High-Temperature Mineral Phases Generated in Natural Clinkers by Spontaneous Combustion of Coal
Abstract
1. Introduction
2. Geological Setting
3. Materials and Methods
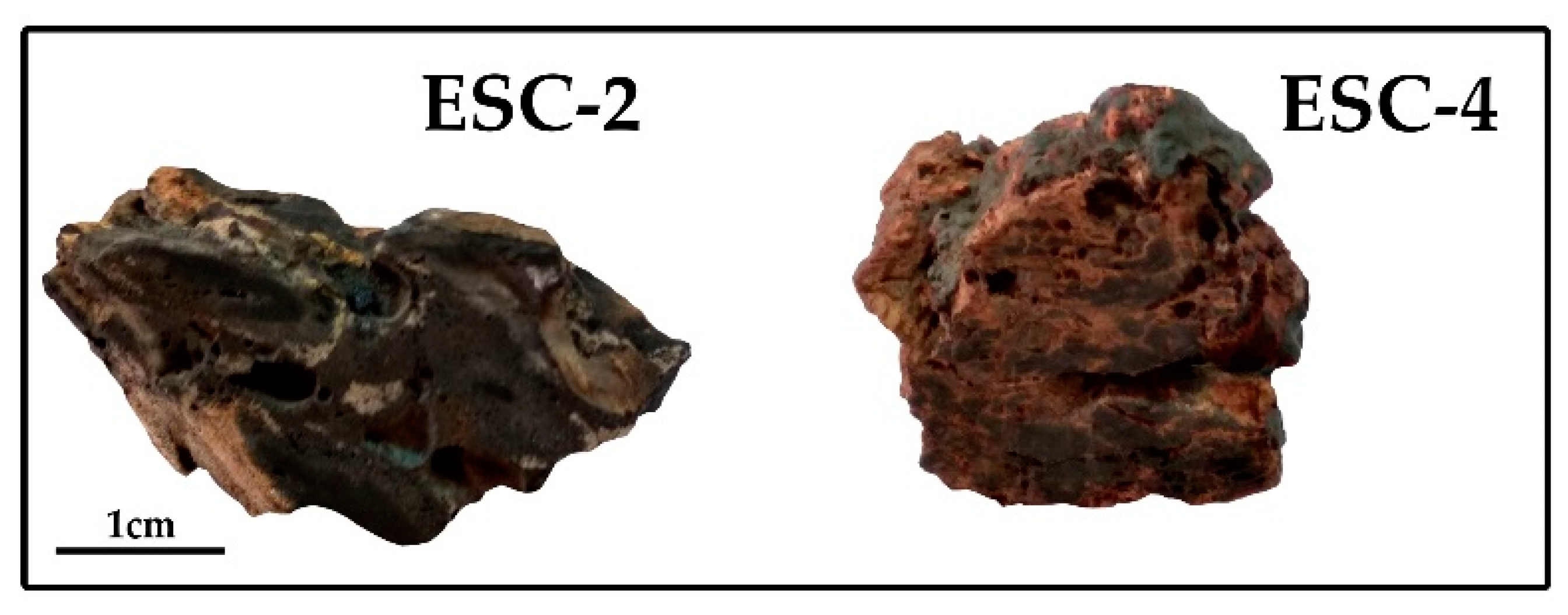
3.1. Description of the Samples
3.2. X-ray Diffraction
3.3. Optical and Electron Microscopy Studies
4. Results
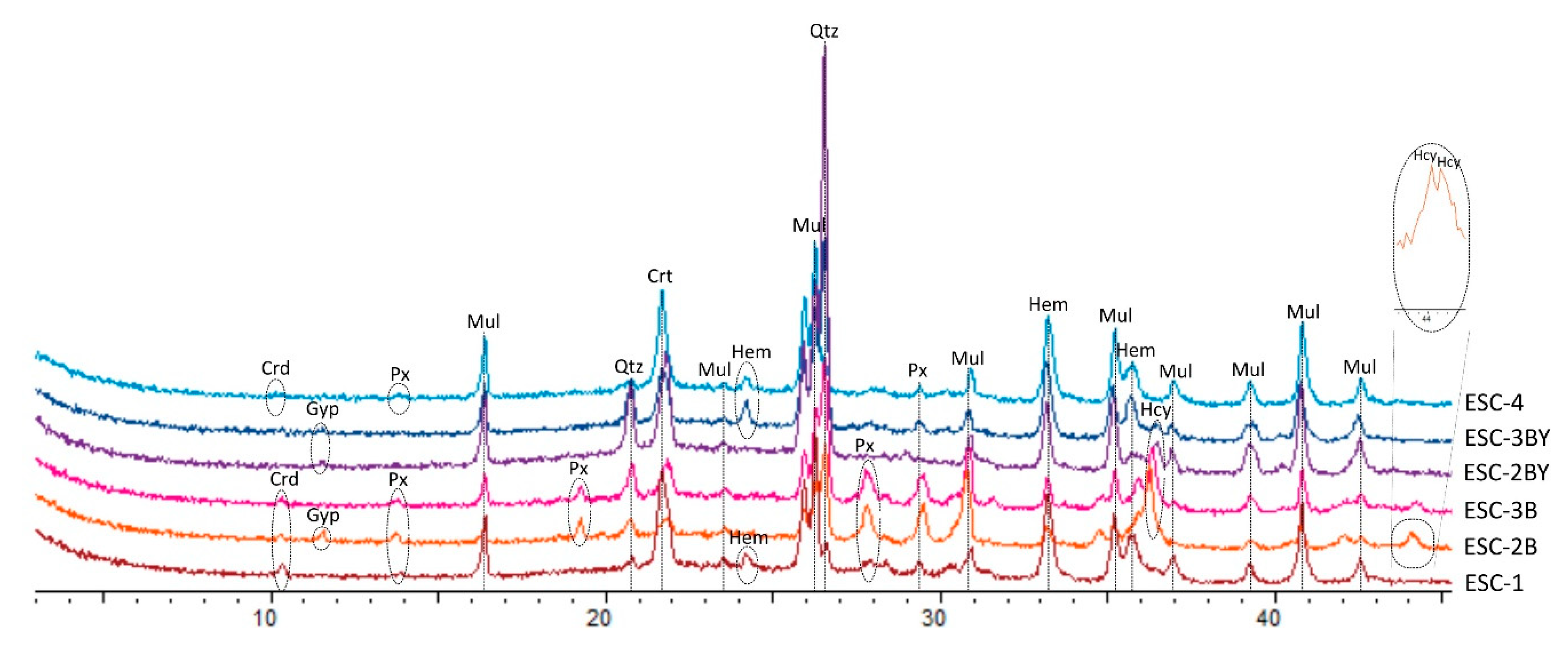
4.1. X-ray Diffraction (XRD): Qualitative Analysis and Mineral Quantification
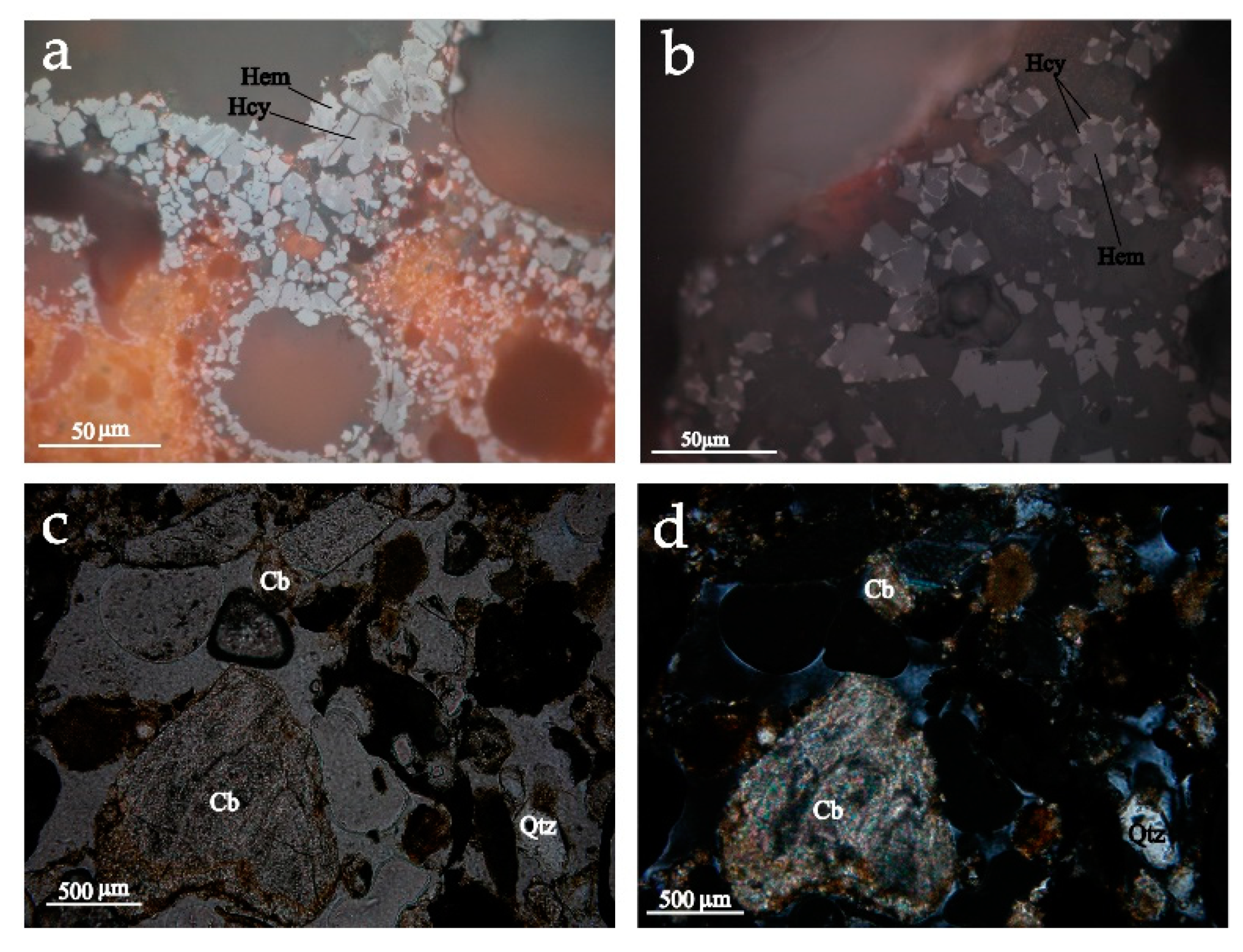
4.2. Optical Microscopy
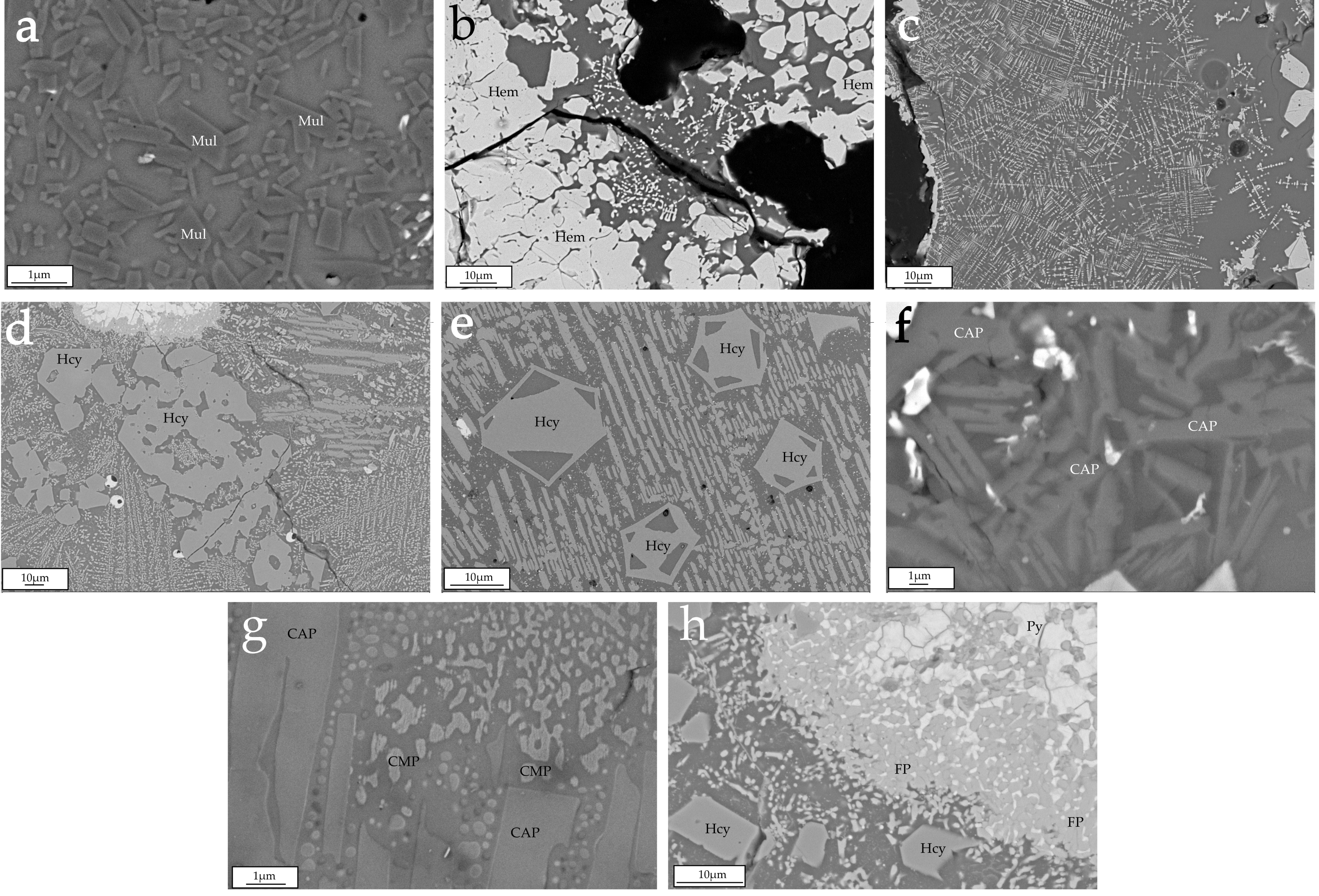
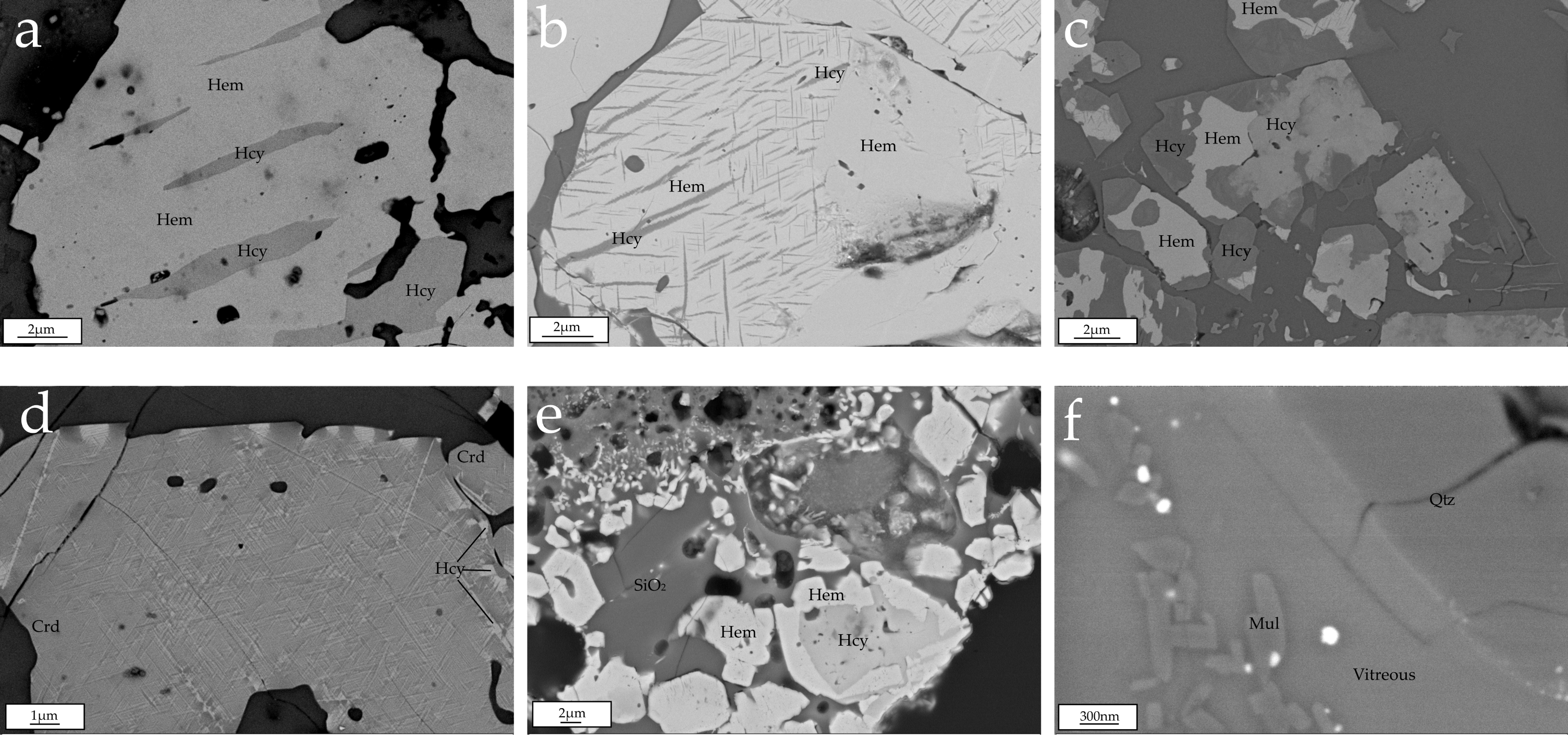
4.3. Field-Emission Scanning Electron Microscopy (FESEM)
4.3.1. Major Phases in the Natural Clinkers
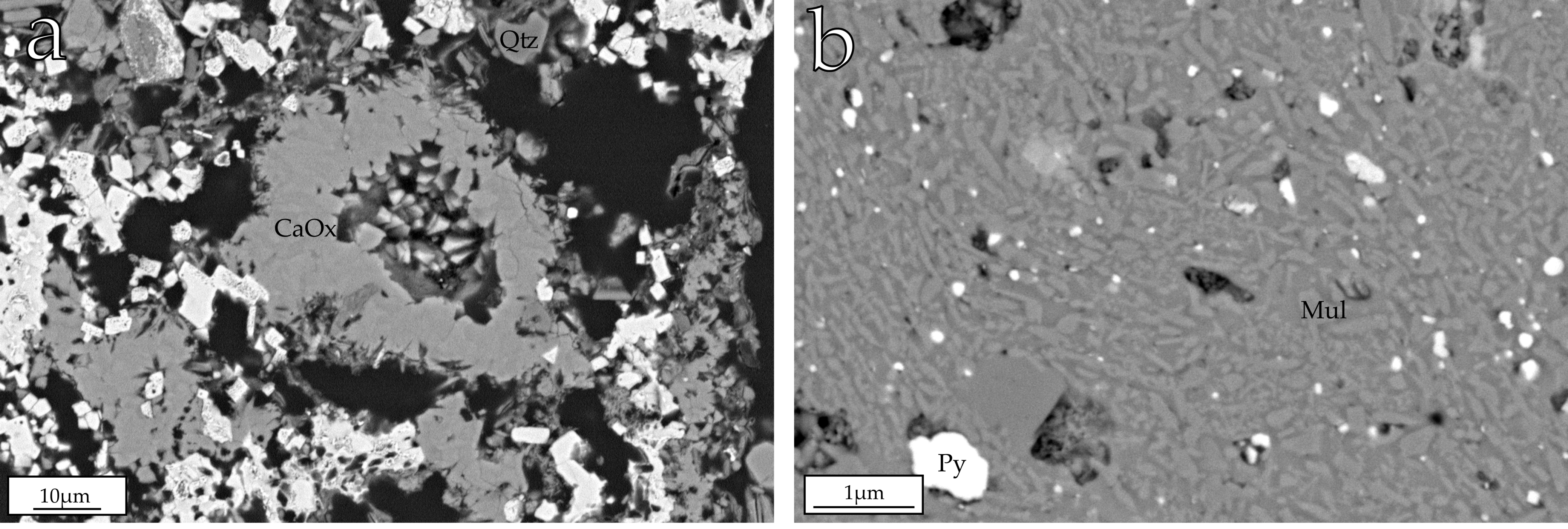
4.3.2. Minor Phases in the Natural Clinkers
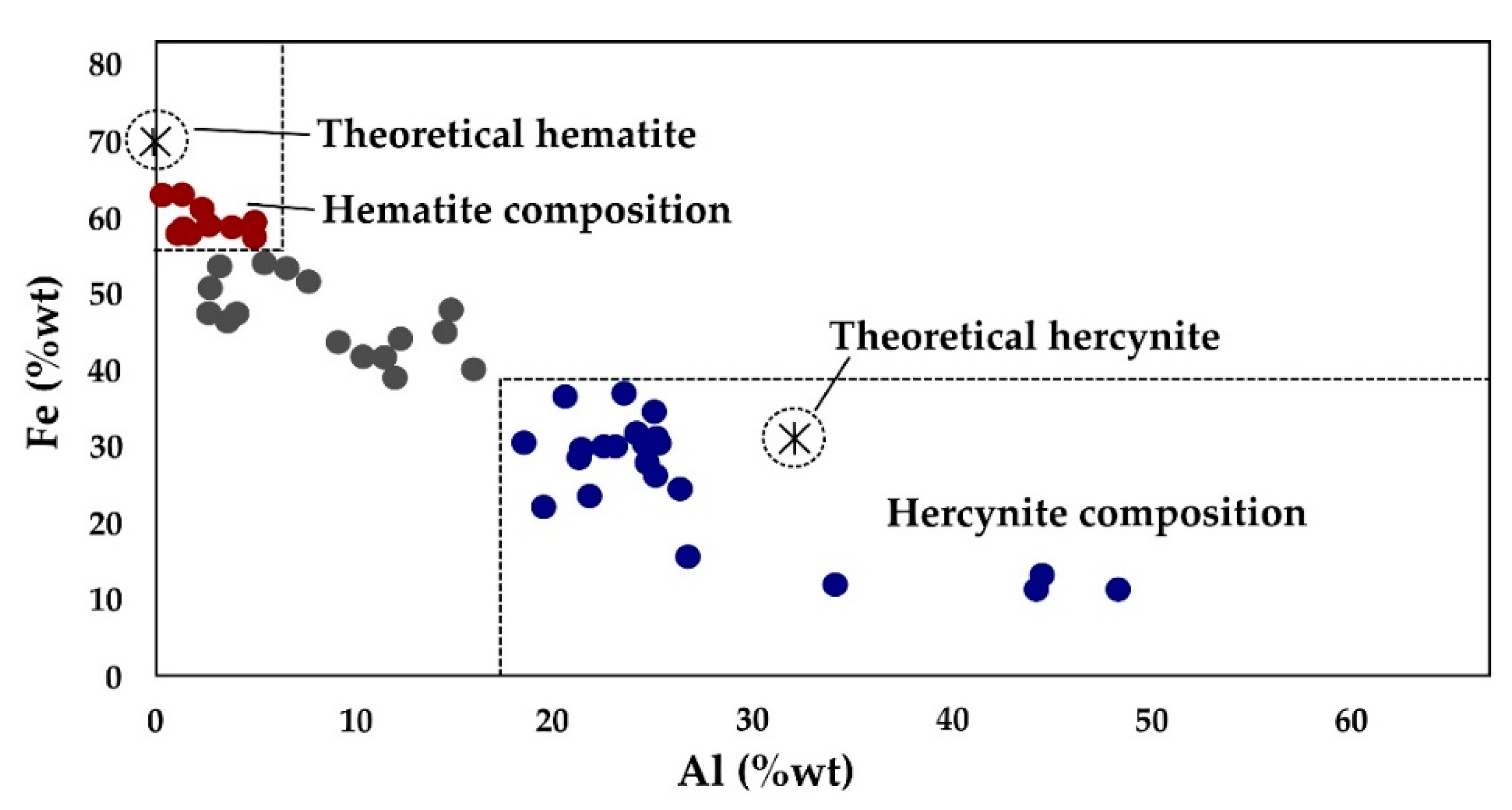
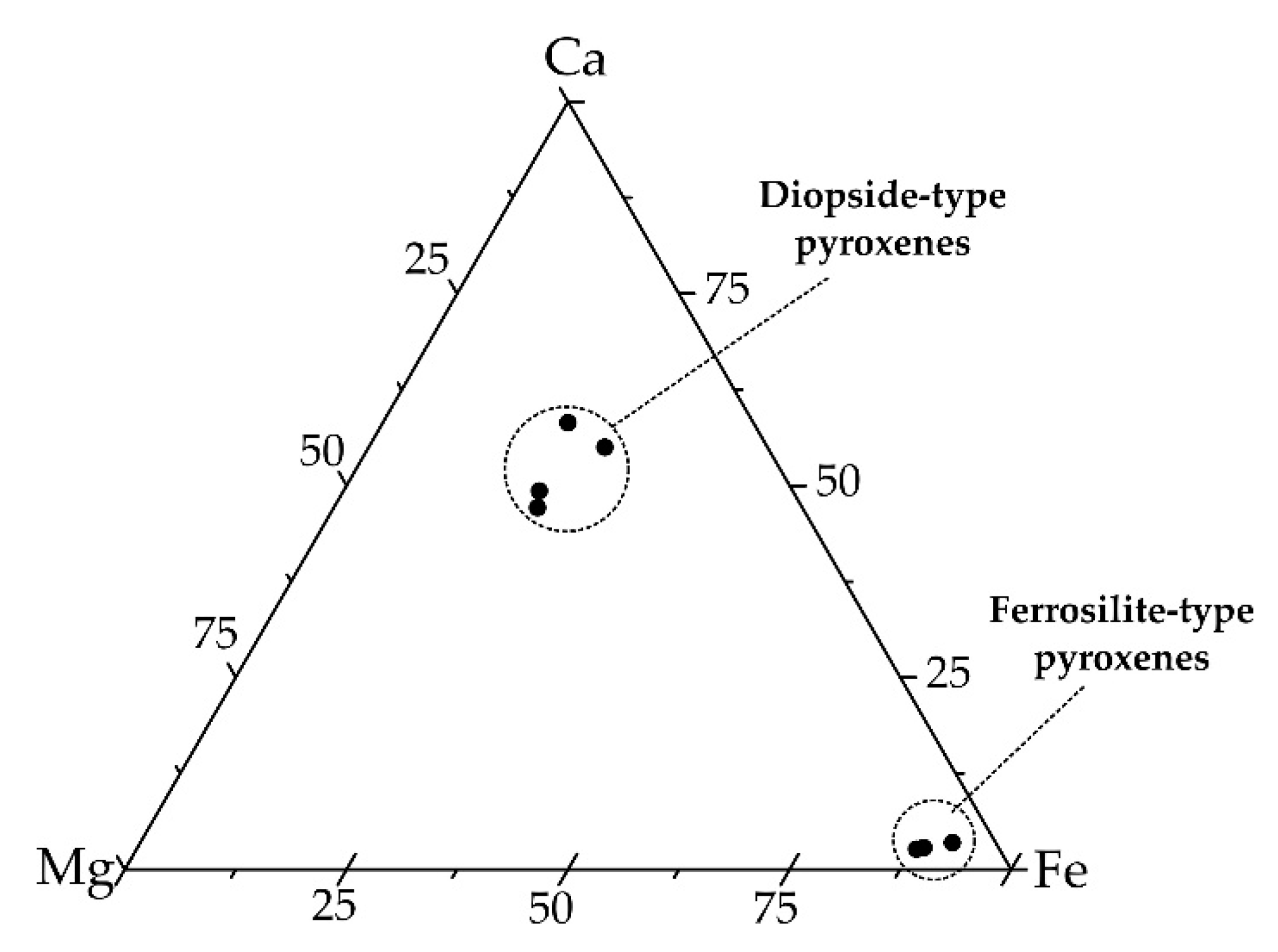
4.3.3. Chemical Analyses
5. Discussion
5.1. Destabilization of the Initial Phases
5.2. Crystallization of New Phases
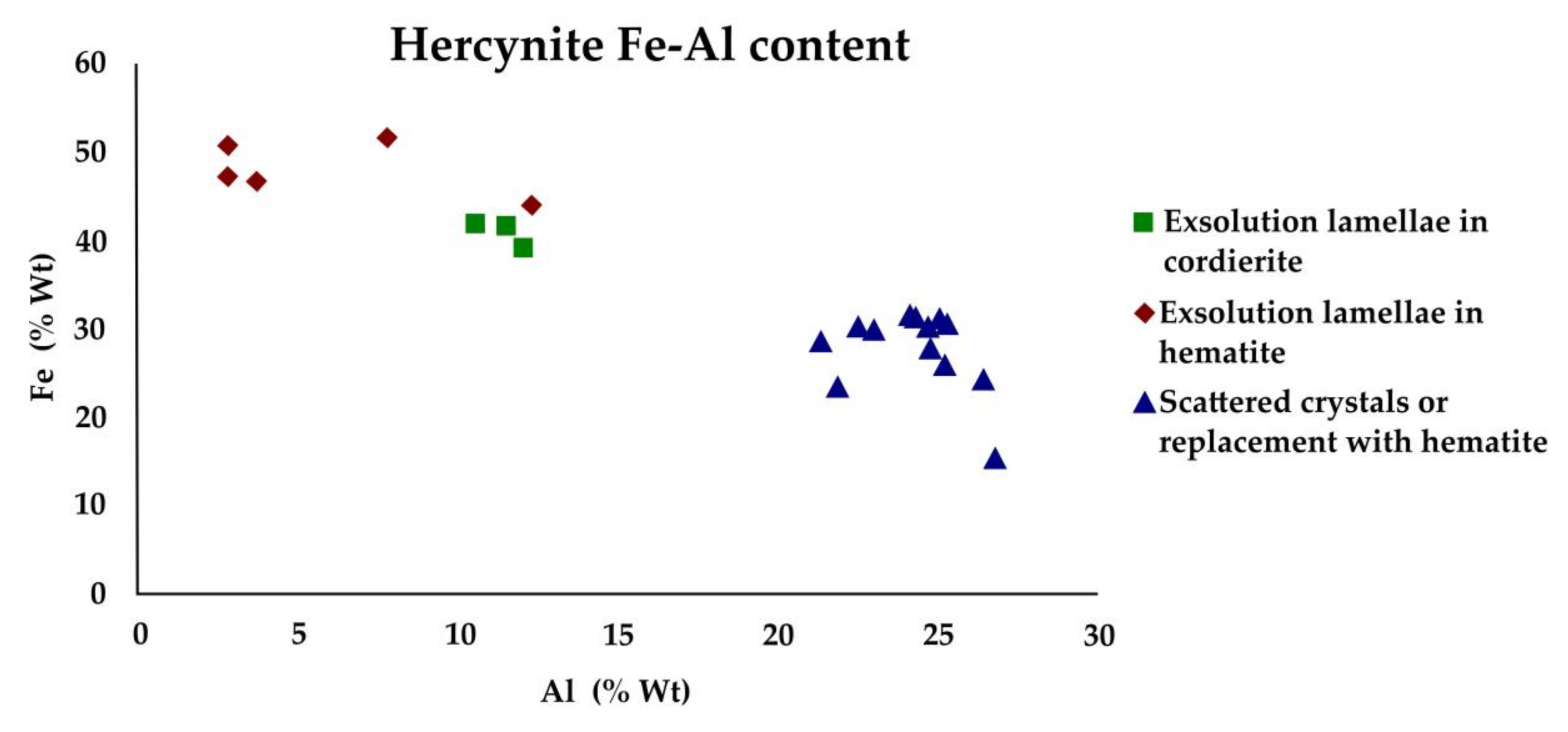
5.2.1. Hematite, Hercynite and Cordierite
5.2.2. Mullite and Pyroxenes
5.3. The Spontaneous Combustion of Coal and the Formation of the Vitreous Phase
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, M.A.; Glasser, D. Spontaneous combustion of carbonaceous stockpiles. Part I. The relative importance of various intrinsic coal properties and properties of the reaction system. Fuel 2005, 84, 1151–1160. [Google Scholar] [CrossRef]
- Querol, X.; Zhuang, X.; Font, O.; Izquierdo, M.; Alstuey, A.; Castro, I.; Van Drooge, B.L.; Moreno, T.; Grimalt, J.O.; Elvira, J.; et al. Influence of soil cover on reducing the environmental impact of spontaneous coal combustion in coal waste gobs: A review and new experimental data. Int. J. Coal Geol. 2011, 85, 2–22. [Google Scholar] [CrossRef]
- Misra, B.K.; Singh, B.D. Susceptibility to spontaneous combustion of Indian coals and lignites: an organic petrographic autopsy. Int. J. Coal Geol. 1994, 25, 265–286. [Google Scholar] [CrossRef]
- Querol, X.; Izquierdo, M.; Monfort, E.; Alvarez, E.; Font, O.; Moreno, T.; Alastuey, A.; Zhuang, X.; Lu, W.; Wang, Y. Environmental characterization of burnt coal gangue banks at Yangquan, Shanxi Province, China. Int. J. Coal Geol. 2008, 75, 93–104. [Google Scholar] [CrossRef]
- Quintero, J.A.; Candela, S.A.; Ríos, C.A.; Montes, C.; Uribe, C. Spontaneous combustion of the Upper Paleocene Cerrejón Formation coal and generation of Clinker in La Guajira Peninsula (Caribbean Region of Colombia. Int. J. Coal Geol. 2009, 80, 196–210. [Google Scholar] [CrossRef]
- Ribeiro, J.; Ferreira da Silva, E.; Flores, D. Burning of coal waste piles from Douro Coalfield (Portugal): Petrological, geochemical and mineralogical characterization. Int. J. Coal Geol. 2010, 81, 359–372. [Google Scholar] [CrossRef]
- Misz-Kennan, M.; Fabiańska, M. Thermal transformation of organic matter in coal waste from Rymer Cones (Upper Silesian Coal Basin, Poland). Int. J. Coal Geol. 2010, 81, 343–358. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Q.; Xiao, H.; Mao, Z.; Chen, P.; Sun, J. Prediction of coal stockpile autoignition delay time using micro-calorimeter technique. Fuel Process. Technol. 2013, 110, 86–93. [Google Scholar] [CrossRef]
- Xu, Y.L.; Wang, L.Y.; Tian, N.; Zhang, J.P.; Yu, M.G.; Delichatsios, M.A. Spontaneous combustion coal parameters for the Crossing-Point Temperature (CPT) method in a Temperature-Programmed System (TPS). Fire Saf. J. 2017, 91, 147–157. [Google Scholar] [CrossRef]
- Sinha, A.; Singh, V.K. Spontaneous Coal Seam Fires: A Global Phenomenon. In Spontaneous Coal Seam Fires: Mitigating a Global Disaster; Voigt, S., Rüter, H., Jiahong, L., Jayakumar, R., Eds.; ERSEC Ecological Book Series-4, Beijing, China, 29 November–1 December 2005; Tsinghua University Press: Beijing, China, 2005; pp. 41–66. [Google Scholar]
- Cosca, M.A.; Essene, E.J.; Geissman, J.W.; Simmons, W.B.; Coates, D.A. Pyrometamorphic rocks associated with naturally burned coal beds, Powder River Basin, Wyoming. Am. Mineral. 1989, 74, 85–100. [Google Scholar]
- Foit, F.F.; Hooper, R.L.; Rosenberg, P.E. An unusual pyroxene, melilite, and iron oxide mineral assemblage in a coal-fire buchite from Buffalo, Wyoming. Am. Mineral. 1987, 72, 137–147. [Google Scholar]
- Henao, J.A.; Carreño, A.M.; Quintero, J.A.; Candela, S.A.; Ríos, C.A.; Ramos, M.A.; Pinilla, J.A. Petrography and application of the Rietveld method to the quantitative analysis of phases of natural Clinker generated by coal spontaneous combustion. Earth Sci. Res. J. 2010, 14, 17–30. [Google Scholar]
- Sokol, E.; Volkova, N.; Lepezin, G. Mineralogy of pyrometamorphic rocks associated with naturally burned coal-bearing spoil-heaps of the Chelyabinsk coal basin, Russia. Eur. J. Mineral. 1998, 10, 1003–1014. [Google Scholar] [CrossRef]
- Ciesielczuk, J.; Misz-Kennan, M.; Hower, J.C.; Fabiańska, M.J. Mineralogy and geochemistry of coal wastes from the Starzykowiec coal-waste dump (Upper Silesia, Poland). Int. J. Coal. Geol. 2014, 127, 42–55. [Google Scholar] [CrossRef]
- Ribeiro, J.; Suárez-Ruiz, I.; Flores, D. Geochemistry of self-burning coal mining residues from El Bierzo Coalfield (NW Spain): Environmental implications. Int. J. Coal. Geol. 2016, 159, 155–168. [Google Scholar] [CrossRef]
- Baboolal, A.A.; Knight, J.; Wilson, B. Petrography and mineralogy of pyrometamorphic combustion metamorphic rocks associated with spontaneous oxidation of lignite seams of the Erin Formation, Trinidad. J. S. Am. Earth. Sci. 2018, 82, 181–192. [Google Scholar] [CrossRef]
- Alastuey, A.; Bastida, J.; Fernández-Turiel, J.L.; Querol, X.; Signes, M. Mineralogía de las arcillas calcinadas de la base de la Fm. Escucha en el área de Foz-Calanda. Cuadernos de Geología Ibérica 1993, 17, 171–184. [Google Scholar]
- Besteiro, J.; Bastida, J.; Amigó, J.M.; Lores, M.T.; López Buendía, A.; Serrano, F.J. Sobre análisis microestructural por DRX y condiciones de formación de mullitas naturales de la cuenca de Oliete (Teruel). Bol. Soc. Esp. Min. 1996, 19, 119–129. [Google Scholar]
- Laita, E.; Bauluz, B. Mineral and textural transformations in aluminium-rich clays during ceramic firing. Appl. Clay Sci. 2018, 152, 284–294. [Google Scholar] [CrossRef]
- Dondi, M.; Ercolani, G.; Fabbri, B.; Marsigli, M. An approach to the chemistry of pyroxenes formed during the firing of Ca-rich silicate ceramics. Clay Miner. 1998, 33, 443–452. [Google Scholar] [CrossRef]
- Bauluz, B.; Mayayo, M.J.; Yuste, A.; Fernandez-Nieto, C.; González López, J.M. Tem study of mineral transformations in fired carbonated clays: Relevance to brick making. Clay Miner. 2004, 39, 333–344. [Google Scholar] [CrossRef]
- Cosca, M.A.; Peacor, D.R. Chemistry and structure of esseneite (CaFe3+AlSiO6) a new pyroxene produced by pyrometamorphism. Am. Mineral. 1987, 72, 148–156. [Google Scholar]
- Sandiford, M.; Neall, F.B.; Powell, R. Metamorphic evolution of aluminous granulites from Labwor Hills, Uganda. Contrib. Mineral. Petrol. 1987, 95, 217–225. [Google Scholar] [CrossRef]
- Brotzu, P.; Gomes, C.B.; Melluso, L.; Morbidelli, L.; Norra, V.; Ruberti, E. Petrogenesis of coexisting SiO2-undersaturated to SiO2-oversaturated felsic igneous rocks: The alkaline complex of Itatiaia, southeastern Brazil. Lithos 1997, 40, 133–156. [Google Scholar] [CrossRef]
- Markl, G. Mullite-corundum-spinel-cordierite-plagioclase xenoliths in the Skaergaard Marginal Border Group: multi-stage interaction between metasediments and basaltic magma. Contrib. Mineral. Petrol. 2005, 149, 196–215. [Google Scholar] [CrossRef]
- Treiman, A.H. Amphibole and hecynite spinel in Shergotty and Zagami: Magmatic water, depth of crystallization, and metasomatism. Meteoritics. 1985, 20, 229–243. [Google Scholar] [CrossRef]
- Pardo, G. Estratigrafía y sedimentología de las formaciones detríticas del Cretácico inferior terminal del Bajo Aragón Turolense. Ph.D. Thesis, University of Zaragoza, Zaragoza, Spain, 1979. (In Spanish). [Google Scholar]
- Cervera, A.; Pardo, G.; Villena, J. Algunas precisiones litoestratigráficas sobre la Formación “lignitos de Escucha”. Tecniterrae 1976, 3, 25–33. [Google Scholar]
- Querol, X. Estudio geológico de la Formación Escucha en la Cueca del Maestrazgo, Cordillera Ibérica oriental. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, 1988. (In Spanish). [Google Scholar]
- Salas, R. El Malm i el Cretaci inferior entre el Massis de Garraf i la Serra d’Espadà: Anàlisi de conca. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, 1987. (In Catalan). [Google Scholar]
- Bauluz, B.; Mayayo, M.J.; Yuste, A.; González López, J.M. Genesis of kaolinite from Albian sedimentary deposits of the Iberian Range (NE Spain): analysis by XRD, SEM and TEM. Clay Miner. 2008, 43, 459–475. [Google Scholar] [CrossRef]
- Hillier, S. Quantitative analysis of clay and other minerals in sandstones by X-ray powder diffraction (XRPD). Int. Assoc. Sedimentol. Spec. Publ. 2003, 34, 213–251. [Google Scholar]
- Schwartz, K.B.; Leong, D.B.; McConville, R.L. Structural chemistry of synthetic cordierite: Evidence for solid solutions and disordered compositional domains in Bi-flux-grown Mg-cordierites. Phys. Chem. Miner. 1994, 20, 563–574. [Google Scholar] [CrossRef]
- Hill, R.J. X-ray powder diffraction profile refinement of synthetic hercynite. Am. Mineral. 1984, 69, 937–942. [Google Scholar]
- Johnson, J.S.; Clark, J.; Miller-Antonio, S.; Robins, D.; Schiffer, M.B.; Skibo, J.M. Effects of Firing Temperature on the Fate of Naturally Occurring Organic Matter in Clays. J. Archaeol. Sci. 1988, 15, 403–414. [Google Scholar] [CrossRef]
- Aras, A. The change of phase composition in kaolinita- and illite-rich clay-based ceramic bodies. Appl. Clay Sci. 2002, 24, 257–269. [Google Scholar] [CrossRef]
- Merriman, R.J.; Peacor, D.R. Very-low grade metapelites: Mineralogy, microfabrics and measuring reaction progress. In Low-Grade Metamorphism, 1st ed.; Frey, M., Robinson, D., Eds.; Blackwell Science: Hoboken, NJ, USA, 1998; pp. 10–60. [Google Scholar] [CrossRef]
- Ram, L.C.; Tripathi, P.S.M.; Mishra, S.P. Mössbauer spectroscopic studies on the transformations of iron-bearing minerals during combustion of coals: Correlation with fouling and slagging. Fuel Process. Technol. 1995, 42, 47–60. [Google Scholar] [CrossRef]
- Kapička, A.; Petrovsky, E.; Ustjak, S.; Macháčková, K. Proxy mapping of fly-ash pollution of soils around a coal-burning power plant: A case study in the Czech Republic. J. Geochem. Explor. 1999, 66, 291–297. [Google Scholar] [CrossRef]
- Floyd, M.; Czerewko, M.A.; Cripps, J.C.; Spears, D.A. Pyrite oxidation in Lower Lias Clay at concrete highway structures affected by thaumasite, Gloucestershire, UK. Cem. Concr. Compos. 2003, 25, 1015–1024. [Google Scholar] [CrossRef]
- Ritsema, C.J.; Groenenberg, J.E. Pyrite Oxidation, Carbonate Weathering, and Gypsum Formation in a Drained Potential Acid Sulfate Soil. Soil Sci. Soc. Am. J. 1993, 57, 968–976. [Google Scholar] [CrossRef]
- Estrada, S.; Piepjohn, K.; Frey, M.J.; Reinhardt, L.; Andruleit, H.; Von Gosen, W. Pliocene coal-seam fires on southern Ellesmere Island, Canadian Artic. N. Jb. Geol. Paläont. 2009, 251, 33–52. [Google Scholar] [CrossRef]
- Müke, A. Magnetite, ilmenite and ulvite in rocks and ore deposits: petrography, microprobe analyses and genetic implications. In Mineralogy and Petrology, 1st ed.; Nasdala, L., Broekmans, M.A.T.M., Eds.; Spinger: Viena, Austria, 2003; Volume 77, pp. 215–234. [Google Scholar] [CrossRef]
- Smith, D.G.W. The chemistry and mineralogy of some emery-like rocks from Sithean Sluaigh, Strachur, Argyllshire. Am. Mineral. 1965, 50, 1982–2022. [Google Scholar]
- Stoddar, E.F. Zinc-rich hercynite in high-grade metamorphic rocks: A product of the dehydration of staurolite. Am. Mineral. 1979, 64, 736–741. [Google Scholar]
- Ridolfi, F.; Braga, R.; Cesare, B.; Renzulli, A.; Perugini, D.; Del Moro, S. Unravelling the complex interaction between mantle and crustal magmas encoded in the lavas of San Vincenzo (Tuscany, Italy). Part I: Petrography and Thermobarometry. Lithos. 2016, 244, 218–232. [Google Scholar] [CrossRef]
- Berg, R. Tungsten skarn mineralizations in a regional metamorphic terrain in northern Norway: A possible metamorphic ore deposit. Miner. Deposita 1991, 26, 281–289. [Google Scholar] [CrossRef]
- Trindade, M.J.; Dias, M.I.; Coroado, J.; Rocha, F. Mineralogical transformations of calcareous rich clays with firing: A comparative study between calcite and dolomite rich clays from Algarve, Portugal. Appl. Clay. Sci. 2009, 42, 345–355. [Google Scholar] [CrossRef]
- D’Souza, M.; Keshava Prasad, A.V.; Ravindra, R. Genesis of Ferropotassic A-Type Granitoids of Mühlig-Hofmannfjella, Central Dronning Maud Land, East Antarctica. In Antarctica: Contributions to Global Earth Sciences, 1st ed.; Fütterer, D.K., Damaske, D., Kleinschmidt, G., Miller, H., Tessensohn, F., Eds.; Springer: Berlin/Heidelberg Germany, 2006; pp. 45–54. [Google Scholar] [CrossRef]
- Huffman, G.P.; Huggins, F.E.; Shah, N. Behavior of basic elements during coal combustion. Prog. Energ. Combust. 1990, 16, 243–251. [Google Scholar] [CrossRef]

| Sample | Mineral Phase (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Quartz | Hematite | Mullite | Cristobalite | Hercynite | Cordierite | Gypsum | Others | |
| ESC-1 | 1.3 | 7.5 | 27.9 | 2.8 | - | 2.2 | - | 58.3 |
| ESC-2B | 3.2 | 4.1 | 16.3 | 1.9 | 5 | 1.3 | 7.6 | 60.6 |
| ESC-2BY | 6.9 | 5.2 | 33.5 | 4.5 | 1.3 | - | 3.3 | 45.3 |
| ESC-3B | 4.6 | 3.7 | 27.8 | 2.6 | 4.3 | 2.5 | - | 54.5 |
| ESC-3BY | 6.9 | 10 | 38 | 4.4 | 1.4 | - | 5.9 | 33.4 |
| ESC-4 | 1.7 | 9.5 | 35.4 | 5.5 | - | 1 | - | 46. 9 |
| Analyzed Phases | wt % | Calculated Average Formula | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | Na | Mg | Al | Si | K | Ca | Ti | Fe | ||
| Hematite (n = 14) | 37.7 | - | - | 2.3 | - | - | - | - | 59.6 | Al0.1Fe1.9O3 |
| (1.7) | - | - | (1.5) | - | - | - | - | (1.8) | ||
| Hercynite (n = 21) | 44.6 | - | 1.8 | 27 | - | - | - | 0.4 | 26.1 | Mg0.1Fe0.8Al1.7O4 |
| (3.8) | - | (1.8) | (7.6) | - | - | - | (0.2) | (7.0) | ||
| Mullite (n = 23) | 51.3 | - | - | 21.4 | 25.9 | - | - | 0.3 | 1.1 | Fe0.1Al2.3Si2.9O9.75 |
| (9.1) | - | - | (5.5) | (4.9) | - | - | (0.1) | (0.9) | ||
| ”Ceramic pyroxenes” (n = 9) | 53.7 | 0.4 | 0.1 | 12.6 | 22.8 | 0.4 | 8.5 | 0.1 | 1.5 | Ca0.5Fe0.1Al1(Al0.1Si1.9)O6 |
| (0.9) | (0.2) | (0.2) | (1.3) | (2.2) | (0.3) | (1.5) | (0.1) | (0.9) | ||
| Cordierite (n = 3) | 45.6 | - | 1.4 | 16 | 5.4 | - | - | 0.8 | 30.9 | (Mg0.5Fe1.6)(Fe1.4Ti0.1)(Si2.6Al2.4)Al2.8O18 |
| (2.2) | - | (0.0) | (0.1) | (3.8) | - | - | (0.1) | (5.8) | ||
| Silica phases (n = 4) | 50.7 | - | - | 1.4 | 48 | - | - | - | - | Al0.1Si0.9O2 |
| (10.1) | - | - | (1.3) | (10.6) | - | - | - | - | ||
| Fe-pyroxenes (n = 3) | 45.6 | - | 1.1 | 4.1 | 18.8 | - | 0.7 | 0.3 | 29.4 | Al0.1Mg0.1Fe1.3(Al0.3Si1.7)O6 |
| (0.2) | - | (0.3) | (0.1) | (0.4) | - | (0.1) | (0.1) | (0.6) | ||
| Ca–Mg pyroxenes (n = 4) | 54.1 | - | 2 | 5.2 | 27.4 | - | 6.9 | 0.1 | 4.3 | (Al0.4Mg0.2Fe0.2Ca0.4)Si2.3O6 |
| (0.3) | - | (0.6) | (1.0) | (1.0) | - | (0.6) | 0 | (0.6) | ||
| Vitreous phase (n = 25) | 48.8 | 0.2 | 1.1 | 11.6 | 30.9 | 2.4 | 1.4 | 0.2 | 2.9 | |
| (9.6) | (0.2) | (1.6) | (7.3) | (6.5) | (1.3) | (1.6) | (0.2) | (2.7) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laita, E.; Bauluz, B.; Yuste, A. High-Temperature Mineral Phases Generated in Natural Clinkers by Spontaneous Combustion of Coal. Minerals 2019, 9, 213. https://doi.org/10.3390/min9040213
Laita E, Bauluz B, Yuste A. High-Temperature Mineral Phases Generated in Natural Clinkers by Spontaneous Combustion of Coal. Minerals. 2019; 9(4):213. https://doi.org/10.3390/min9040213
Chicago/Turabian StyleLaita, Elisa, Blanca Bauluz, and Alfonso Yuste. 2019. "High-Temperature Mineral Phases Generated in Natural Clinkers by Spontaneous Combustion of Coal" Minerals 9, no. 4: 213. https://doi.org/10.3390/min9040213
APA StyleLaita, E., Bauluz, B., & Yuste, A. (2019). High-Temperature Mineral Phases Generated in Natural Clinkers by Spontaneous Combustion of Coal. Minerals, 9(4), 213. https://doi.org/10.3390/min9040213





