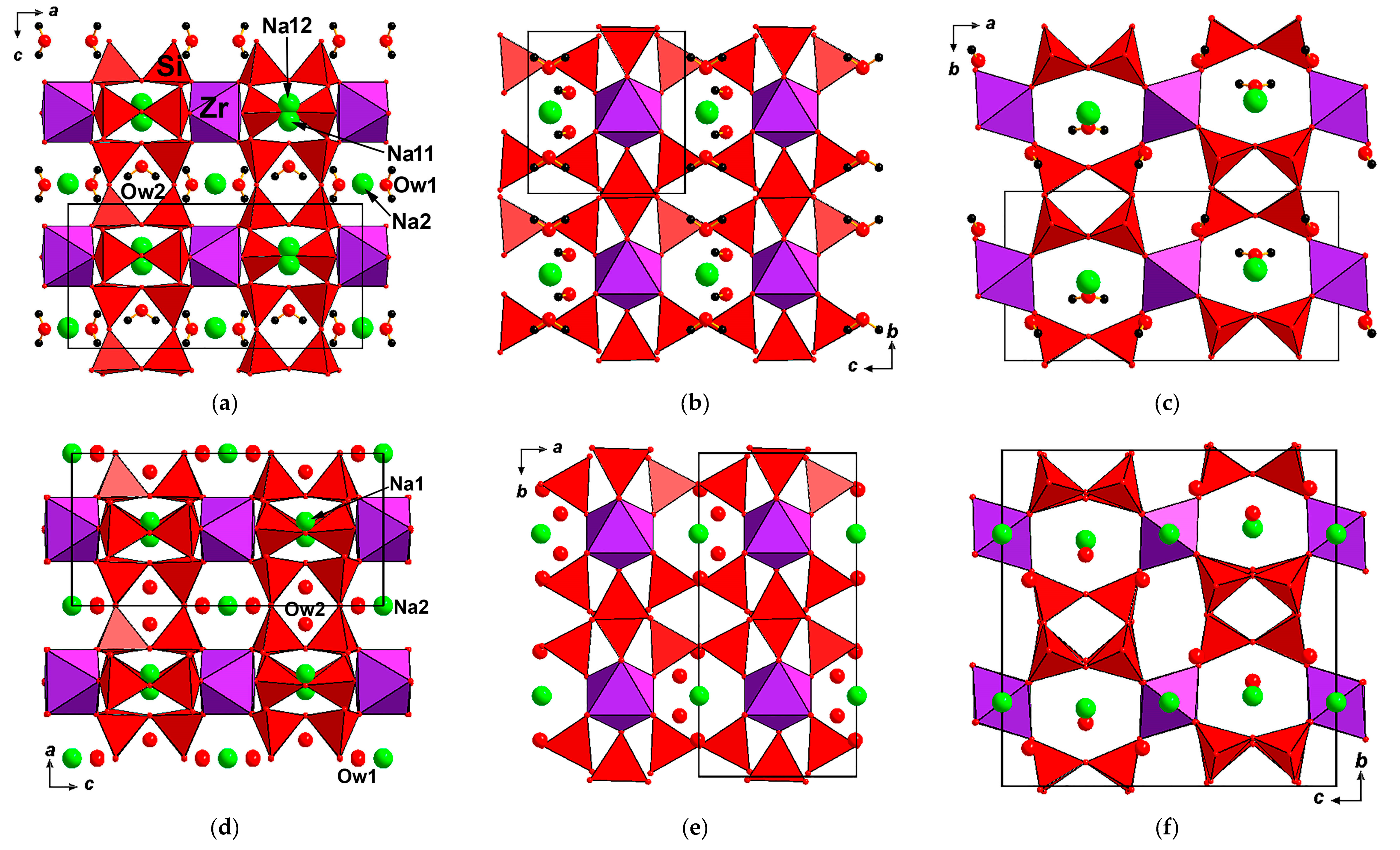
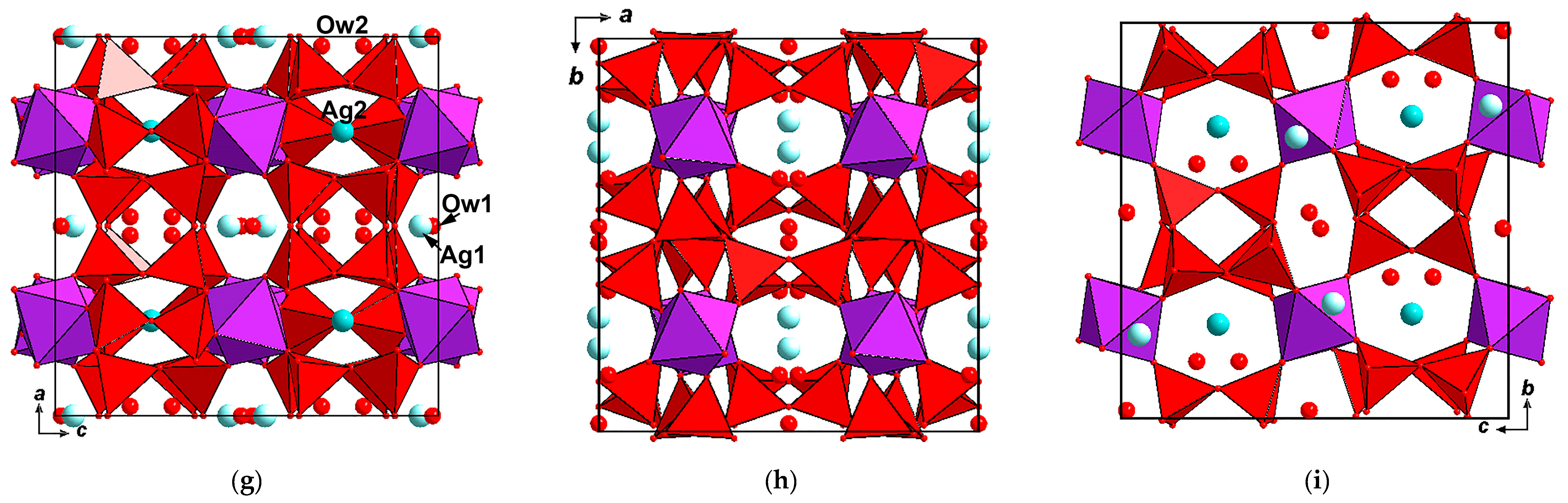
| Sample/Locality | Sp.gr., Z | a (Å) | b (Å) | c (Å) | V (Å3) | Ref. |
|---|---|---|---|---|---|---|
| Elpidite/Lovozero, Kola peninsula, Russia | Pbmm, 2 | 7.4 | 14.4 | 7.05 | 751.3 | [5] |
| Elpidite/Lovozero, Kola peninsula, Russia | Pbm2, 2 | 7.4 | 14.4 | 7.05 | 751.3 | [6] |
| Elpidite/Mont Saint-Hilaire, Québec, Canada | Pbcm, 4 | 7.14(2) | 14.68(1) | 14.65(1) | 1535.5 | [7] |
| Elpidite (Ca-enriched variety)/Khan Bogdo, Mongolia | Pbm2, 2 | 7.31 | 14.68 | 7.13 | 765.1 | [8] |
| Elpidite (Ca-enriched variety), dehydrated/init. Khan Bogdo, Mongolia | Bba2, 8 | 14.48 | 14.28 | 14.09 | 2913.5 | [9] |
| Elpidite/Lovozero, Kola peninsula, Russia | Pbcm, 4 | 7.1136(1) | 14.6764(2) | 14.5977(2) | 1524.02(4) | [10] |
| Elpidite, dehydrated/init. Lovozero, Kola peninsula, Russia | Cmce, 8 | 14.0899(1) | 14.4983(1) | 14.3490(1) | 2931.23(4) | [10] |
| Elpidite/Khan Bogdo, Mongolia | Pbcm, 4 | 7.1312(12) | 14.6853(12) | 14.6349(15) | 1532.6(3) | [3] |
| K-exch. (90 °C)/init. Khan Bogdo, Mongolia | Cmce, 8 | 14.054(3) | 14.308(3) | 14.553(3) | 2926.4(11) | [3] |
| K-exch. (150 °C)/init. Khan Bogdo, Mongolia | Cmce, 8 | 14.037(3) | 14.266(3) | 14.552(3) | 2914.1(11) | [3] |
| Rb-exch. (90 °C)/init. Khan Bogdo, Mongolia | Pbcm, 4 | 7.1280(10) | 14.644(3) | 14.642(3) | 1528.4(5) | [3] |
| Rb-exch. (150 °C)/init. Khan Bogdo, Mongolia | Cmce, 8 | 14.2999(12) | 14.4408(15) | 14.7690(12) | 3049.8(5) | [3] |
| Yusupovite/Darai-Pioz, Tajikistan | C2/m, 8 | 14.5975(4) | 14.1100(4) β 90.0399(4)° | 14.4394(4) | 2974.1(3) | [13] |
| Elpidite/Mont Saint-Hilaire, Québec, Canada | Pbcm, 4 | 7.11340(10) | 14.6796(2) | 14.6030(2) | 1524.87(4) | [11] |
| Elpidite, partially dehydrated/Mont Saint-Hilaire, Québec, Canada | Cmce, 8 | 14.1260(5) | 14.5734(5) | 14.3627(5) | 2956.76(18) | [11] |
| Elpidite, dehydrated/Mont Saint-Hilaire, Québec, Canada | Cmce, 8 | 14.1271(4) | 14.5110(4) | 14.3533(4) | 2942.40(14) | [11] |
| Elpidite/Lovozero, Kola peninsula, Russia | Pbm2 * 2 | 7.3383(4) | 14.6127(7) | 7.1148(3) | 762.94(6) | This work |
| Ag-exch. I/init. Lovozero, Kola peninsula, Russia | Cmce, 8 | 14.1755(7) | 14.6306(9) | 14.2896(7) | 2963.6(3) | This work |
| Ag-exch. II/init. Khan Bogdo, Mongolia | Cmce, 8 | 14.1411(5) | 14.5948(4) | 14.3035(5) | 2952.04(17) | This work |
| Sample | Elpidite (Lovozero) | I | II |
|---|---|---|---|
| Formula weight * | 599.79 | 734.72 | 711.78 |
| Temperature, K | 293(2) | 150.0(1) | |
| Radiation and wavelength, Å | MoKα; 0.71073 | ||
| Crystal system, space group, Z | Orthorhombic, Pma2 **, 2 | Orthorhombic, Cmce, 8 | |
| Unit–cell dimensions, Å | a = 14.6127(7) b = 7.3383(4) c = 7.1148(3) | a = 14.1755(7) b = 14.6306(9) c = 14.2896(7) | a =14.1411(5) b = 14.5948(4) c = 14.3035(5) |
| V, Å3 | 762.94(6) | 2963.6(3) | 2952.04(17) |
| Absorption coefficient μ, mm−1 * | 1.35 | 3.85 | 3.59 |
| F000 * | 592 | 2782 | 2702 |
| Crystal size, mm | 0.12 × 0.25 × 0.30 | 0.007 × 0.007 × 0.013 | 0.10 × 0.13 × 0.15 |
| Diffractometer | Oxford Diffraction, SuperNova | Xcalibur S CCD | |
| θ range for data collection, ° | 2.788–28.769 | 2.784–28.176 | 2.881–27.857 |
| Index ranges | −19 ≤ h ≤ 15 −9 ≤ k ≤ 7, −9 ≤ l ≤ 9 | −18 ≤ h ≤ 18 −15 ≤ k ≤ 17, −16 ≤ l ≤ 18 | −18 ≤ h ≤ 11 −15 ≤ k ≤ 19, −15 ≤ l ≤ 18 |
| Reflections collected | 3681 | 6530 | 4935 |
| Independent reflections | 1653 (Rint = 0.0239) | 1718 (Rint = 0.0636) | 1823 (Rint = 0.0202) |
| Independent reflections [I > 2σ(I)] | 1437 | 1195 | 1707 |
| Absorption correction | Multi-scan | ||
| Refinement method | Full-matrix least-squares on F2 | ||
| Number of refined parameters | 132 | 150 | 147 |
| Final R indices [I > 2σ(I)] R1/wR2 | 0.0275/0.0644 | 0.0514/0.0986 | 0.0539/0.1140 |
| R indices (all data) R1/wR2 | 0.0349/0.0695 | 0.0871/0.1145 | 0.0581/0.1158 |
| GoF | 1.158 | 1.045 | 1.201 |
| Largest diff. peak and hole, e/Å3 | 0.805 and −0.495 | 1.282 and −1.401 | 1.357 and −1.486 |
| Component | Elpidite Lovozero (after [10]) | Elpidite Khan Bogdo | Sample I | Sample II, zone 1 | Sample II, zone 2 | |||
|---|---|---|---|---|---|---|---|---|
| Na2O | 10.17 | 8.64 | 0.64 | 0.87 | 0 | 0.08 | 0 | 0.22 |
| CaO | - | 1.41 | - | - | 0.07 | 0.35 | 3.46 | 2.81 |
| Ag2O | - | - | 28.22 | 26.73 | 29.87 | 31.81 | 20.28 | 21.25 |
| ZrO2 | 20.89 | 20.94 | 16.63 | 15.94 | 17.19 | 15.34 | 17.89 | 17.04 |
| SiO2 | 59.02 | 61.05 | 47.61 | 48.67 | 46.08 | 46.94 | 50.94 | 50.83 |
| Total | 91.32 * | 92.12 ** | 93.10 | 92.21 | 93.21 | 94.52 | 92.57 | 92.15 |
| H2O (by TG data) | 10.16 | 8.43 | 7.00 | 7.49 | ||||
| Site | x | y | z | Ueq |
|---|---|---|---|---|
| Zr | 0.5 | 0.5 | 0.3619(6) | 0.00597(16) |
| Si1 | 0.35828(7) | 0.90523(15) | 0.3600(9) | 0.0082(2) |
| Si2 | 0.3548(3) | 0.2214(4) | −0.3624(3) | 0.0071(8) |
| Si3 | 0.3552(3) | 0.2228(4) | 0.0844(3) | 0.0095(8) |
| Na11 | 0.25 | 0.537(2) | 0.421(3) | 0.0324(13) |
| Na12 | 0.25 | 0.5367(19) | 0.303(3) | 0.0324(13) |
| Na2 | 0.5 | 0.5 | −0.141(3) | 0.0196(6) |
| O1 | 0.3603(2) | 0.1926(5) | −0.133(2) | 0.0134(8) |
| O2 | 0.4202(6) | 0.3797(10) | 0.1528(12) | 0.012(2) |
| O3 | 0.4202(6) | 0.3813(11) | 0.5690(13) | 0.016(2) |
| O4 | 0.25 | 0.2706(16) | 0.143(2) | 0.022(3) |
| O5 | 0.3803(8) | 0.0290(12) | −0.4516(15) | 0.019(3) |
| O6 | 0.3822(7) | 1.0226(10) | 0.1782(16) | 0.018(3) |
| O7 | 0.25 | 0.2664(16) | 0.576(2) | 0.021(4) |
| O8 | 0.25 | 0.8579(7) | 0.358(3) | 0.0272(15) |
| O9 | 0.4117(2) | 0.7189(4) | 0.3685(16) | 0.0137(7) * |
| Ow1 | 0.4202(3) | 0.7750(6) | −0.133(2) | 0.0289(11) |
| H1a | 0.4006 | 0.8393 | −0.2373 | 0.043 * |
| H1b | 0.4006 | 0.8393 | −0.0373 | 0.043 * |
| Ow2 | 0.25 | 0.6213(15) | 0.7385(13) | 0.054(2) |
| H2 | 0.2028(9) | 0.637(13) | 0.807(5) | 0.081 * |
| Bond | Length | Bond | Length | ||||
|---|---|---|---|---|---|---|---|
| Zr–O9 | 2.061(3) × 2 | Na11–O7 | 2.27(2) | ||||
| –O3 | 2.071(9) × 2 | –Ow2 | 2.35(3) | ||||
| –O2 | 2.071(9) × 2 | –O8 | 2.398(19) | ||||
| –O9 | 2.741(11) × 2 | ||||||
| Si1–O9 | 1.576(3) | –O4 | 2.78(2) | ||||
| –O6 | 1.593(11) | –O3 | 2.933(15) × 2 | ||||
| –O8 | 1.6200(16) | ||||||
| –O5 | 1.651(11) | ||||||
| Na12–O4 | 2.26(2) | ||||||
| Si2–O3 | 1.590(9) | –O8 | 2.389(16) | ||||
| –O5 | 1.592(10) | –O9 | 2.755(8) × 2 | ||||
| –O7 | 1.626(6) | –O4 | 2.78(2) | ||||
| –O1 | 1.647(14) | –O3 | 2.943(12) × 2 | ||||
| Si3–O1 | 1.565(15) | Na2–Ow1 | 2.331(4) × 2 | ||||
| –O2 | 1.570(9) | –O3 | 2.524(19) × 2 | ||||
| –O4 | 1.631(6) | –O2 | 2.552(19) × 2 | ||||
| –O6 | 1.661(9) | ||||||
| H–Bonding | |||||||
| D–H···A | d(D–H) (Å) | d(H···A) (Å) | d(D···A) (Å) | ∠DHA (°) | |||
| Ow1–H1a···O5 | 0.925 | 2.086 | 2.992 | 166.2 | |||
| Ow1–H1b···O6 | 0.876 | 2.058 | 2.918 | 166.8 | |||
| Ow2–H2···Ow1 | 0.853 | 2.107 | 2.880 | 150.4 | |||
| Site | x | y | z | Ueq | s.o.f. |
|---|---|---|---|---|---|
| Zr | 0.25 0.25 | 0.25 0.25 | 0.5 0.5 | 0.0068(3) 0.0049(2) | 1 |
| Si1 | 0.39106(12) 0.39092(13) | 0.09674(14) 0.09659(13) | 0.37813(13) 0.37839(14) | 0.0087(4) 0.0083(4) | 1 |
| Si2 | 0.38918(13) 0.38874(13) | 0.38122(14) 0.38052(13) | 0.66004(13) 0.66137(14) | 0.0091(4) 0.0080(4) | 1 |
| Si3 | 0.23591(13) 0.23511(13) | 0.04900(15) 0.04904(12) | 0.64023(13) 0.64087(13) | 0.0093(4) 0.0079(4) | 1 |
| O1 | 0.1674(3) 0.1677(4) | 0.1972(4) 0.1968(4) | 0.3929(3) 0.3926(4) | 0.0156(12) 0.0163(11) | 1 |
| O2 | 0.1444(3) 0.1437(4) | 0.0177(4) 0.0170(4) | 0.3690(4) 0.3673(4) | 0.0202(13) 0.0167(11) | 1 |
| O3 | 0.25 0.25 | 0.0754(6) 0.0756(5) | 0.75 0.75 | 0.0213(18) 0.0171(16) | 1 |
| O4 | 0.2166(3) 0.2154(4) | 0.1406(4) 0.1412(3) | 0.5848(3) 0.5844(4) | 0.0143(11) 0.0133(10) | 1 |
| O5 | 0.0 0.0 | 0.1253(6) 0.1272(5) | 0.3655(5) 0.3637(6) | 0.0183(18) 0.0151(16) | 1 |
| O6 | 0.3283(3) 0.3279(4) | −0.0060(4) −0.0054(4) | 0.6071(3) 0.6064(4) | 0.0173(12) 0.0153(11) | 1 |
| O7 | 0.3747(3) 0.3735(4) | 0.3671(4) 0.3666(4) | 0.7715(3) 0.7729(4) | 0.0184(12) 0.0172(12) | 1 |
| O8 | 0.3655(3) 0.3677(4) | 0.1763(4) 0.1762(4) | 0.4494(3) 0.4513(4) | 0.0142(12) 0.0138(11) | 1 |
| O9 | 0.5 0.5 | 0.0640(5) 0.0631(5) | 0.3884(5) 0.3864(6) | 0.0156(17) 0.0140(15) | 1 |
| Ag1 | 0.5 0.5 | 0.2163(6) 0.2106(12) | 0.5428(3) 0.5471(9) | 0.0422(16) 0.0256(17) | 0.80(2) 0.51(4) |
| Ag11 | 0.5 0.5 | 0.249(4) 0.233(2) | 0.498(4) 0.533(3) | 0.0422(16) 0.0256(17) | 0.040(7) 0.19(4) |
| Ag11a | - 0.5 | - 0.252(2) | - 0.493(3) | - 0.0256(17) | - 0.053(6) |
| Ag12 | 0.5 - | 0.178(5) - | 0.558(3) - | 0.0422(16) - | 0.07(2) - |
| Ag13 | 0.5 0.5 | 0.3530(13) 0.3634(16) | 0.4040(14) 0.4148(16) | 0.0422(16) 0.0256(17) | 0.081(4) 0.055(3) |
| Ag14 | 0.542(2) 0.5187(7) | 0.164(2) 0.1688(11) | 0.578(2) 0.5736(11) | 0.0422(16) 0.0256(17) | 0.0280(19) 0.068(3) |
| Ag15 | 0.5 0.5 | 0.332(2) 0.3352(12) | 0.370(2) 0.3866(12) | 0.0422(16) 0.0256(17) | 0.053(4) 0.069(3) |
| Ag2 | 0.25 0.25 | 0.2393(4) 0.23686(7) | 0.75 0.75 | 0.0206(19) 0.0202(4) | 0.69(5) 0.743(5) |
| Ag21 | 0.273(3) - | 0.231(2) - | 0.753(2) - | 0.0206(19) - | 0.09(3) - |
| Ow1 | 0.0 - | 0.0 - | 0.5 - | 0.051(7) * - | 0.5 ** - |
| Ow11 | 0.0 0.0 | 0.039(3) 0.0201(9) | 0.527(3) 0.5147(10) | 0.051(7) * 0.010(3) * | 0.25 ** 0.5 ** |
| Ow2 | 0.5261(12) 0.5247(16) | 0.3549(12) 0.3569(12) | 0.1934(11) 0.1954(19) | 0.064(8) 0.080(10) | 0.435 ** 0.43 ** |
| Sample I | Sample II | ||||||
|---|---|---|---|---|---|---|---|
| Bond | Length | Bond | Length | Bond | Length | Bond | Length |
| Zr1–O4 | 2.062(5) × 2 | Zr1–O4 | 2.054(5) × 2 | Ag1–Ow2 | 2.37(3) | ||
| –O1 | 2.075(5) × 2 | –O1 | 2.077(5) × 2 | –O8 | 2.373(8) × 2 | ||
| –O8 | 2.090(5) × 2 | –O8 | 2.102(5) × 2 | –O5 | 2.690(15) | ||
| Ag1–O8 | 2.399(5) × 2 | –O1 | 2.863(9) × 2 | ||||
| Si1–O8 | 1.589(5) | –Ow2 | 2.420(17) | Si1–O8 | 1.595(5) | –Ag13 | 2.92(3) |
| –O6 | 1.612(5) | –O5 | 2.663(10) | –O6 | 1.617(5) | –Ag15 | 2.93(3) |
| –O9 | 1.624(3) | –Ag13 | 2.82(2) | –O7 | 1.621(6) | ||
| –O7 | 1.630(5) | –O1 | 2.843(6) × 2 | –O9 | 1.622(3) | Ag11–O8 | 2.36(2) × 2 |
| –Ag15 | 3.00(3) | –O5 | 2.52(3) | ||||
| Si2–O1 | 1.592(5) | Si2–O1 | 1.583(5) | –Ag13 | 2.55(5) | ||
| –O2 | 1.608(6) | –O2 | 1.617(6) | –Ag15 | 2.57(5) | ||
| –O5 | 1.615(2) | Ag2–O3 | 2.398(10) | –O5 | 1.618(3) | –Ow2 | 2.69(5) |
| –O7 | 1.619(5) | –O1 | 2.531(5) × 2 | –O7 | 1.622(6) | –O1 | 2.795(17) × 2 |
| –O7 | 2.591(7) × 2 | ||||||
| Si3–O4 | 1.581(5) | –O4 | 2.808(6) × 2 | Si3–O4 | 1.593(5) | Ag2–O3 | 2.354(8) |
| –O6 | 1.608(5) | –O6 | 1.611(5) | –O1 | 2.540(6) × 2 | ||
| –O3 | 1.628(3) | –O2 | 1.617(5) | –O7 | 2.597(6) × 2 | ||
| –O2 | 1.629(5) | –O3 | 1.622(3) | –O4 | 2.792(5) × 2 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubkova, N.V.; Nikolova, R.P.; Chukanov, N.V.; Kostov-Kytin, V.V.; Pekov, I.V.; Varlamov, D.A.; Larikova, T.S.; Kazheva, O.N.; Chervonnaya, N.A.; Shilov, G.V.; et al. Crystal Chemistry and Properties of Elpidite and Its Ag-Exchanged Forms. Minerals 2019, 9, 420. https://doi.org/10.3390/min9070420
Zubkova NV, Nikolova RP, Chukanov NV, Kostov-Kytin VV, Pekov IV, Varlamov DA, Larikova TS, Kazheva ON, Chervonnaya NA, Shilov GV, et al. Crystal Chemistry and Properties of Elpidite and Its Ag-Exchanged Forms. Minerals. 2019; 9(7):420. https://doi.org/10.3390/min9070420
Chicago/Turabian StyleZubkova, Natalia V., Rositsa P. Nikolova, Nikita V. Chukanov, Vladislav V. Kostov-Kytin, Igor V. Pekov, Dmitry A. Varlamov, Tatiana S. Larikova, Olga N. Kazheva, Nadezhda A. Chervonnaya, Gennadiy V. Shilov, and et al. 2019. "Crystal Chemistry and Properties of Elpidite and Its Ag-Exchanged Forms" Minerals 9, no. 7: 420. https://doi.org/10.3390/min9070420
APA StyleZubkova, N. V., Nikolova, R. P., Chukanov, N. V., Kostov-Kytin, V. V., Pekov, I. V., Varlamov, D. A., Larikova, T. S., Kazheva, O. N., Chervonnaya, N. A., Shilov, G. V., & Pushcharovsky, D. Y. (2019). Crystal Chemistry and Properties of Elpidite and Its Ag-Exchanged Forms. Minerals, 9(7), 420. https://doi.org/10.3390/min9070420








