Physico–Chemical Interaction between Clay Minerals and Albumin Protein according to the Type of Clay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
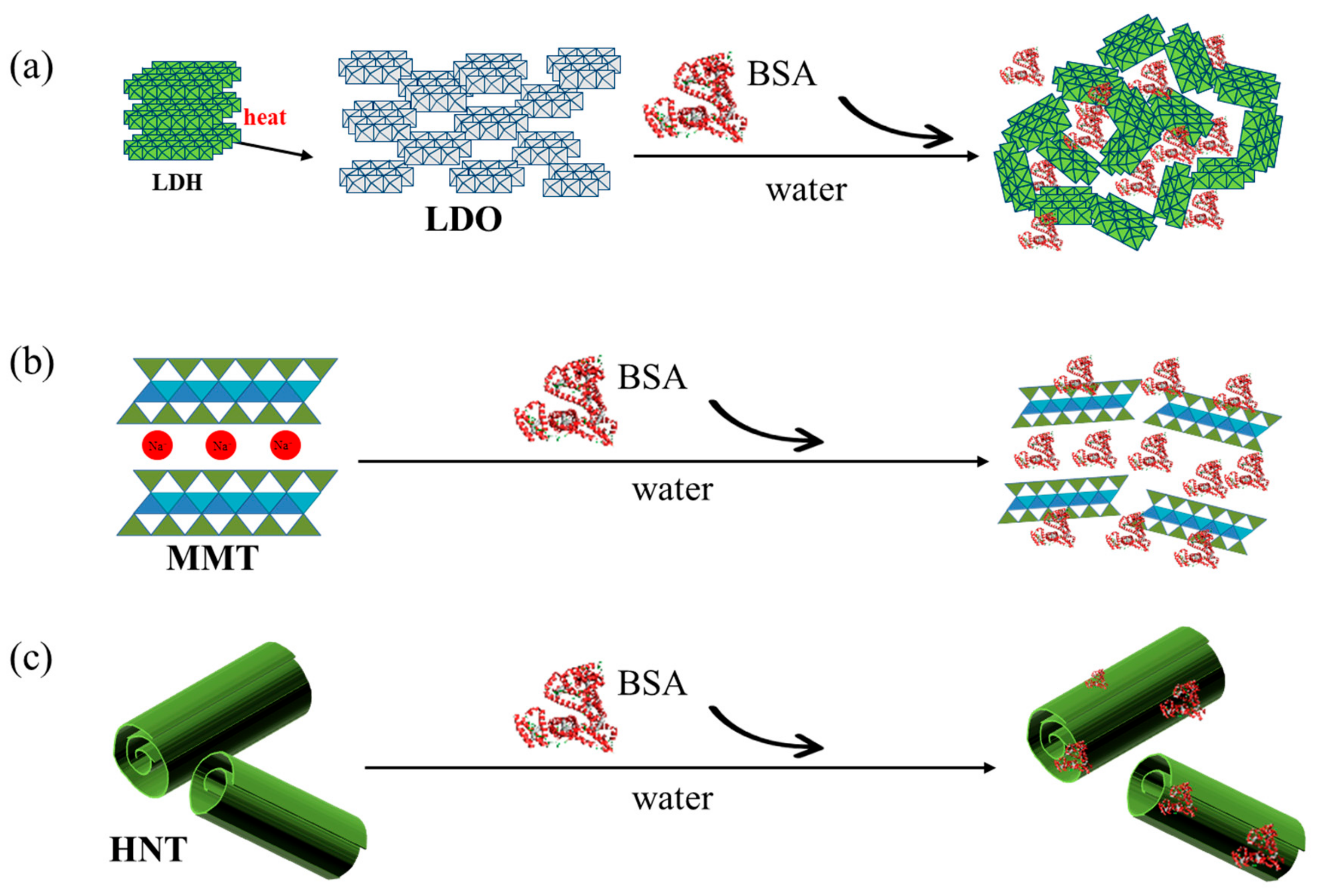
2.2. Preparation of the Protein–Clay Interactant
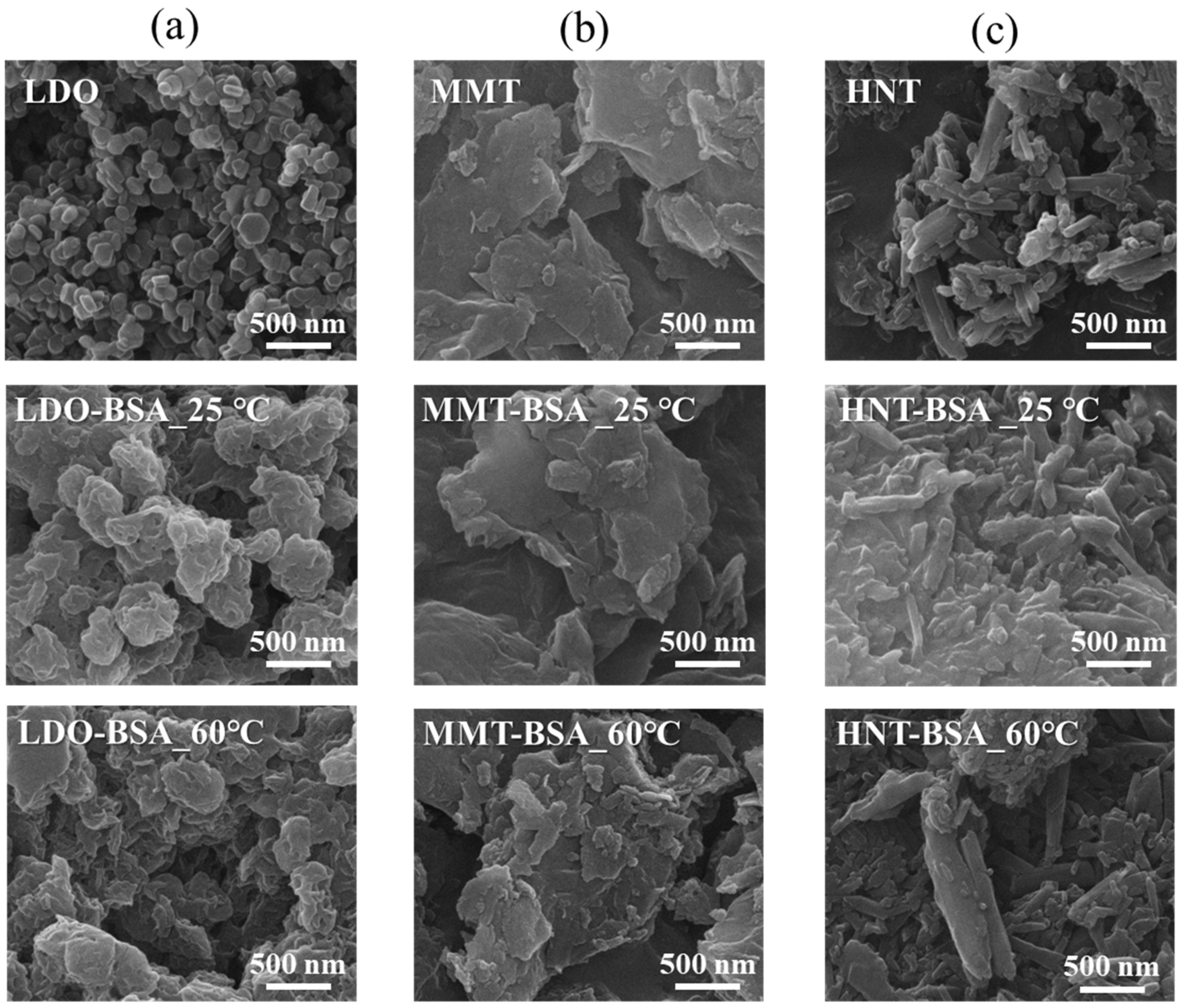
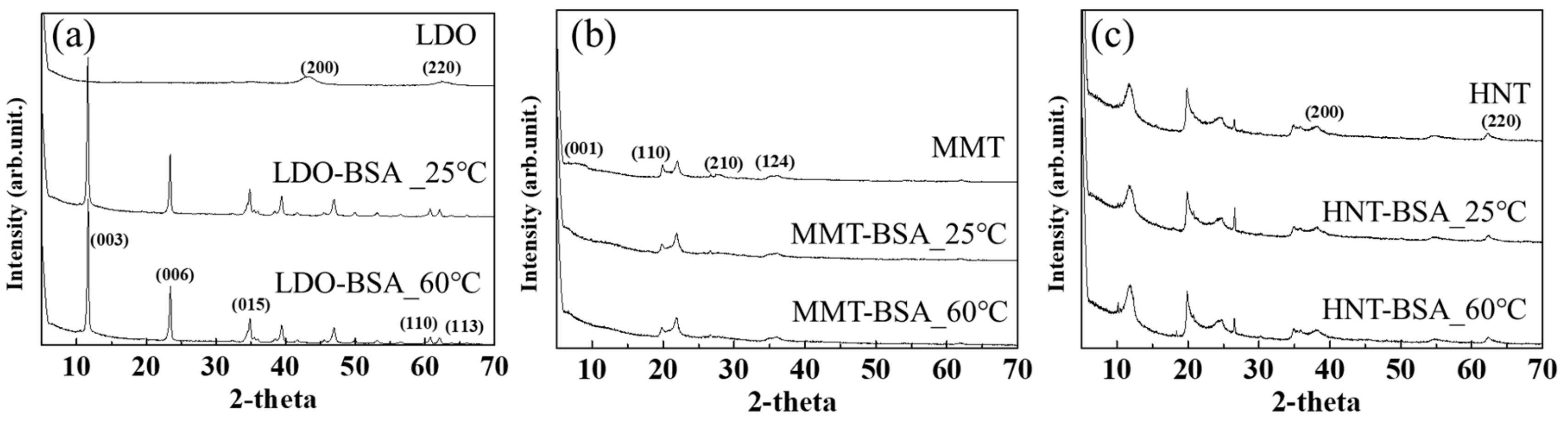
2.3. Surface Morphology and Crystal Structure Analyses
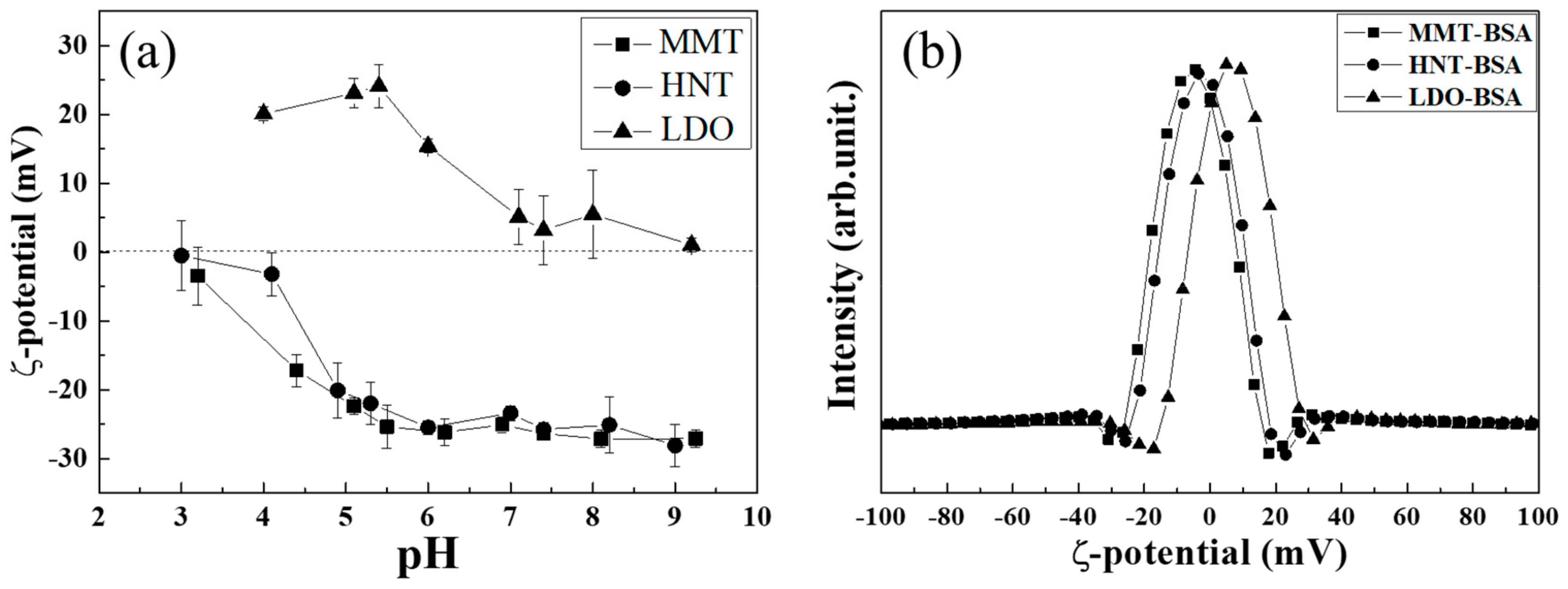
2.4. Quantification of Adsorbed Protein on Clays
2.5. Protein Fluorescence Quenching Assay
2.6. Circular Dichroism Spectroscopy
3. Results
3.1. Protein–Clay Interactant: Surface Morphology and Crystal Structure
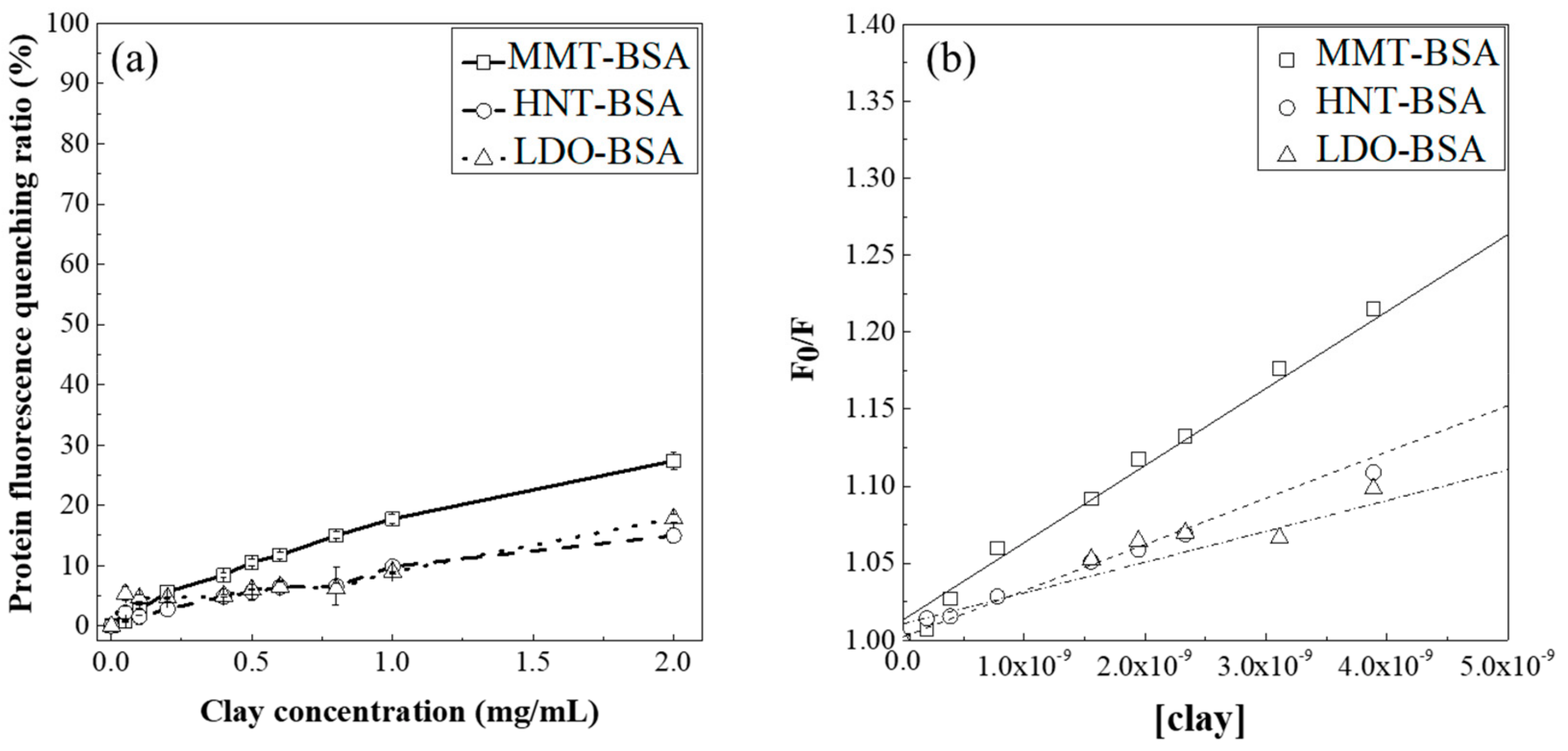
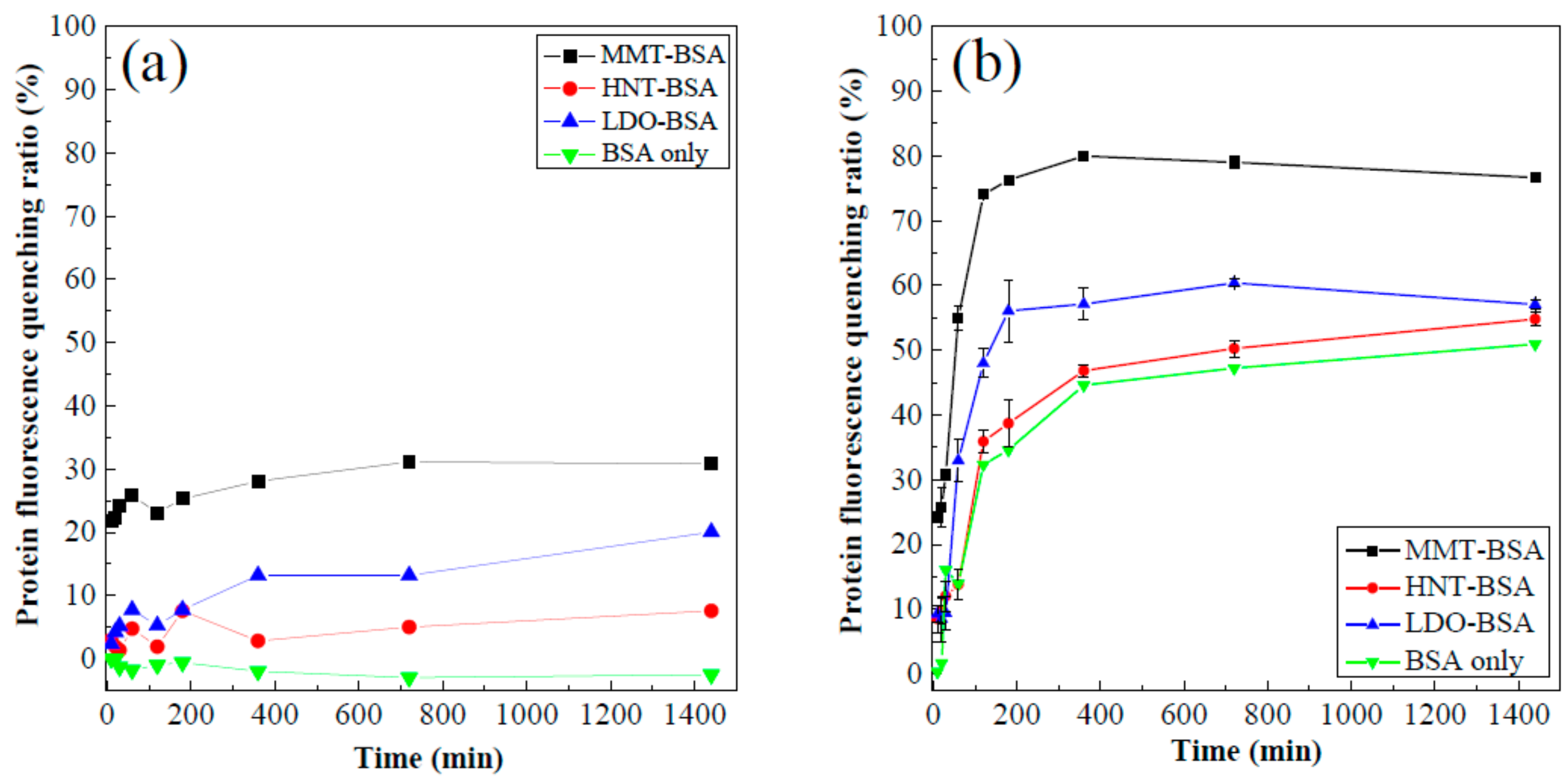
3.2. Protein Fluorescence Quenching
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Aguzzi, C.; Cerezo, P.; Viseras, C.; Caramella, C. Use of clays as drug delivery systems: Possibilities and limitations. Appl. Clay Sci. 2007, 36, 22–36. [Google Scholar] [CrossRef]
- Carretero, M.I. Clay minerals and their beneficial effects upon human health. A review. Appl. Clay Sci. 2002, 21, 155–163. [Google Scholar] [CrossRef]
- Schiller, L.R. Antidiarrheal drug therapy. Curr. Gastroenterol. Rep. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Carretero, M.I.; Pozo, M. Clay and non-clay minerals in the pharmaceutical and cosmetic industries Part II. Active ingredients. Appl. Clay Sci. 2010, 47, 171–181. [Google Scholar] [CrossRef]
- Reddy, K.R.; Darko-Kagya, K.; Al-Hamdan, A.Z. Electrokinetic remediation of pentachlorophenol contaminated clay soil. Water Air Soil Pollut. 2011, 221, 35–44. [Google Scholar] [CrossRef]
- Pushpaletha, P.; Rugmini, S.; Lalithambika, M. Correlation between surface properties and catalytic activity of clay catalysts. Appl. Clay Sci. 2005, 30, 141–153. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Z.; Hu, N.; Zeng, Y.; Rusling, J.F. Layer-by-layer assembly of ultrathin films of hemoglobin and clay nanoparticles with electrochemical and catalytic activity. Langmuir 2002, 18, 8573–8579. [Google Scholar] [CrossRef]
- Reichle, W.T. Catalytic reactions by thermally activated, synthetic, anionic clay minerals. J. Catal. 1985, 94, 547–557. [Google Scholar] [CrossRef]
- Theng, B.K.G. Clay-polymer interactions: Summary and perspectives. Clays Clay Miner. 1982, 30, 1–10. [Google Scholar] [CrossRef]
- Zare, Y.; Garmabi, H. Thickness, modulus and strength of interphase in clay/polymer nanocomposites. Appl. Clay Sci. 2015, 105–106, 66–70. [Google Scholar] [CrossRef]
- Osman, M.A.; Mittal, V.; Suter, U.W. Poly(propylene)-layered silicate nanocomposites: Gas permeation properties and clay exfoliation. Macromol. Chem. Phys. 2007, 208, 68–75. [Google Scholar] [CrossRef]
- Mittal, V. Gas permeation and mechanical properties of polypropylene nanocomposites with thermally-stable imidazolium modified clay. Eur. Polym. J. 2007, 43, 3727–3736. [Google Scholar] [CrossRef]
- Villaluenga, J.P.G.; Khayet, M.; López-Manchado, M.A.; Valentin, J.L.; Seoane, B.; Mengual, J.I. Gas transport properties of polypropylene/clay composite membranes. Eur. Polym. J. 2007, 43, 1132–1143. [Google Scholar] [CrossRef]
- Park, J.K.; Choy, Y.B.; Oh, J.-M.; Kim, J.Y.; Hwang, S.-J.; Choy, J.-H. Controlled release of donepezil intercalated in smectite clays. Int. J. Pharm. 2008, 359, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-M.; Choi, S.-J.; Kim, S.-T.; Choy, J.-H. Cellular uptake mechanism of an inorganic nanovehicle and its drug conjugates: Enhanced efficacy due to clathrin-mediated endocytosis. Bioconjug. Chem. 2006, 17, 1411–1417. [Google Scholar] [CrossRef]
- Oh, J.-M.; Choi, S.-J.; Lee, G.-E.; Kim, J.-E.; Choy, J.-H. Inorganic metal hydroxide nanoparticles for targeted cellular uptake through clathrin-mediated endocytosis. Chem. Asian J. 2009, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-M.; Park, C.-B.; Choy, J.-H. Intracellular drug delivery of layered double hydroxide nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 1632–1635. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Huang, Q.; Zhang, X.; Chen, H. Adsorption of DNA on clay minerals and various colloidal particles from an Alfisol. Soil Biol. Biochem. 2006, 38, 471–476. [Google Scholar] [CrossRef]
- Saeki, K.; Kunito, T. Adsorptions of DNA molecules by soils and variable-charged soil constituents. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 1, 188–195. [Google Scholar]
- Poly, F.; Chenu, C.; Simonet, P.; Rouiller, J.; Jocteur Monrozier, L. Differences between linear chromosomal and supercoiled plasmid DNA in their mechanisms and extent of adsorption on clay minerals. Langmuir 2000, 16, 1233–1238. [Google Scholar] [CrossRef]
- Park, D.-H.; Han, C.J.; Shul, Y.-G.; Choy, J.-H. Avatar DNA nanohybrid system in Chip-on-a-Phone. Sci. Rep. 2014, 4, 4879. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-M.; Kwak, S.-Y.; Choy, J.-H. Intracrystalline structure of DNA molecules stabilized in the layered double hydroxide. J. Phys. Chem. Solids 2006, 67, 1028–1031. [Google Scholar] [CrossRef]
- Nielsen, K.M.; Calamai, L.; Pietramellara, G. Stabilization of Extracellular DNA and Proteins by Transient Binding to Various Soil Components. In Nucleic Acids and Proteins in Soil; Nannipieri, P., Smalla, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 141–157. [Google Scholar]
- Sun, X.; Li, C.; Wu, Z.; Xu, X.; Ren, L.; Zhao, H. Adsorption of protein from model wine solution by different bentonites* *supported by the National Natural Science Foundation of China (No.20466002), the program of ministry of education for New Century Excellent Talents (NCET-04-0989), the doctor funds of Xinjiang Bingtuan (04BSZJ04) and the Shihezi University’s Key scientific and technological project (ZDGG2004-01). Chin. J. Chem. Eng. 2007, 15, 632–638. [Google Scholar]
- Johnston, C.T.; Premachandra, G.S.; Szabo, T.; Lok, J.; Schoonheydt, R.A. Interaction of biological molecules with clay minerals: A combined spectroscopic and sorption study of lysozyme on saponite. Langmuir 2012, 28, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Tully, J.; Yendluri, R.; Lvov, Y. Halloysite clay nanotubes for enzyme immobilization. Biomacromolecules 2016, 17, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Cavallaro, G.; Colletti, C.G.; D’Azzo, G.; Guernelli, S.; Lazzara, G.; Pieraccini, S.; Riela, S. Halloysite nanotubes for efficient loading, stabilization and controlled release of insulin. J. Colloid Interface Sci. 2018, 524, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Duce, C.; Della Porta, V.; Bramanti, E.; Campanella, B.; Spepi, A.; Tiné, M.R. Loading of halloysite nanotubes with BSA, α-Lac and β-Lg: A Fourier transform infrared spectroscopic and thermogravimetric study. Nanotechnology 2016, 28, 055706. [Google Scholar] [CrossRef] [PubMed]
- Della Porta, V.; Bramanti, E.; Campanella, B.; Tiné, M.R.; Duce, C. Conformational analysis of bovine serum albumin adsorbed on halloysite nanotubes and kaolinite: A Fourier transform infrared spectroscopy study. RSC Adv. 2016, 6, 72386–72398. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lee, B.-J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Thyparambil, A.A.; Latour, R.A. Protein helical structure determination using CD spectroscopy for solutions with strong background absorbance from 190 to 230 nm. Biochim. Biophys. Acta 2014, 1844, 2331–2337. [Google Scholar] [CrossRef]
- Causserand, C.; Kara, Y.; Aimar, P. Protein fractionation using selective adsorption on clay surface before filtration. J. Membr. Sci. 2001, 186, 165–181. [Google Scholar] [CrossRef]
- Lacerda, S.H.D.P.; Park, J.J.; Meuse, C.; Pristinski, D.; Becker, M.L.; Karim, A.; Douglas, J.F. Interaction of Gold nanoparticles with common human blood proteins. ACS Nano 2010, 4, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, Q.; Chen, S.; Yu, Z. Adsorption of the insecticidal protein of Bacillus thuringiensis on montmorillonite, kaolinite, silica, goethite and Red soil. Appl. Clay Sci. 2005, 30, 87–93. [Google Scholar] [CrossRef]
- Helassa, N.; Quiquampoix, H.; Noinville, S.; Szponarski, W.; Staunton, S. Adsorption and desorption of monomeric Bt (Bacillus thuringiensis) Cry1Aa toxin on montmorillonite and kaolinite. Soil Biol. Biochem. 2009, 41, 498–504. [Google Scholar] [CrossRef]
- Fiorito, T.M.; Icoz, I.; Stotzky, G. Adsorption and binding of the transgenic plant proteins, human serum albumin, β-glucuronidase, and Cry3Bb1, on montmorillonite and kaolinite: Microbial utilization and enzymatic activity of free and clay-bound proteins. Appl. Clay Sci. 2008, 39, 142–150. [Google Scholar] [CrossRef]
- Barral, S.; Villa-García, M.A.; Rendueles, M.; Díaz, M. Interactions between whey proteins and kaolinite surfaces. Acta Mater. 2008, 56, 2784–2790. [Google Scholar] [CrossRef]
- Kim, T.-H.; Hong, I.T.; Oh, J.-M. Size- and surface charge-controlled layered double hydroxides for efficient algal flocculation. Environ. Sci. Nano 2018, 5, 183–190. [Google Scholar] [CrossRef]
- Gilanizadeh, M.; Zeynizadeh, B. Synthesis and characterization of the immobilized Ni–Zn–Fe layered double hydroxide (LDH) on silica-coated magnetite as a mesoporous and magnetically reusable catalyst for the preparation of benzylidenemalononitriles and bisdimedones (tetraketones) under green conditions. New J. Chem. 2018, 42, 8553–8566. [Google Scholar]
- Oueslati, W.; Ammar, M.; Chorfi, N. Quantitative XRD analysis of the structural changes of ba-exchanged montmorillonite: Effect of an in situ hydrous perturbation. Minerals 2015, 5, 507–526. [Google Scholar] [CrossRef]
- Mokhtar, M.; Inayat, A.; Ofili, J.; Schwieger, W. Thermal decomposition, gas phase hydration and liquid phase reconstruction in the system Mg/Al hydrotalcite/mixed oxide: A comparative study. Appl. Clay Sci. 2010, 50, 176–181. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, K.-M.; Lee, K.; Kim, Y.S.; Oh, J.-M. Nano–Bio Interaction between graphite oxide nanoparticles and human blood components. Eur. J. Inorg. Chem. 2012, 2012, 5343–5349. [Google Scholar] [CrossRef]
- Lu, N.; Sui, Y.; Ding, Y.; Tian, R.; Li, L.; Liu, F. Adsorption of human serum albumin on functionalized single-walled carbon nanotubes reduced cytotoxicity. Chem. Biol. Interact. 2018, 295, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Orts-Gil, G.; Natte, K.; Thiermann, R.; Girod, M.; Rades, S.; Kalbe, H.; Thünemann, A.F.; Maskos, M.; Österle, W. On the role of surface composition and curvature on biointerface formation and colloidal stability of nanoparticles in a protein-rich model system. Colloids Surf. B Biointerfaces 2013, 108, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Goy-López, S.; Juárez, J.; Alatorre-Meda, M.; Casals, E.; Puntes, V.F.; Taboada, P.; Mosquera, V. Physicochemical characteristics of protein–NP bioconjugates: The role of particle curvature and solution conditions on human serum albumin conformation and fibrillogenesis inhibition. Langmuir 2012, 28, 9113–9126. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Kita, Y. Protection of bovine serum albumin from aggregation by Tween 80. J. Pharm. Sci. 2000, 89, 646–651. [Google Scholar] [CrossRef]
- Borzova, V.A.; Markossian, K.A.; Chebotareva, N.A.; Kleymenov, S.Y.; Poliansky, N.B.; Muranov, K.O.; Stein-Margolina, V.A.; Shubin, V.V.; Markov, D.I.; Kurganov, B.I. Kinetics of thermal denaturation and aggregation of bovine serum albumin. PLoS ONE 2016, 11, e0153495. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-M.; Oh, J.-M. Physico–Chemical Interaction between Clay Minerals and Albumin Protein according to the Type of Clay. Minerals 2019, 9, 396. https://doi.org/10.3390/min9070396
Kim H-M, Oh J-M. Physico–Chemical Interaction between Clay Minerals and Albumin Protein according to the Type of Clay. Minerals. 2019; 9(7):396. https://doi.org/10.3390/min9070396
Chicago/Turabian StyleKim, Hyoung-Mi, and Jae-Min Oh. 2019. "Physico–Chemical Interaction between Clay Minerals and Albumin Protein according to the Type of Clay" Minerals 9, no. 7: 396. https://doi.org/10.3390/min9070396
APA StyleKim, H.-M., & Oh, J.-M. (2019). Physico–Chemical Interaction between Clay Minerals and Albumin Protein according to the Type of Clay. Minerals, 9(7), 396. https://doi.org/10.3390/min9070396




