3.2. Time-Recovery and Grade-Recovery Curves
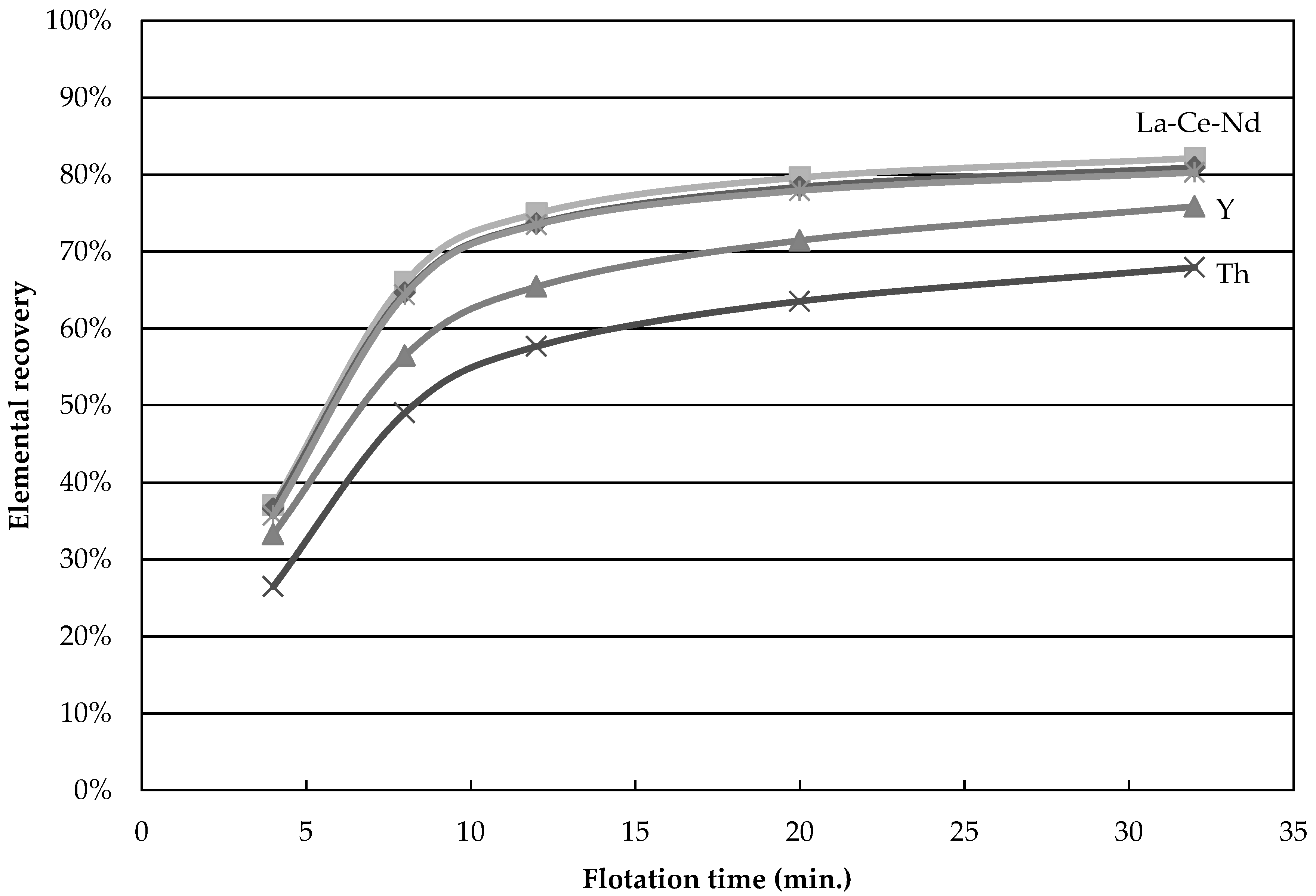
The recovery of Nd, La, Y, and Th as a function of time for test 4 is shown in
Figure 1. Nd and La nearly fall on the same curve while Y and Th behave differently, indicating that La and Nd are carried by the same REE minerals in the same proportions, while Y and Th are distributed among minerals that respond differently to flotation. This result is a clear indication that more than one element should be considered when a concentration process is studied for a REE ore. Indeed, incorrect conclusions would have been drawn if yttrium was used here to assess the flotation performances. Ideally one should always look at the mineral behavior in the concentration process, as done below.
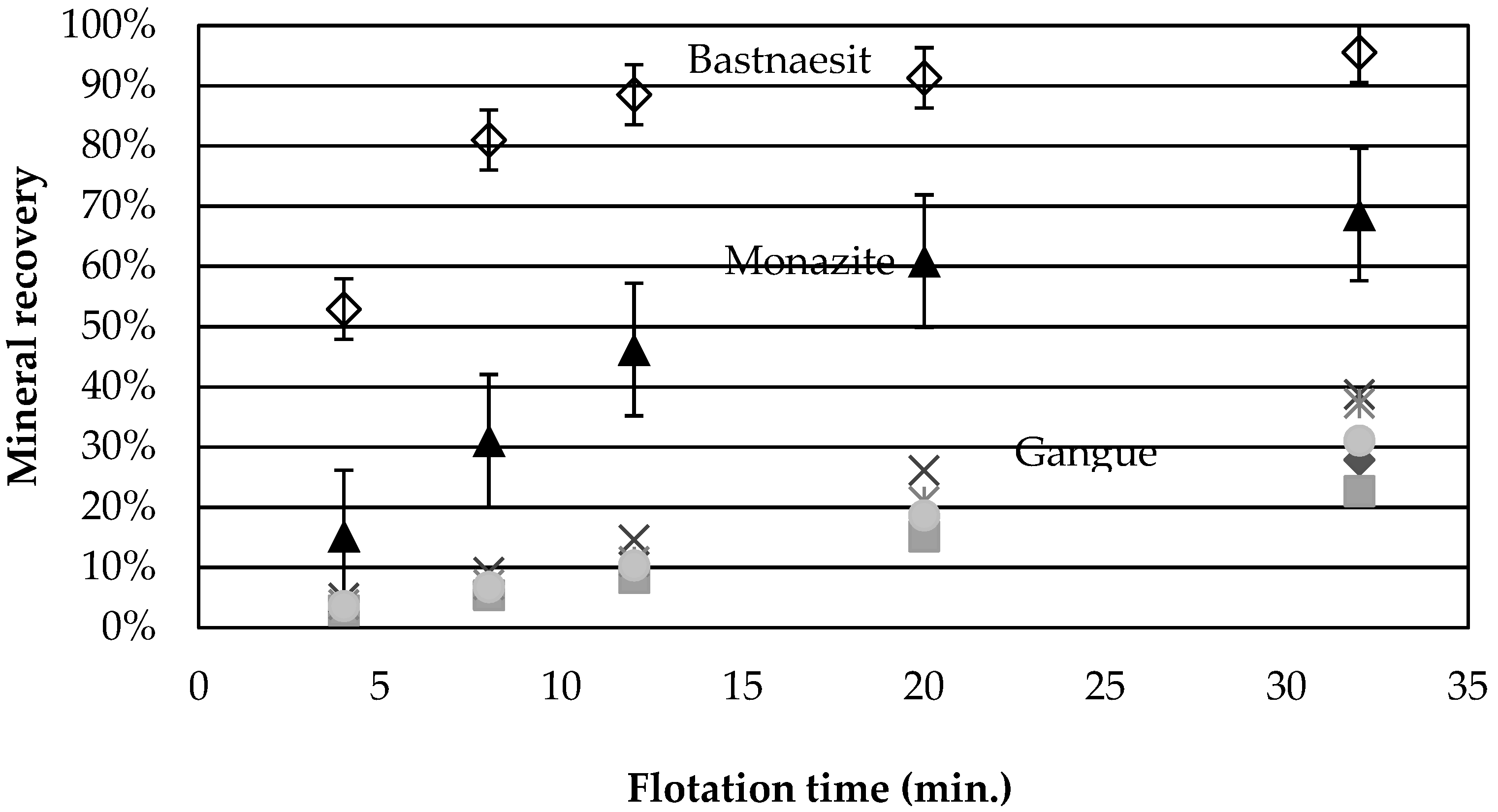
Figure 2 presents the average cumulative mineral recoveries as a function of time for tests 1, 7, and 10, the central points. The recovery is greater for bastnaesite than for monazite, a behaviour observed for all experiments. The faster flotation rate of bastnaesite over monazite was also observed for the Bayan Obo ore [
9], and this behaviour was attributed to the better adsorption of the collector onto the bastnaesite surface than onto the monazite. In the present case, the behaviour is also attributed to the liberation characteristics of the ore. According to QEMScan results, at a
d80 of 120 µm, 47 wt % of bastnaesite is free or liberated compared to only 27 wt % for monazite. Monazite grains are also smaller, with a
d50 of 19 µm compared to 25 µm for bastnaesite, which can further decrease the recovery kinetics. Depression of the monazite by calcium ions [
23] is another phenomenon which may be at play in this complex system.
None of the gangue minerals in
Figure 2 appear to follow a 1st order recovery rate. Calcite and Fe-oxide recoveries are greater than those for chlorite and dolomite. The same behaviour is obtained for all the tests.
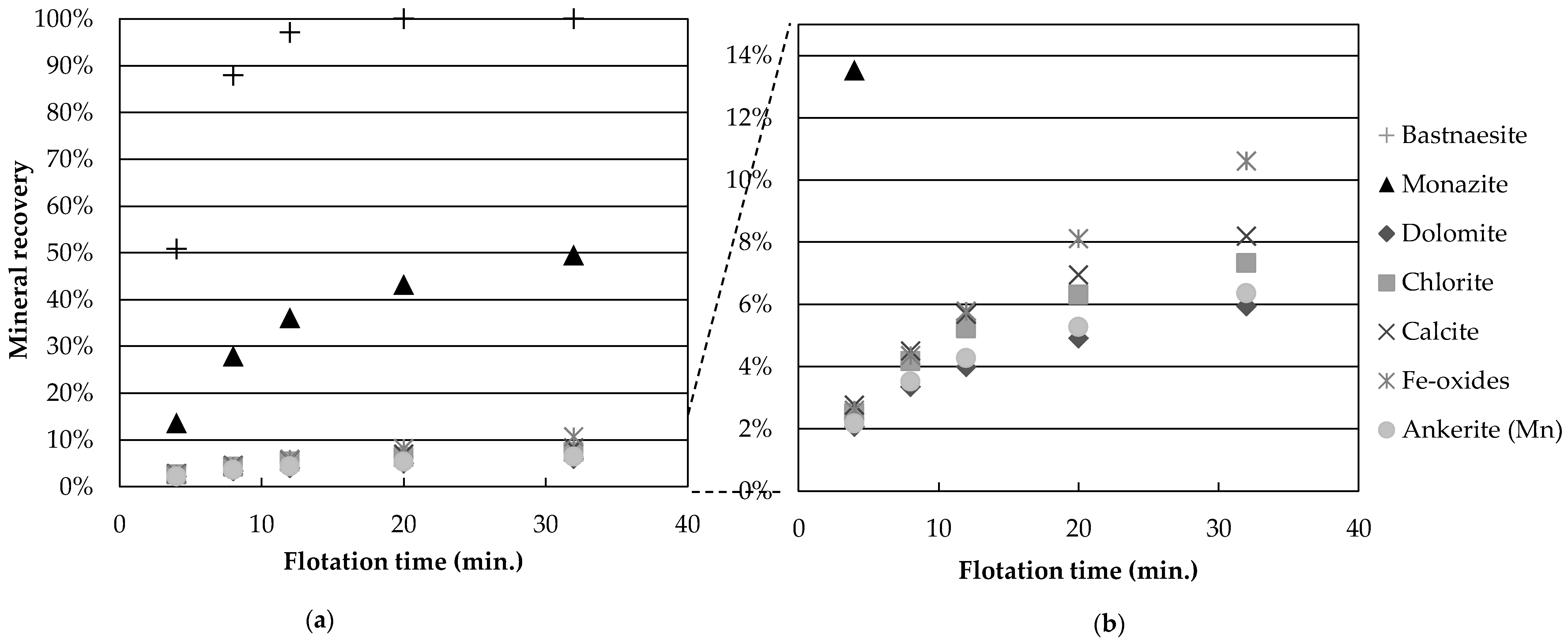
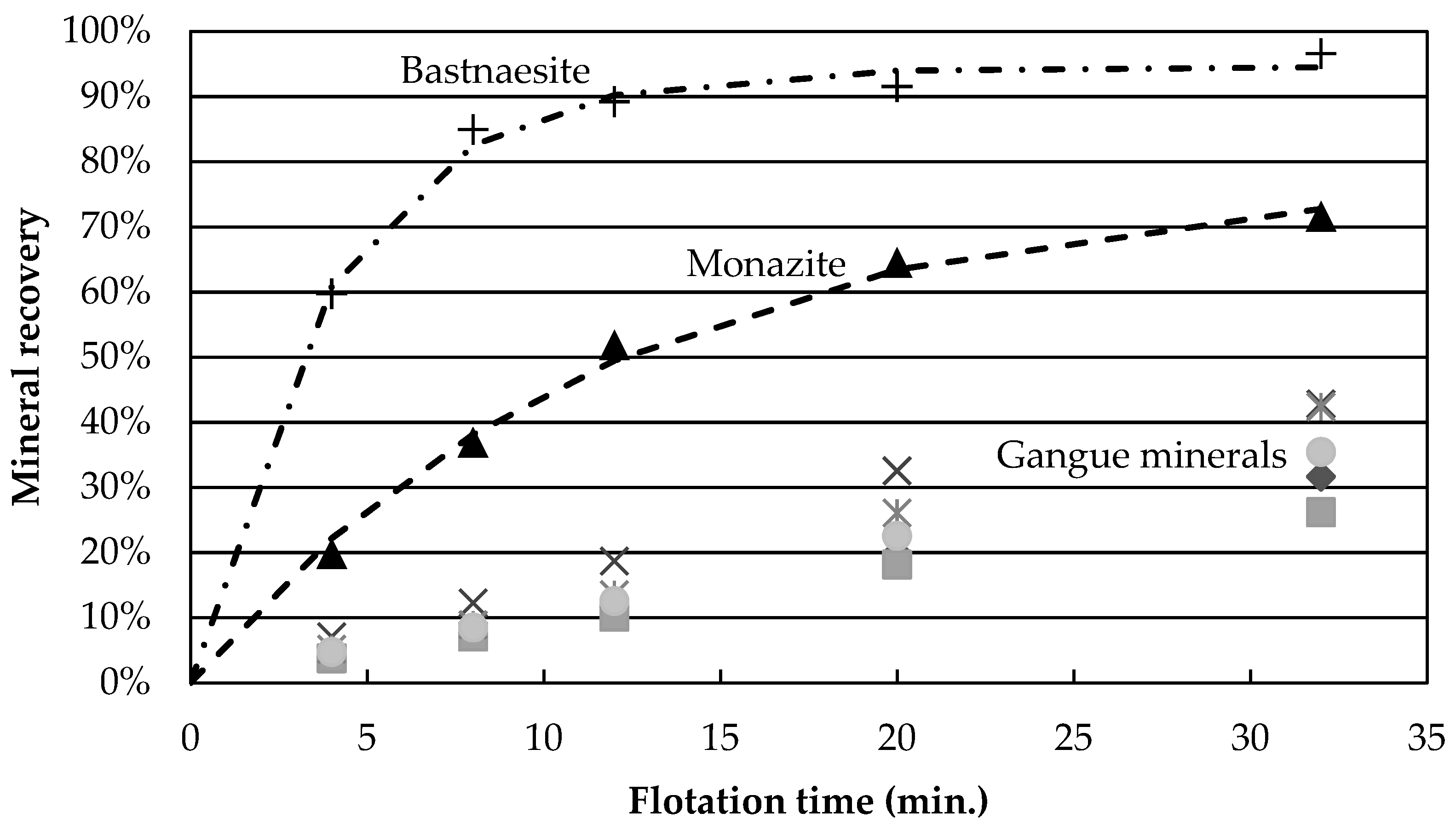
Figure 3 presents the mineral recoveries as a function of time for test 4 (high temperature, high Na
2SiO
3, no guar gum). The recovery of all gangue minerals is reduced by a four-fold factor, compared to the central points. This grouping of gangue mineral recovery is observed for gangue recoveries below 25% (experiments 2, 4, 5, 9 and 11). Thus, it appears that the depression caused by the flotation conditions of experiment 4 (temperature and depressants) affects all gangue minerals independently of species. One hypothesis explaining this would be a reduction in unselective entrainment of gangue particles with water. However, the low degree of correlation (
R2 = 0.34) between dolomite and water recovery suggests that entrainment alone is unlikely to explain the variation in gangue mineral recovery and that, instead, actual depression of gangue minerals occurs. This agrees with the work of other authors who have shown that hydroxamic acid type collectors do adsorb onto calcite, one of the minerals present in this study [
23,
24].
Figure 3b shows that calcite and Fe-oxides have the largest recovery values. Tests at reduced wt % solids, as well as concentrate cleaning tests, could help determine whether the recovered gangue minerals are still attached to the valuable minerals or if they report to the concentrate through entrainment.
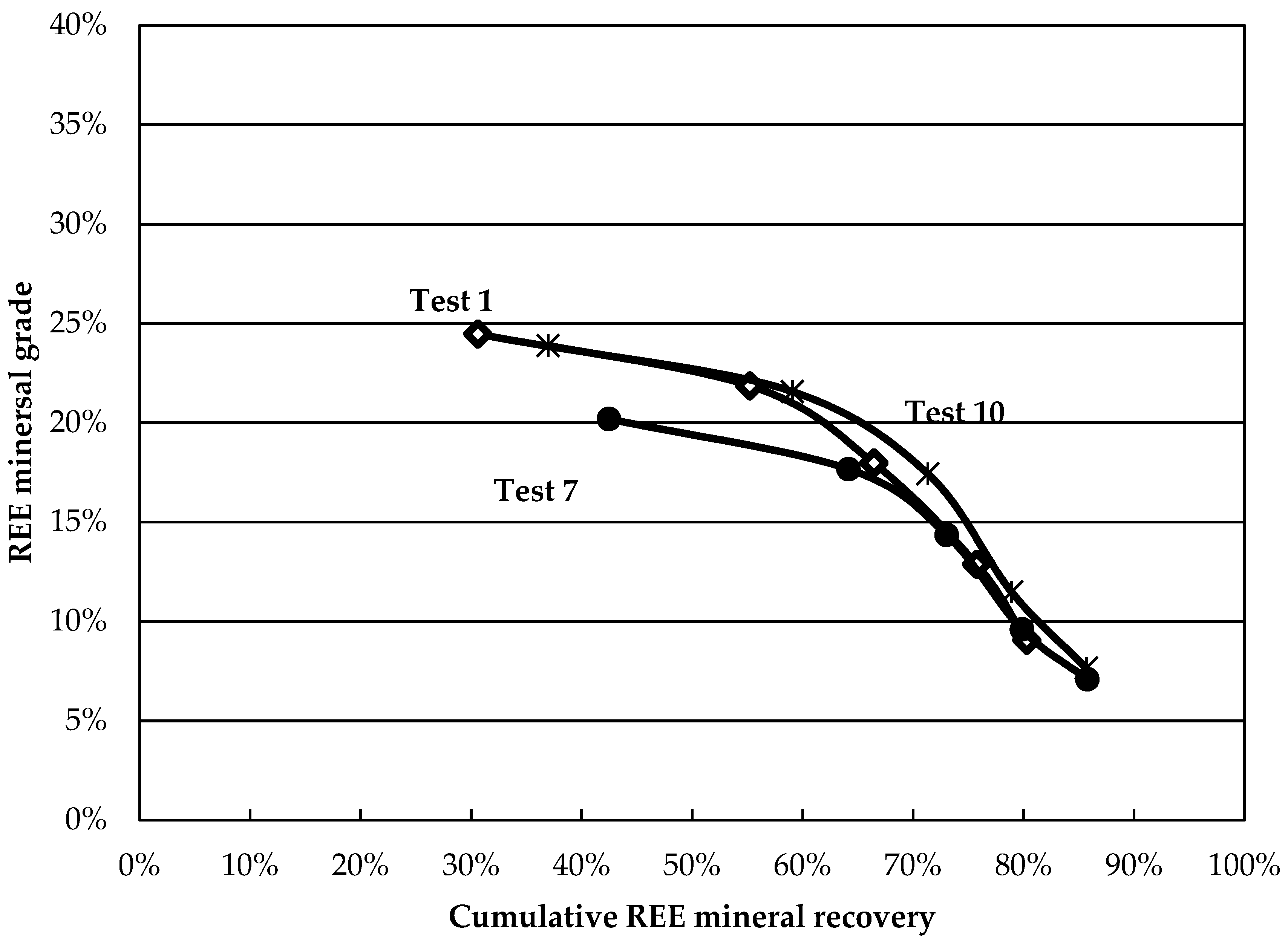
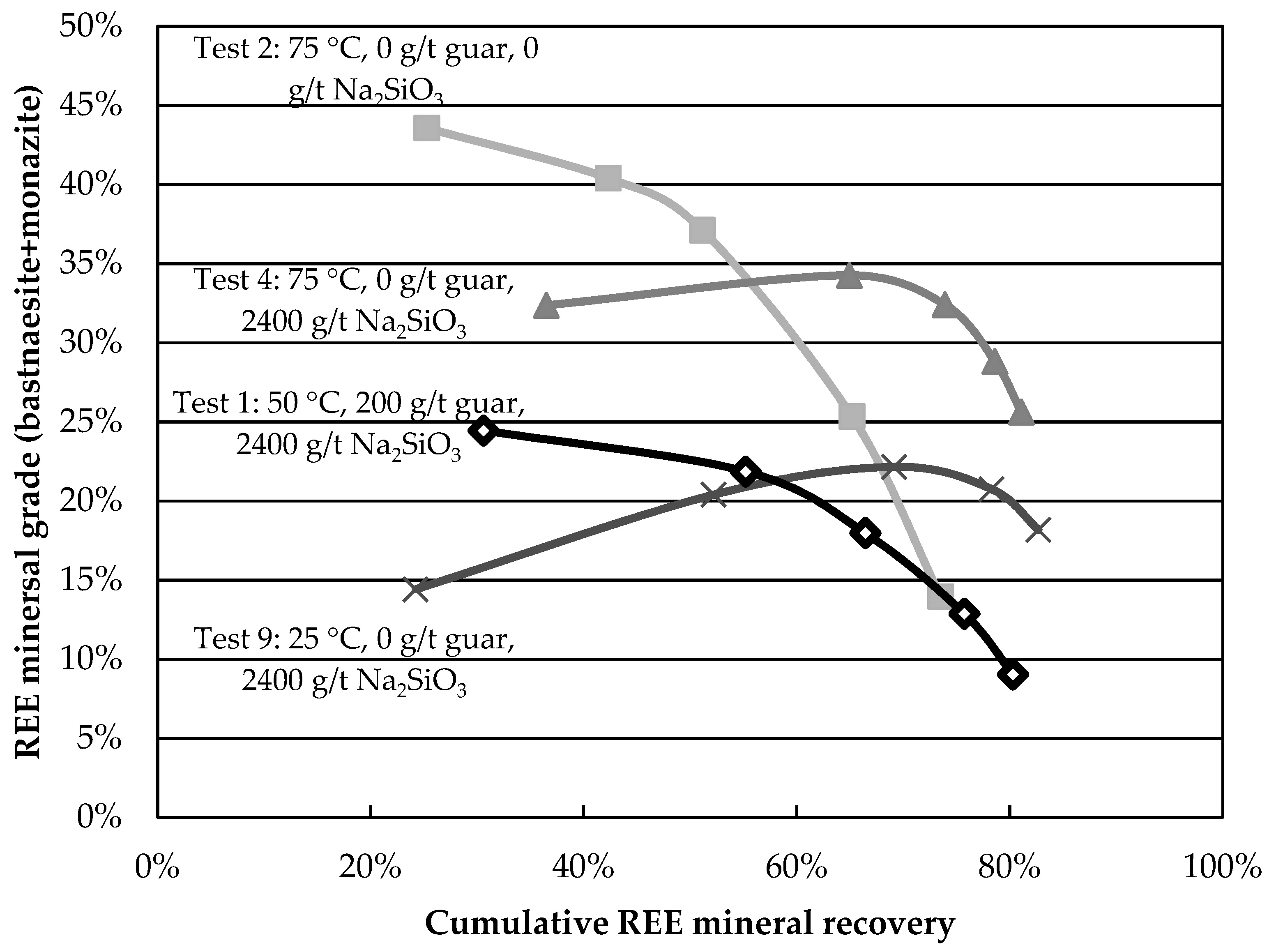
Although these results reveal information about the behaviour of minerals in the various tests, comparative analysis remains tedious. Grade-recovery curves are proposed as simple and rapid tools to resolve this. The higher and further to the right a curve is, the better the separation. The curves for the central points are presented in
Figure 4. The curves show good reproducibility, especially at larger recovery values. The grade-recovery curves for some factorial tests are shown in
Figure 5. Tests 2, 4, and 9 present an improvement over the central point at recoveries above 60%. Test 4 shows the greatest REE mineral content for a REE recovery above 60%. The results obtained in this test (8-fold grade increase at a recovery above 80%) surpass those obtained on most Canadian REE ores for a single rougher flotation stage and compare favourably with others reported using hydroxamic acids [
11], despite lower feed grades.
The grade-recovery curves shown here, while helpful in identifying optimal conditions, make a thorough analysis difficult. For instance, they do not provide information regarding the kinetics of bastnaesite and monazite recovery. Furthermore, it is difficult to quantify the degree of improvement provided by one curve over another, making modelling difficult. The means of quantifying these aspects are proposed in the next section.
3.3. Modelling of the Kinetic Curves for the REE Minerals and Analysis of the Factorial Design
First-order models have been used in flotation for over 80 years [
25] and are still in use today [
26]. Thus, a first-order kinetic flotation model, shown in Equation (1) for bastnaesite, was calibrated for each of the 11 tests, where
R∞, expressed in %, represents a mineral’s ultimate flotation recovery, achieved after an infinite flotation time, and
k, expressed in min
−1, is the flotation rate constant used to quantify the rate of recovery. Separate
R∞ and
k values were fitted for each REE mineral (
R∞,bast. and
kbast. for bastnaesite and
R∞,monaz. and
kmonaz for monazite) to minimize a criterion
J, shown in Equation (2), comprising the sum of the squared differences between the measured (
Rbast.) and fitted
() cumulative REE mineral recoveries for concentrate
i at cumulative flotation time
t. An equation similar to Equation (1) is calibrated for monazite, using
R∞,monaz. and
kmonaz:
Figure 6 shows the agreement between the data and the model (dotted line) for test 7, conducted at the baseline conditions. The first-order model is able to describe the flotation kinetics of bastnaesite and monazite. As previously observed, gangue mineral recoveries are typically grouped. Since they also tend to follow a zero-order (constant) rate of increase with time, a single value,
, i.e., the recovery of gangue after 32 min of flotation, will be used to quantify gangue mineral recovery in the next section.
The fitted parameters (R∞,bast., kbast., R∞,monaz. and kmonaz) of the kinetic flotation model discussed above are used as performance indicators. To further define the impact of the three factors tested, several response variables are calculated to be used as additional performance indicators:
Test conditions and results are summarized in
Table 7. The reproducibility of the test results is quantified using the triplicate center-point, i.e., tests 1, 7, and 10, at the bottom of the table. Since many of the performance indicator values fall well outside the ±2 standard deviation, test conditions likely have a significant impact on performance.
The data shows that gangue recovery (
) varies widely, from 7% to 68%. This is an expected result since two of the three factors tested are depressants for gangue minerals. Monazite ultimate recovery
R∞,monaz. is systematically lower than that of bastnaesite, in agreement with the above results and observations by other researchers [
9].
The analysis of the factorial design begins with the calculation of the main effects of the factors [
27] shown in
Table 8. These quantify the impact of changing a factor from its baseline value (0) to its larger value (+1) [
27]. Higher temperatures appear to decrease REE ultimate recovery
and gangue recovery
Rg,32, but promote the recovery kinetics
k. In the range tested, temperature appears to be the most important factor affecting the rate of REE mineral flotation. Increasing the sodium silicate addition improves the bastnaesite ultimate recovery
R∞,bast. but reduces
R∞,monaz. and gangue recovery. Guar gum promotes a larger recovery value for both REE and gangue minerals.
In order to confirm the significance of these effects, it is necessary to conduct statistical analysis. The data in
Table 7 was analyzed using a hierarchical stepwise regression at a significance level α = 5%. Among the seven responses shown in
Table 8, only the bastnaesite ultimate recovery
R∞,bast., the kinetic constant
kbast. and the separation efficiency
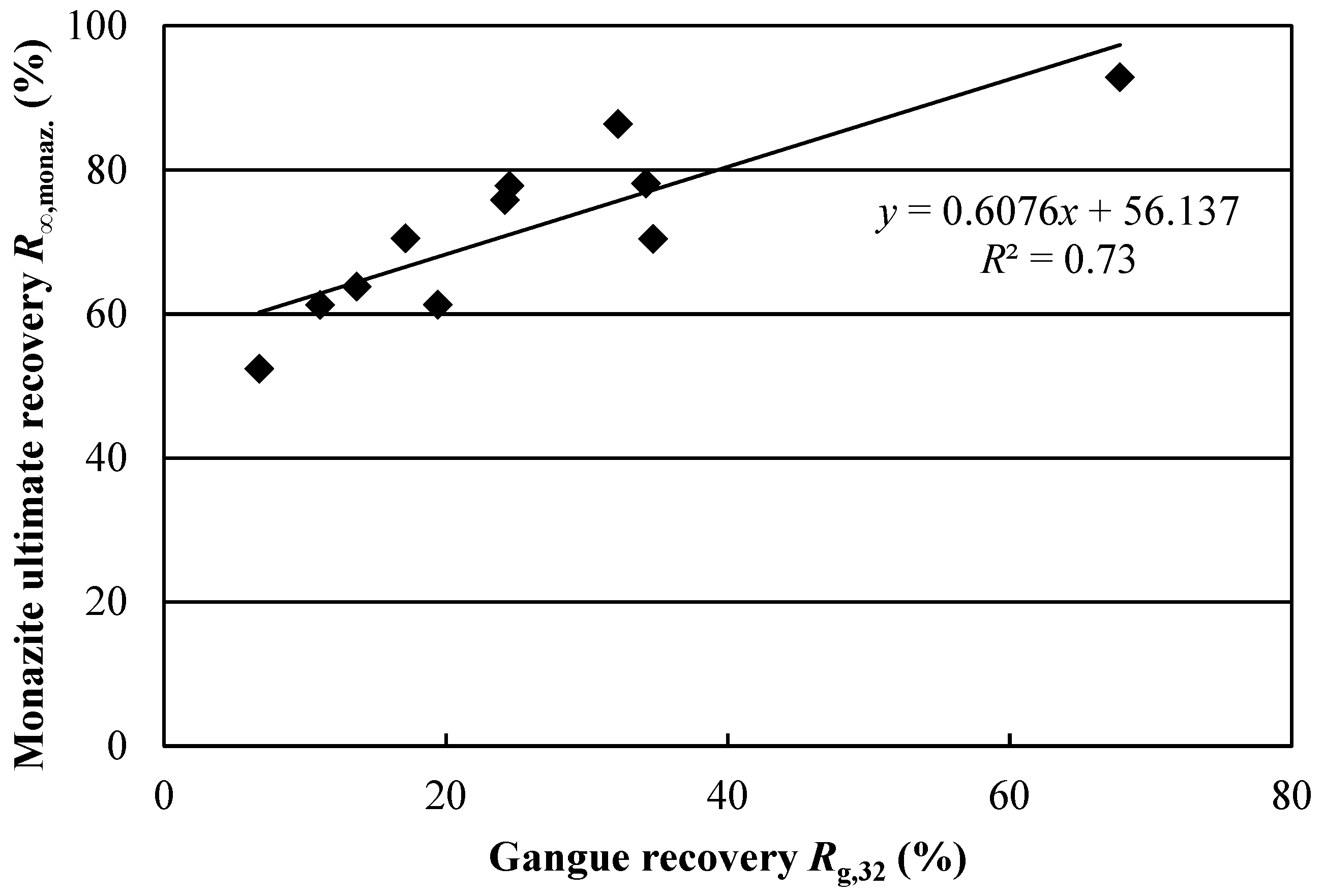
S yielded significant regression models. This indicates that the changes in test conditions either had no effect on monazite recovery or that the effects were masked by experimental error. Furthermore, the correlation between monazite and gangue recovery, shown in
Figure 7, suggests that high monazite recoveries may simply result from gangue-associated monazite recovery. These conclusions show how dividing REE mineral recovery between bastnaesite and monazite through mineral reconstruction allows for more accurate conclusions to be drawn. Indeed, if global REE recovery had been used, the issue of lower monazite recovery may have been overlooked.
Equations (4)–(6) are the empirical regression models predicting the responses based on the levels of the tested factors, expressed in engineering units. In these models, T and N, respectively, stand for temperature in °C and Na2SiO3 dosage in g/t.
Bastnaesite ultimate recovery,
increases with sodium silicate dosage, as shown in Equation (4). Since depressants typically do not improve mineral recovery, the effect is likely explained by the higher pH values observed at larger Na
2SiO
3 dosages (see
Table 7). This is in agreement with other studies [
28,
29], which show that bastnaesite recovery, when using hydroxamic acid type collectors, improves upon going from pH 7 to 9.
The flotation rate constant for bastnaesite
kbast. increases with temperature, as shown in Equation (5). This supports the work of other researchers [
8], which has shown that increasing temperature increases collector adsorption onto bastnaesite more than on calcite and barite. This observation is also consistent with the results of [
9], although in that case, the increase in flotation rate was associated to the production of smaller air bubbles at higher temperatures. A larger flotation kinetic constant for a valuable mineral improves separation efficiency, although other factors can play an important role, as shown in Equation (6).
The model presented in Equation (6) shows that higher temperatures and sodium silicate additions improve separation efficiency. This is not surprising: part of the improvement arises from increases in
and
kbast. values. However, since sodium silicate has the largest effect on gangue recovery
Rg,32 (see
Table 8), its capacity to depress gangue minerals [
10] likely contributes to its effect on separation efficiency. The dispersing properties of this reagent may add to the improvement. The use of separation efficiency as a performance index helps in fully assessing the effect of Na
2SiO
3 and better defining the optimal conditions.
Guar gum dosage has a negative effect on all responses, although these effects are not statistically significant. Upon guar gum addition, the slurry appeared more viscous, indicating a possible change in rheological properties. This change could lead to less particle dropback from the froth phase to the slurry phase, thereby promoting gangue mineral entrainment. Given these observations, future testing should be conducted without guar gum or at dosages smaller than 200 g/t.
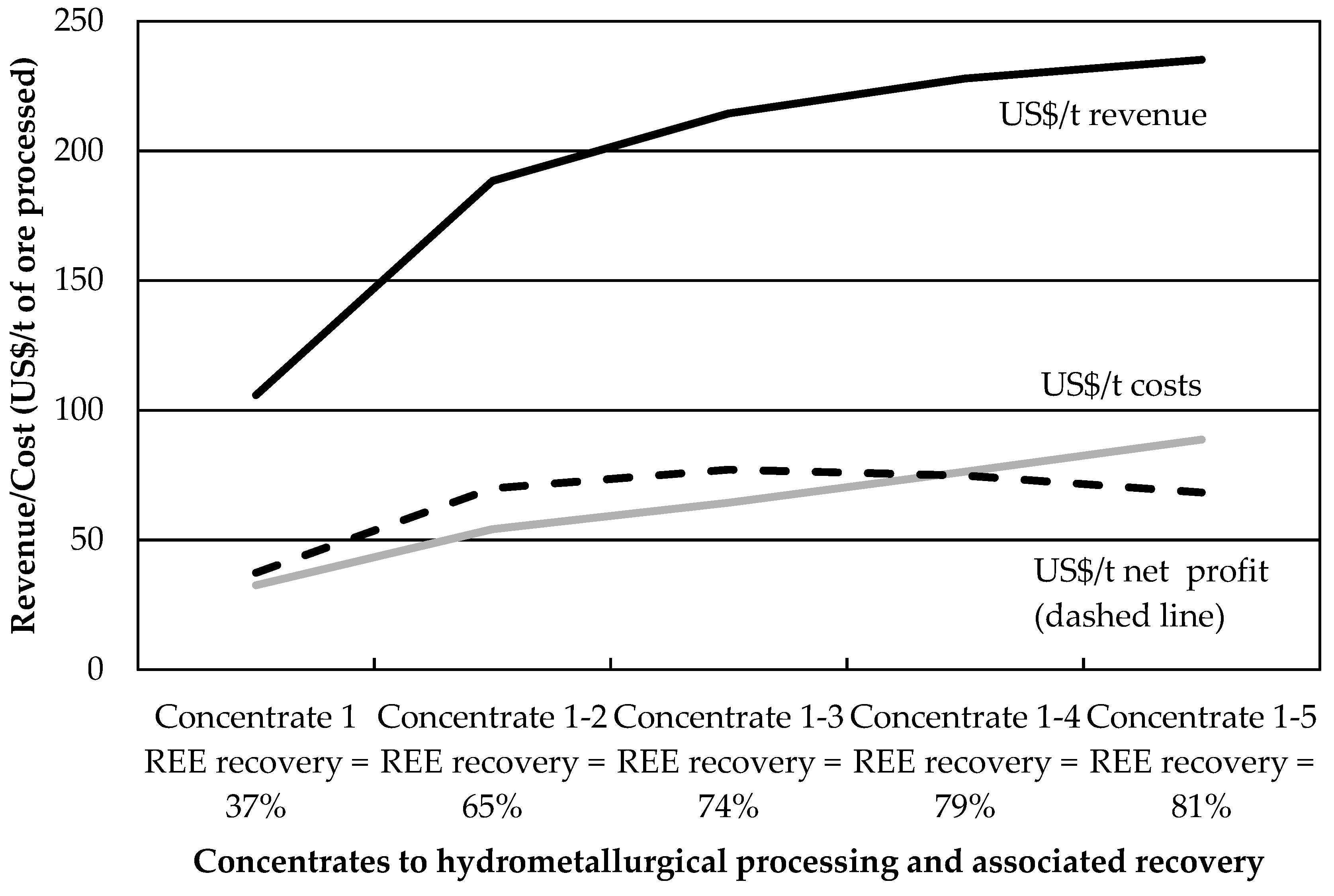
Exploratory tests using Na
2SiO
3 dosages above 3000 g/t and no guar gum at 75 °C yielded reduced REE mineral recoveries, partly due to low froth stability. The optimum separation efficiency conditions for this ore sample may thus lie within the factorial design presented in this paper. The economic optimum, however, may lie elsewhere, owing to the increased costs associated with hydrometallurgical processing of REE from flotation concentrates. This is especially true when large amounts of acid-consuming carbonate gangue minerals are present [
30]. The next section uses an economic performance indicator to quantify the impact of gangue recovery on the profitability of REE mineral flotation.