The Atomic Arrangement of Cr-rich Tourmaline from the #1 Mine, Balmat, St. Lawrence County, New York, USA
Abstract
:1. Introduction and Geologic Setting
2. Methods
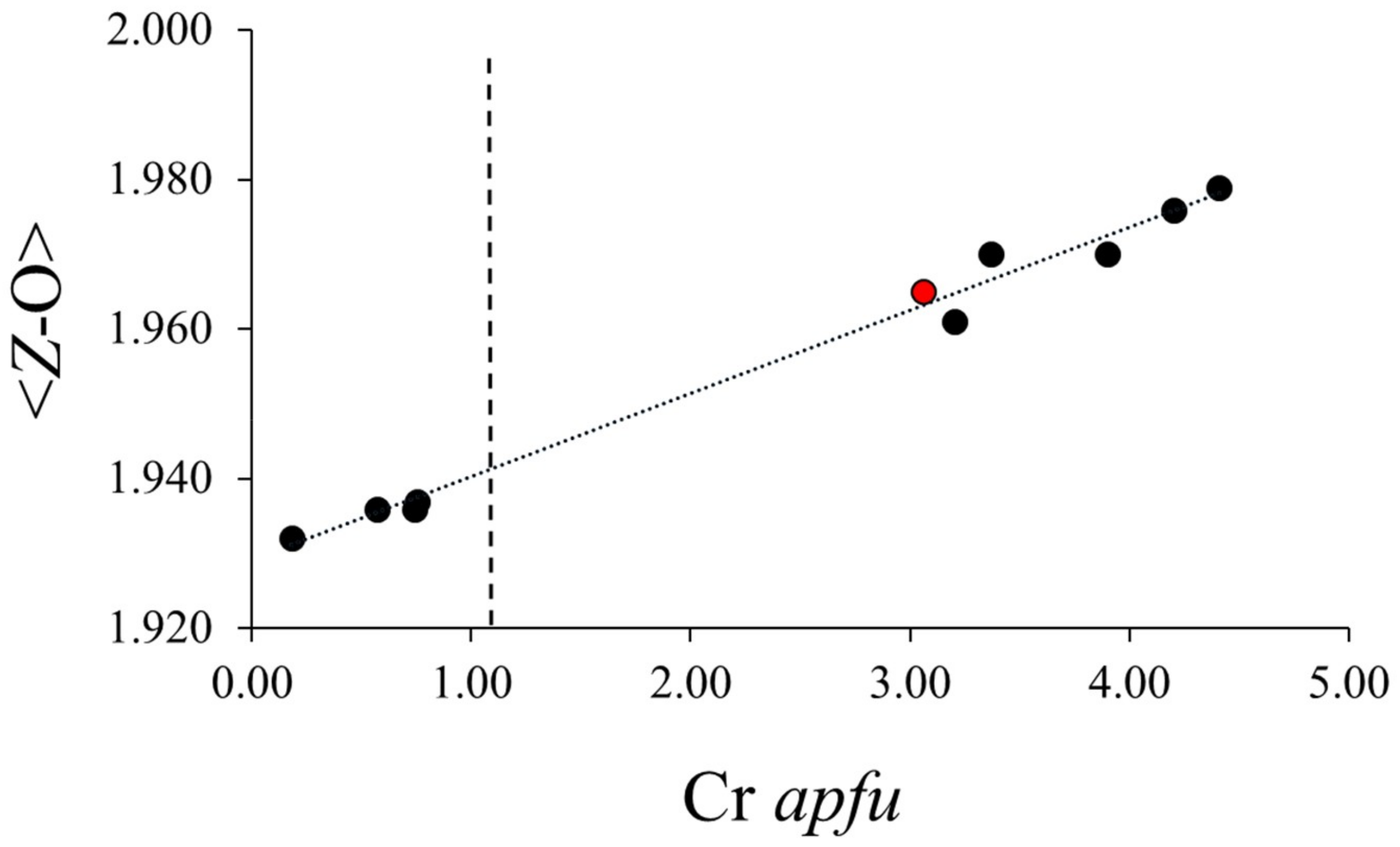
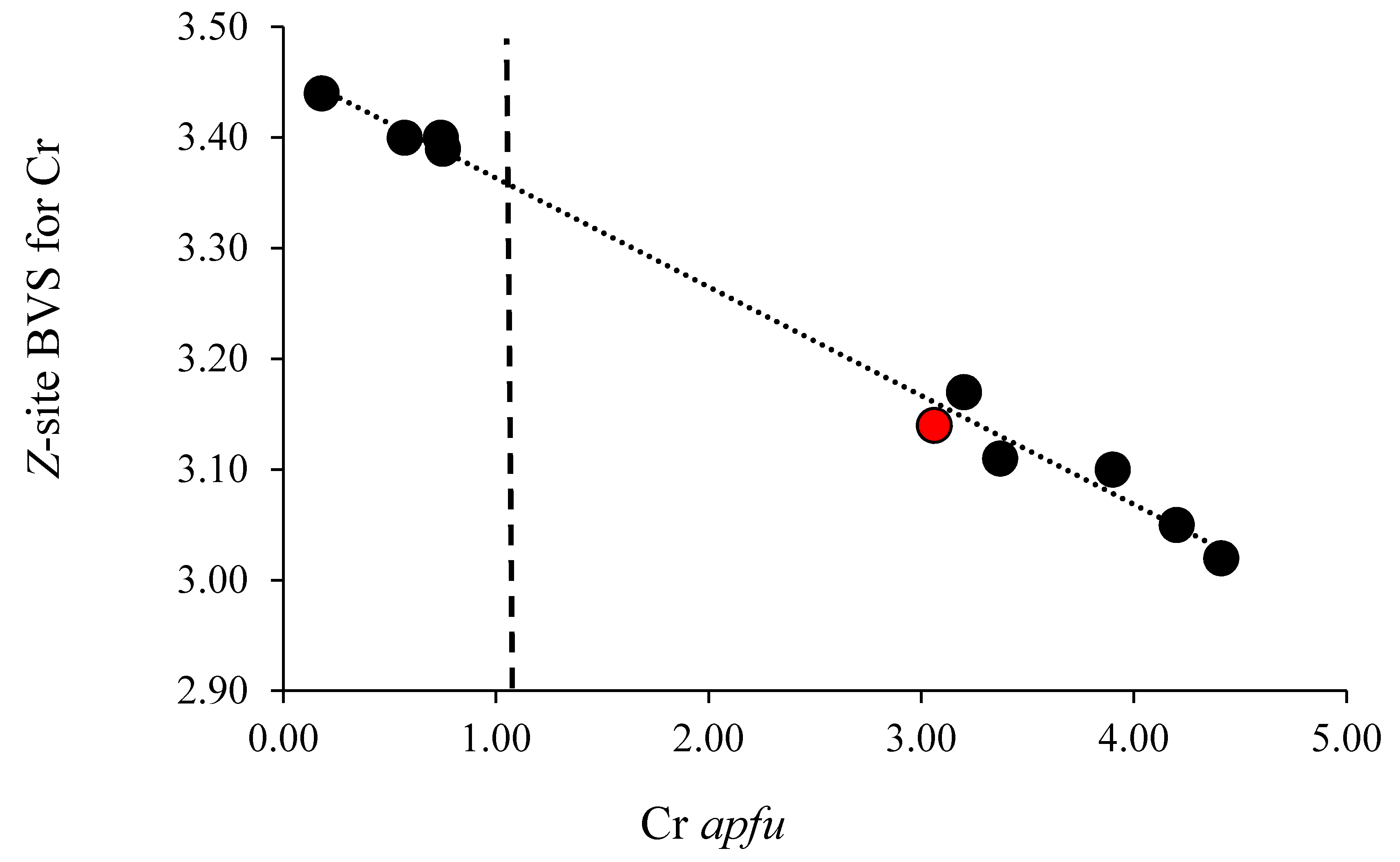
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vereschagin, O.S.; Rozhdestvenksa, I.V.; Frank-Kamenetskaya, O.V.; Zolotarev, A.A. Ion substitutions and structural adjustment in Cr-bearing tourmalines. Eur. J. Mineral. 2014, 26, 309–321. [Google Scholar] [CrossRef]
- Bosi, F.; Reznitskii, L.; Halenius, U.; Skoby, H. Crystal chemistry of Al-V-Cr oxy-tourmalines from Sludyanka complex, Lake Baikal, Russia. Eur. J. Mineral. 2017, 29, 457–472. [Google Scholar] [CrossRef]
- Vereshchagin, O.S.; Frank-Kamenetskaya, O.V.; Rozhdestvenskaya, I.V.; Zolotarev, A.A. Incorporation of 3d elements in tourmalines: structural adjustments and stability. Eur. J. Mineral. 2018, 30, 917–928. [Google Scholar] [CrossRef]
- Lupulescu, M.V.; Rowe, R. Al-rich chromium-dravite from the #1 Mine, Balmat, St. Lawrence County, New York. Can. Mineral. 2011, 49, 1189–1198. [Google Scholar]
- Henry, D.J.; Novák, M.; Hawthorne, F.C.; Ertl, A.; Dutrow, B.L.; Uher, P.; Pezzotta, F. Nomenclature of the tourmaline-supergroup minerals. Am. Mineral. 2011, 96, 895–913. [Google Scholar] [CrossRef]
- Bosi, F.; Reznitskii, L.; Skogby, H. Oxy-chromium-dravite, NaCr3(Cr4Mg2)(Si6O18) (BO3)3 (OH)3O, a new mineral species of the tourmaline supergroup. Am. Mineral. 2014, 97, 2023–2030. [Google Scholar] [CrossRef]
- Brown, C.E. Mineralization, Mining, and Mineral Resources in the Beaver Creek Area of the Grenville Lowlands in St. Lawrence County, New York; USGS Prof. Paper 1279; USGS Publications Warehouse: Reston, VA, USA, 1983. [CrossRef]
- Brown, C.E.; Ayuso, R.A. Significance of Tourmaline-Rich Rocks in the Grenville Complex of St. Lawrence County, New York; USGS Bulletin 1626-C; U.S. Government Printing Office: Washington, DC, USA, 1984; pp. 1–33.
- Slack, J.F.; Herriman, N.; Barnes, R.G.; Plimer, I.R. Stratiform tourmalinites in metamorphic terranes and their geologic significance. Geology 1984, 12, 713–716. [Google Scholar] [CrossRef]
- Chiarenzelli, J.; Lupulescu, M.; Thren, E.; Cousens, B. Tectonic implications of the discovery of Shawinigan ophiolite (Pyrites Complex) in the Adirondack Lowlands. Geosphere 2011, 7, 333–356. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure refinement with SHELX. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Bosi, F.; Lucchesi, S.; Reanitsku, L. Crystal chemistry of the dravite-chromdravite series. Eur. J. Mineral. 2004, 16, 345–352. [Google Scholar] [CrossRef]
- Ertl, A.; Hughes, J.M.; Pertlik, F.; Foit, F.F., Jr.; Wright, S.E.; Brandstátter, F.; Marler, B. Polyhedron distortions in tourmaline. Can. Mineral. 2002, 40, 153–162. [Google Scholar] [CrossRef]
- Wright, S.E.; Foley, J.A.; Hughes, J.M. Optimization of site occupancies in minerals using quadratic programming. Am. Mineral. 2000, 85, 524–531. [Google Scholar] [CrossRef]
| Space group | R3m |
| Unit cell dimensions | a = 16.0242(3) Å |
| c = 7.3002(2) Å | |
| Volume | 1623.37(8) Å3 |
| F(000) | 1545 |
| Theta range | 2.54° to 33.00° |
| Index ranges | −23 ≤ h ≤ 24, −24 ≤ k ≤ 24, −11 ≤ ℓ ≤ 10 |
| Reflections | 11,837 |
| Independent reflections | 1438 [Rint = 0.0215] |
| Refinement method | Full-matrix least-squares on F2 |
| Refinement program | SHELXL-2014/7 (Sheldrick, 2015) |
| Function minimized | Σw(Fo2 − Fc2)2 |
| Data/restraints/parameters | 1438/0/94 |
| Goodness-of-fit on F2 | 1.080 |
| Final R indices | 1422 data; I > 2σ(I) |
| all data | |
| Weighting scheme | w = 1/[σ2(Fo2) + (0.0191P)2 + 0.5287P] where P = (Fo2 + 2Fc2)/3 |
| Extinction coefficient | 0.0000(1) |
| Largest diff. peaks | 0.369 and −0.313 eÅ−3 |
| Atom | x/a | y/b | z/c | U(eq) | Occ. |
|---|---|---|---|---|---|
| NaX | 0 | 0 | 3/4 | 0.0112(4) | Na1.27(3) |
| Si | 0.80930(3) | 0.81101(3) | 0.9755(2) | 0.00579(11) | Si1.00 |
| B | 0.89016(10) | 0.78032(19) | 0.5218(4) | 0.0075(4) | B1.00 |
| CrY | 0.87614(3) | 0.93807(2) | 0.3381(2) | 0.00587(15) | Cr0.588(6)Mg0.412 |
| AlZ | 0.70206(3) | 0.73811(3) | 0.3651(2) | 0.00620(11) | Al0.782(3)Cr0.218 |
| O1 | 0 | 0 | 0.2074(4) | 0.0081(5) | O1.00 |
| O2 | 0.93967(6) | 0.87933(13) | 0.4896(3) | 0.0094(3) | O1.00 |
| O3 | 0.74056(14) | 0.87028(7) | 0.4644(3) | 0.0118(3) | O1.00 |
| H3 | 0.749(4) | 0.8744(18) | 1.577(7) | 0.054(16) | H1.00 |
| O4 | 0.90761(7) | 0.81522(14) | 0.9037(3) | 0.0113(3) | O1.00 |
| O5 | 0.81814(14) | 0.90907(7) | 0.8839(3) | 0.0108(3) | O1.00 |
| O6 | 0.80721(9) | 0.81648(9) | 0.1954(3) | 0.0090(2) | O1.00 |
| O7 | 0.71637(9) | 0.71698(8) | 0.8994(3) | 0.0105(2) | O1.00 |
| O8 | 0.79206(9) | 0.73157(9) | 0.5360(3) | 0.0117(2) | O1.00 |
| Na(X)- | Distance |
| O2 (×3) | 2.533(2) |
| O5 (×3) | 2.7065(19) |
| O4 (×3) | 2.799(2) |
| Mean | 2.680 |
| B- | Distance |
| O8 (×2) | 1.3653(18) |
| O2 | 1.394(3) |
| Mean | 1.375 |
| Al(Z)- | Distance |
| O8 | 1.9330(13) |
| O7 | 1.9457(13) |
| O8 | 1.9493(14) |
| O6 | 1.9580(13) |
| O7 | 1.9828(13) |
| O3 | 2.0211(9) |
| Mean | 1.964 |
| Si- | Distance |
| O7 | 1.5979(12) |
| O6 | 1.6089(14) |
| O4 | 1.6295(8) |
| O5 | 1.6473(9) |
| Mean | 1.621 |
| Cr(Y)- | Distance |
| O1 | 1.9659(15) |
| O6 (×2) | 1.9872(13) |
| O2 (×2) | 2.0256(12) |
| O3 | 2.0951(19) |
| Mean | 2.014 |
| Oxide | wt. % | Range | Standard |
|---|---|---|---|
| SiO2 | 34.72 | 34.40–35.01 | kyanite |
| TiO2 | 0.97 | 0.90–1.10 | rutile |
| Al2O3 | 15.85 | 15.61–16.20 | kyanite |
| Cr2O3 | 21.08 | 20.60–21.60 | chromite |
| V2O3 | 0.33 | 0.27–0.38 | V2O5 |
| B2O3 * | 10.18 | NA | NA |
| FeO | 0.15 | 0.09–0.22 | Syn Fayalite |
| MgO | 9.87 | 9.70–9.98 | P140 forsterite |
| CaO | 1.19 | 1.12–1.26 | Di2Ti ** |
| MnO | 0.06 | 0.04–0.07 | P140 forsterite |
| Na2O | 2.09 | 1.99–2.21 | jadeite |
| K2O | 0.05 | 0.05–0.07 | OR-1 *** |
| Li2O * | 0.47 | NA | NA |
| F | 0.02 | 0.01–0.04 | topaz |
| H2O * | 3.50 | NA | NA |
| O=F | 0.01 | ||
| Total | 100.54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dannenberg, S.G.; Di Paolo, D.; Ehlers, A.M.; McCarthy, K.P.; Mancini, M.T.; Reuter, M.B.; Seth, D.M.; Song, Z.; Valladares, M.I.; Zhu, X.; et al. The Atomic Arrangement of Cr-rich Tourmaline from the #1 Mine, Balmat, St. Lawrence County, New York, USA. Minerals 2019, 9, 398. https://doi.org/10.3390/min9070398
Dannenberg SG, Di Paolo D, Ehlers AM, McCarthy KP, Mancini MT, Reuter MB, Seth DM, Song Z, Valladares MI, Zhu X, et al. The Atomic Arrangement of Cr-rich Tourmaline from the #1 Mine, Balmat, St. Lawrence County, New York, USA. Minerals. 2019; 9(7):398. https://doi.org/10.3390/min9070398
Chicago/Turabian StyleDannenberg, Steven G., Devany Di Paolo, Alix M. Ehlers, Kyle P. McCarthy, Mark T. Mancini, Matthew B. Reuter, Dennis M. Seth, Zihui Song, Maria I. Valladares, Xuanfu Zhu, and et al. 2019. "The Atomic Arrangement of Cr-rich Tourmaline from the #1 Mine, Balmat, St. Lawrence County, New York, USA" Minerals 9, no. 7: 398. https://doi.org/10.3390/min9070398
APA StyleDannenberg, S. G., Di Paolo, D., Ehlers, A. M., McCarthy, K. P., Mancini, M. T., Reuter, M. B., Seth, D. M., Song, Z., Valladares, M. I., Zhu, X., Hughes, J. M., & Lupulescu, M. V. (2019). The Atomic Arrangement of Cr-rich Tourmaline from the #1 Mine, Balmat, St. Lawrence County, New York, USA. Minerals, 9(7), 398. https://doi.org/10.3390/min9070398




