Application of Red Mud in Wastewater Treatment
Abstract
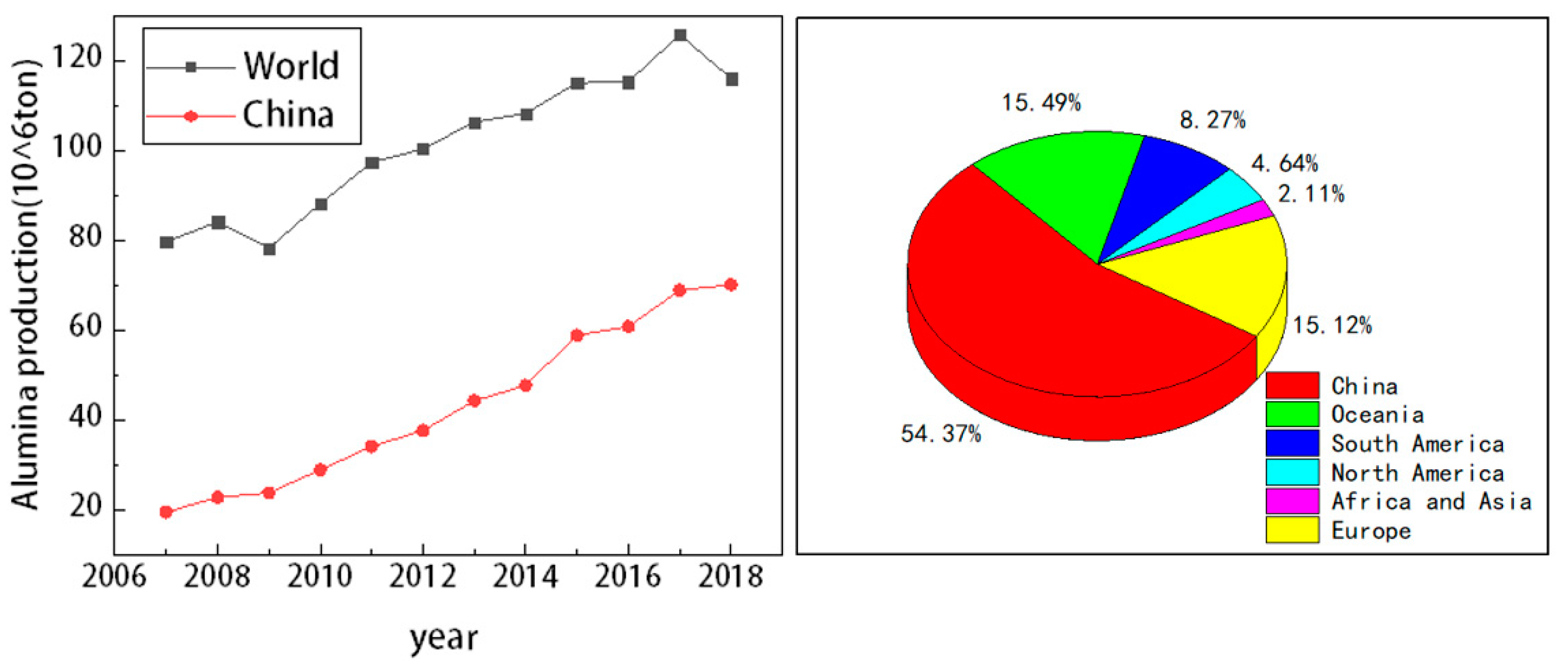
1. Introduction
2. Properties of RM
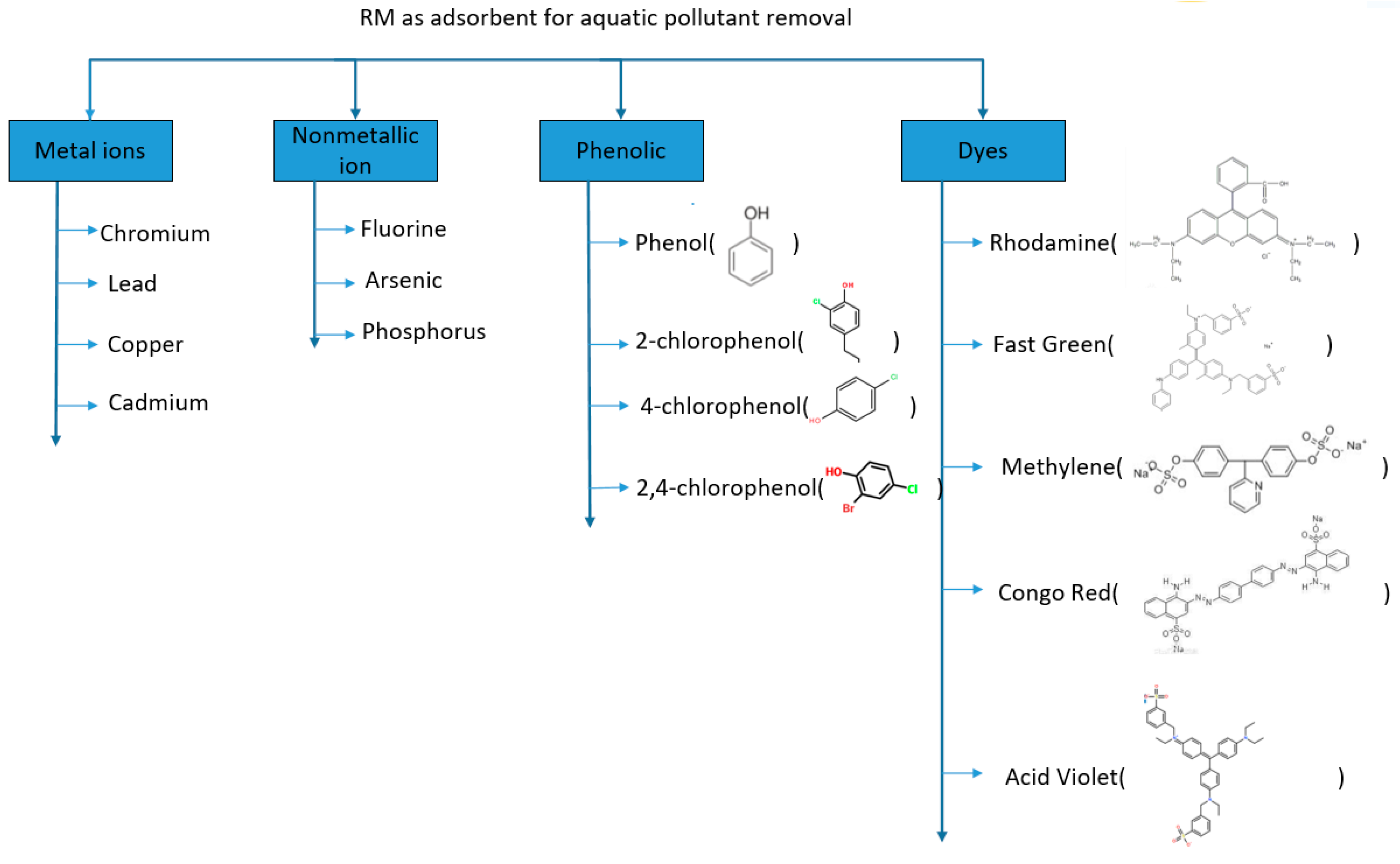
3. Application in Water Treatment
3.1. Removal of Metal Ions from Water
3.1.1. Removal of Chromium (Cr)
3.1.2. Removal of Lead (Pb)
3.1.3. Removal of Copper (Cu)
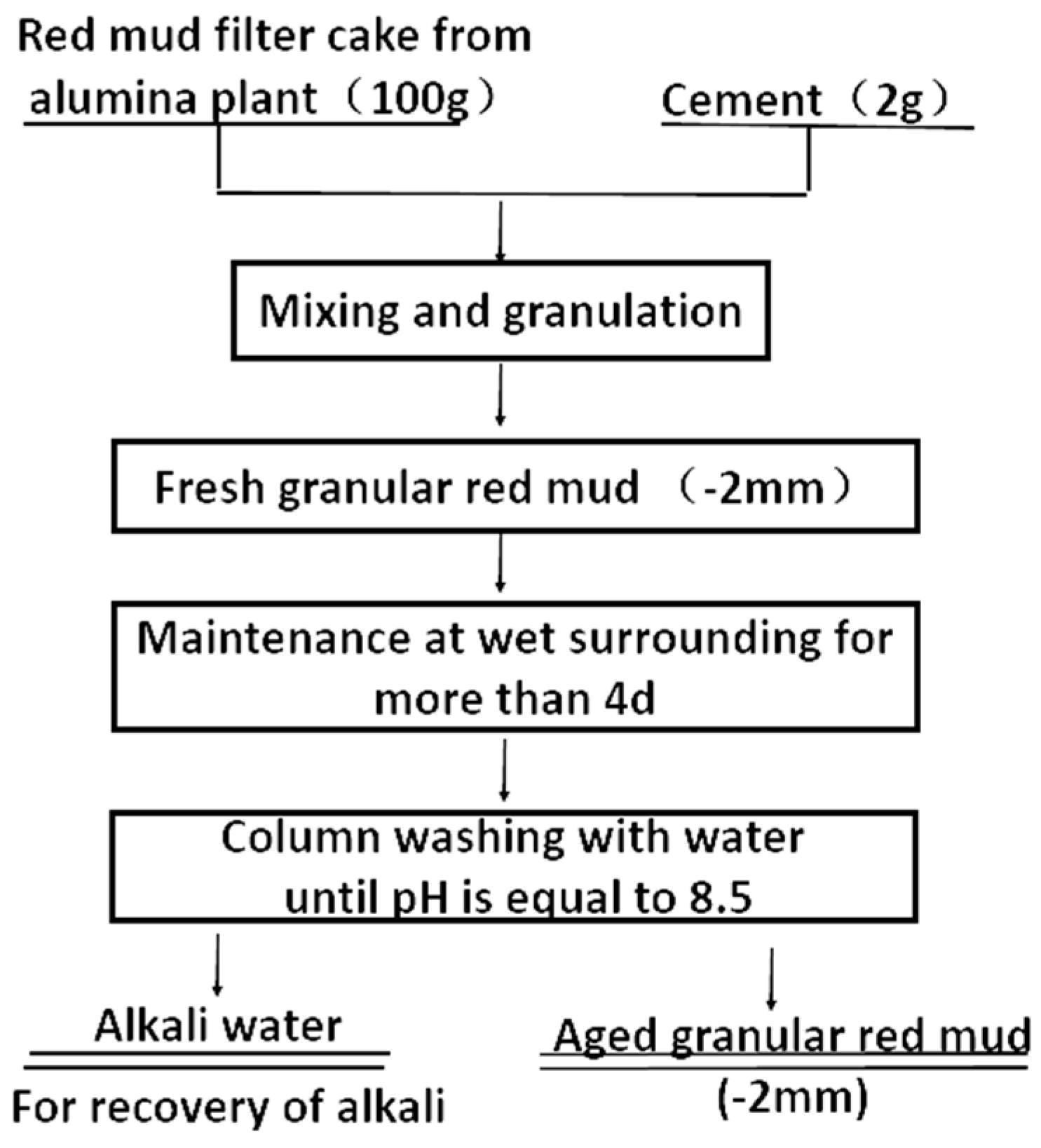
3.1.4. Removal of Cadmium (Cd)
3.2. Removal of Non-Metallic Ions
3.2.1. Removal of Fluorine (F)
3.2.2. Removal of arsenic (As)
3.2.3. Removal of Phosphorous (P)
3.3. Removal of Phenolic Pollutant
3.4. Removal of Dyes from Water
4. Treatment of Waste Gas
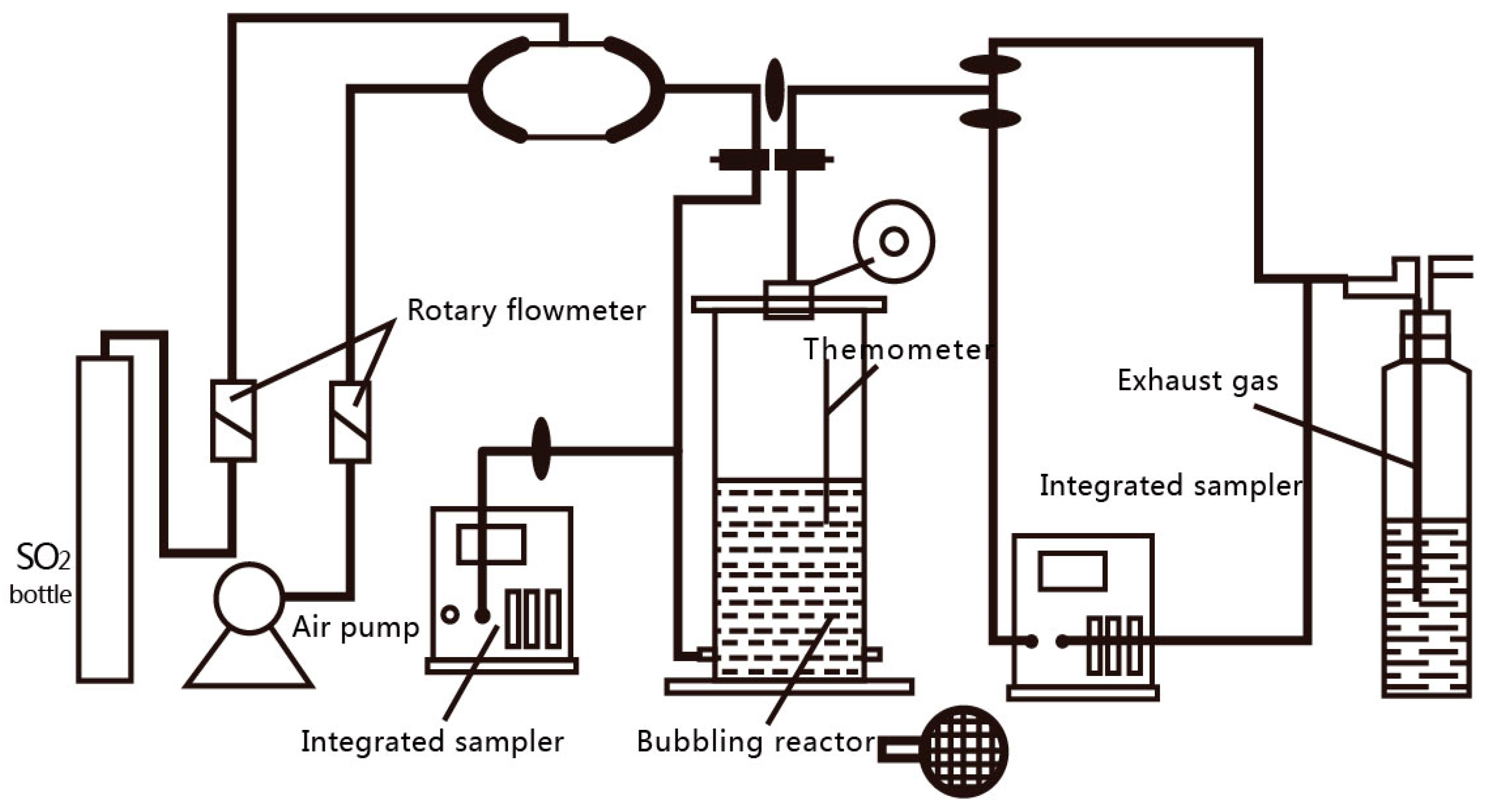
4.1. Desulfurization Process
4.1.1. Dry Desulfurization
4.1.2. Wet Desulfurization
4.2. Decarburizing Process
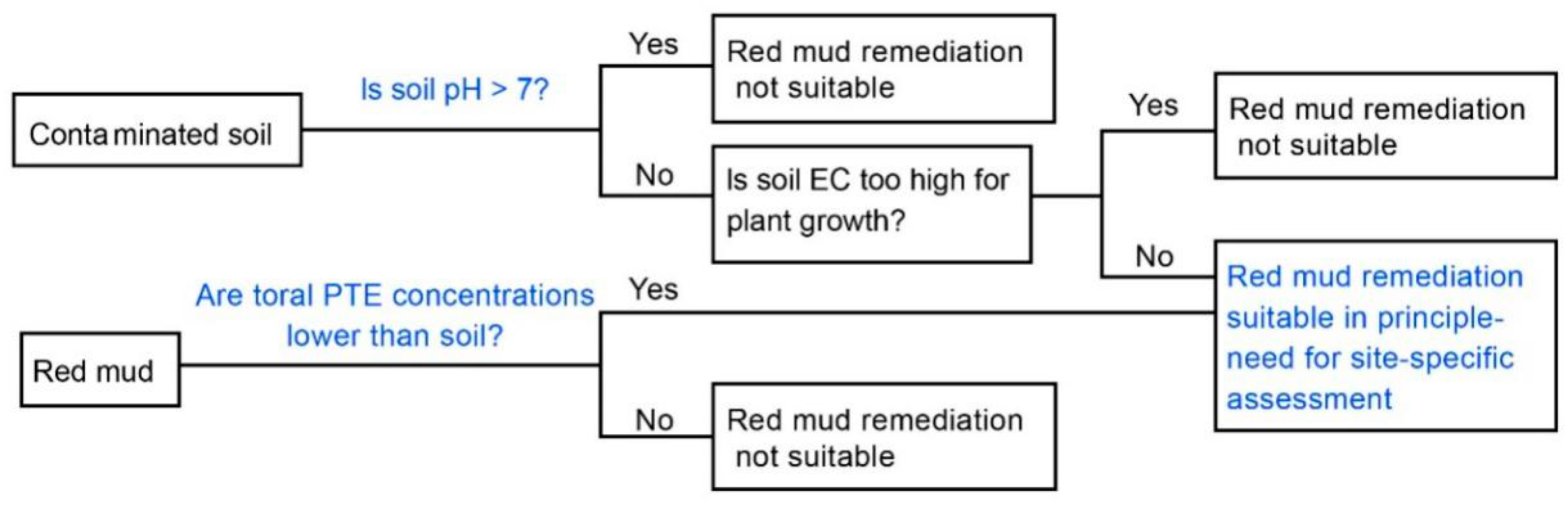
5. Remediation of Heavy Metal Contaminated Soils
6. Conclusions
- Methods of recycling or disposal after adsorption of heavy metals to prevent secondary pollution.
- Study of adsorbents that are more conducive to storage and transportation.
- Further improvement of adsorption performance (such as making porous nanostructures).
- Strategies to avoid secondary pollution of RM (leaching of heavy metals) in adsorption.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.; Zhang, Y.; Liu, J.; Hu, P.; Meng, K.; Lv, F.; Tong, W.; Chu, P.K. Dealkalization of red mud by carbide slag and flue gas. CLEAN Soil Air Water 2017, 46, 1700634. [Google Scholar] [CrossRef]
- Lyu, F.; Sun, N.; Sun, W.; Khoso, S.A.; Tang, H.-H.; Wang, L. Preliminary assessment of revegetation potential through ryegrass growing on bauxite residue. J. Cent. South Univ. 2019, 26, 404–409. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Q.; Yan, K.; Cheng, F.; Lou, H. Novel Process for Alumina Extraction via the Coupling Treatment of Coal Gangue and Bauxite Red Mud. Ind. Eng. Chem. Res. 2014, 53, 4518–4521. [Google Scholar] [CrossRef]
- Lyu, F.; Gao, J.-D.; Sun, W.; Liu, R.-Q.; Sun, X.-D.; Cao, X.-F.; Wang, L.; Sun, W. Utilisation of propyl gallate as a novel selective collector for diaspore flotation. Miner. Eng. 2019, 131, 66–72. [Google Scholar] [CrossRef]
- Hua, Y.; Heal, K.V.; Friesl-Hanl, W. The use of red mud as an immobiliser for metal/metalloid-contaminated soil: A review. J. Hazard. Mater. 2016, 325, 17. [Google Scholar] [CrossRef]
- Oliveira, A.A.S.; Tristão, J.C.; Ardisson, J.D.; Dias, A.; Lago, R.M. Production of nanostructured magnetic composites based on Fe0 nuclei coated with carbon nanofibers and nanotubes from red mud waste and ethanol. Appl. Catal. B Environ. 2011, 105, 163–170. [Google Scholar] [CrossRef]
- Kinnarinen, T.; Huhtanen, M.; Holliday, L.; Häkkinen, A. Challenges related to solute analysis of bauxite residue filter cakes. Miner. Eng. 2018, 120, 1–6. [Google Scholar] [CrossRef]
- Liu, S.; Rehren, T.; Chen, J.; Xu, C.; Venunan, P.; Larreina-Garcia, D.; Martinón-Torres, M. Bullion production in imperial china and its significance for sulphide ore smelting world-wide. J. Archaeol. Sci. 2015, 55, 151–165. [Google Scholar] [CrossRef]
- Fu, J.; Song, R.; Mao, W.J.; Wang, Q.; An, S.Q.; Zeng, Q.F.; Zhu, H.L. Adsorption of disperse blue 2BLN by microwave activated red mud. Environ. Prog. Sustain. Energy 2011, 30, 558–566. [Google Scholar] [CrossRef]
- Boubakri, A.; Hafiane, A.; Bouguecha, S.A.T. Direct contact membrane distillation: Capability to desalt raw water. Arab. J. Chem. 2017, 10, S3475–S3481. [Google Scholar] [CrossRef]
- Mehdilo, A.; Zarei, H.; Irannajad, M.; Arjmandfar, H.J.M.E. Flotation of zinc oxide ores by cationic and mixed collectors. Miner. Eng. 2012, 36–38, 331–334. [Google Scholar] [CrossRef]
- Çoruh, S.; Ergun, O.N. Use of fly ash, phosphogypsum and red mud as a liner material for the disposal of hazardous zinc leach residue waste. J. Hazard. Mater. 2010, 173, 468–473. [Google Scholar] [CrossRef]
- Rafiq, Z.; Nazir, R.; Shahwar, D.-e.; Shah, M.R.; Ali, S. Utilization of magnesium and zinc oxide nano-adsorbents as potential materials for treatment of copper electroplating industry wastewater. J. Environ. Chem. Eng. 2014, 2, 642–651. [Google Scholar] [CrossRef]
- András, G. The red mud accident in Ajka (Hungary): Characterization and potential health effects of fugitive dust. Environ. Sci. Technol. 2011, 4, 1608–1615. [Google Scholar]
- Kushwaha, S.S.; Kishan, D.; Chauhan, M.S.; Khetawath, S. Stabilization of red mud using eko soil enzyme for highway embankment. Mater. Today Proc. 2018, 5, 20500–20512. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kumar, R.; Jeon, B.-H. Struvite precipitation under changing ionic conditions in synthetic wastewater: Experiment and modeling. J. Colloid Interface Sci. 2016, 474, 93–102. [Google Scholar] [CrossRef]
- Sahu, M.K.; Mandal, S.; Yadav, L.S.; Dash, S.S.; Patel, R.K. Equilibrium and kinetic studies of Cd(II) ion adsorption from aqueous solution by activated red mud. Desalin. Water Treat. 2016, 57, 14251–14265. [Google Scholar] [CrossRef]
- Narayanan, R.P.; Ma, L.-C.; Kazantzis, N.K.; Emmert, M.H. Cost analysis as a tool for the development of Sc recovery processes from bauxite residue (red mud). ACS Sustain. Chem. Eng. 2018, 6, 5333–5341. [Google Scholar] [CrossRef]
- Wu, C.-S.; Liu, D.-Y. Mineral phase and physical properties of red mud calcined at different temperatures. J. Nanomater. 2012, 2012, 2. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, X.; Pan, J.; Luo, Y. Direct reduction and beneficiation of a refractory siderite lump. Miner. Process. Extr. Metall. 2014, 123, 246–250. [Google Scholar] [CrossRef]
- Deilami-nezhad, L.; Moghaddam-Banaem, L.; Sadeghi, M. Development of bone seeker radiopharmaceuticals by scandium-47 and estimation of human absorbed dose. Appl. Radiat. Isot. 2017, 129, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Rees, E.E.; Petukhova, T.; Mascarenhas, M.; Pelcat, Y.; Ogden, N.H. Environmental and social determinants of population vulnerability to zika virus emergence at the local scale. Parasites Vectors 2018, 11, 290. [Google Scholar] [CrossRef]
- Ascensão, G.; Seabra, M.P.; Aguiar, J.B.; Labrincha, J.A. Red mud-based geopolymers with tailored alkali diffusion properties and ph buffering ability. J. Clean. Prod. 2017, 148, 23–30. [Google Scholar] [CrossRef]
- Novais, R.M.; Carvalheiras, J.; Seabra, M.P.; Pullar, R.C.; Labrincha, J.A. Innovative application for bauxite residue: Red mud-based inorganic polymer spheres as pH regulators. J. Hazard. Mater. 2018, 358, 69–81. [Google Scholar] [CrossRef]
- Zheng, P.; Zhang, K.; Dang, Y.; Bai, B.; Guan, W.; Suo, Y. Adsorption of organic dyes by TiO2@yeast-carbon composite microspheres and their in situ regeneration evaluation. J. Nanomater. 2015, 3, 6. [Google Scholar]
- Reddy, N.G.; Rao, B.H. Characterization of settled particles of the red mud waste exposed to different aqueous environmental conditions. Indian Geotech. J. 2018, 48, 1–15. [Google Scholar]
- Liang, G.; Chen, W.; Nguyen, A.V.; Nguyen, T.A.H. Red mud carbonation using carbon dioxide: Effects of carbonate and calcium ions on goethite surface properties and settling. J. Colloid Interface Sci. 2018, 517, 230–238. [Google Scholar] [CrossRef]
- Christoforakos, N.P.R.; Lazaridis, N.K. Μelanoidin removal from aqueous systems by a hybrid flotation-filtration technique. J. Chem. Technol. Biotechnol. 2018, 93, 2422–2428. [Google Scholar] [CrossRef]
- Arbabi, M.; Golshani, N. Removal of copper ions Cu (II) from industrial wastewater: A review of removal methods. Int. J. Epidemiol. Res. 2016, 3, 283–293. [Google Scholar]
- Taheri, M.; Alavi Moghaddam, M.R.; Arami, M. Techno-economical optimization of reactive blue 19 removal by combined electrocoagulation/coagulation process through Mopso using RSM and Anfis models. J. Environ. Manag. 2013, 128, 798–806. [Google Scholar] [CrossRef]
- Golder, A.K.; Samanta, A.N.; Ray, S. Anionic reactive dye removal from aqueous solution using a new adsorbent—Sludge generated in removal of heavy metal by electrocoagulation. Chem. Eng. J. 2006, 122, 107–115. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Boukli-Hacene, N. Weighted pseudo-almost automorphic solutions for some partial functional differential equations. Nonlinear Anal. Real World Appl. 2011, 12, 562–570. [Google Scholar] [CrossRef]
- Górka, A.; Zamorska, J.; Antos, D. Coupling Ion Exchange and Biosorption for Copper(II) Removal From Wastewaters. Ind. Eng. Chem. Res. 2011, 50, 3494–3502. [Google Scholar] [CrossRef]
- Barron, O.E.; Qu, H.J.A.R. Information Asymmetry and the Ex Ante Impact of Public Disclosure Quality on Price Efficiency and the Cost of Capital: Evidence from a Laboratory Market. Account. Rev. 2014, 89, 1269–1297. [Google Scholar] [CrossRef]
- Yan, Z.G.; Meng, W.; Liu, Z.T.; Feng-Chang, W.U.; Wang, H.; Zhou, J.L.; Yang, N.Y.; Zhang, Y.H. Development of aquatic life criteria and lash-up standard for ammonia in liao river basin. China Environ. Sci. 2011, 31, 1829–1835. [Google Scholar]
- Bhatnagar, A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater. Environ. Technol. 2011, 32, 231–249. [Google Scholar] [CrossRef]
- Tsamo, C.; Djomou Djonga, P.N.; Dangwang Dikdim, J.M.; Kamga, R. Kinetic and equilibrium studies of Cr(VI), Cu(II) and Pb(II) removal from aqueous solution using red mud, a low-cost adsorbent. Arab. J. Sci. Eng. 2018, 43, 2353–2368. [Google Scholar] [CrossRef]
- Kim, S.C.; Nahm, S.W.; Park, Y.-K. Property and performance of red mud-based catalysts for the complete oxidation of volatile organic compounds. J. Hazard. Mater. 2015, 300, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, J.; Li, W.; He, Y.; Qiu, Y.; Li, P.; Wang, C.; Huang, F.; Wang, D.; Gao, S. Immobilization, enrichment and recycling of Cr(VI) from wastewater using a red mud/carbon material to produce the valuable chromite (FeCr2O4). Chem. Eng. J. 2018, 350, 1103–1113. [Google Scholar] [CrossRef]
- Sahu, R.C.; Patel, R.; Ray, B.C. Removal of hydrogen sulfide using red mud at ambient conditions. Fuel Process. Technol. 2011, 92, 1587–1592. [Google Scholar] [CrossRef]
- Cao, J.-L.; Yan, Z.-L.; Deng, Q.-F.; Wang, Y.; Yuan, Z.-Y.; Sun, G.; Jia, T.-K.; Wang, X.-D.; Bala, H.; Zhang, Z.-Y. Mesoporous modified-red-mud supported Ni catalysts for ammonia decomposition to hydrogen. Int. J. Hydrog. Energy 2014, 39, 5747–5755. [Google Scholar] [CrossRef]
- Oliveira, A.A.S.; Costa, D.A.S.; Teixeira, I.F.; Moura, F.C.C. Gold nanoparticles supported on modified red mud for biphasic oxidation of sulfur compounds: A synergistic effect. Appl. Catal. B Environ. 2015, 162, 475–482. [Google Scholar] [CrossRef]
- Grujicic, M.; Yavari, R.; Snipes, J.; Ramaswami, S. A linear friction welding process model for Carpenter Custom 465 precipitation-hardened martensitic stainless steel: A weld microstructure-evolution analysis. Proc. Inst. Mech. Eng. Part B: J. Eng. Manuf. 2015, 229. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, H.; Hu, Y.; Zhang, Y.; Ouyang, L.; Gao, M. Fates of microcystis aeruginosa cells and associated microcystins in sediment and the effect of coagulation process on them. Toxins 2013, 6, 152–167. [Google Scholar] [CrossRef]
- Yu, L. Biodegradation of decabromodiphenyl ether (BDE-209) by crude enzyme extract from pseudomonas aeruginosa. Int. J. Environ. Res. Public Health 2015, 9, 11829–11847. [Google Scholar]
- Chiang, Y.W.; Ghyselbrecht, K.; Santos, R.M.; Martens, J.A.; Swennen, R.; Cappuyns, V.; Meesschaert, B. Adsorption of multi-heavy metals onto water treatment residuals: Sorption capacities and applications. Chem. Eng. J. 2012, 200–202, 405–415. [Google Scholar] [CrossRef]
- Zhao, Y.; Wendling, L.A.; Wang, C.; Pei, Y. Use of Fe/Al drinking water treatment residuals as amendments for enhancing the retention capacity of glyphosate in agricultural soils. J. Environ. Sci. 2015, 34, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Xiao, R.; Zhang, K.; Gao, H. Arsenic and heavy metal pollution in wetland soils from tidal freshwater and salt marshes before and after the flow-sediment regulation regime in the yellow river delta, China. J. Hydrol. 2012, 450–451, 244–253. [Google Scholar] [CrossRef]
- Bai, J.; Xiao, R.; Cui, B.; Zhang, K.; Wang, Q.; Liu, X.; Gao, H.; Huang, L. Assessment of heavy metal pollution in wetland soils from the young and old reclaimed regions in the pearl river estuary, South China. Environ. Pollut. 2011, 159, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Sudduth, E.; Perakis, S.; Bernhardt, E. Nitrate in watersheds: Straight from soils to streams? J. Geophys. Res. Biogeosci. 2014, 118, 291–302. [Google Scholar] [CrossRef]
- Do Prado, N.T.; Heitmann, A.P.; Mansur, H.S.; Mansur, A.A.; Oliveira, L.C.A.; de Castro, C.S. Pet-modified red mud as catalysts for oxidative desulfurization reactions. J. Environ. Sci. 2017, 57, 312–320. [Google Scholar] [CrossRef]
- Belviso, C.; Agostinelli, E.; Belviso, S.; Cavalcante, F.; Pascucci, S.; Peddis, D.; Varvaro, G.; Fiore, S. Synthesis of magnetic zeolite at low temperature using a waste material mixture: Fly ash and red mud. Microporous Mesoporous Mater. 2015, 202, 208–216. [Google Scholar] [CrossRef]
- McNevin, A.A.; Boyd, C.E. Copper concentrations in channel catfish ictalurus punctatus ponds treated with copper sulfate. J. World Aquacult. Soc. 2007, 35, 16–24. [Google Scholar] [CrossRef]
- Yu, Y.; Paul Chen, J. Key factors for optimum performance in phosphate removal from contaminated water by a Fe–Mg–La tri-metal composite sorbent. J. Colloid Interface Sci. 2015, 445, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Mahani, H.; Levy Keya, A.; Berg, S.; Bartels, W.-B.; Nasralla, R.; Rossen, W.R. Insights into the mechanism of wettability alteration by low-salinity-flooding (LSF) in carbonates. Energy Fuels 2015, 29, 1352–1367. [Google Scholar] [CrossRef]
- Kumar Yadav, K.; Gupta, N.; Kumar, A.; Reece, L.M.; Singh, N.; Rezania, S.; Ahmad Khan, S. Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecol. Eng. 2018, 120, 274–298. [Google Scholar] [CrossRef]
- Duan, F.; Chen, C.; Zhao, X.; Yang, Y.; Liu, X.; Qin, Y. Water-compatible surface molecularly imprinted polymers with synergy of bi-functional monomers for enhanced selective adsorption of bisphenol a from aqueous solution. Environ. Sci. Nano 2016, 3, 213–222. [Google Scholar] [CrossRef]
- Guo, L.-X.; Xu, X.-M.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Characterization and authentication of significant chinese edible oilseed oils by stable carbon isotope analysis. J. Am. Oil Chem. Soc. 2010, 87, 839–848. [Google Scholar] [CrossRef]
- Wang, L.; Ji, B.; Hu, Y.; Liu, R.; Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Yusuf, N.M.; Ooi, B.S. Preparation and modification of poly (vinyl) alcohol membrane: Effect of crosslinking time towards its morphology. Desalination 2012, 287, 35–40. [Google Scholar] [CrossRef]
- Liang, W.; Couperthwaite, S.J.; Kaur, G.; Yan, C.; Johnstone, D.W.; Millar, G.J. Effect of strong acids on red mud structural and fluoride adsorption properties. J. Colloid Interface Sci. 2014, 423, 158–165. [Google Scholar] [CrossRef]
- Ju, S.H.; Lu, S.D.; Peng, J.H.; Zhang, L.B.; Srinivasakannan, C.; Guo, S.H.; Wei, L. Removal of cadmium from aqueous solutions using red mud granulated with cement. Trans. Nonferrous Met. Soc. China. 2012, 22, 3140–3146. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Kumar, S.; Chadd, R.P.; Kumar, A. Trace elements in soil-vegetables interface: Translocation, bioaccumulation, toxicity and amelioration—A review. Sci. Total Environ. 2019, 651, 2927–2942. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zeng, H.; Liu, P.; Yu, J.; Guo, F.; Xu, G.; Zhang, Z.-G. The recycle of red mud as excellent SCR catalyst for removal of NOx. RSC Adv. 2017, 7, 53622–53630. [Google Scholar] [CrossRef]
- Liu, T.; Li, F.; Jin, Z.; Yang, Y. Acidic leaching of potentially toxic metals cadmium, cobalt, chromium, copper, nickel, lead, and zinc from two Zn smelting slag materials incubated in an acidic soil. Environ. Pollut. 2018, 238, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Panda, I.; Jain, S.; Das, S.K.; Jayabalan, R. Characterization of red mud as a structural fill and embankment material using bioremediation. Int. Biodeterior. Biodegrad. 2017, 119, 368–376. [Google Scholar] [CrossRef]
- Yan, S.; Song, W. Photo-transformation of pharmaceutically active compounds in the aqueous environment: A review. Environ. Sci. Process. Impacts 2014, 16, 697–720. [Google Scholar] [CrossRef]
- Smiljanić, S.; Smičiklas, I.; Perić-Grujić, A.; Lončar, B.; Mitrić, M. Rinsed and thermally treated red mud sorbents for aqueous Ni2+ ions. Chem. Eng. J. 2010, 162, 75–83. [Google Scholar] [CrossRef]
- Li, Z.; Din, J.; Xu, J.; Liao, C.; Yin, F.; Lǚ, T.; Cheng, L.; Li, J. Discovery of the REE minerals in the Wulong–Nanchuan bauxite deposits, Chongqing, China: Insights on conditions of formation and processes. J. Geochem. Explor. 2013, 133, 88–102. [Google Scholar] [CrossRef]
- Li, W.; Ning, S.; Zhen, W.; Haisheng, H.; Yue, Y.; Runqing, L.; Yuehua, H.; Honghu, T.; Wei, S. Self-assembly of mixed dodecylamine–dodecanol molecules at the air/water interface based on large-scale molecular dynamics. J. Mol. Liq. 2019, 276, 867–874. [Google Scholar]
- Zhang, L.; Zhang, H.; Guo, W.; Tian, Y. Removal of malachite green and crystal violet cationic dyes from aqueous solution using activated sintering process red mud. Appl. Clay Sci. 2014, 93–94, 85–93. [Google Scholar] [CrossRef]
- Ghosh, I.; Guha, S.; Balasubramaniam, R.; Kumar, A.V.R. Leaching of metals from fresh and sintered red mud. J. Hazard. Mater. 2011, 185, 662–668. [Google Scholar] [CrossRef]
- Yordanova, G.; Godjevargova, T.; Nenkova, R.; Ivanova, D. Biodegradation of phenol and phenolic derivatives by a mixture of immobilized cells of aspergillus awamori and trichosporon cutaneum. Biotechnol. Biotechnol. Equip. 2013, 27, 3681–3688. [Google Scholar] [CrossRef]
- Gupta, V.; Ali, P.I.; Saini, V. Removal of rhodamine b, fast green, and methylene blue from wastewater using red mud, an aluminum industry waste. Ind. Eng. Chem. Res. 2004, 43, 1740–1747. [Google Scholar] [CrossRef]
- Abu-El-Halawa, R.; Zabin, S.A. Removal efficiency of Pb, Cd, Cu and Zn from polluted water using dithiocarbamate ligands. J. Taibah Univ. Sci. 2017, 11, 57–65. [Google Scholar] [CrossRef]
- Tor, A.; Cengeloglu, Y.; Ersoz, M. Increasing the phenol adsorption capacity of neutralized red mud by application of acid activation procedure. Desalination 2009, 242, 19–28. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saini, V.K. Removal of Chlorophenols from Wastewater Using Red Mud: An Aluminum Industry Waste. Environ. Sci. Technol. 2004, 38, 4012–4018. [Google Scholar] [CrossRef]
- Tang, Z.X.; Chen, Y.; Xue, J.; Yue, S. Adsorption and removal of Congo red dye from aqueous solution by using nano-Fe3O4. Adv. Mater. Res. 2012, 503–504, 262–265. [Google Scholar] [CrossRef]
- Shenvi, S.S.; Isloor, A.M.; Ismail, A.F.; Shilton, S.J.; Ahmad, A.A. Humic acid based biopolymeric membrane for effective removal of methylene blue and rhodamine B. Ind. Eng. Chem. Res. 2015, 54, 4965–4975. [Google Scholar] [CrossRef]
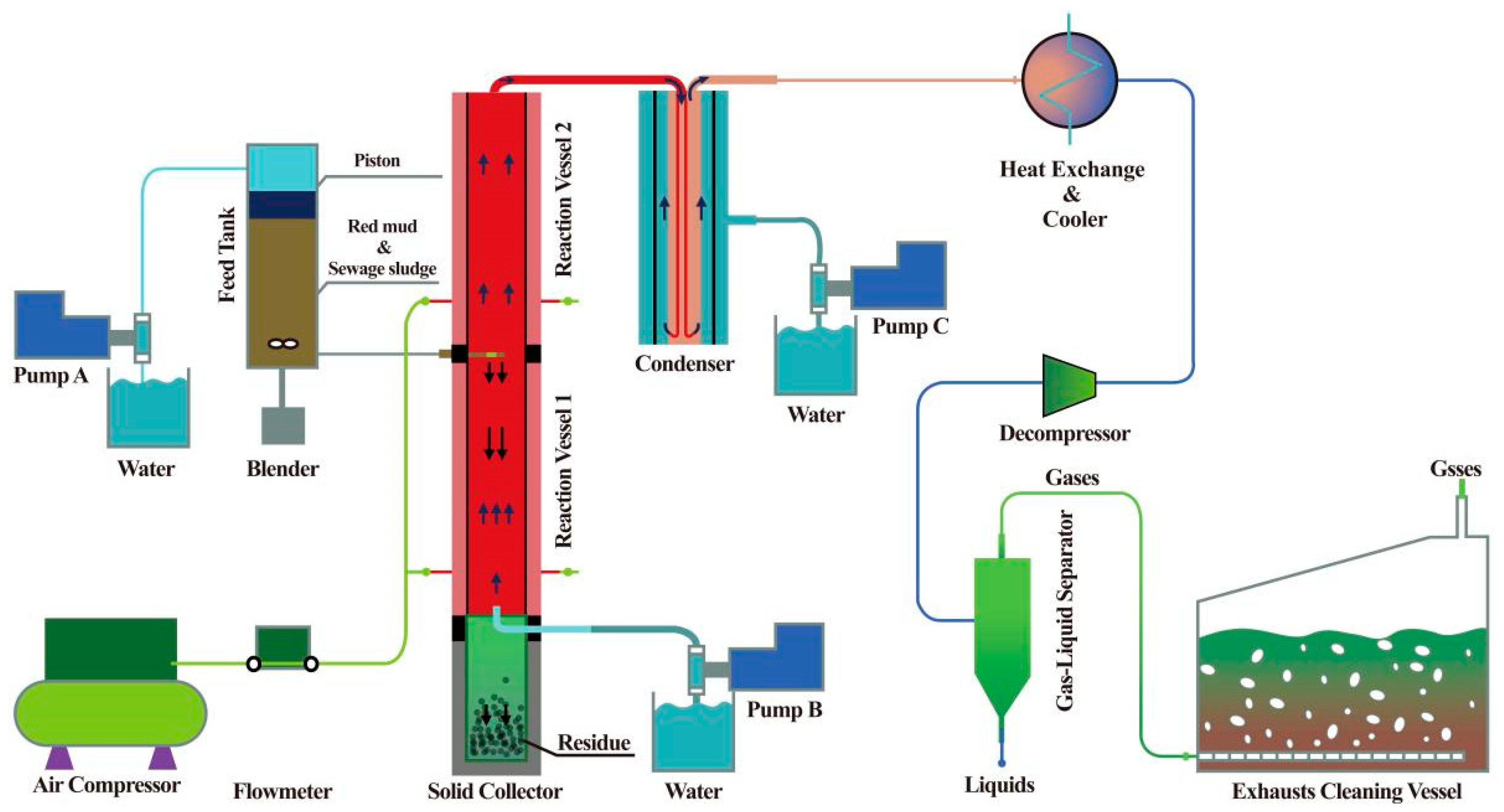
- Qian, L.; Wang, S.; Xu, D.; Guo, Y.; Tang, X.; Wang, L. Treatment of municipal sewage sludge in supercritical water: A review. Water Res. 2016, 89, 118–131. [Google Scholar] [CrossRef]
- Sahu, R.C.; Patel, R.; Ray, B.C. Utilization of activated CO2-neutralized red mud for removal of arsenate from aqueous solutions. J. Hazard. Mater. 2010, 179, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Y.; Zou, L.; Deng, T.; Zhang, J.; Sun, Y.; Ruan, X.; Zhu, P.; Qian, G. Simultaneous wastewater decoloration and fly ash dechlorination during the dye wastewater treatment by municipal solid waste incineration fly ash. Desalin. Water Treat. 2011, 32, 179–186. [Google Scholar] [CrossRef]
- Samanta, A.; Das, S.; Jana, S. Exploring β-Feooh nanorods as an efficient adsorbent for arsenic and organic dyes. Chem. Sel. 2018, 3, 2467–2473. [Google Scholar] [CrossRef]
- Namasivayam, C.; Arasi, D.J.S.E. Removal of Congo red from wastewater by adsorption onto waste red mud. Chemosphere 1997, 34, 401–417. [Google Scholar] [CrossRef]
- Namasivayam, C.; Yamuna, R.; Arasi, D. Removal of acid violet from wastewater by adsorption on waste red mud. Environ. Geol. 2001, 41, 269–273. [Google Scholar]
- Tor, A.; Cengeloglu, Y. Removal of Congo red from aqueous solution by adsorption onto acid activated red mud. J. Hazard. Mater. 2006, 138, 409–415. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Mesoporous titania photocatalysts: Preparation, characterization and reaction mechanisms. J. Mater. Chem. 2011, 21, 11686–11707. [Google Scholar] [CrossRef]
- Liu, H.; Han, K.; Niu, S.; Lu, C.; Liu, M.; Li, H. Experimental Study and Mechanism Analysis of Modified Limestone by Red Mud for Improving Desulfurization. Int. Symp. Coal Combust. 2013, 465–477. [Google Scholar] [CrossRef]
- Zhou, M.; Ji, Y.S.; Chang, Z.; Zhang, C.; Yan, X.T. Experimental study of alkali-activated red mud cement material. Appl. Mech. Mater. 2013, 357–360, 705–709. [Google Scholar] [CrossRef]
- Ghosh, S.; Paul, A.K. Bioleaching of nickel by Aspergillus humicola SKP102 isolated from indian lateritic overburden. J. Sustain. Min. 2016, 15, 108–114. [Google Scholar] [CrossRef]
- Sushil, S.; Alabdulrahman, A.M.; Balakrishnan, M.; Batra, V.S.; Blackley, R.A.; Clapp, J.; Hargreaves, J.S.J.; Monaghan, A.; Pulford, I.D.; Rico, J.L.; et al. Carbon deposition and phase transformations in red mud on exposure to methane. J. Hazard. Mater. 2010, 180, 409–418. [Google Scholar] [CrossRef]
- Leal, P.V.B.; Magriotis, Z.M.; Sales, P.F.; Papini, R.M.; Viana, P.R.d.M. Effect of the acid treatment conditions of kaolinite on etheramine adsorption: A comparative analysis using chemometric tools. J. Environ. Manag. 2017, 197, 393. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Lian, B.; Mo, B.; Liu, C. Bioleaching of heavy metals from red mud using aspergillus niger. Hydrometallurgy 2013, 136, 71–77. [Google Scholar] [CrossRef]
- Buda, A.R.; Koopmans, G.F.; Bryant, R.B.; Chardon, W.J. Emerging technologies for removing nonpoint phosphorus from surface water and groundwater: Introduction. J. Environ. Qual. 2012, 41, 621–627. [Google Scholar] [CrossRef]
- Bryant, R.B.; Buda, A.R.; Kleinman, P.J.; Church, C.D.; Saporito, L.S.; Folmar, G.J.; Bose, S.; Allen, A.L. Using flue gas desulfurization gypsum to remove dissolved phosphorus from agricultural drainage waters. J. Environ. Qual. 2012, 41, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Fois, E.; Antonio Lallai, A.; Mura, G. Sulfur dioxide absorption in a bubbling reactor with suspensions of bayer red mud. Ind. Eng. Chem. Res. 2006, 46, 6770–6776. [Google Scholar] [CrossRef]
- Lu, J.; Liu, D.; Hao, J.; Zhang, G.; Lu, B. Phosphate removal from aqueous solutions by a nano-structured Fe–Ti bimetal oxide sorbent. Chem. Eng. Res. Des. 2015, 93, 652–661. [Google Scholar] [CrossRef]
- Alharthi, A.I.; Hargreaves, J.S.J.; Pulford, I.D.; Gupta, N.; Balakrishnan, M.; Batra, V.S.; Singh, R.K. Hydrocarbon cracking over red mud and modified red mud samples. J. Sustain. Metall. 2016, 2, 387–393. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Lu, R.; Zhou, F.; An, Q.; Meng, Z.; Fei, B.; Lv, F. Novel multiple coagulant from bayer red mud for oily sewage treatment. Desalin. Water Treat. 2015, 54, 690–698. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on fenton and improvements to the fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Chaerun, S.K.; Sulistyo, R.S.; Minwal, W.P.; Mubarok, M.Z. Indirect bioleaching of low-grade nickel limonite and saprolite ores using fungal metabolic organic acids generated by aspergillus niger. Hydrometallurgy 2017, 174, 29–37. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, W.; Jin, X.; Wang, G.; Lu, H.; Wang, H.; Chen, D.; Fan, B.; Hou, T.; Zhang, R. Effect of the particle size of quartz powder on the synthesis and CO2 absorption properties of Li4SiO4 at high temperature. Ind. Eng. Chem. Res. 2013, 52, 1886–1891. [Google Scholar] [CrossRef]
- Nereson, N.A.; Raymond, C.F.; Jacobel, R.W.; Waddington, E.D. The accumulation pattern across siple dome, west antarctica, inferred from radar-detected internal layers. J. Glaciol. 2017, 46, 75–87. [Google Scholar] [CrossRef]
- Hamdi, N.; Srasra, E. Acid-base properties of organosmectite in aqueous suspension. Appl. Clay Sci. 2014, 99, 1–6. [Google Scholar] [CrossRef]
- Fontes, V.J.B.; Simões, S.D.M.; Fernandes, T.D.S.; Varjão, A.E.L.; Santos, S.L.D.O.; Franco, J.M.; Santos, M.A.D. Pulmonary function and symptoms in asthmatics adolescents. World Allergy Organ. J. 2015, 8 (Suppl. 1), A266. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Hao, X.W. Effect of red mud addition on the fractionation and bio-accessibility of Pb, Zn and as in combined contaminated soil. Chem. Ecol. 2012, 28, 37–48. [Google Scholar] [CrossRef]
- Huang, Y.; Hao, X. The influence of red mud, bone char and lime on uptake and accumulation of Pb, Zn and as by maize (zea mays) planted in contaminated soil. In Proceedings of the Third International Conference on Digital Manufacturing & Automation, Guilin, China, 31 July–2 August 2012; pp. 922–926. [Google Scholar]
- Zhou, R.; Wei, J.; Luo, L.; Zhang, J.; Zhou, Y.; Wang, Y. Effects of red mud addition on fractions of Cd, Pb and wheat root growth in calcareous soil. Chin. J. Environ. Eng. 2017, 11, 2560–2567. (In Chinese) [Google Scholar]
- Eid, E.M.; El-Bebany, A.F.; Alrumman, S.A.; Hesham, A.E.-L.; Taher, M.A.; Fawy, K.F. Effects of different sewage sludge applications on heavy metal accumulation, growth and yield of spinach (Spinacia oleracea L.). Int. J. Phytoremediat. 2017, 19, 340–347. [Google Scholar] [CrossRef]
- Li, B.; Yang, J.; Wei, D.; Chen, S.; Li, J.; Ma, Y. Field evidence of cadmium phytoavailability decreased effectively by rape straw and/or red mud with zinc sulphate in a Cd-contaminated calcareous soil. PLoS ONE 2014, 9, e109967. [Google Scholar] [CrossRef]

| Chemical Constituent | Fe2O3 | Al2O3 | SiO2 | CaO | Na2O | TiO2 | K2O | MgO | Sc2O3 | Nb2O5 | Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayer process | 13.69 | 7.02 | 18.10 | 42.21 | 2.38 | 2.1 | 0.30 | - | - | - | - |
| Combined process | 10.97 | 7.68 | 22.67 | 40.78 | 2.93 | 3.26 | 0.38 | 1.77 | - | - | 11.77 |
| Sintering process | 11.4 | 10.66 | 21.06 | 40.62 | 1.49 | - | 0.45 | 0.93 | - | - | 6.86 |
| Mineral Composition (Chemical Formula) | Sintering Process | Combined Process | Bayer Process |
|---|---|---|---|
| β-2CaO·SiO2 | 46 | 43 | - |
| Sodium aluminosilicate (Na2O·Al2O3·1.7SiO2·nH2O)·NaX or Na2X | 4 | 4 | 20 |
| Anorthite 3CaO·Al2O3·3Si2O2 or 3CaO·Al2O3·xSiO2·(6–2x)H2O | 5 | 2 | 20 |
| Calcite CaCO3 | 14 | 14 | 19 |
| Limonite Fe2O3·H2O | 7 | 4 | 4 |
| Boehmite Al2O3·H2O | - | 1 | 21 |
| Perovskite CaO·TiO2 | 7 | 12 | 15 |
| 4CaO·Al2O3·Fe2O3 | 8 | 12 | - |
| Na2O·Al2O3·2SiO2 | 7 | 8 | - |
| FeS2 | 1 | - | - |
| Others | 1 | - | 1 |
| Total | 100 | 100 | 100 |
| Chemical Constituent | Fe2O3 | Al2O3 | SiO2 | CaO | Na2O | TiO2 | K2O | MgO | Sc2O3 | Nb2O5 | Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayer process | 13.69 | 7.02 | 18.10 | 42.21 | 2.38 | 2.1 | 0.30 | - | - | - | - |
| Combined process | 10.97 | 7.68 | 22.67 | 40.78 | 2.93 | 3.26 | 0.38 | 1.77 | - | - | 11.77 |
| Sintering process | 11.4 | 10.66 | 21.06 | 40.62 | 1.49 | - | 0.45 | 0.93 | - | - | 6.86 |
| Adsorbent | Adsorbate | Amount Adsorbed | Reference |
|---|---|---|---|
| RM | Phenol | 0.63–0.74 mol/g | [77] |
| RM | 2-Chlorophenol | 0.72–0.79 mol/g | [77] |
| RM | 4-Chlorophenol | 0.78–0.82 mol/g | [77] |
| RM | 2,4-Dichlorophenol | 0.80–0.85 mol/g | [77] |
| Neutralized RM | Phenol | 2.50 × 10−5 mol/g | [75] |
| Acid-ARM | Phenol | 2.98 × 10−5 mol/g | [76] |
| Adsorbent | Adsorbate | Amount Adsorbed | Reference |
|---|---|---|---|
| RM | Rhodamine B | (1.01–1.16) × 10−5 mol/g | [83] |
| RM | Fast Green | (7.25–9.35) × 10−6 mol/g | [83] |
| RM | Methylene blue | (4.35–5.23) × 10−5 mol/g | [83] |
| RM | Congo red | 5.81 × 10−6 mol/g | [84] |
| RM | Acid violet | 2.42 × 10−6 mol/g | [85] |
| Acid-ARM | Congo red | 1.02 × 10−5 mol/g | [86] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Hu, G.; Lyu, F.; Yue, T.; Tang, H.; Han, H.; Yang, Y.; Liu, R.; Sun, W. Application of Red Mud in Wastewater Treatment. Minerals 2019, 9, 281. https://doi.org/10.3390/min9050281
Wang L, Hu G, Lyu F, Yue T, Tang H, Han H, Yang Y, Liu R, Sun W. Application of Red Mud in Wastewater Treatment. Minerals. 2019; 9(5):281. https://doi.org/10.3390/min9050281
Chicago/Turabian StyleWang, Li, Guangyan Hu, Fei Lyu, Tong Yue, Honghu Tang, Haisheng Han, Yue Yang, Runqing Liu, and Wei Sun. 2019. "Application of Red Mud in Wastewater Treatment" Minerals 9, no. 5: 281. https://doi.org/10.3390/min9050281
APA StyleWang, L., Hu, G., Lyu, F., Yue, T., Tang, H., Han, H., Yang, Y., Liu, R., & Sun, W. (2019). Application of Red Mud in Wastewater Treatment. Minerals, 9(5), 281. https://doi.org/10.3390/min9050281






