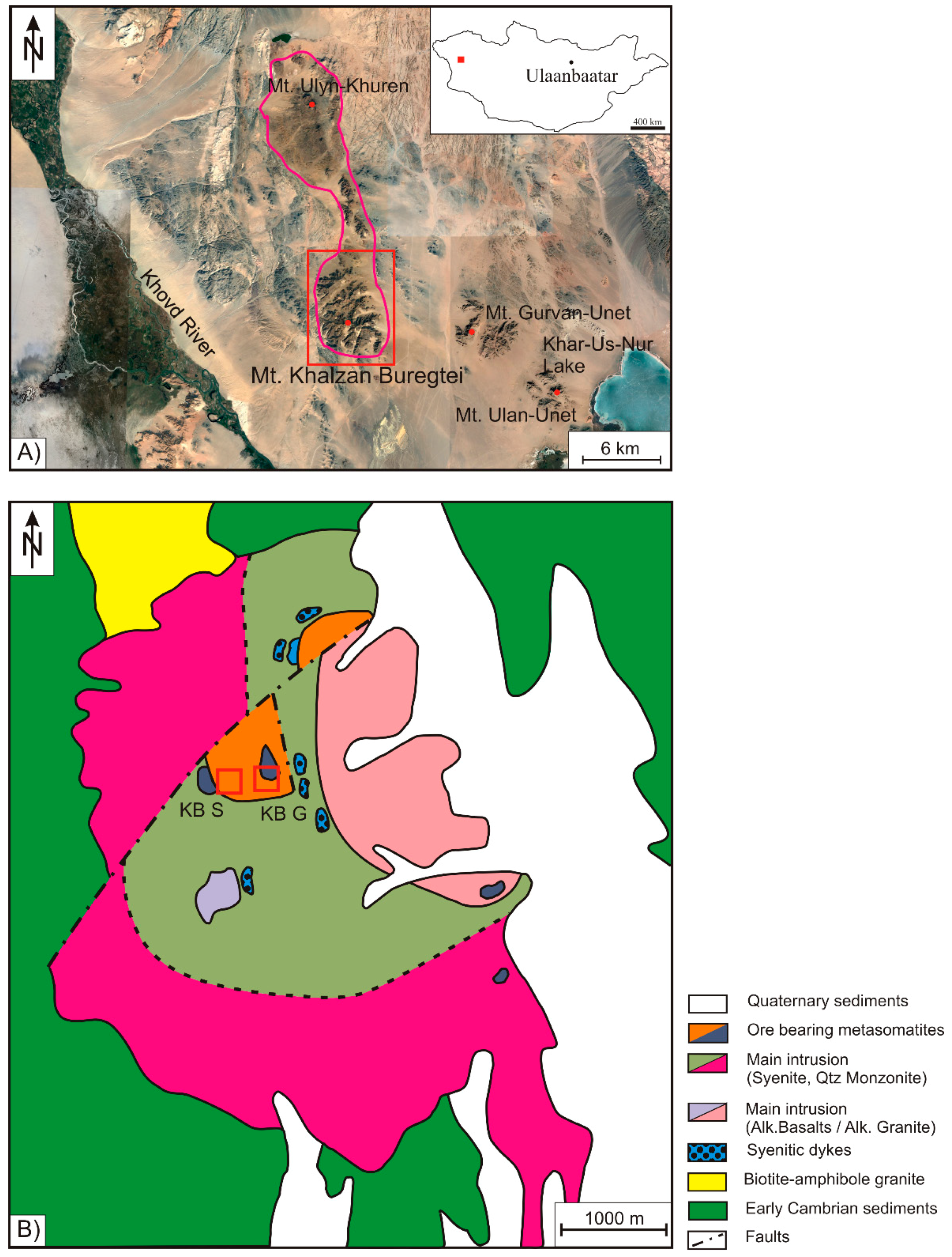
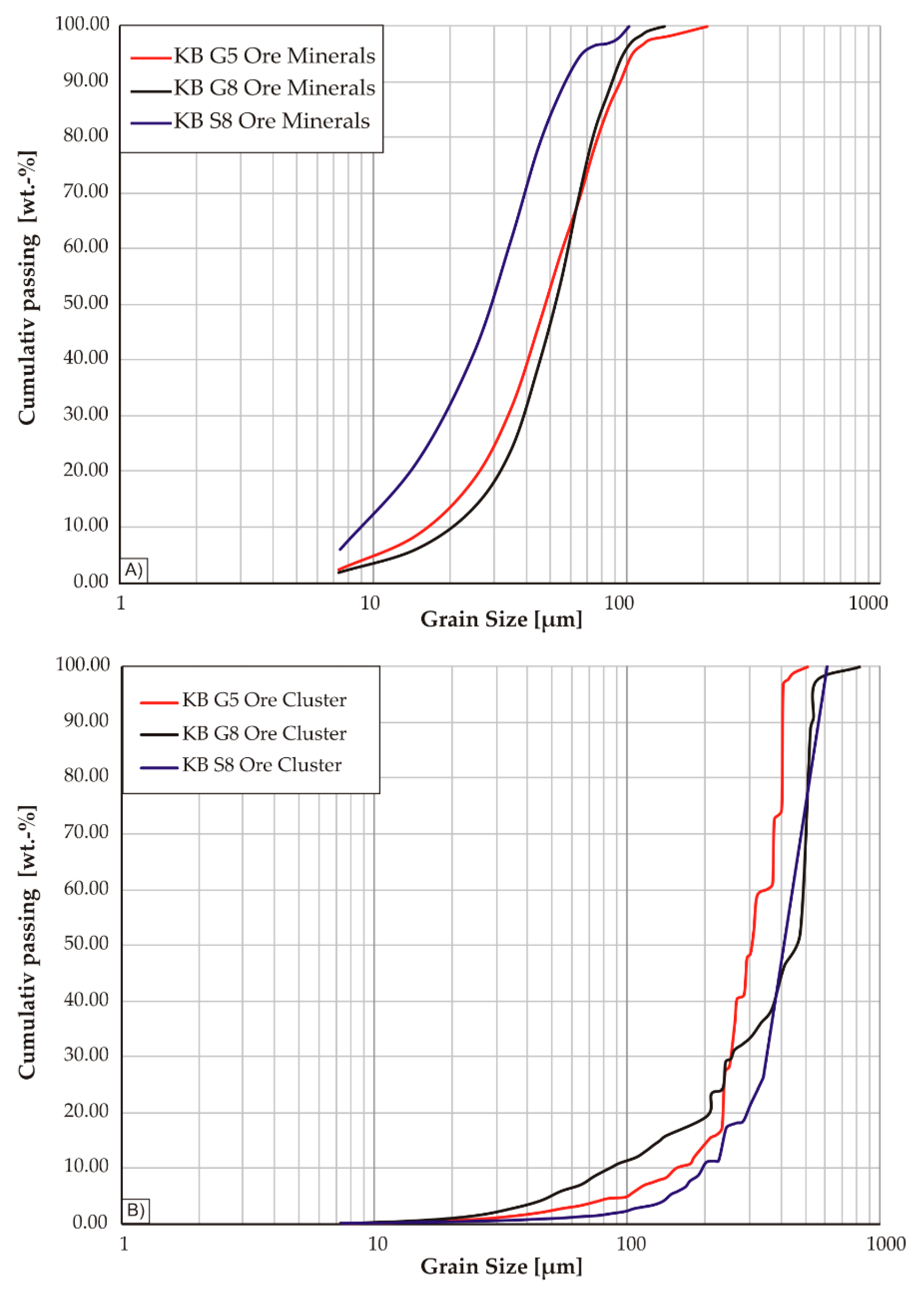
4.1. Petrography
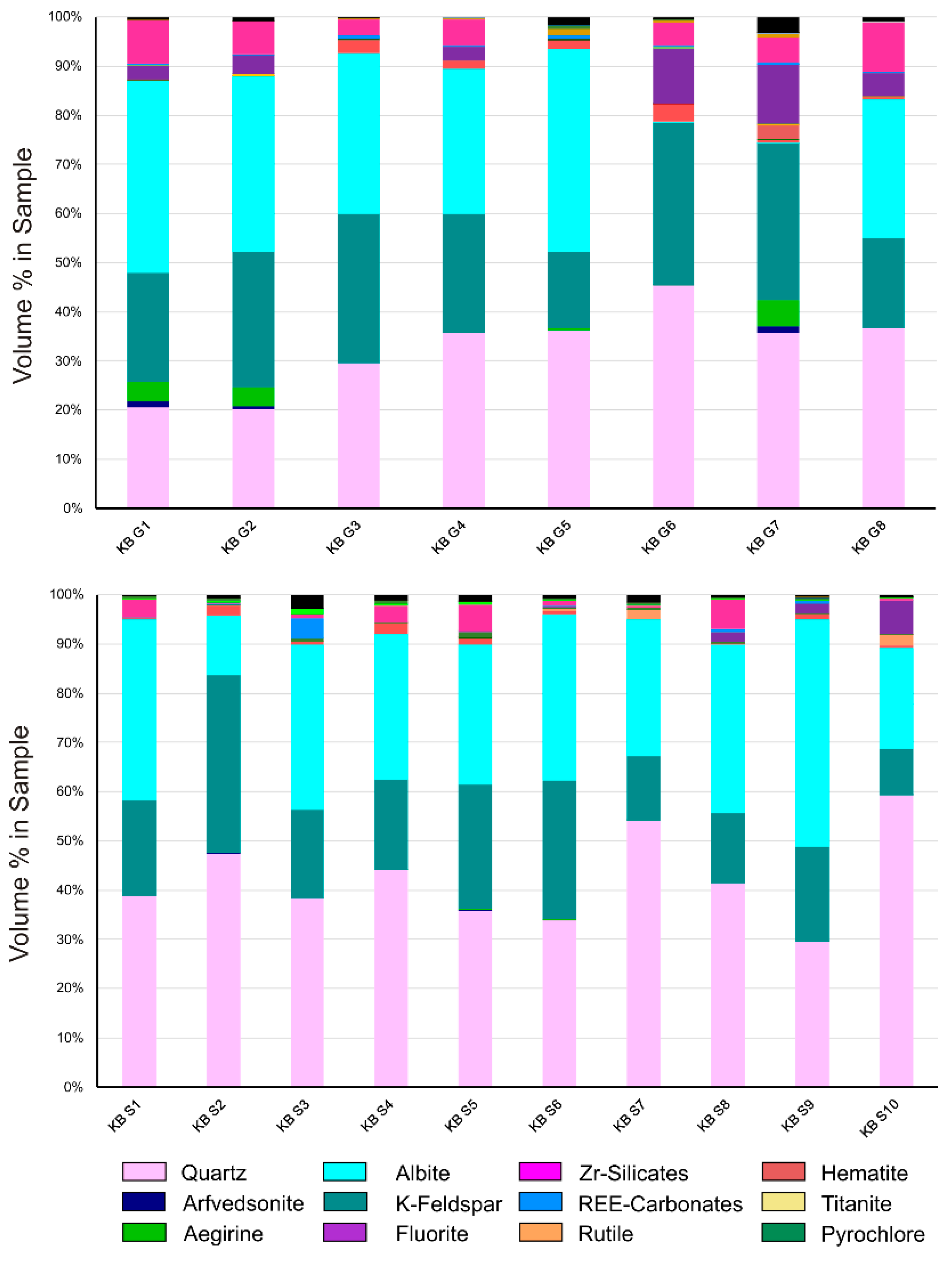
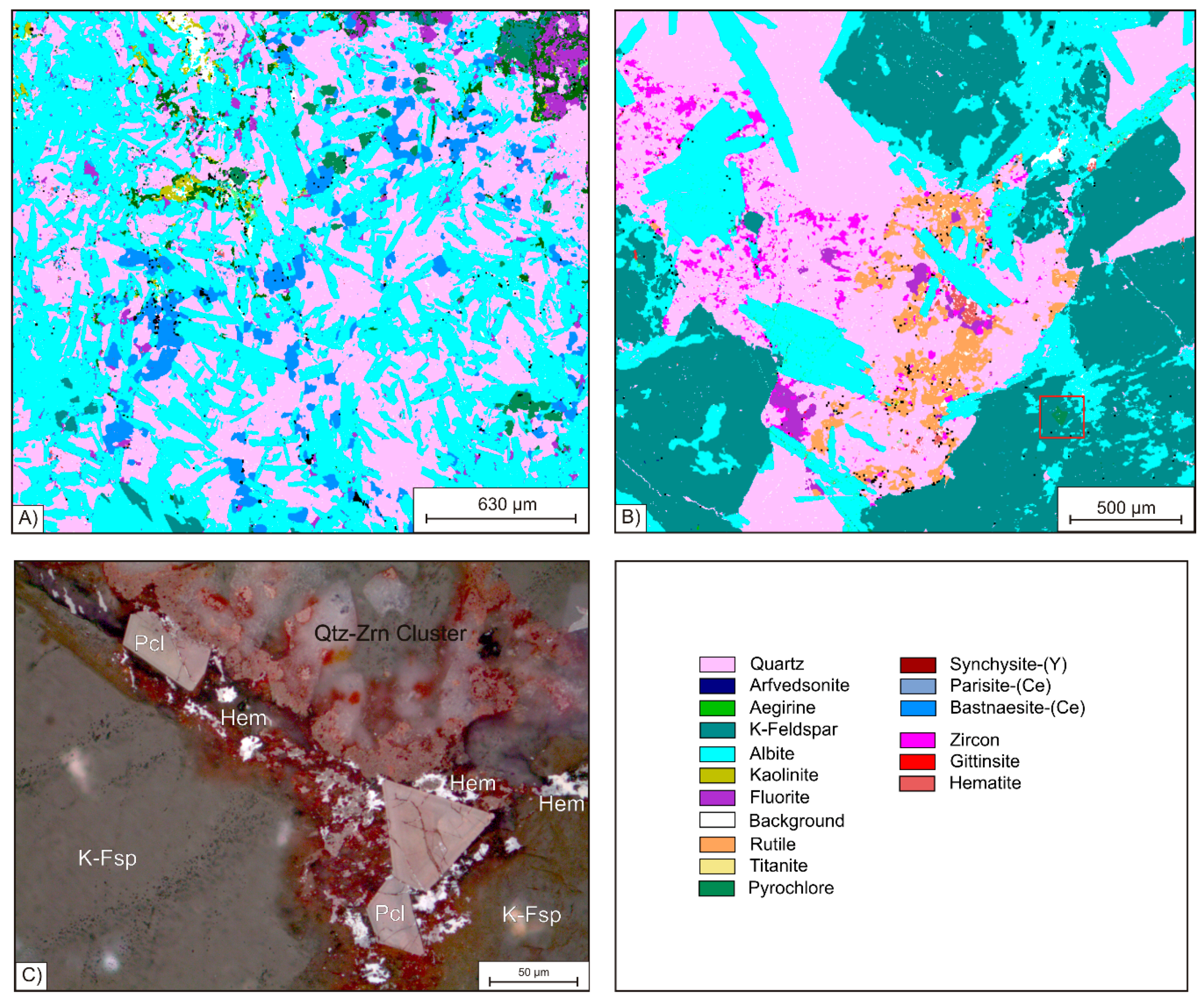
Quartz, K-feldspar, and albite are the dominant rock-forming minerals and in total account for more than 70 vol. % in all samples. The mafic minerals arfvedsonite and aegirine are less abundant (<5 vol. %) and can only locally be observed (
Figure 2). Despite the variable proportions of the rock forming minerals, mainly showing higher quartz abundances in the KB-S sampling area, all samples, excluding one, can be classified as alkali granites in terms of the terminology of [
20] for magmatic rocks. Only the sample KB S10 was classified as quartz rich granitoid rock due to a significantly higher abundance of quartz. All samples show variable proportions of HFSE ore minerals, such as Zr silicates, REE-carbonates, pyrochlore, as well as of hematite, rutile, titanite, or fluorite (
Figure 2).
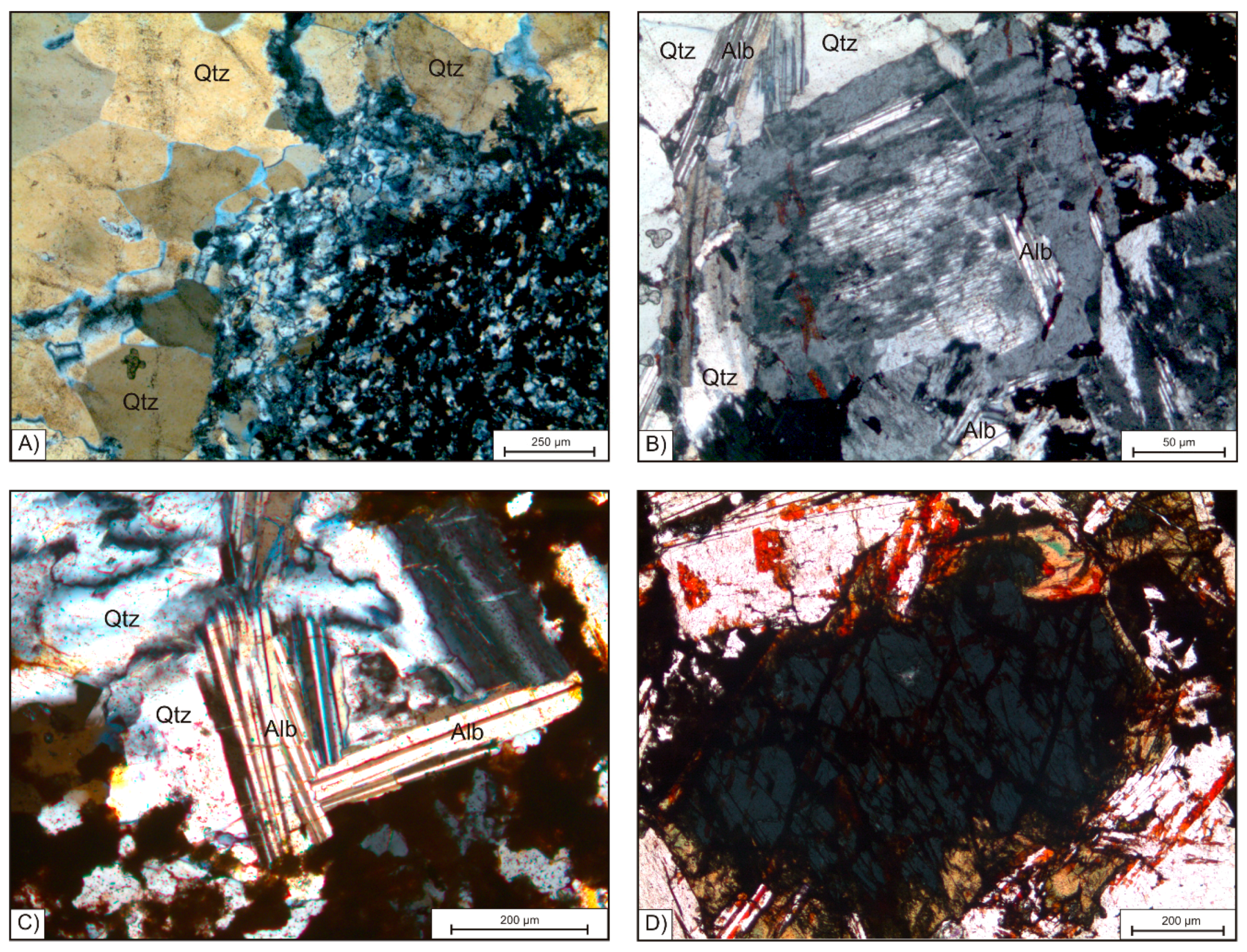
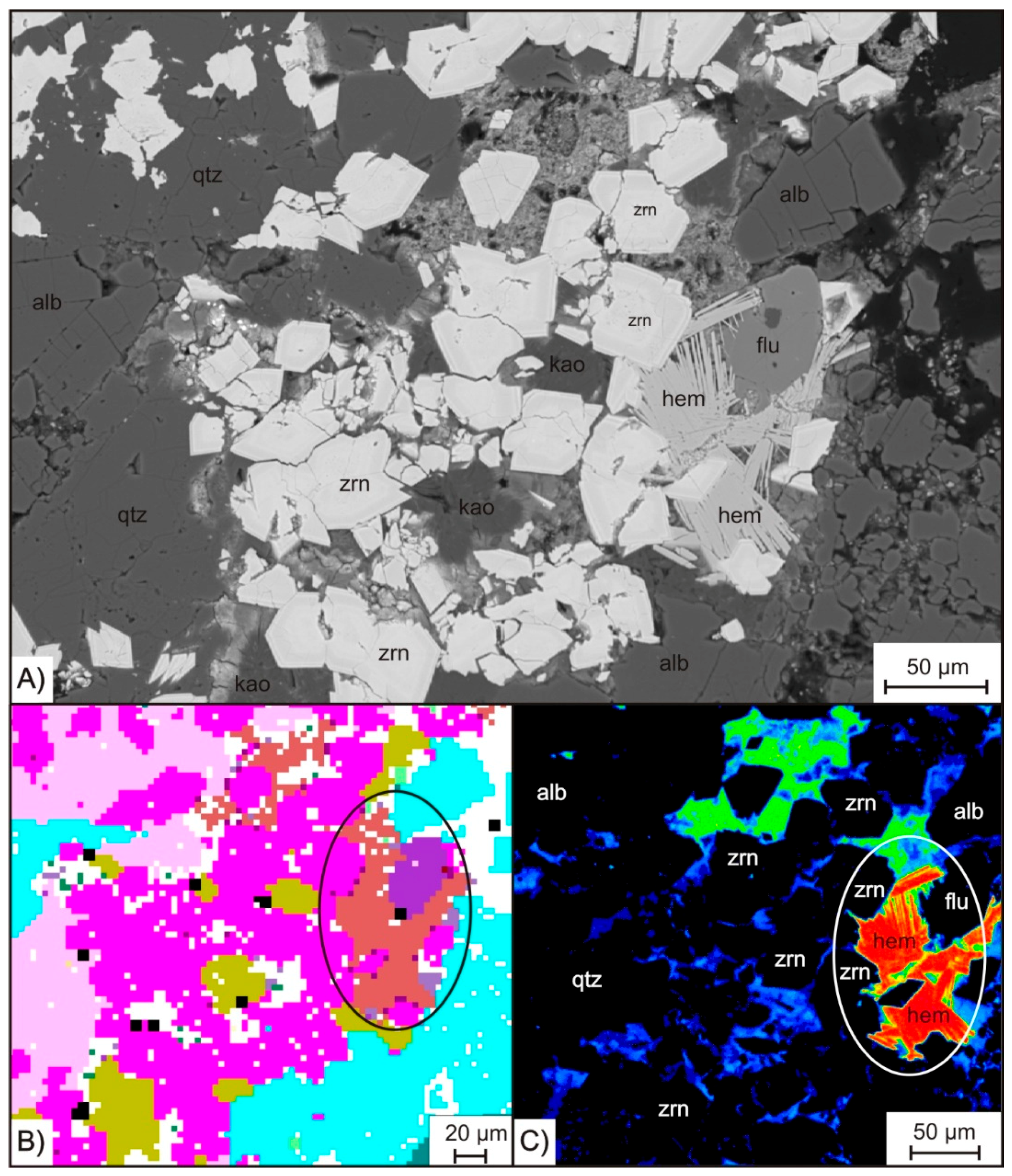
Quartz is present in all samples and shows a variation in grain size. The coarse fraction is represented by quartz grains up to 2 mm in KB G and up to 5 mm in KB S. Some grains show snowball structures enclosing other mineral phases like K-feldspar and albite. Further, big crystals of quartz show evidence of ductile deformation, like bulging and the formation of sub grains (
Figure 3A, yellowish). In addition to the coarse fraction, a fraction of fine quartz grains can be observed. The latter is strongly intergrown with HFSE minerals, as well as with hematite and fluorite (
Figure 3A).
K-feldspar shows a size distribution similar to the coarse grained quartz in both sample suites. Irregularly shaped crystal domains inside of K-feldspar grains may represent relict hatch-twin domains formed in earlier microclinization (
Figure 3B). Euhedral K-feldspar is in close association to albite, which can be observed parallel to grain boundaries. K-feldspar often shares straight boundaries with coarse grained quartz (
Figure 3B).
Albite forms lath shaped crystals varying in grain size from 50 µm to 400 µm (
Figure 3C). Coarse laths of albite, however, are found at the edges of coarse grained snowball quartz and K-feldspar grains. Fine albite crystals can be observed in interstitial volumes of quartz and K-feldspar. Evidence of the replacement of quartz and K-feldspar by albite is dominant in all samples from the centre of the Khalzan Buregtei deposit. The close intergrowth of lath-shaped albite crystals with each other leads to the formation of a matrix-like texture observable in all samples containing high amounts of albite.
The mafic minerals arfvedsonite and aegirine occur in the rocks with a grain size up to 1 mm but with low modal abundance. As
Figure 2 shows, significant amounts are only observed in the samples KB G1, G2, and G9. Arfvedsonite is euhedral and shows good cleavage. Aegirine forms halos surrounding arfvedsonite as illustrated in
Figure 3D. Ongoing replacement reaction from arfvedsonite to aegirine is observed propagating along amphibole cleavage plains. Aegirine is further replaced by albite laths. Albite grains in contact with arfvedsonite and aegirine often show red staining (
Figure 3D).
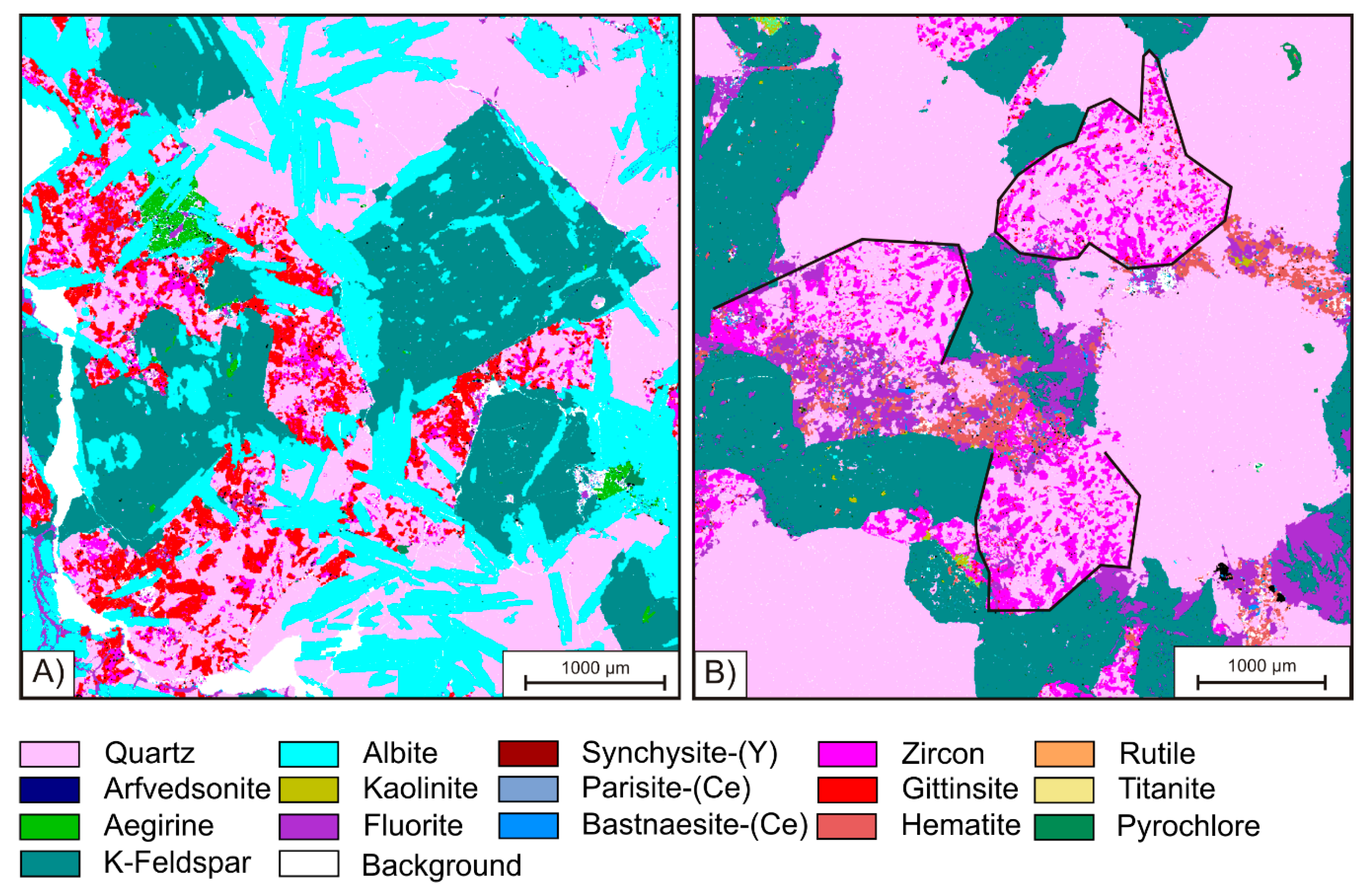
The group of HFSE-bearing minerals comprises Zr-silicates, REE-carbonates, and the Nb mineral pyrochlore. Zircon is the most abundant mineral with concentrations as high as 11 vol. %. The Zr-silicate gittinsite [CaZrSi
2O
7], in contrast, is only present in samples KB G1 and G2, in which it occurs in aggregates with zircon clustering around relicts of aegirine (green in
Figure 4A). According to [
14,
16] and [
6] more Zr-silicate minerals like elpidite [Na
2ZrSi
6O
15∙H
2O] and armstrongite [CaZrSi
6O
15∙H
2O] occur at the Khalzan Buregtei deposit; however, at the studied location, these minerals were found only on a very rare level. Due to the low abundance of these minerals at the sampled parts of the deposit, they are not considered as ore minerals in this study.
The REE-carbonates are represented by bastnaesite-(Ce) as the most abundant phase, as well as by parasite-(Ce) and synchysite-(Y). In total, their modal volume is <5 vol. %. Pyrochlore can be observed in nearly all samples, but with low abundance compared to zircon or REE-carbonates.
Except for pyrochlore, the HFSE minerals occur concentrated within cluster-like aggregates together with fine grained quartz. It can be recognized in
Figure 4B that such clusters form pseudmorphs after amphibole indicated by the subhedral shape of the aggregates, which have straight grain boundaries against coarse grained quartz and K-feldspar. These polymineralic ore clusters, which can be observed through the entire suite of samples from KB G and KB S, have variable sizes from 250 µm up to 2 mm, whereas the minerals within the clusters are mainly anhedral and show smaller diameters. While the REE-carbonates vary in grain-size from 90 to 200 µm, Zr-silicates and quartz grains are even smaller and rarely exceed 50 µm. In addition to Zr-silicate and the REE- and Y-carbonates, the ore clusters also contain abundant hematite and fluorite, as well as, in rare cases, the Ti-phases rutile and titanite. The latter two minerals are strongly intergrown with fine grained quartz. Rutile forms euhedral prismatic crystals characterized by oscillatory zoning, which occasionally reach a maximum diameter exceeding 100 µm (
Figure 5A). Locally, hematite also forms individual clusters with fine grained quartz. Fibrous hematite aggregates can be observed exceeding diameters >50 µm of irregular fan or spherulite texture. However, it can be detected as very fine grained (<5 µm) pigments in interstitial volume of mineral phases like zircon, as illustrated by blue colors at the high-resolution Fe mapping in
Figure 5C.
Purple colored fluorite can be observed in the cluster assemblage as well as associated with coarse grained quartz, K-feldspar, and albite (
Figure 4B,
Figure 6A). It is mostly of xenomorphic shape and can locally exceed a grain size of 500 µm. Besides the abundance in interstitial volume, purple fluorite is found in veins cross-cutting the central part of the Khalzan Buregtei deposit. Further, fluorite can be observed as a replacement of K-feldspar and coarse grained quartz.
The Ti-phases rutile and titanite are strongly intergrown with fine grained quartz. Rutile forms euhedral prismatic crystals characterized by oscillatory zoning, which occasionally reach a maximum diameter exceeding 100 µm (
Figure 6B). In contrast to the other ore minerals, pyrochlore is not accumulated in clusters, but is mostly associated with K-feldspar and coarse grained quartz. All grains of pyrochlore are euhedral (
Figure 6C) and most of the crystals show evidence of brittle deformation. Furthermore, pyrochlore grains are observed being cross-cut by lath shaped albite. Like the other ore minerals, pyrochlore crystals are dominantly <50 µm but can exceed 140 µm in rare cases. As illustrated in
Figure 6C, joints within pyrochlore are also healed by hematite precipitation.
Based in the observation of textural properties such as grain size, mineral assemblage, crystal shape, and grain boundaries indicative of equilibrium or replacement reactions, mineral phases can be differentiated into groups of primary or post-magmatic formation. Coarse grained minerals of subhedral shape sharing straight grain boundaries are considered as minerals formed under magmatic conditions. K-feldspar and arfvedsonite are two phases with coarse grain size as well as, in part, semihedral shape. Furthermore, the coarse grains of quartz are observed to share straight grain boundaries with K-feldspar. Thus, the precursor rocks of the metasomatites of the Khalzan Buregtei deposit can be identified as alkali syenite to alkali granites (QAPF, [
20]). Owing to their coarse grain size, these rocks had a porphyritic appearance, which is in accordance with the observation of [
5,
13,
14,
15] regarding the unaltered rock suite of the main intrusive phase.
Post-magmatic phases are identified by significant change to finer grain size and irregular grain shape. Furthermore, the formation of polymineralic aggregates pseudomorphically replacing magmatic phases (e.g., amphibole) can also best be explained in a reaction of a solid mineral with a fluid, i.e., in a post-magmatic reaction. Thus, aegirine and albite, which grew at the expense of magmatic arfvedsonite and K-feldspar, respectively, are classified as post-magmatic minerals. Fine grained quartz, which shares straight grain boundaries with albite, is considered as cogenetic and thus also as post-magmatic. Post-magmatic formation is also indicated for ore and accessory minerals (e.g., hematite and fluorite) found in clusters intergrown with post-magmatic quartz. The semihedral shape of ore mineral clusters and their straight grain boundaries shared with magmatic phases are evidence that ore clusters mainly represent aggregates pseudomorphically replacing arfvedsonite and aegirine, which itself replaces arfvedsonite before (
Figure 3D and
Figure 4B). The occurrence of clusters of post-magmatic minerals thus mirrors the texture of precursor porphyritic alkaline granite rock. Veins filled with carbonates were found cross-cutting all other mineralogical and textural features of the rocks and were interpreted to be late stage post-magmatic. It is important to note that these textural observations show that even with the enrichment of Zr, the REE or Nb were probably caused by magmatic fractionation [
15]; the textural properties of the rare metal ores of Khalzan Buregtei are predominantly of post-magmatic metasomatic character, which is also in line with the characterization by [
5].
4.2. Geochemistry
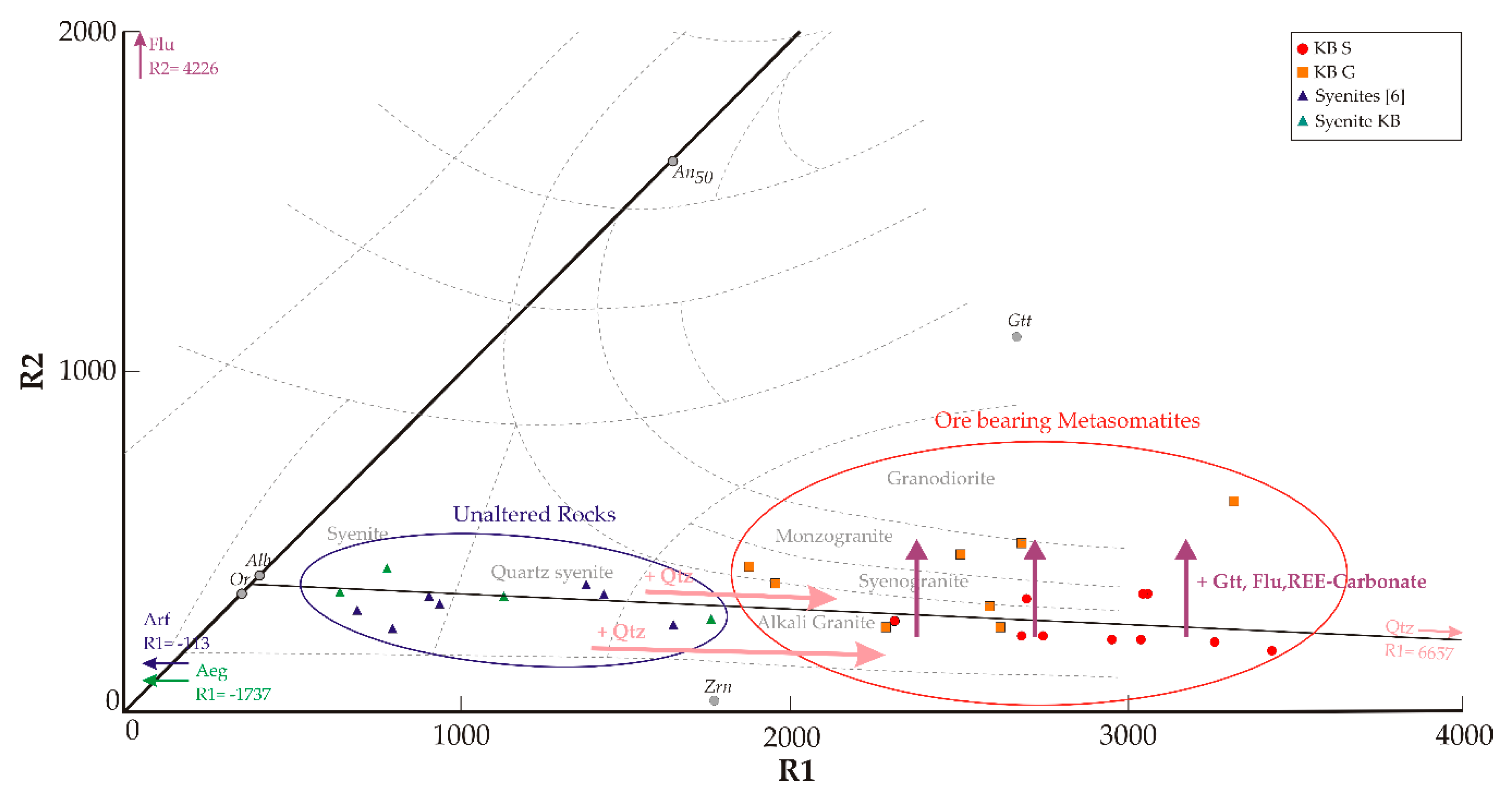
Whole rock major element composition as well as multication parameters (R1, R2 [
21]) and rock classification based on major element signatures (TAS for plutonic rocks [
22], R1-R2) are listed in
Table 1 and
Table 2 for both sample series KB G and KB S.
As is indicated by the SiO
2 values, all rocks are silica oversaturated. The samples of KB S, especially, show an increased concentration of SiO
2 compared to KB G, which is in accordance to the increased quartz content indicated by the modal composition
Figure 2. Other major elements are more variably distributed in the KBG sample suite, in which KBG 6 and 7 show elevated amounts of fluorite, but are in contrast to all other samples containing no albite in
Figure 2. This leads to significantly decreased Na
2O and Al
2O
3 concentrations, but enhanced values of CaO and LOI (
Figure 2,
Table 1 and
Table 2). In addition to fluorite, gittinsite, which is present among the Zr-silicates in some samples (e.g., KB G1), is a carrier of CaO, too. Variations in major element composition are mirrored in element-based rock classification. Lower SiO
2 content in KB G samples but variable sums of Na
2O + K
2O result in TAS classifications of monzonite, granodiorite, and granite (
Table 1). In contrast, the de la Roche classification, which, in addition to Si and the alkalis, includes Fe, Ti, Ca, Mg, and Al, reflects element variability by assigning the classes syenogranite, alkali granite, granodiorite, and monzogranite to the KB G samples. While all KB S samples are entirely classified as granites in the TAS scheme, the de la Roche scheme leads to classification as alkali granite and syeno granite in some cases or does not allow classification at all. The latter can be observed in samples with highest SiO
2 concentrations and R1 multication parameter > 3000. Therefore, these samples are classified as “Qtz rich” in this study.
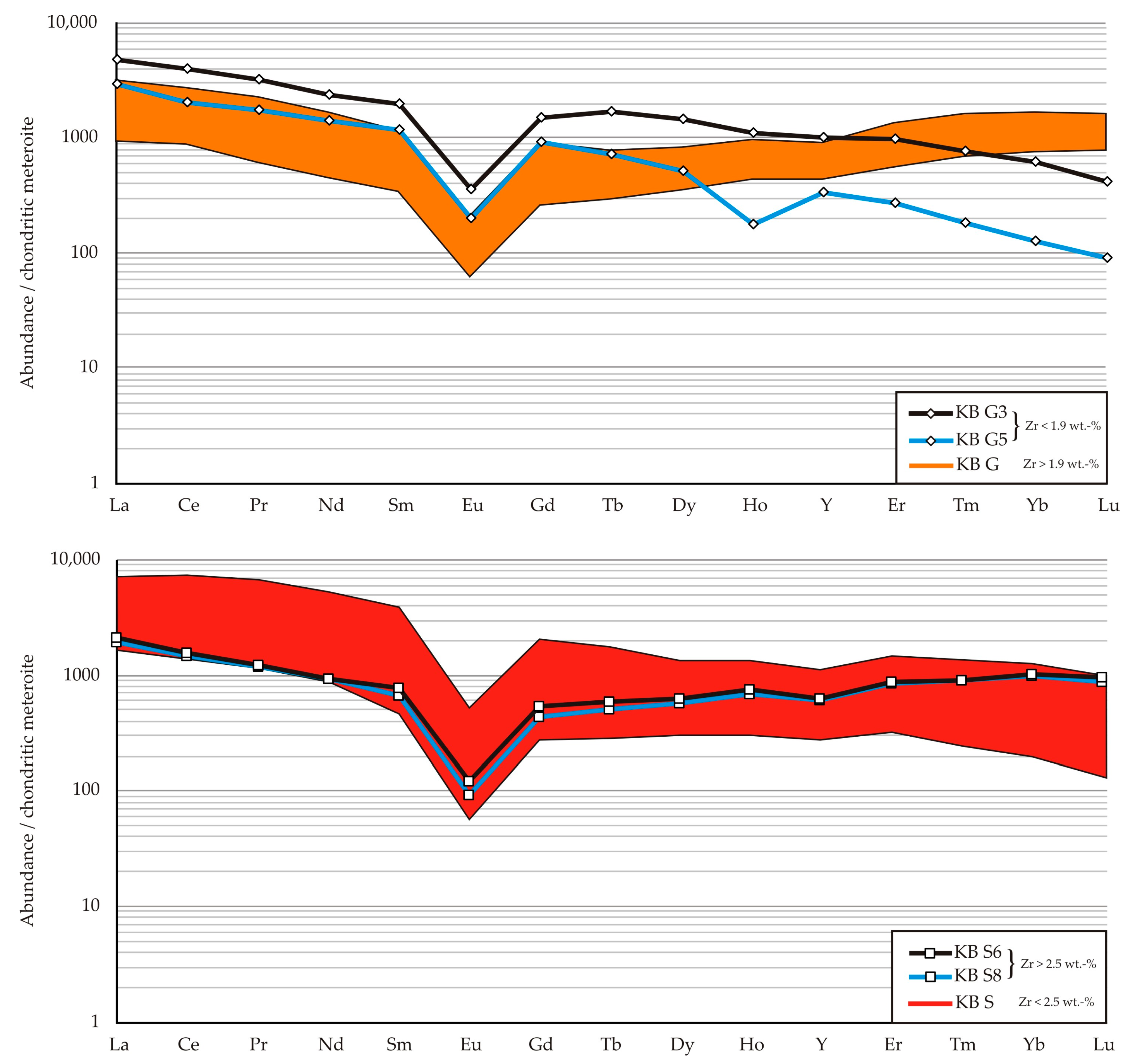
The results of the REE analyses of the bulk rock samples are given in
Table 3 and
Table 4. Chondrite normalized distribution patterns are given in
Figure 7. All samples show REE concentrations strongly enriched in comparison to chondritic composition [
23] and they have negative Eu anomalies, which are more pronounced in KB G samples (Eu/Eu* < 0.3) in contrast to KB S (Eu/Eu* < 0.2), see
Table 3 and
Table 4. The HREE (heavy rare earth elements Gd-Lu) elements show a significantly higher enrichment in the KB G samples in comparison to KB S. The latter samples, on the other hand, show a steeper pattern, with elevated enrichment of LREE (light rare earth-elements La-Eu) ratios up to 7000 but a generally lower enrichment of HREE ≤ 1000 (
Figure 7). KB G samples, instead, are less enriched in LREE < 3000, which leads to flat patterns.
Zirconium has variable concentrations across the data set. KB G samples contain Zr up to 3.3 wt. % (
Figure 7 orange range) whereas only two samples have concentrations < 1.9 wt. %. Within both sample suites, those of low Zr concentrations show decreasing enrichment of the HREE. In contrast, samples with high Zr concentration are characterized by increased HREE enrichment.
Niobium concentrations show a distribution similar to the Zr. maximum values given by the method’s upper limit of quantification at 2500 mg/kg. Minimum concentrations of Nb in KB G samples generally are two times higher than the concentrations of KB S samples ranging between 490 and 790 mg/kg. Concentrations of Nb are generally higher in KB G and show a variation between 1000 mg/kg and 1900 mg/kg. In comparison, the concentration of Nb in KB S samples, excluding the samples exceeding the upper limit of quantification, cover the range from 490 mg/kg to 1945 mg/kg.
4.3. Elemental Deportment
Petrographic and geochemical investigation points out that the major ore mineral classes are Zr-silicates, REE-carbonates, and Ti/Nb oxides that occur within the metasomatic rocks forming the centre of the Khalzan Buregtei deposit. Zircon is the most abundant ore mineral. It can be detected in all samples of ore metasomatites. Further, it is identified as the major carrier for the rare metals Zr, Hf, Y, and HREE due to the high concentrations of Y, Dy, and Er across the analysed zircon crystals, as demonstrated by the representative analyses of different zircon and gittinsite grains in
Table 5.Table 6 summarizes EPMA analyses of bastnaesite-(Ce), parisite-(Ce), and synchysite-(Y), which are the most abundant minerals of the REE-carbonate group. It can be seen that the LREEs show a high concentration in the first two minerals, whereas synchysite-Y has got elevated concentrations of Y and is thereby suspected to contain unquantified amounts of HREE as well. Low total sums can be explained by unquantified amounts of HREE incorporated into the analysed minerals. The individual concentration levels of these elements are below the limit of quantification of the applied EPMA.
Pyrochlore occurs as a major carrier for Nb in the Khalzan Buregtei deposit. Eight pyrochlore EPMA analyses are given in
Table 7. They show that pyrochlore is enriched in the LREEs Ce, La, and Nd. Heavier REE elements were not detected. Due to the content of Ce, pyrochlore is identified as ceriopyrochlore-Ce, which contains only very small amounts of betafite [Ca
2(Ti,Nb)
2O
6O] and microlite [(Na,Ca)
2Ta
2O
6(O,OH,F)], as indicated by the low concentrations of Ti and Ta, respectively. Pyrochlore also shows a capability to enrich U to concentrations up to 1.16 wt. %. Semi-quantitative analyses indicate that, in addition to pyrochlore, rutile can be another carrier of Nb
2O
5 with variable concentrations from 2 to 5 wt. %.