In Situ Chalcophile and Siderophile Element Behavior in Sulfides from Moroccan Middle Atlas Spinel Peridotite Xenoliths during Metasomatism and Weathering
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Analytical Techniques
2.2.1. Electron Microprobe
2.2.2. Laser Ablation ICP-MS
3. Results
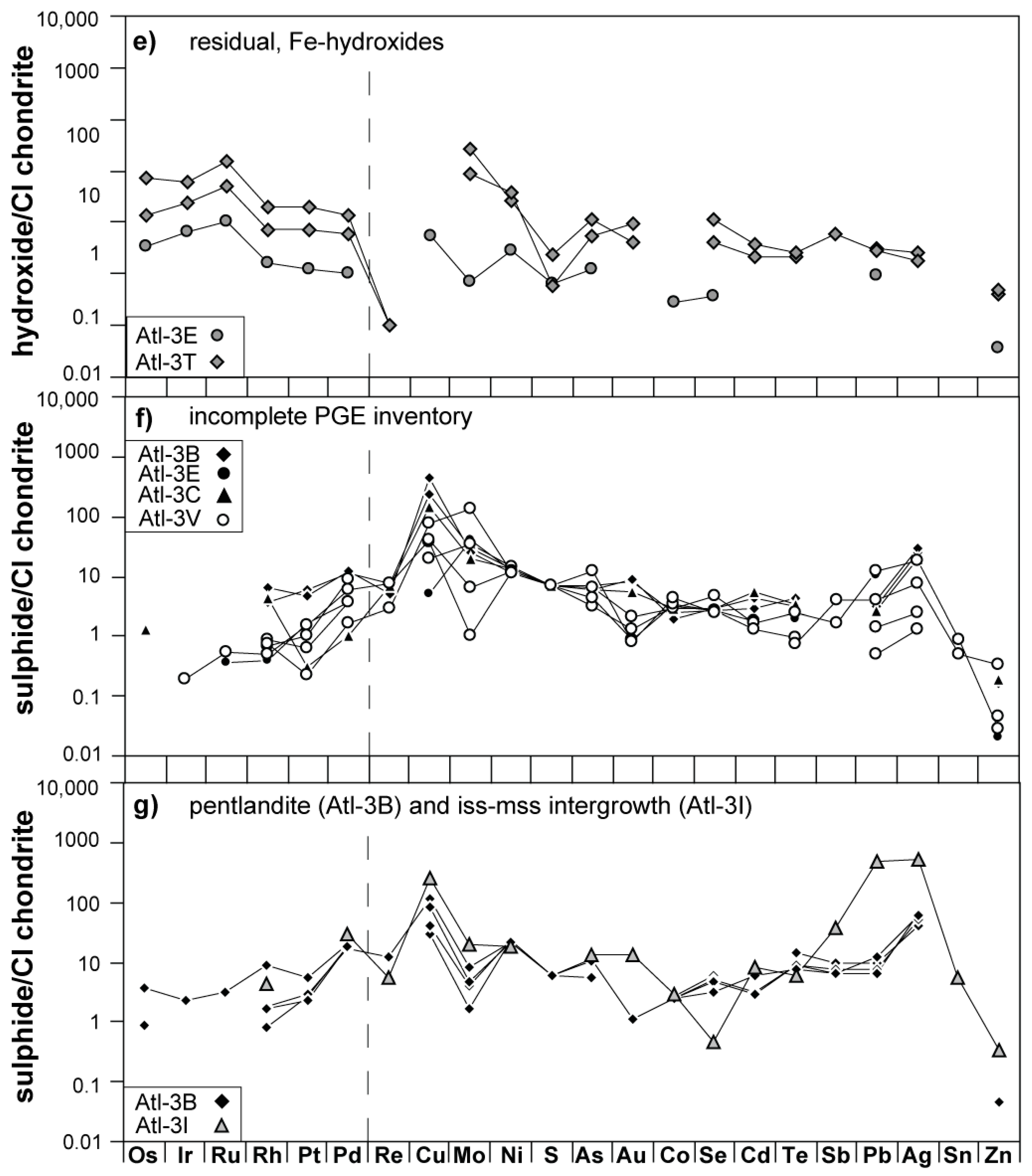
3.1. Petrographic Description of Sulfide Phases and Associated Fe-Hydroxides
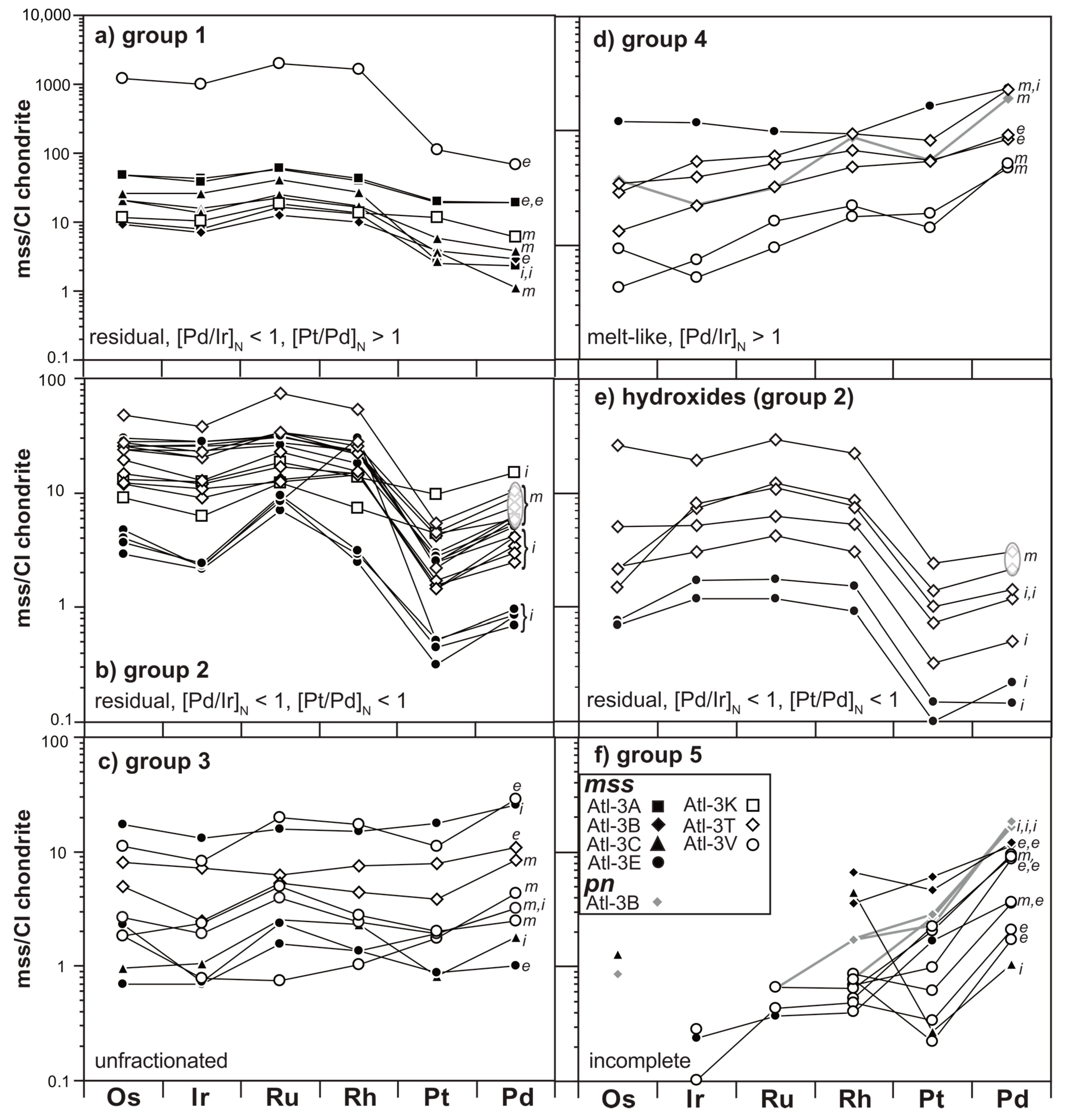
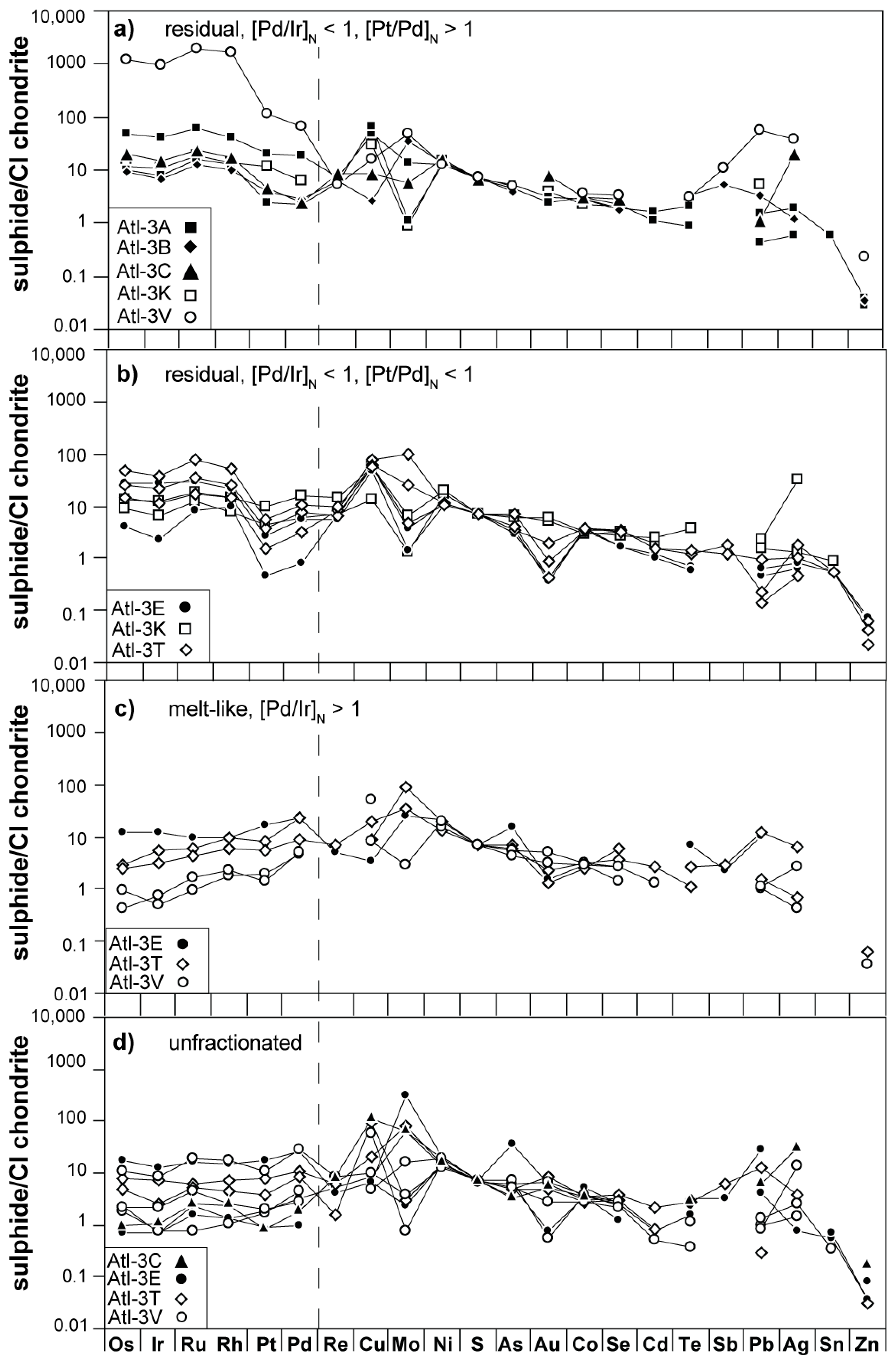
3.2. Siderophile/Chalcophile Major and Trace Element Composition of Sulfides
3.3. Siderophile/Chalcophile Major and Trace Element Composition of Fe-Hydroxides
4. Discussion
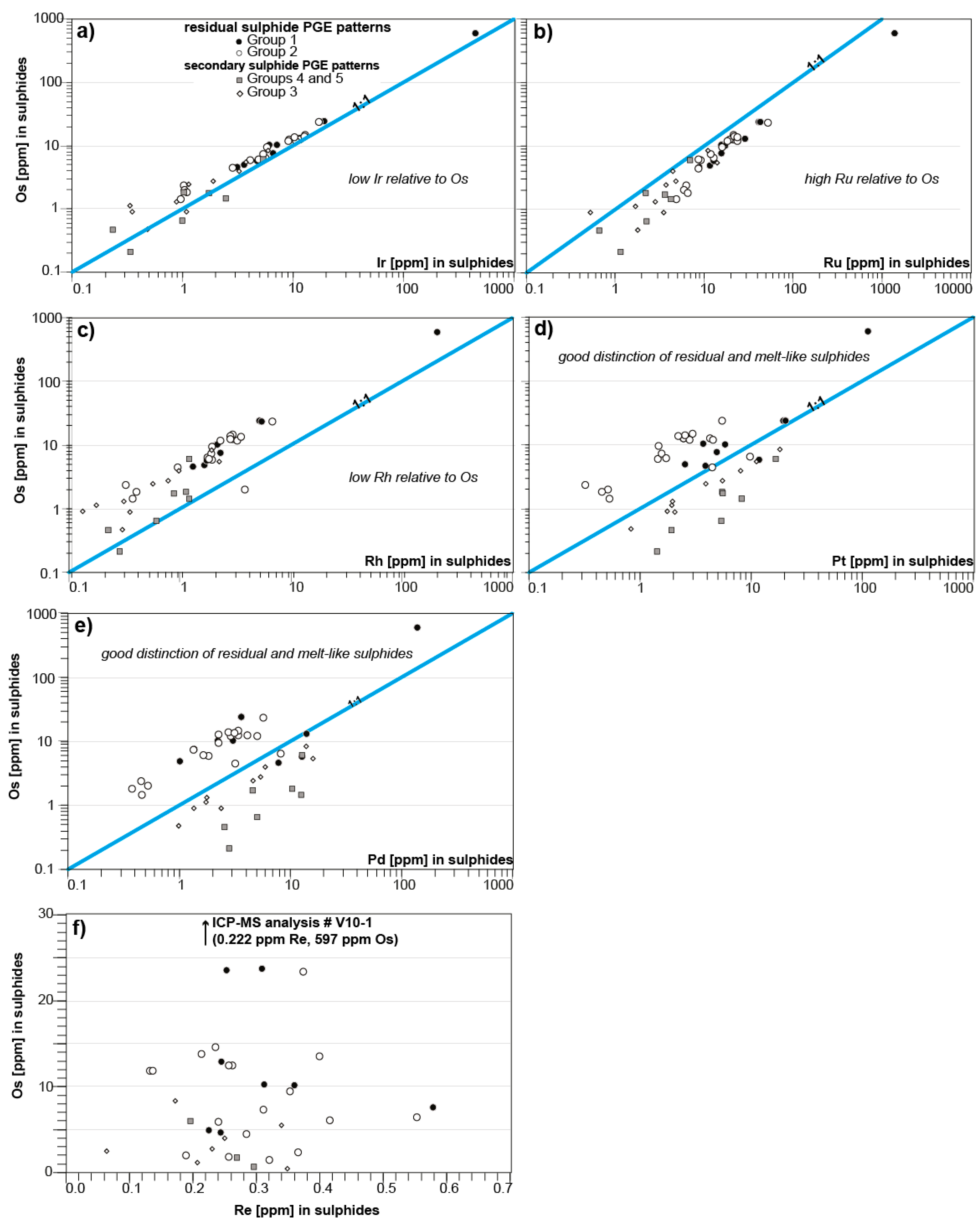
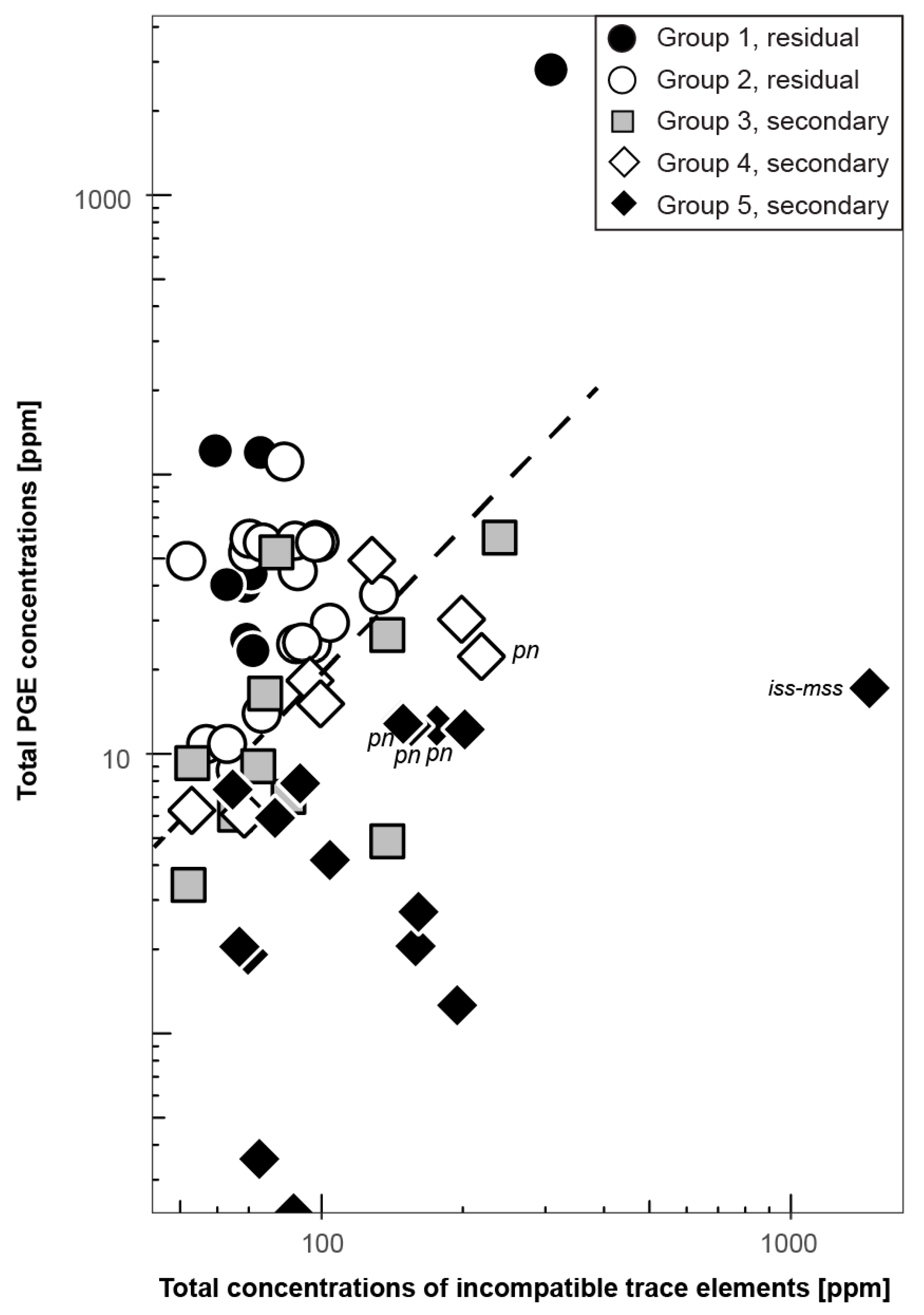
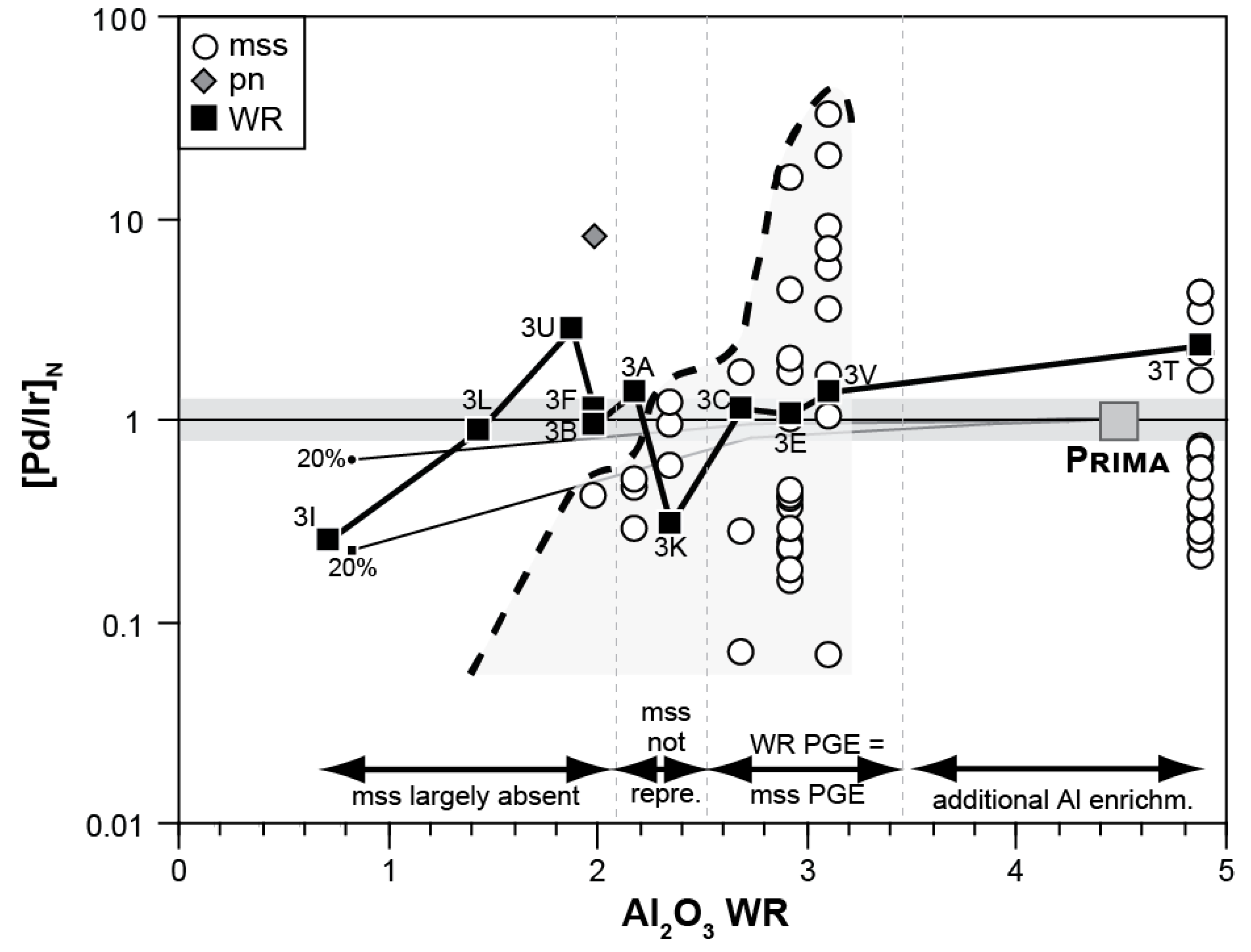
4.1. The Relationship of Sulfide Inventory and Whole-Rock PGE Compositions
4.1.1. Late-Stage Exsolution, Elemental Mobility, and Breakdown of Sulfides
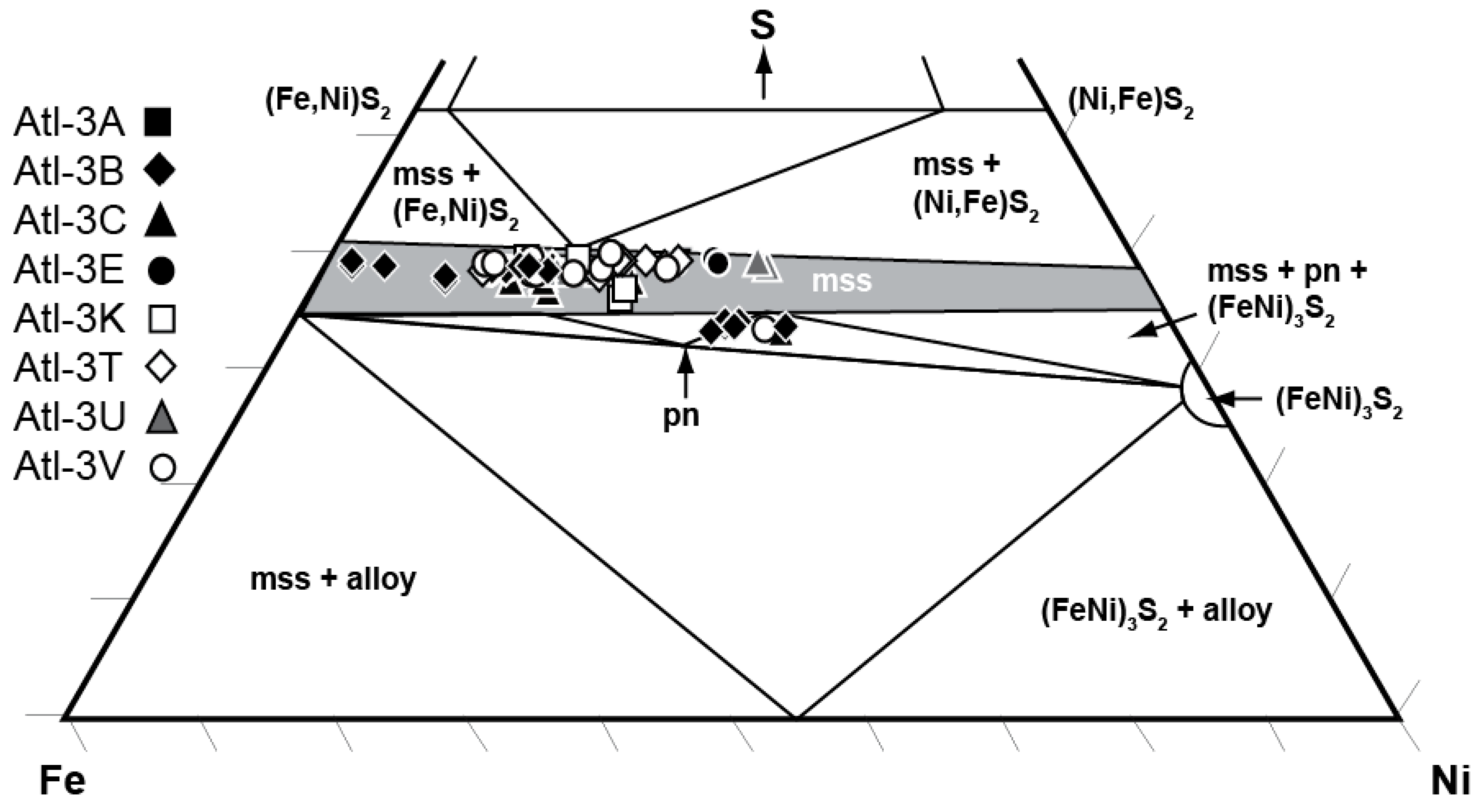
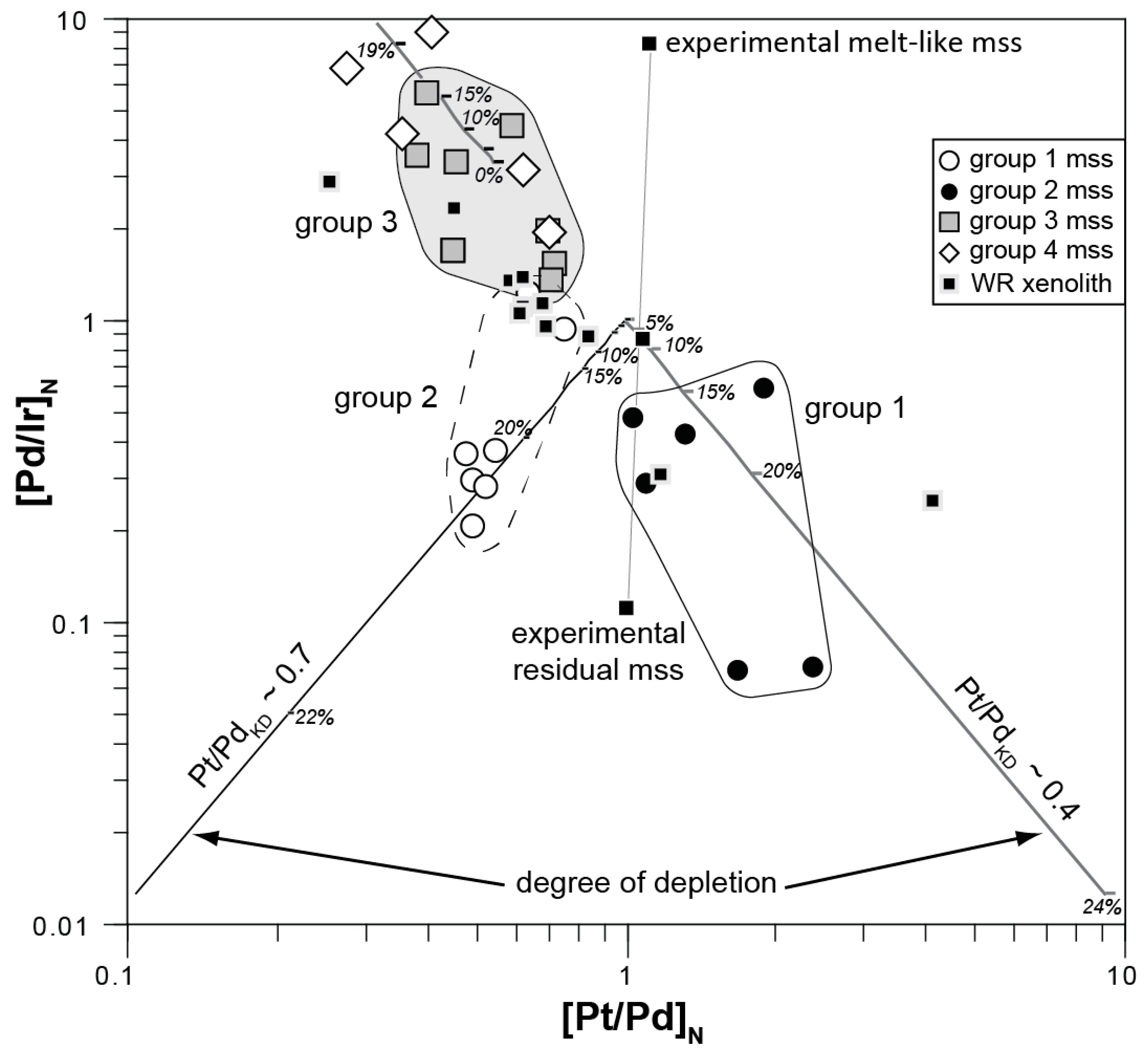
4.1.2. Implications of Elemental Heterogeneity in mss
4.1.3. The PGE Relationship of mss and Peridotite Whole Rocks
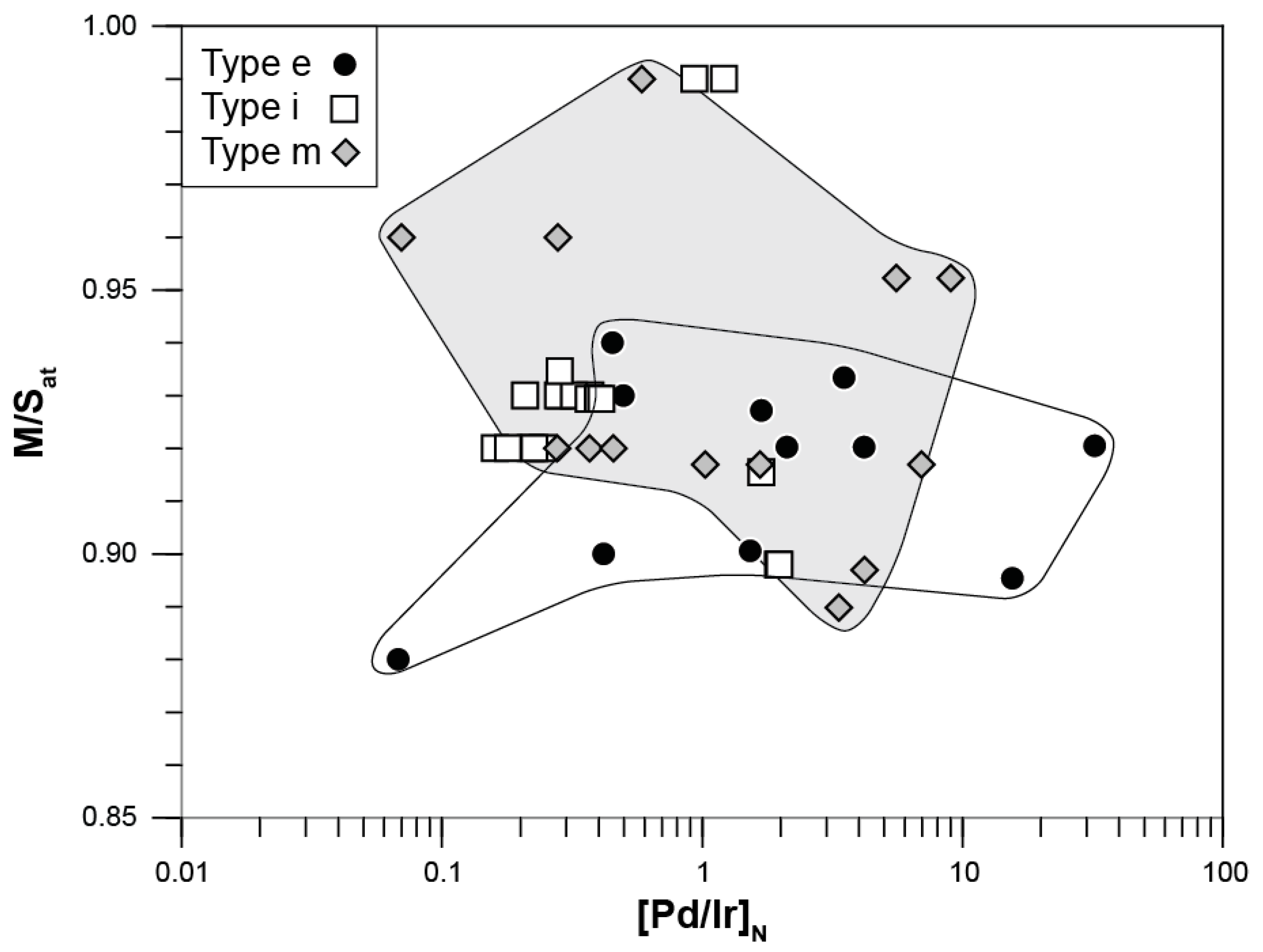
4.2. Evidence for Mantle Depletion and Metasomatic Enrichment in mss
4.2.1. Melt Extraction Recorded in Middle Atlas Xenolith mss PGE Signatures
4.2.2. Precipitation of Melt-Like mss during the Initial Melting Event or from Ascending Basaltic Melts?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Petrography of Middle Atlas Xenoliths
Appendix B. Composition of Silicates and Spinel
References
- Pearson, D.G.; Wittig, N. The Formation and Evolution of Cratonic Mantle Lithosphere—Evidence from Mantle Xenoliths. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 255–292. ISBN 978-0-08-098300-4. [Google Scholar]
- Aulbach, S.; Gerdes, A.; Viljoen, K.S. Formation of diamondiferous kyanite–eclogite in a subduction mélange. Geochim. Cosmochim. Acta 2016, 179, 156–176. [Google Scholar] [CrossRef]
- Luguet, A.; Reisberg, L. Highly Siderophile Element and Os-187 Signatures in Non-cratonic Basalt-hosted Peridotite Xenoliths: Unravelling the Origin and Evolution of the Post-Archean Lithospheric Mantle. Rev. Mineral. Geochem. 2016, 81, 305–367. [Google Scholar] [CrossRef]
- Lee, C.T.A.; Lenardic, A.; Thiagarajan, N.; Agranier, A.; O’Neill, C.J.; Yin, Q.Z. Remnant iron oxide/sulfide mattes from a Hadean magma ocean at the core-mantle boundary: Insights from a small scale post-Archean analog. Geochim. Cosmochim. Acta 2006, 70, A347. [Google Scholar] [CrossRef]
- Griffin, W.L.; O’Reilly, S.Y.; Afonso, J.C.; Begg, G.C. The Composition and Evolution of Lithospheric Mantle: A Re-evaluation and its Tectonic Implications. J. Petrol. 2009, 50, 1185–1204. [Google Scholar] [CrossRef]
- Griffin, W.L.; O’Reilly, S.Y.; Abe, N.; Aulbach, S.; Davies, R.M.; Pearson, N.J.; Doyle, B.J.; Kivi, K. The origin and evolution of Archean lithospheric mantle. Precambrian Res. 2003, 127, 19–41. [Google Scholar] [CrossRef]
- Carlson, R.W.; Pearson, D.G.; James, D.E. Physical, chemical, and chronological characteristics of continental mantle. Rev. Geophys. 2005, 43. [Google Scholar] [CrossRef]
- Lorand, J.P.; Luguet, A.; Alard, O. Platinum-group element systematics and petrogenetic processing of the continental upper mantle: A review. Lithos 2013, 164, 2–21. [Google Scholar] [CrossRef]
- Lorand, J.P.; Barrat, J.A.; Chevrier, V.; Sautter, V.; Pont, S. Metal-saturated sulfide assemblages in NWA 2737: Evidence for impact-related sulfur devolatilization in Martian meteorites. Meteorit. Planet. Sci. 2012, 47, 1830–1841. [Google Scholar] [CrossRef]
- Day, J.M.D.; Brandon, A.D.; Walker, R.J. Highly Siderophile Elements in Earth, Mars, the Moon, and Asteroids. Rev. Mineral. Geochem. 2016, 81, 161–238. [Google Scholar] [CrossRef]
- Kiseeva, E.S.; Kamenetsky, V.S.; Yaxley, G.M.; Shee, S.R. Mantle melting versus mantle metasomatism—“The chicken or the egg” dilemma. Chem. Geol. 2017, 455, 120–130. [Google Scholar] [CrossRef]
- Brenan, J.M. Re-Os fractionation by sulfide melt-silicate melt partitioning: A new spin. Chem. Geol. 2008, 248, 140–165. [Google Scholar] [CrossRef]
- Lorand, J.P.; Luguet, A.; Alard, O. Platinum-group elements: A new set of key tracers for the earth’s interior. Elements 2008, 4, 247–252. [Google Scholar] [CrossRef]
- Luguet, A.; Alard, O.; Lorand, J.P.; Pearson, N.J.; Ryan, C.; O’Reilly, S.Y. Laser-ablation microprobe (LAM)-ICPMS unravels the highly siderophile element geochemistry of the oceanic mantle. Earth Planet. Sci. Lett. 2001, 189, 285–294. [Google Scholar] [CrossRef]
- Luguet, A.; Lorand, J.P.; Seyler, M. Sulfide petrology and highly siderophile element geochemistry of abyssal peridotites: A coupled study of samples from the Kane Fracture Zone (45 degrees W 23 degrees 20N, MARK Area, Atlantic Ocean). Geochim. Cosmochim. Acta 2003, 67, 1553–1570. [Google Scholar] [CrossRef]
- Luguet, A.; Shirey, S.B.; Lorand, J.P.; Horan, M.F.; Carlson, R.W. Residual platinum-group minerals from highly depleted harzburgites of the Lherz massif (France) and their role in HSE fractionation of the mantle. Geochim. Cosmochim. Acta 2007, 71, 3082–3097. [Google Scholar] [CrossRef]
- Pearson, D.G.; Carlson, R.W.; Shirey, S.B.; Boyd, F.R.; Nixon, P.H. Stabilization of Archean Lithospheric Mantle—A Re-Os Isotope Study of Peridotite Xenoliths from the Kaapvaal Craton. Earth Planet. Sci. Lett. 1995, 134, 341–357. [Google Scholar] [CrossRef]
- Schilling, M.E.; Carlson, R.W.; Conceicao, R.V.; Dantas, C.; Bertotto, G.W.; Koester, E. Re-Os isotope constraints on subcontinental lithospheric mantle evolution of southern South America. Earth Planet. Sci. Lett. 2008, 268, 89–101. [Google Scholar] [CrossRef]
- Tsuru, A.; Walker, R.J.; Kontinen, A.; Peltonen, P.; Hanski, E. Re-Os isotopic systematics of the 1.95 Ga Jormua Ophiolite Complex, northeastern Finland. Chem. Geol. 2000, 164, 123–141. [Google Scholar] [CrossRef]
- Wittig, N.; Pearson, D.G.; Baker, J.A.; Duggen, S.; Hoernle, K. A major element, PGE and Re–Os isotope study of Middle Atlas (Morocco) peridotite xenoliths: Evidence for coupled introduction of metasomatic sulphides and clinopyroxene. Lithos 2010, 115, 15–26. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhi, X.; Chen, L.; Saunders, A.D.; Reichow, M.K. Re-Os isotopic compositions of picrites from the Emeishan flood basalt province, China. Earth Planet. Sci. Lett. 2008, 276, 30–39. [Google Scholar] [CrossRef]
- Gonzalez-Jimenez, J.M.; Villaseca, C.; Griffin, W.L.; O’Reilly, S.Y.; Belousova, E.; Ancochea, E.; Pearson, N.J. Significance of ancient sulfide PGE and Re-Os signatures in the mantle beneath Calatrava, Central Spain. Contrib. Mineral. Petrol. 2014, 168. [Google Scholar] [CrossRef]
- Aulbach, S.; Luchs, T.; Brey, G.P. Distribution and behaviour during metasomatism of PGE-Re and Os isotopes in off-craton mantle xenoliths from Namibia. Lithos 2014, 184, 478–490. [Google Scholar] [CrossRef]
- Pearson, D.G.; Irvine, G.J.; Ionov, D.A.; Boyd, F.R.; Dreibus, G.E. Re-Os isotope systematics and platinum group element fractionation during mantle melt extraction: A study of massif and xenolith peridotite suites. Chem. Geol. 2004, 208, 29–59. [Google Scholar] [CrossRef]
- Becker, H.; Horan, M.F.; Walker, R.J.; Gao, S.; Lorand, J.-P.; Rudnick, R.L. Highly siderophile element composition of the Earth’s primitive upper mantle: Constraints from new data on peridotite massifs and xenoliths. Geochim. Cosmochim. Acta 2006, 70, 4528–4550. [Google Scholar] [CrossRef]
- Pernet-Fisher, J.F.; Howarth, G.H.; Pearson, D.G.; Woodland, S.; Barry, P.H.; Pokhilenko, N.P.; Pokhilenko, L.N.; Agashev, A.M.; Taylor, L.A. Plume impingement on the Siberian SCLM: Evidence from Re-Os isotope systematics. Lithos 2015, 218, 141–154. [Google Scholar] [CrossRef]
- Lorand, J.P.; Luguet, A. Chalcophile and Siderophile Elements in Mantle Rocks: Trace Elements Controlled by Trace Minerals. Rev. Mineral. Geochem. 2016, 81, 441–488. [Google Scholar] [CrossRef]
- Griffin, W.L.; O’Reilly, S.Y.; Pearson, N.J.; Graham, S. In situ Re-Os dating of sulfides in Kaapvaal xenoliths. Geochim. Cosmochim. Acta 2002, 66, A292. [Google Scholar]
- Malarkey, J.; Wittig, N.; Pearson, D.G.; Davidson, J.P. Characterising modal metasomatic processes in young continental lithospheric mantle: A microsampling isotopic and trace element study on xenoliths from the Middle Atlas Mountains, Morocco. Contrib. Mineral. Petrol. 2011, 162, 289–302. [Google Scholar] [CrossRef]
- Wittig, N.; Pearson, D.G.; Duggen, S.; Baker, J.A.; Hoernle, K. Tracing the metasomatic and magmatic evolution of continental mantle roots with Sr, Nd, Hf and and Pb isotopes: A case study of Middle Atlas (Morocco) peridotite xenoliths. Geochim. Cosmochim. Acta 2010, 74, 1417–1435. [Google Scholar] [CrossRef]
- Wittig, N.; Pearson, D.G.; Downes, H.; Baker, J.A. The U, Th and Pb elemental and isotope compositions of mantle clinopyroxenes and their grain boundary contamination derived from leaching and digestion experiments. Geochim. Cosmochim. Acta 2009, 73, 469–488. [Google Scholar] [CrossRef]
- Hellebrand, E.; Snow, J.E.; Dick, H.J.B.; Hofmann, A.W. Coupled major and trace elements as indicators of the extent of melting in mid-ocean-ridge peridotites. Nature 2001, 410, 677–681. [Google Scholar] [CrossRef]
- Armstrong, J.T. Citzaf—A Package of Correction Programs for the Quantitative Electron Microbeam X-Ray-Analysis of Thick Polished Materials, Thin-Films, and Particles. Microbeam Anal. 1995, 4, 177–200. [Google Scholar]
- van Achterbergh, E.; Ryan, C.G.; Jackson, S.E.; Griffin, W.L. Data reduction software for LA-ICP-MS. In Laser-Ablation-ICPMS in the Earth Sciences: Principles and Applications; Sylvester, P., Ed.; Mineralogical Association of Canada: Ottawa, ON, Canada, 2001. [Google Scholar]
- Wohlgemuth-Ueberwasser, C.C.; Ballhaus, C.; Berndt, J.; Paliulionyte, V.S.N.; Meisel, T. Synthesis of PGE sulfide standards for laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Contrib. Mineral. Petrol. 2007, 154, 607–617. [Google Scholar] [CrossRef]
- Wittig, N. Application of Novel U-Th-Pb and Lu-Hf Isotopic Techniques to Tracing the Melting and Metasomatic History of Mantle Rocks. Ph.D. Thesis, Copenhagen University, Copenhagen, Denmark, 2006. [Google Scholar]
- Westner, K.J. Identifying Clinopyroxene-Sulphide Co-Precipitation, Rejuvenation Mechanisms and Platinum-Group Element Fractionation Processes in Young Continental Mantle Roots. Diploma Thesis, Friedrich-Alexander-Universität, Erlangen-Nürnberg, Germany, 2011. [Google Scholar]
- Barnes, S.J.; Lightfoot, P.C. Formation of magmatic nickel-sulfide ore deposits and processes affecting their copper and platinum-group element contents. In Economic Geology 100th Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 179–213. [Google Scholar]
- Bockrath, C.; Ballhaus, C.; Holzheid, A. Fractionation of the platinum-group elements during mantle melting. Science 2004, 305, 1951–1953. [Google Scholar] [CrossRef] [PubMed]
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Liu, Y.N.; Brenan, J. Partitioning of platinum-group elements (PGE) and chalcogens (Se, Te, As, Sb, Bi) between monosulfide-solid solution (MSS), intermediate solid solution (ISS) and sulfide liquid at controlled fO2-fS2 conditions. Geochim. Cosmochim. Acta 2015, 159, 139–161. [Google Scholar] [CrossRef]
- Patten, C.; Barnes, S.-J.; Mathez, E.A.; Jenner, F.E. Partition coefficients of chalcophile elements between sulfide and silicate melts and the early crystallization history of sulfide liquid: LA-ICP-MS analysis of MORB sulfide droplets. Chem. Geol. 2013, 358, 170–188. [Google Scholar] [CrossRef]
- Li, Y.; Audétat, A. Partitioning of V, Mn, Co, Ni, Cu, Zn, As, Mo, Ag, Sn, Sb, W, Au, Pb, and Bi between sulfide phases and hydrous basanite melt at upper mantle conditions. Earth Planet. Sci. Lett. 2012, 355–356, 327–340. [Google Scholar] [CrossRef]
- Li, Y.; Audétat, A. Effects of temperature, silicate melt composition, and oxygen fugacity on the partitioning of V, Mn, Co, Ni, Cu, Zn, As, Mo, Ag, Sn, Sb, W, Au, Pb, and Bi between sulfide phases and silicate melt. Geochim. Cosmochim. Acta 2015, 162, 25–45. [Google Scholar] [CrossRef]
- Helmy, H.M.; Ballhaus, C.; Wohlgemuth-Ueberwasser, C.; Fonseca, R.O.C.; Laurenz, V. Partitioning of Se, As, Sb, Te and Bi between monosulfide solid solution and sulfide melt—Application to magmatic sulfide deposits. Geochim. Cosmochim. Acta 2010, 74, 6174–6179. [Google Scholar] [CrossRef]
- Barnes, S.J.; Prichard, H.M.; Cox, R.A.; Fisher, P.C.; Godel, B. The location of the chalcophile and siderophile elements in platinum-group element ore deposits (a textural, microbeam and whole rock geochemical study): Implications for the formation of the deposits. Chem. Geol. 2008, 248, 295–317. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.J.; Prichard, H.M. The distribution of platinum group elements (PGE) and other chalcophile elements among sulfides from the Creighton Ni-Cu-PGE sulfide deposit, Sudbury, Canada, and the origin of palladium in pentlandite. Mineralium Deposita 2010, 45, 765–793. [Google Scholar] [CrossRef]
- Godel, B.; Barnes, S.J.; Maier, W.D. Platinum-group elements in sulphide minerals, platinum-group minerals, and whole-rocks of the Merensky Reef (Bushveld Complex, South Africa): Implications for the formation of the reef. J. Petrol. 2007, 48, 1569–1604. [Google Scholar] [CrossRef]
- Godel, B.; Barnes, S.J. Platinum-group elements in sulfide minerals and the whole rocks of the J-M Reef (Stillwater Complex): Implication for the formation of the reef. Chem. Geol. 2008, 248, 272–294. [Google Scholar] [CrossRef]
- Osbahr, I.; Oberthur, T.; Klemd, R.; Josties, A. Platinum-group element distribution in base-metal sulfides of the UG2 chromitite, Bushveld Complex, South Africa-a reconnaissance study. Mineralium Deposita 2014, 49, 655–665. [Google Scholar] [CrossRef]
- Osbahr, I.; Klemd, R.; Oberthür, T.; Brätz, H.; Schouwstra, R. Platinum-group element distribution in base-metal sulfides of the Merensky Reef from the eastern and western Bushveld Complex, South Africa. Mineralium Deposita 2013, 48, 211–232. [Google Scholar] [CrossRef]
- Grokhovskaya, T.L.; Bakaev, G.F.; Shelepina, E.P.; Lapina, M.I.; Laputina, I.P.; Muravitskaya, G.N. PGE Mineralization in the Vuruchuaivench Gabbronorite Massif, Monchegorsk Pluton (Kola Peninsula, Russia). Geol. Ore Depos. 2000, 42, 133–146. [Google Scholar]
- Barnes, S.-J.; Cox, R.A.; Zientek, M.L. Platinum-group element, Gold, Silver and Base Metal distribution in compositionally zoned sulfide droplets from the Medvezky Creek Mine, Noril’sk, Russia. Contrib. Mineral. Petrol. 2006, 152, 187–200. [Google Scholar] [CrossRef]
- Lorand, J.P.; Alard, O. Platinum-group element abundances in the upper mantle: New constraints from in situ and whole-rock analyses of Massif Central xenoliths (France). Geochim. Cosmochim. Acta 2001, 65, 2789–2806. [Google Scholar] [CrossRef]
- Locmelis, M.; Melcher, F.; Oberthür, T. Platinum-group element distribution in the oxidized Main Sulfide Zone, Great Dyke, Zimbabwe. Mineralium Deposita 2010, 45, 93–109. [Google Scholar] [CrossRef]
- Lorand, J.P. Are spinel Iherzolite xenoliths representative of the abundance of sulfur in the upper mantle? Geochim. Cosmochim. Acta 1990, 54, 1487–1492. [Google Scholar] [CrossRef]
- Oberthür, T. The Fate of Platinum-Group Minerals in the Exogenic Environment-From Sulfide Ores via Oxidized Ores into Placers: Case Studies Bushveld Complex, South Africa, and Great Dyke, Zimbabwe. Minerals 2018, 8, 581. [Google Scholar] [CrossRef]
- Oberthür, T.; Melcher, F. Behaviour of PGE and PGM in the supergene environment: A case study of persistence and redistribution in the Main Sulfide Zone of The Great Dyke, Zimbabwe. In Exploration for Platinum-Group Element Deposits; Mungall, J.E., Ed.; Short Course Series Volume 35; Mineralogical Association of Canada: Ottawa, ON, Canada, 2005; pp. 97–111. [Google Scholar]
- Suarez, S.; Prichard, H.M.; Velasco, F.; Fisher, P.C.; McDonald, I. Alteration of platinum-group minerals and dispersion of platinum-group elements during progressive weathering of the Aguablanca Ni-Cu deposit, SW Spain. Mineralium Deposita 2010, 45, 331–350. [Google Scholar] [CrossRef]
- Traoré, D.; Beauvais, A.; Auge, T.; Chabaux, F.; Parisot, J.C.; Cathelineau, M.; Peiffert, C.; Colin, F. Platinum and palladium mobility in supergene environment: The residual origin of the Pirogues River mineralization, New Caledonia. J. Geochem. Explor. 2006, 88, 350–354. [Google Scholar] [CrossRef]
- Frei, M. Composition, Formation and Leaching Behaviour of Supergene, Polymetallic Ores from the Sanyati Deposit (Zimbabwe); Schweizerbart Science Publishers: Stuttgart, Germany, 2011; ISBN 978-3-510-95992-1. [Google Scholar]
- Cabri, L.J. New Data on Phase Relations in Cu-Fe-S System. Econ. Geol. 1973, 68, 443–454. [Google Scholar] [CrossRef]
- Etschmann, B.; Pring, A.; Putnis, A.; Grguric, B.A.; Studer, A. A kinetic study of the exsolution of pentlandite (Ni,Fe)(9)S8 from the monosulfide solid solution (Fe,Ni)S. Am. Mineral. 2004, 89, 39–50. [Google Scholar] [CrossRef]
- Alard, O.; Griffin, W.L.; Lorand, J.P.; Jackson, S.E.; O’Reilly, S.Y. Non-chondritic distribution of the highly siderophile elements in mantle sulphides. Nature 2000, 407, 891–894. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, B.; Gonzalez-Jimenez, J.M. Petrogenesis of the Platinum-Group Minerals. Rev. Mineral. Geochem. 2016, 81, 489–578. [Google Scholar] [CrossRef]
- Wittig, N.; Webb, M.; Pearson, D.G.; Dale, C.W.; Ottley, C.J.; Hutchison, M.; Jensen, S.M.; Luguet, A. Formation of the North Atlantic Craton: Timing and mechanisms constrained from Re–Os isotope and PGE data of peridotite xenoliths from S.W. Greenland. Chem. Geol. 2010, 276, 166–187. [Google Scholar] [CrossRef]
- Shaw, D.M. Trace element fractionation during anatexis. Geochim. Cosmochim. Acta 1970, 34, 237–243. [Google Scholar] [CrossRef]
- Johnson, K.T.M.; Dick, H.J.B.; Shimizu, N. Melting in the oceanic upper mantle: An ion microprobe study of diopsides in abyssal peridotites. J. Geophys. Res. Solid Earth 1990, 95, 2661–2678. [Google Scholar] [CrossRef]
- Pearson, N.J.; Alard, O.; Griffin, W.L.; Jackson, S.E.; O’Reilly, S.Y. In situ measurement of Re-Os isotopes in mantle sulfides by laser ablation multicollector-inductively coupled plasma mass spectrometry: Analytical methods and preliminary results. Geochim. Cosmochim. Acta 2002, 66, 1037–1050. [Google Scholar] [CrossRef]
- Griffin, W.L.; O’Reilly, S.Y.; Ryan, C.G. The composition and origin of sub-continental lithospheric mantle. In Mantle Petrology: Field Observations and High Pressure Experimentation: A Tribute to Francis R. (Joe) Boyd; Fei, Y., Bertka, C.M., Mysen, B.O., Eds.; Geochemical Society: Houston, TX, USA, 1999; pp. 13–45. [Google Scholar]
- Zindler, A.; Jagoutz, E. Mantle Cryptology. Geochim. Cosmochim. Acta 1988, 52, 319–333. [Google Scholar] [CrossRef]
- Bodinier, J.L.; Menzies, M.A.; Shimizu, N.; Frey, F.A.; McPherson, E. Silicate, hydrous and carbonate metasomatism at Lherz, France: Contemporaneous derivatives of silicate melt-harzburgite reaction. J. Petrol. 2004, 45, 299–320. [Google Scholar] [CrossRef]
- Gregoire, M.; Bell, D.R.; Le Roex, A.P. Trace element geochemistry of phlogopite-rich mafic mantle xenoliths: Their classification and their relationship to phlogopite-bearing peridotites and kimberlites revisited. Contrib. Mineral. Petrolol. 2002, 142, 603–625. [Google Scholar] [CrossRef]
- Menzies, M.; Hawkesworth, C. Mantle Metasomatism; Academic Press Inc.: Orlando, FL, USA, 1986. [Google Scholar]
- Downes, H. Formation and modification of the shallow sub-continental lithospheric mantle: A review of geochemical evidence from ultramafic xenolith suites and tectonically emplaced ultramafic massifs of western and central Europe. J. Petrol. 2001, 42, 233–250. [Google Scholar] [CrossRef]
- Irvine, G.J.; Pearson, D.G.; Kjarsgaard, B.; Carlson, R.; Kopylova, M.; Dreibus, G. A Re–Os isotope and PGE study of kimberlite-derived peridotite xenoliths from Somerset Island and a comparison to the Slave and Kaapvaal cratons. Lithos 2003, 71, 461–488. [Google Scholar] [CrossRef]
- Wang, K.L.; O’Reilly, S.Y.; Griffin, W.L.; Pearson, N.J.; Zhang, M. Sulfides in mantle peridotites from Penghu Islands, Taiwan: Melt percolation, PGE fractionation, and the lithospheric evolution of the South China block. Geochim. Cosmochim. Acta 2009, 73, 4531–4557. [Google Scholar] [CrossRef]
- Reisberg, L.; Meisel, T. The Re-Os isotopic system: A review of analytical techniques. Geostand. Newsl. 2002, 26, 249–267. [Google Scholar] [CrossRef]
- Pearson, D.G.; Canil, D.; Shirey, S.B. Mantle Samples Included in Volcanic Rocks: Xenoliths and Diamonds. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; pp. 171–275. ISBN 978-0-08-043751-4. [Google Scholar]
- Droop, G.T.R. A general equation for estimating Fe3+ concentrations in ferromagnesian silicates and oxides from microprobe analyses, using stoichiometric criteria. Miner. Mag. 1987, 51, 431–435. [Google Scholar] [CrossRef]
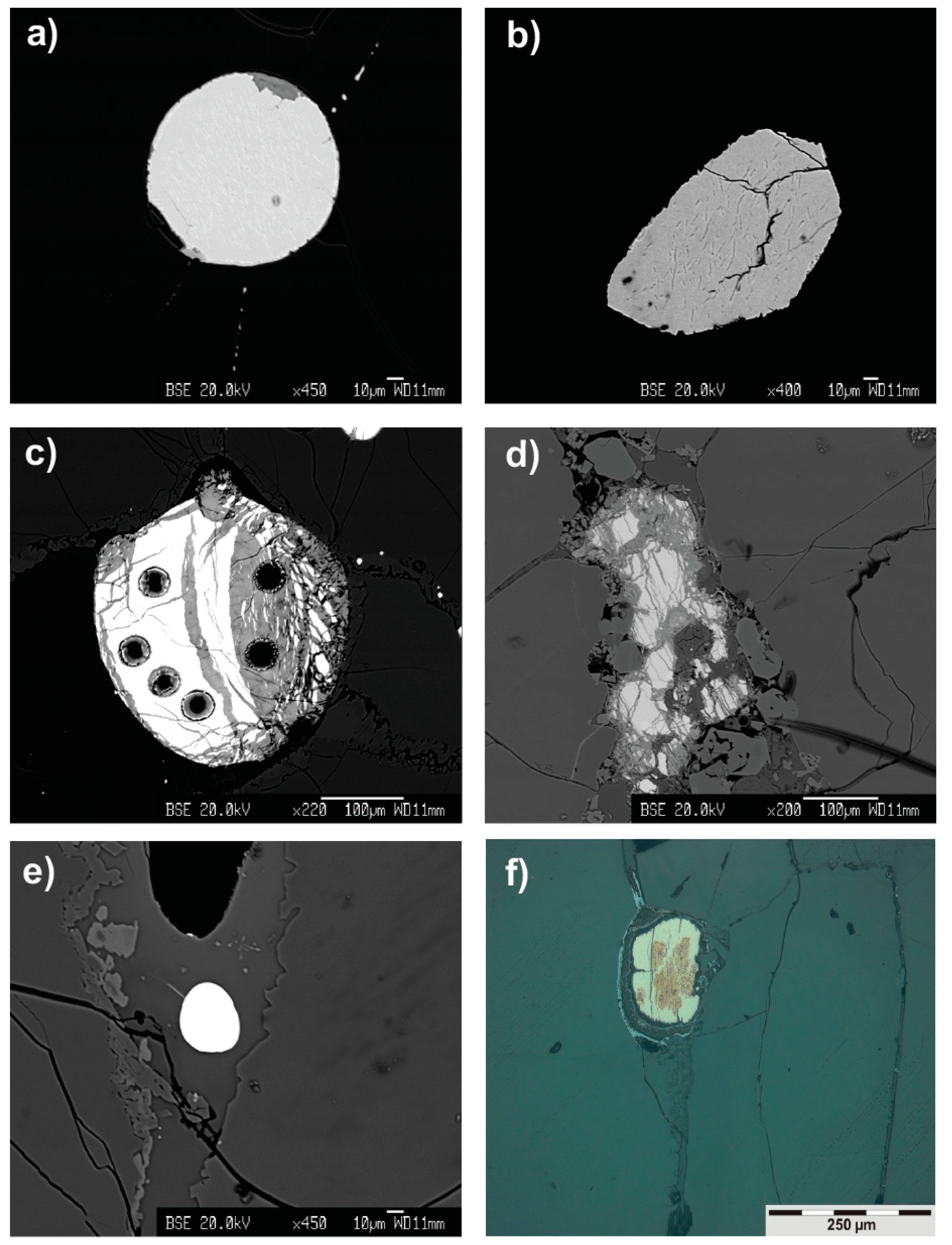
| Sample | Olivine | Orthopyroxene | Clinopyroxene | Spinel | Sulfide | Al2O3 (wt %) |
|---|---|---|---|---|---|---|
| Atl-3A | 70 | 14 | 16 | 0.2 | 0.005 | 2.18 |
| Atl-3B | 78 | 13 | 8 | 0.2 | 0.005 | 1.98 |
| Atl-3C | 67 | 20 | 12 | 0.3 | 0.006 | 2.69 |
| Atl-3E | 65 | 18 | 16 | 0.4 | 0.02 | 2.92 |
| Atl-3F | 72 | 14 | 13 | 0.2 | 0.0001 | 1.99 |
| Atl-3I | 90 | 7 | 3 | 0.1 | 0.002 | 0.72 |
| Atl-3K | 68 | 21 | 11 | 0.3 | 0.004 | 2.36 |
| Atl-3L | 75 | 20 | 4 | 0.1 | - | 1.44 |
| Atl-3T | 57 | 20 | 23 | 0.58 | 0.009 | 4.88 |
| Atl-3U | 77 | 16 | 7 | 0.4 | 0.0001 | 1.87 |
| Atl-3V | 64 | 21 | 14 | 0.5 | 0.004 | 3.11 |
| EMP Analysis # | Type | Cu-Rich Lamellae | mss Lamellae | Associated with pn | Fe | Ni | Cu | S | Total | Fe/S | Fe/Ni | Fe/Cu | Ni/(Ni + Fe)at | M/Sat |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3a-4-15 | e | x | - | - | 45.04 | 16.88 | 0.41 | 37.88 | 100.21 | 1.19 | 2.67 | 109.69 | 0.26 | 0.93 |
| 3a-15-47 | i | - | - | - | 33.03 | 29.16 | 0.11 | 38.39 | 100.69 | 0.86 | 1.13 | 301.82 | 0.46 | 0.91 |
| 3a-18-52 | m | - | - | - | 48.05 | 14.44 | - | 37.84 | 100.39 | 1.27 | 3.33 | - | 0.22 | 0.94 |
| atl-3b-10 | e | - | - | - | 44.00 | 17.45 | - | 37.99 | 99.48 | 1.16 | 2.52 | - | 0.27 | 0.92 |
| 3b-13-103 | i | - | - | x | 59.82 | 2.08 | - | 39.38 | 101.37 | 1.52 | 28.76 | - | 0.03 | 0.90 |
| 3c-2-4 | m | - | - | - | 40.88 | 21.17 | - | 38.06 | 100.11 | 1.07 | 1.93 | - | 0.33 | 0.92 |
| 3c-8-19 | i | - | x | - | 45.42 | 16.86 | 0.11 | 37.61 | 100.00 | 1.21 | 2.69 | 403.25 | 0.26 | 0.94 |
| 3c-10-21 | e | - | - | - | 47.85 | 14.77 | - | 37.42 | 100.10 | 1.28 | 3.24 | - | 0.23 | 0.95 |
| 3e-1-71 | i | - | - | - | 31.31 | 29.30 | - | 38.04 | 98.65 | 0.82 | 1.07 | - | 0.47 | 0.89 |
| 3e-6-85 | e | - | - | - | 42.97 | 19.35 | - | 38.02 | 100.38 | 1.13 | 2.22 | - | 0.30 | 0.93 |
| 3k-5-35 | e | - | x | - | 40.34 | 24.04 | - | 35.28 | 99.72 | 1.14 | 1.68 | - | 0.36 | 1.03 |
| 3k-9-45 | i | - | x | - | 43.33 | 18.68 | - | 37.99 | 100.03 | 1.14 | 2.32 | - | 0.29 | 0.92 |
| 3k-17-49 | m | - | x | - | 41.63 | 19.24 | - | 38.99 | 99.86 | 1.07 | 2.16 | - | 0.31 | 0.88 |
| 3t-4-61 | i | x | - | - | 48.87 | 12.92 | 0.10 | 38.14 | 100.03 | 1.28 | 3.78 | 477.95 | 0.20 | 0.92 |
| 3t-11-77 | m | x | - | - | 38.44 | 22.23 | 0.35 | 38.64 | 99.65 | 0.99 | 1.73 | 111.39 | 0.35 | 0.89 |
| 3t-13-79 | e | - | - | - | 34.73 | 26.42 | 0.21 | 38.78 | 100.13 | 0.90 | 1.31 | 169.18 | 0.42 | 0.89 |
| 3v-1-38 | i | - | - | - | 39.43 | 21.35 | - | 39.81 | 100.58 | 0.99 | 1.85 | - | 0.34 | 0.86 |
| 3v-1-40 | m | - | - | - | 48.68 | 12.31 | 0.75 | 38.47 | 100.22 | 1.27 | 3.96 | 64.56 | 0.19 | 0.91 |
| 3v-3-43 | e | x | - | - | 36.80 | 19.85 | 5.88 | 36.68 | 99.21 | 1.00 | 1.85 | 6.26 | 0.34 | 0.95 |
| ICP-MS Analysis # | Type | Host | Group | Phase | S (wt %) | Ni | Co | Cu | Zn | Mo | Cd | Os | Ir | Ru | Rh |
| A 4-1 | e | ol | 1 | mss | 37.91 | 132,787 | 1432 | 7411 | 11.8 | 20.1 | 1.16 | 23.7 | 19.2 | 41.7 | 5.23 |
| A 4-2 | e | ol | 1 | mss | 37.88 | 138,488 | 1475 | 3806 | - | 4.67 | - | 23.5 | 17.6 | 43.8 | 5.56 |
| A 6 | i | - | 1 | mss | 37.83 | 126,278 | 1571 | 8195 | 8.92 | 1.02 | 0.77 | 4.87 | 3.65 | 11.7 | 1.67 |
| B 11 | e | ol | 1 | mss | 38.14 | 148,896 | 1414 | 317 | 10.9 | 32.1 | - | 4.58 | 3.16 | 8.85 | 1.32 |
| C 9-3 | m | - | 1 | mss | 37.18 | 156,804 | 1562 | 994 | - | 4.94 | - | 10.2 | 7.27 | 15.8 | 2.15 |
| C 9-4 | m | - | 1 | mss | 37.18 | 148,763 | 1523 | 1036 | - | 5.55 | - | 10.1 | 6.2 | 17.6 | 2.19 |
| C 9a | i | - | 1 | mss | - | 184,311 | 1680 | 1131 | - | 12.5 | - | 12.9 | 11.7 | 29.2 | 3.52 |
| K 12-2 | m | - | 1 | mss | 36.71 | 152,741 | 1170 | 3476 | - | 0.819 | - | 5.7 | 4.77 | 12.9 | 1.76 |
| V 10-1 | e | cpx | 1 | mss | 38.39 | 131,970 | 1838 | 1933 | 72.3 | 42.8 | - | 597 | 448 | 1406 | 211 |
| E 5-2 | i | - | 2 | mss | 38.24 | 116,185 | 1719 | 5149 | 26.1 | 1.6 | - | 12.6 | 11.9 | 21.7 | 2.99 |
| E 5-3 | i | - | 2 | mss | 38.00 | 111,350 | 1723 | 3163 | 23.4 | 1.19 | 0.884 | 12.4 | 11.6 | 19.5 | 3 |
| E 5-4 | i | - | 2 | mss | 38.00 | 113,874 | 1776 | 11,511 | 27.5 | 1.08 | 0.818 | 14.6 | 12.9 | 21.9 | 3.01 |
| E 5-5 | i | - | 2 | mss | 38.00 | 102,412 | 1837 | 3272 | 20.5 | 0.771 | 0.752 | 11.8 | 10.7 | 18.4 | 2.36 |
| E 5-6 | i | - | 2 | mss | 38.24 | 121,258 | 1765 | 5681 | 25.2 | 1.72 | 1.06 | 13.8 | 12.8 | 22.2 | 2.86 |
| E 14-1 | i | - | 2 | mss | 38.10 | 120,004 | 1926 | 5008 | 16.7 | 4.03 | 0.969 | 2.34 | 1.04 | 6.35 | 0.326 |
| E 14-2 | i | - | 2 | mss | 38.10 | 113,326 | 1795 | 7759 | 21.7 | 1.25 | 0.632 | 1.42 | 0.974 | 4.97 | 0.376 |
| E 14-3 | i | - | 2 | mss | 38.10 | 111,828 | 1789 | 7891 | 25.9 | 6.73 | 0.546 | 1.98 | 1.06 | 5.99 | 3.87 |
| E 14-4 | i | - | 2 | mss | 37.80 | 121,696 | 1978 | 5673 | 23.8 | 1.62 | - | 1.81 | 1.11 | 6.68 | 0.411 |
| K 9-1 | i | - | 2 | mss | 37.99 | 173,185 | 1397 | 1643 | - | 1.16 | 1.36 | 4.41 | 2.85 | 8.76 | 0.958 |
| K 9-2 | i | - | 2 | mss | 37.99 | 207,507 | 1545 | 6837 | - | 5.67 | 1.81 | 6.4 | 5.67 | 13.1 | 1.8 |
| T 3-1 | i | - | 2 | mss | 38.01 | 110,122 | 1591 | 11,382 | 12.7 | 29.2 | 0.99 | 5.89 | 4.16 | 9.35 | 1.96 |
| T 3-2 | i | - | 2 | mss | 38.01 | 112,494 | 1629 | 12,074 | 13.1 | 36 | 0.988 | 7.27 | 5.39 | 11.9 | 1.93 |
| T 3-3 | i | - | 2 | mss | 38.01 | 108,393 | 1627 | 10,966 | 18.4 | 31 | - | 6.01 | 4.91 | 8.82 | 1.86 |
| T 3-7 | i | - | 2 | mss | 38.01 | 125,898 | 1856 | 3111 | 31.9 | 266 | 1.19 | 9.41 | 5.86 | 16.1 | 1.98 |
| T 4-1 | m | - | 2 | mss | 38.14 | 110,765 | 1688 | 10,773 | 15.3 | 12.1 | 1.14 | 12.4 | 9.29 | 24.2 | 2.9 |
| T 4-2 | m | - | 2 | mss | 38.14 | 120,113 | 1737 | 6687 | 8.34 | 21 | 0.611 | 11.8 | 9.17 | 24 | 3.3 |
| T 4-4 | m | - | 2 | mss | - | 130,452 | 1900 | 3989 | 15.3 | 36.6 | 1.61 | 13.5 | 10.4 | 24.1 | 3.62 |
| T7-3 | m | - | 2 | mss | 38.00 | 111,383 | 1837 | 6944 | 7.13 | 4.05 | - | 23.3 | 17.2 | 52.4 | 6.92 |
| E5-7 | i | - | 2 | hy | 0.26 | 2991 | 14.3 | 83.1 | 1.75 | 0.075 | - | 0.373 | 0.781 | 1.24 | 0.198 |
| E5-8 | i | - | 2 | hy | 0.44 | 2808 | 12.8 | 46.1 | 0.616 | 0.052 | - | 0.34 | 0.538 | 0.841 | 0.115 |
| T3-4 | i | - | 2 | hy | 0.29 | 26,251 | 94.3 | 613 | 10.9 | 12.6 | 0.116 | 2.47 | 2.39 | 4.41 | 0.689 |
| T3-5 | i | - | 2 | hy | 0.30 | 46,564 | 373 | 937 | 19.4 | 19.4 | 0.219 | 0.729 | 3.67 | 7.92 | 0.986 |
| T3-6 | i | - | 2 | hy | 3.10 | 10,402 | 53.9 | 368 | 6.87 | 38.3 | 0.114 | 1.1 | 1.4 | 3.03 | 0.403 |
| T4-3 | m | - | 2 | hy | 0.28 | 19,758 | 63.6 | 423 | 12 | 3.66 | 0.261 | 1.07 | 3.34 | 8.61 | 1.12 |
| T4-5 | m | - | 2 | hy | 0.35 | 60,688 | 228 | 5067 | 18.9 | 11.4 | - | 12.9 | 8.91 | 21.1 | 2.9 |
| C 8a-2 | i | - | 3 | mss | 38.26 | 146,337 | 1714 | 13,717 | 49.8 | 55.4 | - | 0.475 | 0.482 | 1.8 | 0.304 |
| E6 | e | opx | 3 | mss | 38.02 | 157,471 | 1745 | 781 | 11.6 | 2.12 | - | 0.344 | 0.32 | 1.11 | 0.178 |
| E 14-6 | i | - | 3 | mss | - | 192,002 | 2671 | 1019 | 11.1 | 55.7 | 0.558 | 1.13 | 0.333 | 1.69 | 0.176 |
| E 16-2 | i | - | 3 | mss | 38.67 | 222,492 | 1476 | 799 | 25.4 | 286 | - | 8.38 | 5.92 | 11.1 | 1.95 |
| T 2-1 | e | - | 3 | mss | 38.66 | 153,138 | 1774 | 2455 | 9.88 | 71.4 | 1.52 | 3.95 | 3.24 | 4.43 | 0.979 |
| T 11-1 | m | - | 3 | mss | 38.54 | 156,006 | 1411 | 11,022 | - | 2.66 | 0.608 | 2.45 | 1.14 | 3.74 | 0.572 |
| V 3-1 | m | - | 3 | mss | 36.68 | 173,357 | 1916 | 6955 | - | 0.695 | 0.368 | 0.906 | 0.354 | 0.531 | 0.134 |
| V 12 | e | cpx | 3 | mss | 37.66 | 132,842 | 1855 | 1193 | - | 3.32 | - | 5.46 | 3.75 | 14 | 2.27 |
| V 15-2 | m | - | 3 | mss | 37.82 | 189,784 | 1330 | 646 | - | 6.63 | - | 1.31 | 0.88 | 2.81 | 0.312 |
| V 15-3 | m | - | 3 | mss | 38.82 | 197,724 | 1378 | 521 | - | 23.1 | - | 0.892 | 1.09 | 3.52 | 0.358 |
| E 16-1 | i | - | 4 | mss | 38.67 | 219,155 | 1457 | 421 | - | 22.5 | - | 5.94 | 5.38 | 7.02 | 1.23 |
| T 1 | m | - | 4 | mss | 38.52 | 200,702 | 1228 | 1027 | 19.1 | 77.8 | - | 1.43 | 2.48 | 4.28 | 1.22 |
| T 2-2 | e | opx | 4 | mss | 38.66 | 135,203 | 1574 | 2372 | - | 50.3 | 1.95 | 1.71 | 1.81 | 3.65 | 0.886 |
| T 2-3 | e | opx | 4 | mss | 38.06 | 140,359 | 1609 | 2453 | - | 12.6 | - | 0.643 | 1 | 2.29 | 0.622 |
| V 3-2 | m | - | 4 | mss | 36.68 | 162,919 | 1550 | 6388 | - | - | 0.908 | 0.457 | 0.236 | 0.673 | 0.229 |
| V 15-1 | m | - | 4 | mss | 37.82 | 202,685 | 1396 | 954 | 10.8 | 2.6 | - | 0.209 | 0.338 | 1.16 | 0.288 |
| B 14 | m | - | 4 | pn | 32.28 | 237,046 | 1384 | 3410 | 13.9 | 1.48 | - | 1.81 | 1.04 | 2.25 | 1.14 |
| B 6 | e | ol | 5 | mss | 37.14 | 136,753 | 1227 | 27,885 | 52.7 | 25.6 | 2.14 | - | - | - | 0.465 |
| B 7 | e | ol | 5 | mss | 36.23 | 116,239 | 994 | 52,535 | 102 | 22.1 | 3.01 | - | - | - | 0.864 |
| C8b-1 | i | - | 5 | mss | 38.26 | 157,668 | 1545 | 52,344 | 58.8 | 26.4 | 4.01 | 0.625 | - | - | 0.58 |
| C8b-2 | i | - | 5 | mss | 38.26 | 133,601 | 1398 | 579 | - | 11.3 | - | - | - | - | - |
| C8b-3 | i | - | 5 | mss | 38.26 | 128,418 | 1452 | 1193 | - | 14.6 | - | - | - | - | - |
| E 11 | e | ol | 5 | mss | 38.60 | 128,640 | 1729 | 623 | 6.14 | 35.5 | - | - | 0.109 | 0.263 | 0.051 |
| V 1 | m | - | 5 | mss | 38.47 | 125,435 | 1611 | 4448 | 14.4 | 0.896 | 1.32 | - | - | - | 0.089 |
| V 4 | m | - | 5 | mss | 37.26 | 148,483 | 1410 | 2426 | 8.45 | 30.8 | 1.19 | - | - | - | 0.113 |
| V 5-1 | e | opx | 5 | mss | 38.30 | 111,711 | 1446 | 3370 | - | 4.93 | 1.22 | - | - | 0.471 | 0.084 |
| V 5-2 | e | opx | 5 | mss | 38.30 | 134,576 | 1646 | 3755 | - | 6.44 | 0.898 | - | 0.128 | - | 0.069 |
| V5-3 | e | ol | 5 | mss | - | 128,530 | 1859 | 6665 | - | 1.41 | 0.6 | - | 0.047 | 0.307 | 0.063 |
| V5-5 | e | opx | 5 | mss | 37.55 | 131,418 | 1818 | 7605 | - | 10.9 | - | - | - | - | 0.053 |
| V 13-1 | e | ol | 5 | mss | 38.11 | 118,863 | 2240 | 9146 | 107 | 120 | - | - | - | - | 0.1 |
| B 2 | i | - | 5 | pn | 32.82 | 240,810 | 1220 | 4719 | - | 3.62 | 2.29 | - | - | - | 0.102 |
| B 4 | i | - | 5 | pn | 33.26 | 222,135 | 1229 | 13,538 | - | 7.35 | 2.12 | 0.422 | - | - | 0.226 |
| B 5 | i | - | 5 | pn | 32.89 | 229,773 | 1223 | 9954 | - | 4.08 | 4.06 | - | - | 0.459 | 0.223 |
| I 3-1 | e | ol | 5 | mss + iss | - | 188,046 | 1461 | 31,278 | 101 | 17.7 | 5.8 | - | - | - | 0.573 |
| ICP-MS # | Pt | Pd | Re | Au | Ag | Pb | As | Sb | Sn | Se | Te | Ni/(Ni + Fe)at | M/Sat | [Pd/Ir]N | [Pt/Pd]N |
| A 4-1 | 19.5 | 10.5 | 0.309 | 0.417 | 0.469 | 3.64 | 9.57 | - | 0.952 | 41.9 | 4.16 | 0.26 | 0.94 | 0.45 | 1.01 |
| A 4-2 | 20.5 | 10.6 | 0.253 | 0.433 | 0.082 | 3.63 | 9.65 | - | - | 40 | 5.58 | 0.26 | 0.93 | 0.50 | 1.05 |
| A 6 | 2.55 | 1.26 | 0.226 | 0.351 | 0.123 | 1.03 | 8.57 | - | - | 47.4 | 2.1 | 0.23 | 0.93 | 0.29 | 1.10 |
| B 11 | 3.89 | 1.6 | 0.244 | - | 0.234 | 7.93 | 7.47 | 0.747 | - | 36.2 | 7.95 | 0.28 | 0.9 | 0.42 | 1.32 |
| C 9-3 | 3.7 | 0.614 | 0.313 | 0.455 | 0.605 | 3.07 | - | - | - | 64.5 | - | 0.26 | 0.96 | 0.07 | 3.28 |
| C 9-4 | 5.83 | 2.09 | 0.361 | 1.7 | 7.66 | 2.24 | - | - | - | 59.2 | - | 0.26 | 0.96 | 0.28 | 1.52 |
| C 9a | 2.74 | 0 | 0.245 | - | 3.1 | 14.2 | - | - | - | 79.8 | - | - | - | - | - |
| K 12-2 | 11.8 | 3.38 | - | 0.561 | - | 12.8 | - | - | - | 43.1 | 6.34 | 0.37 | 0.99 | 0.59 | 1.90 |
| V 10-1 | 113 | 36.8 | 0.222 | - | 7.33 | 140 | 8.81 | 1.47 | - | 71.4 | 6.92 | 0.25 | 0.88 | 0.07 | 1.67 |
| E 5-2 | 2.48 | 2.3 | - | - | 0.075 | 2.17 | 6.35 | - | - | 35.3 | - | 0.22 | 0.92 | 0.16 | 0.59 |
| E 5-3 | 2.5 | 3.42 | 0.263 | 0.073 | 0.134 | 1.56 | 5.22 | - | - | 38.5 | - | 0.22 | 0.92 | 0.24 | 0.40 |
| E 5-4 | 3 | 3.43 | 0.236 | 0.049 | 0.192 | 0.521 | 4.29 | - | - | 36.9 | - | 0.22 | 0.92 | 0.22 | 0.48 |
| E 5-5 | 2.82 | 2.93 | 0.133 | - | 0.121 | 0.844 | 7.36 | - | - | 21.9 | - | 0.22 | 0.92 | 0.23 | 0.52 |
| E 5-6 | 2.57 | 2.78 | 0.214 | - | - | 0.632 | 4.47 | - | - | 42 | 1.62 | 0.22 | 0.92 | 0.18 | 0.50 |
| E 14-1 | 0.322 | 0.461 | 0.366 | - | - | 3.54 | 7.88 | - | 0.903 | 26.8 | - | 0.22 | 0.93 | 0.37 | 0.38 |
| E 14-2 | 0.535 | 0.468 | 0.321 | - | 0.06 | 1.33 | 6.63 | - | - | 35.3 | - | 0.22 | 0.93 | 0.40 | 0.62 |
| E 14-3 | 0.515 | 0.525 | 0.189 | - | 0.253 | 0.838 | 5.17 | - | - | 40.5 | 1.37 | 0.22 | 0.93 | 0.41 | 0.53 |
| E 14-4 | 0.458 | 0.381 | 0.257 | 0.051 | - | 0.625 | 5.54 | - | - | 32.9 | - | 0.2 | 0.93 | 0.28 | 0.65 |
| K 9-1 | 4.48 | 3.22 | 0.285 | 0.722 | 0.269 | 3.9 | 12.1 | - | 1.48 | 68.5 | - | 0.37 | 0.99 | 0.93 | 0.76 |
| K 9-2 | 9.77 | 8.31 | 0.553 | 0.823 | 6.43 | 5.61 | 11.4 | - | - | 54.1 | 8.84 | 0.37 | 0.99 | 1.21 | 0.64 |
| T 3-1 | 1.46 | 1.87 | 0.241 | 0.075 | 0.369 | 0.82 | 8.04 | - | - | 68.6 | 3.75 | 0.22 | 0.93 | 0.37 | 0.43 |
| T 3-2 | 1.57 | 1.36 | 0.312 | 0.13 | 0.43 | 0.42 | 8.71 | - | - | 77.9 | 2.52 | 0.22 | 0.93 | 0.21 | 0.63 |
| T 3-3 | 1.74 | 1.66 | 0.416 | 0.167 | 0.288 | 0.288 | 5.69 | - | 0.872 | 62.9 | 2.33 | 0.22 | 0.93 | 0.28 | 0.57 |
| T 3-7 | 1.48 | 2.28 | 0.353 | - | - | 0.683 | 27.9 | 0.254 | - | 70.7 | - | 0.22 | 0.93 | 0.32 | 0.35 |
| T 4-1 | 4.31 | 4.14 | 0.257 | 0.286 | - | 0.548 | 9.11 | - | 0.894 | 68.5 | 3.23 | 0.2 | 0.92 | 0.37 | 0.57 |
| T 4-2 | 4.56 | 5.05 | 0.137 | 0.346 | 0.194 | 0.972 | 5.03 | - | - | 72.3 | - | 0.2 | 0.92 | 0.46 | 0.49 |
| T 4-4 | 2.21 | 3.18 | 0.4 | 0.161 | 0.228 | 5.41 | 5.51 | 0.17 | - | 68.5 | - | - | - | 0.25 | 0.38 |
| T7-3 | 5.54 | 5.76 | 0.375 | 0.06 | 0.095 | 0.354 | 7.25 | - | - | 68.4 | - | 0.19 | 0.92 | 0.28 | 0.52 |
| E5-7 | 0.151 | 0.082 | - | - | - | 0.302 | 0.234 | - | - | 0.793 | - | - | - | 0.09 | 1.00 |
| E5-8 | 0.096 | 0.115 | - | - | - | 0.181 | - | - | - | 0.791 | - | - | - | 0.18 | 0.45 |
| T3-4 | 0.737 | 0.651 | 0.013 | - | 0.068 | 0.805 | 1.02 | - | - | 11.7 | 0.537 | - | - | 0.23 | 0.62 |
| T3-5 | 1.02 | 0.786 | - | 0.062 | 0.043 | 1.15 | 3 | - | - | 13.1 | 0.453 | - | - | 0.18 | 0.71 |
| T3-6 | 0.329 | 0.282 | - | - | 0.054 | 0.441 | 2.07 | - | - | 1.87 | - | - | - | 0.17 | 0.64 |
| T4-3 | 1.41 | 1.19 | 0.012 | 0.079 | 0.024 | 1.16 | 1.02 | - | - | 10.1 | 0.4 | - | - | 0.29 | 0.65 |
| T4-5 | 2.43 | 1.68 | - | 0.175 | 0.048 | 0.265 | - | 0.08 | - | 38.8 | 0.757 | - | - | 0.16 | 0.79 |
| C 8a-2 | 0.823 | 0.986 | 0.349 | 0.743 | 5.99 | 15.2 | 6.06 | - | - | 54.9 | 5.46 | 0.25 | 0.92 | 1.69 | 0.45 |
| E6 | 0.892 | 0.553 | - | 0.105 | - | 3.37 | 10.5 | - | 1.19 | 25.3 | - | 0.3 | 0.93 | 1.43 | 0.88 |
| E 14-6 | 1.93 | 1.76 | 0.207 | - | 0.154 | 10.2 | 6.28 | - | 0.911 | 52.2 | 3.64 | - | - | 4.37 | 0.60 |
| E 16-2 | 18 | 14 | 0.172 | 0.891 | - | 71.5 | 66.4 | 0.467 | - | 67.7 | 7.28 | 0.42 | 0.9 | 1.96 | 0.70 |
| T 2-1 | 8 | 5.99 | 0.25 | 1.15 | 0.778 | 31.2 | 9.06 | 0.884 | - | 77.1 | 6.51 | 0.27 | 0.9 | 1.53 | 0.73 |
| T 11-1 | 3.91 | 4.61 | 0.064 | 0.703 | - | 0.697 | 9.25 | - | - | 64.6 | - | 0.35 | 0.89 | 3.35 | 0.46 |
| V 3-1 | 1.76 | 2.38 | - | 0.911 | 0.288 | 2.34 | 11.3 | - | - | 49.3 | 0.886 | 0.34 | 0.95 | 5.56 | 0.40 |
| V 12 | 11.2 | 15.9 | 0.34 | 0.079 | 0.512 | 3.23 | 13.2 | - | 0.586 | 56.2 | 6.37 | 0.26 | 0.93 | 3.51 | 0.38 |
| V 15-2 | 1.95 | 1.77 | - | 0.474 | 5.07 | 1.32 | 12.6 | - | - | 51.1 | 2.74 | 0.37 | 0.92 | 1.66 | 0.60 |
| V 15-3 | 2.05 | 1.35 | - | 0.328 | 0.272 | 2.93 | 7.33 | - | - | 42.2 | - | 0.37 | 0.92 | 1.02 | 0.83 |
| E 16-1 | 16.7 | 12.9 | 0.196 | 0.217 | - | 25.7 | 29.6 | 0.314 | - | 56.4 | 15.9 | 0.42 | 0.9 | 1.98 | 0.70 |
| T 1 | 8.26 | 12.6 | - | 0.188 | 1.24 | 29.9 | 13 | 0.406 | - | 129 | 6.02 | 0.41 | 0.9 | 4.20 | 0.36 |
| T 2-2 | 5.58 | 4.64 | 0.27 | 0.468 | 0.204 | 6.04 | 10.4 | - | - | 75.4 | - | 0.26 | 0.92 | 2.12 | 0.65 |
| T 2-3 | 5.47 | 5.07 | 0.296 | 0.187 | 0.07 | 1.79 | 10.9 | - | - | 83.9 | 2.65 | 0.26 | 0.92 | 4.19 | 0.59 |
| V 3-2 | 1.92 | 2.57 | - | 0.73 | 0.088 | 2.63 | 9.87 | - | - | 54.2 | - | 0.34 | 0.95 | 9.01 | 0.41 |
| V 15-1 | 1.44 | 2.84 | - | 0.44 | 0.541 | 2.79 | 7.94 | - | - | 30.4 | - | 0.37 | 0.92 | 6.95 | 0.28 |
| B 14 | 5.53 | 10.5 | - | - | 7.85 | 24.1 | 9.97 | 1.36 | - | 129 | 33.1 | 0.46 | 1.16 | 8.35 | 0.29 |
| B 6 | 6.13 | 6.22 | 0.296 | 1.19 | 4.97 | 7.77 | 12.6 | - | - | 57.8 | 10.1 | 0.23 | 0.93 | - | 0.54 |
| B 7 | 4.71 | 6.66 | 0.204 | 1.27 | 5.95 | 9.96 | 11 | - | - | 61.7 | 7.11 | 0.23 | 0.93 | - | 0.39 |
| C8b-1 | 0.276 | 0.573 | 0.258 | 0.807 | 11.1 | 9.53 | - | - | - | 64.4 | 10 | 0.26 | 0.94 | - | 0.26 |
| C8b-2 | 0.356 | 0 | 0.324 | 0.804 | 0.44 | 2.66 | - | - | - | 65.3 | 4.51 | 0.26 | 0.94 | - | - |
| C8b-3 | 0 | 0 | 0.241 | 0.711 | 0.2 | 6.88 | 10.9 | - | - | 59.7 | 8.82 | 0.26 | 0.94 | - | - |
| E 11 | 1.7 | 2.05 | - | - | - | 26.2 | 10.7 | - | - | 56.6 | 4.52 | 0.24 | 0.9 | 15.56 | 0.45 |
| V 1 | 1 | 4.81 | - | 0.184 | 0.264 | 1.24 | 5.89 | - | - | 56.3 | - | 0.19 | 0.91 | - | 0.11 |
| V 4 | 0.629 | 1.98 | - | 0.303 | 3.63 | 30.9 | 11.9 | - | 1.48 | 97.1 | 5.94 | 0.3 | 0.95 | - | 0.17 |
| V 5-1 | 2.09 | 5.2 | 0.386 | - | 1.04 | 2.63 | 11.1 | - | - | 71.6 | 2.45 | 0.25 | 0.91 | - | 0.22 |
| V 5-2 | 2.27 | 4.99 | 0.365 | 0.159 | 0.459 | 3.59 | 6.66 | - | - | 50 | 2.91 | 0.25 | 0.92 | 32.25 | 0.25 |
| V5-3 | 0.339 | 1.16 | 0.212 | 0.09 | 0.214 | 4.7 | 9.88 | - | - | 52 | 2.24 | - | - | 20.42 | 0.16 |
| V5-5 | 0 | 1.99 | 0.234 | - | 0.229 | 2.65 | 5.31 | - | - | 57.2 | 1.46 | 0.25 | 0.94 | - | - |
| V 13-1 | 0.223 | 0.939 | 0.119 | 0.11 | 1.46 | 10.2 | 22.3 | 0.55 | 0.819 | 50.4 | 1.76 | 0.2 | 0.91 | - | 0.13 |
| B 2 | 2.71 | 9.81 | - | 0.157 | 9.91 | 18 | 20.4 | 1.07 | - | 104 | 20.1 | 0.45 | 1.14 | - | 0.15 |
| B 4 | 2.9 | 9.02 | 0.5 | - | 13.4 | 15.7 | - | 0.895 | - | 102 | 21.2 | 0.39 | 1.13 | - | 0.18 |
| B 5 | 2.31 | 10.2 | - | - | 12.1 | 29.1 | 18.6 | 0.889 | - | 65.3 | 18 | 0.45 | 1.15 | - | 0.12 |
| I 3-1 | 0 | 16.6 | 0.226 | 1.82 | 105 | 1196 | 24.1 | 5.11 | 9.01 | 9.97 | 13.5 | - | - | - | - |
| EMP # | 3a-1-3 | 3a-2-7 | 3e-5-4 | 3k-13-10 | 3t-8-77 | 3t-11-81 | 3b-5a-70 | 3k-9-30 |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 24.80 | 22.74 | 10.56 | 6.83 | 15.39 | 25.47 | 0.20 | 1.35 |
| Al2O3 | 3.00 | 0.36 | 0.08 | 2.74 | 5.66 | 3.48 | 0.06 | 0.82 |
| Cr2O3 | - | 0.13 | 0.10 | - | - | - | - | - |
| Fe2O3 | 48.26 | 63.22 | 58.07 | 39.11 | 66.51 | 17.50 | 86.74 | 77.40 |
| MgO | 0.39 | 0.13 | - | 0.95 | 1.53 | 4.74 | - | - |
| CaO | 4.28 | 4.21 | 1.45 | 0.86 | 1.59 | 0.82 | 0.07 | 0.11 |
| NiO | 6.25 | 1.47 | 15.92 | 25.75 | 1.99 | 32.27 | 5.58 | 2.27 |
| CuO | 0.14 | 0.11 | 0.24 | 3.09 | 0.43 | 1.64 | - | - |
| S | - | - | 0.34 | 0.20 | 0.18 | - | 0.13 | 0.23 |
| Cl | - | - | 0.11 | 0.30 | 0.15 | 0.25 | 0.76 | 0.47 |
| Total | 87.12 | 92.37 | 86.87 | 79.83 | 93.43 | 86.17 | 93.54 | 82.65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westner, K.J.; Beier, C.; Klemd, R.; Osbahr, I.; Brooks, N. In Situ Chalcophile and Siderophile Element Behavior in Sulfides from Moroccan Middle Atlas Spinel Peridotite Xenoliths during Metasomatism and Weathering. Minerals 2019, 9, 276. https://doi.org/10.3390/min9050276
Westner KJ, Beier C, Klemd R, Osbahr I, Brooks N. In Situ Chalcophile and Siderophile Element Behavior in Sulfides from Moroccan Middle Atlas Spinel Peridotite Xenoliths during Metasomatism and Weathering. Minerals. 2019; 9(5):276. https://doi.org/10.3390/min9050276
Chicago/Turabian StyleWestner, Katrin J., Christoph Beier, Reiner Klemd, Inga Osbahr, and Nadine Brooks. 2019. "In Situ Chalcophile and Siderophile Element Behavior in Sulfides from Moroccan Middle Atlas Spinel Peridotite Xenoliths during Metasomatism and Weathering" Minerals 9, no. 5: 276. https://doi.org/10.3390/min9050276
APA StyleWestner, K. J., Beier, C., Klemd, R., Osbahr, I., & Brooks, N. (2019). In Situ Chalcophile and Siderophile Element Behavior in Sulfides from Moroccan Middle Atlas Spinel Peridotite Xenoliths during Metasomatism and Weathering. Minerals, 9(5), 276. https://doi.org/10.3390/min9050276





