U-Pb Ages, O Isotope Compositions, Raman Spectrum, and Geochemistry of Cassiterites from the Xi’ao Copper-Tin Polymetallic Deposit in Gejiu District, Yunnan Province
Abstract
:1. Introduction
2. Geological Setting
3. Ore Geology of the Xi’ao Deposit
4. Samples and Analytical Methods
5. Results
5.1. Color and CL Images
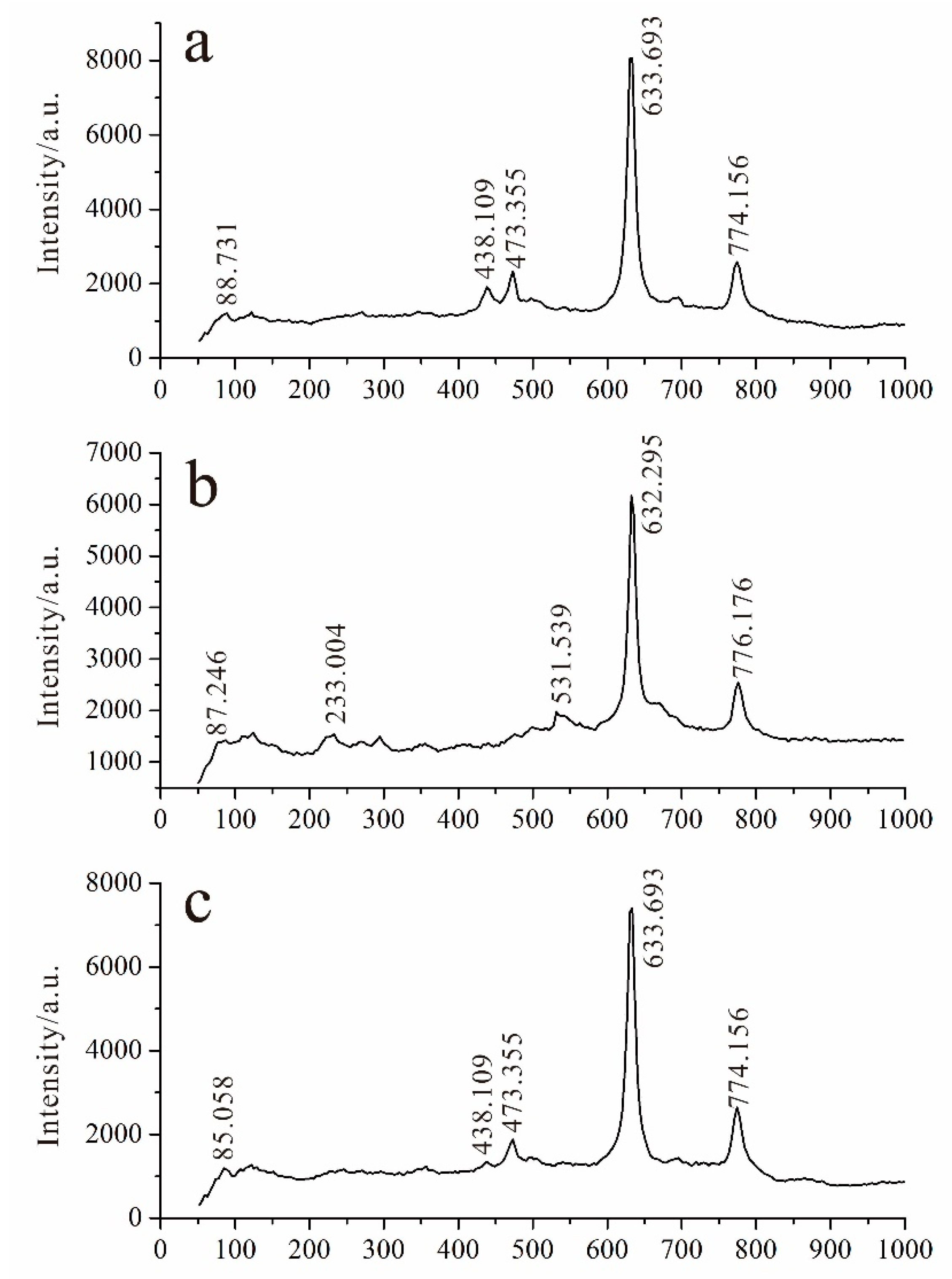
5.2. Raman Spectrum
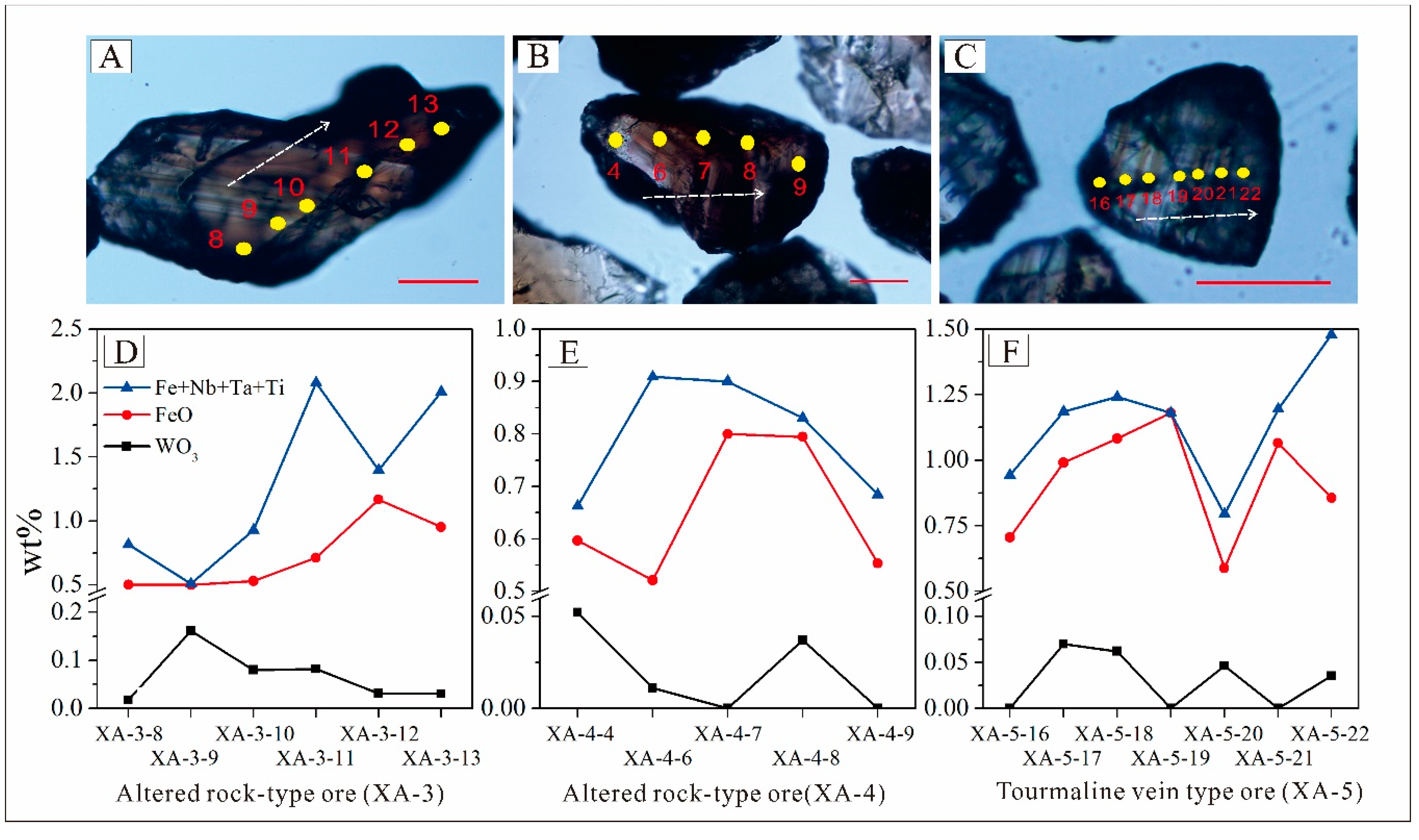
5.3. Cassiterite Composition
5.4. Oxygen Isotopes
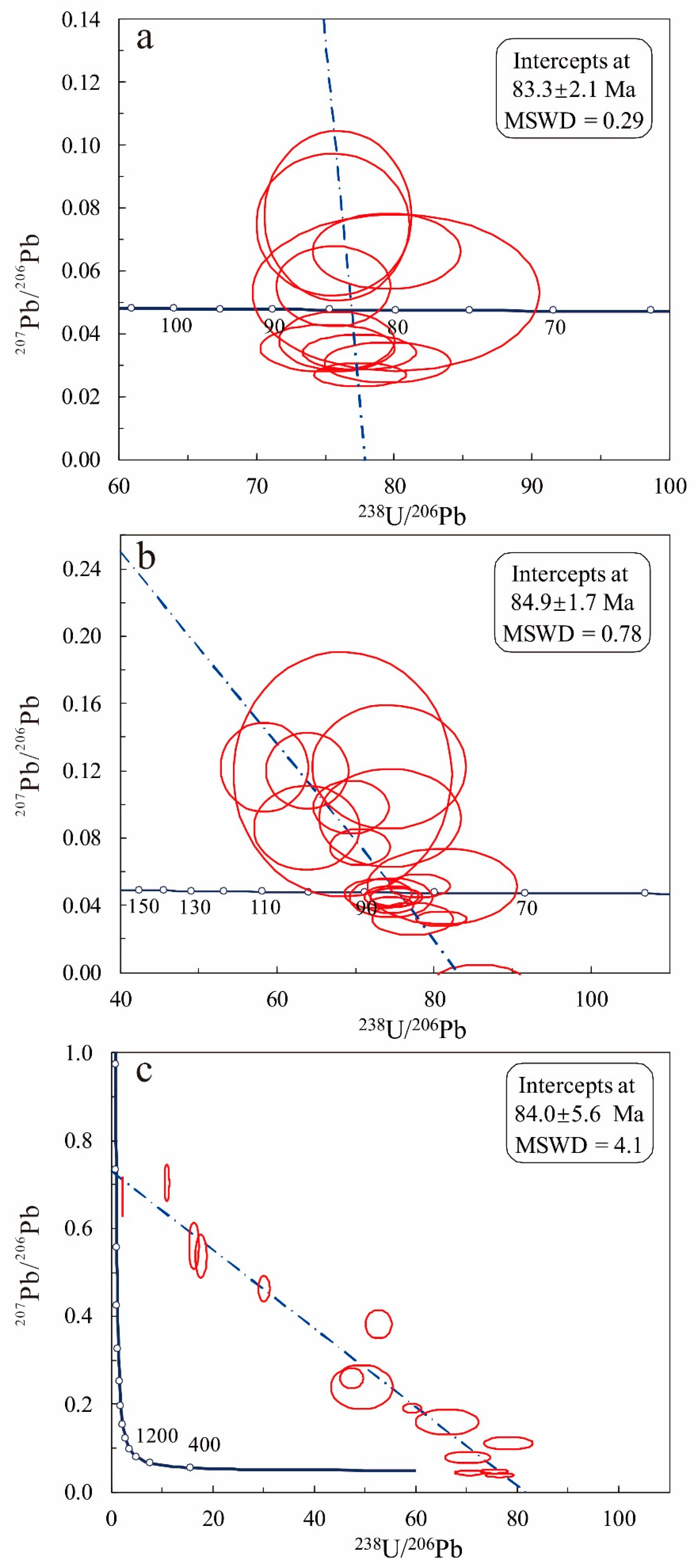
5.5. U-Pb Ages
6. Discussion
6.1. Timing of Sn Mineralization
6.2. Color, CL Images and Raman Spectrum
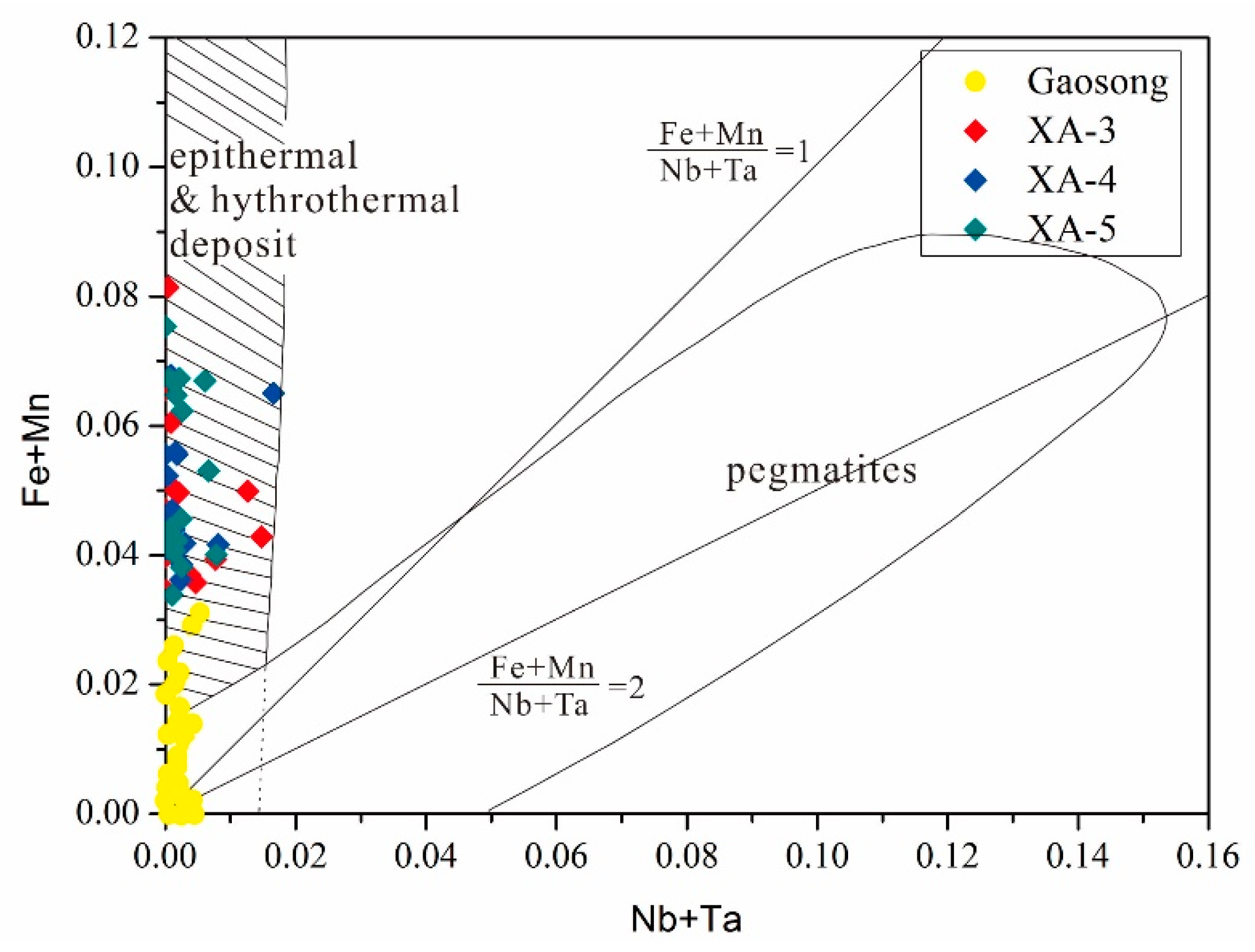
6.3. Metallogenic Conditions
6.4. Ore Genesis of the Xi’ao Deposit
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plimer, I.R.; Lu, J.; Kleeman, J.D. Trace and rare earth elements in cassiterite-sources of components for the tin deposits of the Mole Granite, Australia. Miner. Depos. 1991, 26, 267–274. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Yu, J.M.; Lu, J.J. Trace and rare-earth element geochemistry in tourmaline and cassiterite from the Yunlong tin deposit, Yunnan, China: Implication for migmatitic-hydrothermal fluid evolution and ore genesis. Chem. Geol. 2004, 209, 193–213. [Google Scholar] [CrossRef]
- Moore, F.; Howie, R.A. Geochemistry of some Cornubian cassiterites. Miner. Depos. 1979, 14, 103–107. [Google Scholar] [CrossRef]
- Murciego, A.; Sanchez, A.G.; Dusausoy, Y.; Pozas, J.M.M.; Ruck, R. Geochemistry and EPR of cassiterites from the Iberian Hercynian Massif. Mineral. Mag. 1997, 61, 357–365. [Google Scholar] [CrossRef]
- Neiva, A.M.R. Geochemistry of cassiterite and its inclusions and exsolution products from tin and tungsten deposits in Portugal. Can. Mineral. 1996, 34, 745–768. [Google Scholar]
- Neiva, A.M.R. Geochemistry of cassiterite and wolframite from tin and tungsten quartz veins in Portugal. Ore Geol. Rev. 2008, 33, 221–238. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Chen, B.; Ma, X.H. In situ LA-ICP-MS U-Pb age and geochemical data of cassiterite of the Furong tin deposit, the Nanling Range: Implications for the origin and evolution of the ore-forming fluid. Chin. Sci. Bull. 2014, 59, 2505–2519. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, D.L.; Peng, J.T.; Hu, R.Z.; Yuan, S.D.; Zheng, D.S. The Closure of U-Pb System in Cassiterite and its Reliability for Dating. Geol. Rev. 2011, 57, 549–554. (In Chinese) [Google Scholar]
- Gulson, B.L.; Jones, M.T. Cassiterite: Potential for direct dating of mineral deposits and a precise age for the Bushveld complex granites. Geology 1992, 20, 355–358. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhang, R.Q.; Ding, X.; Ling, M.X.; Fan, W.M.; Sun, W.D. Dating cassiterite using laser ablation ICP-MS. Ore Geol. Rev. 2016, 72, 313–322. [Google Scholar] [CrossRef]
- Liu, Y.P.; Li, Z.X.; Li, H.M.; Guo, L.G.; Xu, W.; Ye, L.; Li, C.Y.; Pi, D.H. U-Pb geochronology of cassiterite and zircon from the DuLong Sn-Zn deposit: Evidence for Cretaceous large-scale granitic magmatism and mineralization events in southeastern Yunnan province, China. Acta Petrol. Sin. 2007, 23, 967–976. (In Chinese) [Google Scholar]
- Wang, X.J.; Liu, Y.P.; Liao, Y.L.; Bao, T.; Ye, L.; Zhang, Q. In-situ LA-MC-ICP-MS cassiterite U-Pb dating of Dulong Sn-Zn polymetallic deposit and its significance. Acta Pet. Sin. 2014, 30, 867–876. (In Chinese) [Google Scholar]
- Yuan, S.D.; Peng, J.T.; Hu, R.Z.; Li, H.M.; Shen, N.P.; Zhang, D.L. A precise U-Pb age on cassiterite from the Xianghualing tin-polymetallic deposit (Hunan, South China). Miner. Depos. 2008, 43, 375–382. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, R.Q.; Li, C.Y.; Sun, W.D.; Hu, Y.B.; Kang, D.M.; Wu, J.D. Genesis of the Gaosong Sn–Cu deposit, Gejiu district, SW China: Constraints from in situ LA-ICP-MS cassiterite U–Pb dating and trace element fingerprinting. Ore Geol. Rev. 2018, 92, 627–642. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Lehmann, B.; Seltmann, R.; Sun, W.D.; Li, C.Y. Cassiterite U–Pb geochronology constrains magmatic-hydrothermal evolution in complex evolved granite systems: The classic Erzgebirge tin province (Saxony and Bohemia). Geology 2017, 45, 1095–1098. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Lu, J.J.; Lehmann, B.; Li, C.Y.; Li, G.L.; Zhang, L.P.; Guo, J.; Sun, W.D. Combined zircon and cassiterite U–Pb dating of the Piaotang granite-related tungsten–tin deposit, southern Jiangxi tungsten district, China. Ore Geol. Rev. 2017, 82, 268–284. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Lu, J.J.; Wang, R.C.; Yang, P.; Zhu, J.C.; Yao, Y.; Gao, J.F.; Li, C.; Lei, Z.H.; Zhang, W.L.; et al. Constraints of in situ zircon and cassiterite U–Pb, molybdenite Re–Os and muscovite 40Ar–39Ar ages on multiple generations of granitic magmatism and related W–Sn mineralization in the Wangxianling area, Nanling Range, South China. Ore Geol. Rev. 2015, 65, 1021–1042. [Google Scholar] [CrossRef]
- Yuan, S.D.; Peng, J.T.; Hao, S.; Li, H.M.; Geng, J.Z.; Zhang, D.L. In situ LA-MC-ICP-MS and ID-TIMS U–Pb geochronology of cassiterite in the giant Furong tin deposit, Hunan Province, South China: New constraints on the timing of tin–polymetallic mineralization. Ore Geol. Rev. 2011, 43, 235–242. [Google Scholar] [CrossRef]
- Zhang, J.B.; Ding, J.H.; Nan, G.L. The characteristics and potential of tin resources in China. Geol. Chin. 2015, 42, 839–852. (In Chinese) [Google Scholar]
- Chen, Y.X.; Li, H.; Sun, W.D.; Ireland, T.; Tian, X.F.; Hu, Y.B.; Yang, W.B.; Chen, C.; Xu, D.R. Generation of late meosozic qianlishan A2-type granite in Nanling Range: Implications for Shizhuyuan W-Sn mineralization and tectonic evolution. Lithos 2016, 266–267, 435–452. [Google Scholar] [CrossRef]
- Bucci, L.A.; McNaughton, N.J.; Fletcher, I.R.; Groves, D.I.; Kositcin, N.; Stein, H.J.; Hagemann, S.G. Timing and duration of high-temperature gold mineralization and spatially associated granitoid magmatism at Chalice, Yilgarn craton, western Australia. Econ. Geol. 2004, 99, 1123–1144. [Google Scholar] [CrossRef]
- Chen, X.C.; Hu, R.Z.; Bi, X.W.; Li, H.M.; Lan, J.B.; Zhao, C.H.; Zhu, J.J. Cassiterite LA-MC-ICP-MS U/Pb and muscovite 40Ar/39Ar dating of tin deposits in the Tengchong-Lianghe tin district, NW Yunnan, China. Miner. Depos. 2014, 49, 843–860. [Google Scholar] [CrossRef]
- Cheng, Y.B.; Mao, J.W.; Rusk, B.; Yang, Z.X. Geology and genesis of Kafang Cu-Sn deposit, Gejiu district, SW China. Ore Geol. Rev. 2012, 48, 180–196. [Google Scholar] [CrossRef]
- Cheng, Y.B.; Mao, J.W.; Chang, Z.S.; Pirajno, F. The origin of the world class tin-polymetallic deposits in the Gejiu district, SW China: Constraints from metal zoning characteristics and 40Ar–39Ar geochronology. Ore Geol. Rev. 2013, 53, 50–62. [Google Scholar] [CrossRef]
- Qin, D.X.; Liu, Y.S.; Tan, S.C.; Chen, A.B.; Xue, C.D.; Fan, Z.G.; Dang, Y.T.; Tong, X.; Wu, J.D.; Li, Y.X.; et al. Metallogenic ages of Gejiu tin ore deposit in Yunnan Province. Chin. J. Geol. 2006, 41, 122–132. (In Chinese) [Google Scholar]
- Yang, Z.X.; Mao, J.W.; Chen, M.H.; Tong, X.; Wu, J.D.; Cheng, Y.B.; Zhao, H.J. Re-Os dating of molybdenite from the Kafang skarn copper (tin) deposit in the Gejiu tin polymetallic ore district and its geological significance. Acta Pet. Sin. 2008, 24, 1937–1944. (In Chinese) [Google Scholar]
- Qin, D.X.; Li, Y.S.; Fan, Z.G.; Chen, A.B.; Tan, S.C.; Hong, T.; Li, L.J.; Lin, X.P. The geochemistry and mineralization evolvement of Gejiu tin ore deposits. Eng. Sci. 2006, 8, 30–39. (In Chinese) [Google Scholar]
- Li, B.L.; Zhu, D.Q.; Xing, X.F.; Zhao, L.; Jia, L.H.; Liu, Y.H. 40Ar/39Ar geochronologic study of mica in the Gejiu tin-copper polymetallic ore district, Yunnan province, and its geological significance. Geol. Bull. China 2015, 34, 2315–2324. (In Chinese) [Google Scholar]
- Chen, S.Y.; Zhao, P.D.; Tong, X.; Wu, J.D.; Mo, G.P.; Chen, X.S. Metallogenic characteristics of westem low altered tin-copper polymetallic deposit and its prospecting significance in east part of Gejiu, Yunnan. Earth Sci. J. China Univ. Geosci. 2011, 36, 277–281. (In Chinese) [Google Scholar]
- Chen, S.Y.; Zhao, P.D.; Tong, X.; Wu, J.D.; Mo, G.P. Mineralizing multiformity and deep prospecting of Gejiu Super Sn-Cu multi-metal deposit, Yunnan, China. Earth Sci. J. China Univ. Geosci. 2009, 34, 319–324. (In Chinese) [Google Scholar]
- Lü, M.; Tan, S.C.; Hao, S.; Li, H.M.; Zhang, Y.H.; Chen, K.Z.; Guo, X.Y. Mineralogical study of cassiterite grains from the Gejiu tin deposit. Northewest. Geol. 2016, 49, 101–108. (In Chinese) [Google Scholar]
- Shi, T.Z. Typomorphic features of cassiterite crystals in Gejiu Laochang deposit and its significance for determining the genetic type of deposit and finding ore shoot in Laochang deposit. Yunnan Metall. 1980, 45–48, 64. (In Chinese) [Google Scholar]
- Shi, T.Z. Typomorphic features of cassiterite crystals in Lao-chang mining area and their geological significance. Geochimica 1980, 2, 200–205. (In Chinese) [Google Scholar]
- Yin, C.Y. The typomorphic characteristic of cassiterites and its application in the study of the source of placer tin deposit. Geol. Prosp. 1981, 11, 24–27. (In Chinese) [Google Scholar]
- Guo, J.; Zhang, R.Q.; Sun, W.D.; Li, C.Y. LA-ICP-MS U-Pb geochronology of cassiterite in the Gejiu tin-polymetallic deposit, Yunnan province. Acta Mineral. Sin. 2015, s1, 698. (In Chinese) [Google Scholar]
- Cheng, Y.B.; Spandler, C.; Kemp, A.; Mao, J.W.; Rusk, B.; Hu, Y.; Blake, K. Controls on cassiterite (SnO2) crystallization: Evidence from cathodoluminescence, trace-element chemistry, and geochronology at the Gejiu Tin District. Am. Mineral. 2019, 104, 118–129. [Google Scholar] [CrossRef]
- Southwest Metallurgical Geological Prospecting Company, Ministry of Metallurgical Industry. Geology of Tin Deposits in the Gejiu Area, 1st ed.; Metallurgical Industry Publishing House: Beijing, China, 1984; pp. 1–100. (In Chinese) [Google Scholar]
- Zhuang, Y.Q.; Wang, R.Z.; Yang, S.P.; Yin, J.M. Gejiu Tin (Cu) Polymetallic Ore Deposit in Yunnan; Seismological Press House: Beijing, China, 1996; pp. 1–184. (In Chinese) [Google Scholar]
- Wang, Q.; Li, J.W.; Jian, P.; Zhao, Z.H.; Xiong, X.L.; Bao, Z.W.; Xu, J.F.; Li, C.F.; Ma, J.L. Alkaline syenites in eastern Cathaysia (South China): Link to Permian–Triassic transtension. Earth Planet. Sci. Lett. 2005, 230, 339–354. [Google Scholar] [CrossRef]
- Mao, J.W.; Cheng, Y.B.; Guo, C.L.; Yang, Z.X.; Feng, J.R. Gejiu tin polymetallic ore-field: Deposit model and discussion for several points concerned. Acta Geol. Sin. 2008, 82, 1455–1467. (In Chinese) [Google Scholar]
- Cheng, Y.B.; Mao, J.W.; Xie, G.Q.; Chen, M.H.; Zhao, C.S.; Yang, Z.X.; Zhao, H.J.; Li, X.Q. Petrogenesis of the Laochang-Kafang granite in the Gejiu Area, Yunnan Province: Constraints from geochemistry and zircon U-Pb dating. Acta Geol. Sin. 2008, 82, 1478–1493. (In Chinese) [Google Scholar]
- Cheng, Y.B.; Mao, J.W.; Xie, G.Q.; Chen, M.H.; Yang, Z.X. Zircon U-Pb dating of granites in Gejiu superlarge tin polymetallic orefield and its significance. Miner. Depos. 2009, 3, 297–312. (In Chinese) [Google Scholar]
- Cheng, Y.B.; Mao, J.W. Age and geochemistry of granites in Gejiu area, Yunnan province, SW China: Constraints on their petrogenesis and tectonic setting. Lithos 2010, 258–276. [Google Scholar] [CrossRef]
- Li, X.L.; Mao, J.W.; Cheng, Y.B.; Zhang, J. Petrogenesis of the Gaofengshan granite in Gejiu area, Yunnan Province: Zircon U–Pb dating and geochemical constraints. Acta Petrol. Sin. 2012, 183–198. (In Chinese) [Google Scholar]
- Zhang, J.W.; Dai, C.G.; Huang, Z.L.; Luo, T.Y.; Qian, Z.K.; Zhang, Y. Age and petrogenesis of Anisian magnesian alkali basalts and their genetic association with the Kafang stratiform Cu deposit in the Gejiu supergiant tin-polymetallic district, SW China. Ore Geol. Rev. 2015, 69, 403–416. [Google Scholar] [CrossRef]
- Chen, Y.C.; Zhu, Y.S. Mineral Deposits of China; Geological Publishing House: Beijing, China, 1993; pp. 209–211. (In Chinese) [Google Scholar]
- Suo, S.T.; Bi, X.H.; Zhou, H.W. Very Low Grade Metamorphism; Geological Publishing House: Beijing, China, 1999; pp. 1–68. (In Chinese) [Google Scholar]
- Yan, D.P.; Zhou, M.F.; Wang, Y.; Wang, C.L.; Zhao, T.P. Structural styles and chronological evidences from Dulong–Song Chay tectonic dome: Earlier spreading of south china sea basin due to late mesozoic to early cenozoic extension of south China block. Earth Sci. J. China Univ. Geosci. 2005, 30, 402–412. (In Chinese) [Google Scholar]
- Liu, S.; Su, W.C.; Hu, R.Z.; Feng, C.X.; Gao, S.; Coulson, I.M.; Wang, T.; Feng, G.Y.; Tao, Y.; Xia, Y. Geochronological and geochemical constraints on the petrogenesis of alkaline ultramafic dykes from southwest Guizhou Province, SW China. Lithos 2010, 114, 253–264. [Google Scholar] [CrossRef]
- Liao, S.L.; Chen, S.Y.; Deng, X.H.; Li, P.; Zhao, J.N.; Liao, R.Y.Z. Fluid inclusion characteristics and geological significance of the Xi’ao copper-tin polymetallic deposit in Gejiu, Yunnan Province. J. Asian Earth Sci. 2014, 79, 455–467. [Google Scholar] [CrossRef]
- Wu, Q.S.; Liu, Q.L. Genetic evolution and mineralization of Gejiu tin-bearing granite. Miner. Resour. Geol. 1985, 4, 22–31. (In Chinese) [Google Scholar]
- Wu, Q.S.; Liu, Q.L. Genesis, evolution and mineralization of a complex formed from Sn-bearing granite magma in Gejiu, Yunnan. J. Guilin Coll. Geol. 1986, 6, 229–238. (In Chinese) [Google Scholar]
- Cheng, Y.B.; Mao, J.W.; Chen, M.H.; Yang, Z.X.; Feng, J.R.; Zhao, H.J. LA-ICP-MS zircon dating of alkaline rocks and lamprophyres in Gejiu area and its implications. Geol. Chin. 2008, 35, 1138–1149. (In Chinese) [Google Scholar]
- Zhang, Y.; Huang, Z.L.; Luo, T.Y.; Qian, Z.K.; Zhang, J.W.; Sun, J.B. The geochmeistry and geochronology of the Jiasha intrusion. Geochimica 2013, 42, 523–543. (In Chinese) [Google Scholar]
- Lü, B.S. A new round of mineral prospecting and prospecting targets in Gejiu, Yunnan province. Geol. Rev. 2005, 51, 640–648. (In Chinese) [Google Scholar]
- Li, J.H. Study of origin and characteristics of the granite in Gejiu Sn deposits. Yunnan Geol. 1985, 4, 327–352. (In Chinese) [Google Scholar]
- Qin, D.X.; Li, Y.S. Studies on the Geology of the Gejiu Sn-Cu Polymetallic Deposit; Science Press: Beijing, China, 2008; pp. 1–180. (In Chinese) [Google Scholar]
- Liao, S.L.; Chen, S.Y.; Deng, X.H.; Li, P. REE characteristics and significance of granite alteration zone of Xi’ao copper-tin polymetallic deposit in Gejiu area. J. Cent. South Univ. 2014, 45, 1555–1565. (In Chinese) [Google Scholar]
- Liao, S.L.; Chen, S.Y.; Yao, T.; Zhao, J.N.; Deng, X.H.; Li, P. Geochemical characteristics and Geological significance of the Xi’ao Cu-Sn polymetallic deposit in the Gejiu area. Geotecton. Metall. 2014, 38, 635–646. (In Chinese) [Google Scholar]
- Zhang, R.X.; Yang, S.Y. A mathematical model for determining carbon coating thickness and its application in electron probe microanalysis. Microsc. Microanal. 2016, 22, 1374–1380. [Google Scholar] [CrossRef]
- Clayton, R.N.; O’Neil, J.R.; Mayeda, T.K. Oxygen isotope exchange between quartz and water. J. Geophys. Res. 1972, 77, 3057–3067. [Google Scholar] [CrossRef]
- Zhang, L.G.; Liu, J.X.; Chen, Z.S.; Zhou, H.B. Experimental investigations of oxygen isotope fractionation in cassiterite and wolframite. Econ. Geol. 1994, 89, 150–157. [Google Scholar] [CrossRef]
- Li, C.Y.; Wang, F.Y.; Hao, X.L.; Ding, X.; Zhang, H.; Ling, M.X.; Zhou, J.B.; Li, Y.L.; Fan, W.M.; Sun, W.D. The formation of the Dabaoshan porphyry molybdenum deposit induced by slab rollback. Lithos 2012, 150, 101–110. [Google Scholar] [CrossRef]
- Tu, X.L.; Zhang, H.; Deng, W.F.; Ling, M.X.; Liang, H.Y.; Liu, Y.; Sun, W.D. Application of RESOlution in-situ laser ablation ICP-MS in trace element analyses. Geochimica 2011, 40, 83–98. [Google Scholar] [CrossRef]
- Lin, J.; Liu, Y.S.; Yang, Y.H.; Hu, Z.C. Calibration and correction of LA-ICP-MS and LA-MC-ICP-MS analyses for element contents and isotopic ratios. Solid Earth Sci. 2016, 1, 5–27. [Google Scholar] [CrossRef]
- Liu, Y.S.; Gao, S.; Hu, Z.C.; Gao, C.G.; Zong, K.Q.; Wang, D.B. Continental and oceanic crust recycling-induced melt–peridotite interactions in the Trans-North China Orogen: U–Pb dating, Hf isotopes and trace elements in zircons from mantle xenoliths. J. Pet. 2010, 51, 537–571. [Google Scholar] [CrossRef]
- Ludwig, K.R. User’s manual for Isoplot/Ex, 3rd ed.; A Geochronology Toolkit for Microsoft Excel, No. 4. Berkeley Geochronological Center, Special Publication; Berkeley Geochronological Center: Berkeley, CA, USA, 2003. [Google Scholar]
- Huang, P.Y.; Wang, X.; Chen, J.; Ren, M.H.; Lai, G.B. Morphological and geochemical studies of the cassiterite in Taoxikeng deposit, southern Jiangxi, China. Geol. Rev. 2012, 58, 987–1000. (In Chinese) [Google Scholar]
- Kaur, J.; Shah, J.; Kotnala, R.K.; Verma, K.C. Raman spectra, photoluminescence and ferromagnetism of pure, Co and Fe doped SnO2 nanoparticles. Ceram. Int. 2012, 38, 5563–5570. [Google Scholar] [CrossRef]
- Wang, R.C.; Wu, J.W. Raman Spectroscopy of Nb, Ta-rich Cassiterite in Beauvoir and Montebras Granites, France. Chin. J. Geochem. 1993, 12, 353–359. [Google Scholar] [CrossRef]
- Katiyar, R.S.; Dawson, P.; Hargreave, M.M.; Wilkinson, G.R. Dynamics of the rutile structure. III. Lattice dynamics, infrared and Raman spectra of SnO2. J. Phys. C Solid State Phys. 1971, 4, 2421–2431. [Google Scholar] [CrossRef]
- Deng, X.H.; Chen, Y.J.; Bagas, L.; Zhou, H.Y.; Zheng, Z.; Yue, S.W.; Chen, H.J.; Li, H.M.; Tu, J.R.; Cui, Y.R. Cassiterite U-Pb geochronology of the Kekekaerde W-Sn deposit in the Baiganhu ore field, East Kunlun Orogen, NW China: Timing and tectonic setting of mineralization. Ore Geol. Rev. 2017, 100, 534–544. [Google Scholar] [CrossRef]
- Goncharov, G.N.; Filatov, S.K. Typical structural features of cassiterite from Sherlovaya Gora. Geochem. Int. 1971, 8, 268–275. [Google Scholar]
- Izoret, L.; Marnier, G.; Dusausoy, Y. Crystallochemical characterization of cassiterite from tin and tungsten deposits in Galicia, Spain. Can. Mineral. 1985, 23, 221–231. (In French) [Google Scholar]
- Greaves, G.; Stevenson, B.G.; Taylor, R.G. Magnetic cassiterites from Herberton, North Queensland, Australia. Econ. Geol. 1971, 66, 480–487. [Google Scholar] [CrossRef]
- Hosking, K.F.G.; Cai, H.L. The characteristics and significance of pleochromatism for cassiterites in the Southeast Asian tin belt. Geol. Explor. 1984, 20, 25–28. (In Chinese) [Google Scholar]
- Grubb, P.L.C.; Hannaford, P. Ferromagnetism and colour zoning in some Malayan cassiterite. Nature 1966, 209, 677–678. [Google Scholar] [CrossRef]
- Swart, P.K.; Moore, F. The occurrence of uranium in association with cassiterite, wolframite, and sulphide mineralization in South-West England. Mineral. Mag. 1982, 46, 211–215. [Google Scholar] [CrossRef]
- Zagruzina, I.A.; Pinskii, E.M.; Savinova, I.B. Uranium in cassiterite of tin deposits. Int. Geol. Rev. 1987, 29, 94–109. [Google Scholar] [CrossRef]
- Marshall, D.J. Cathodoluminescence of Geological Materials; Unwin Hyman: Boston, MA, USA, 1988; pp. 1–146. [Google Scholar]
- Götze, J. Potential of cathodoluminescence (CL) microscopy and spectroscopy for the analysis of minerals and materials. Anal. Bioanal. Chem. 2002, 374, 703–708. [Google Scholar] [CrossRef]
- Xu, H.F.; Cui, J.G.; Qiu, X.P. Application of Cathodoluminescence in Petrology and Mineral Deposit Geology; Geology Publishing House: Beijing, China, 2006; pp. 1–78. (In Chinese) [Google Scholar]
- Hall, M.R.; Ribbe, P.H. An electron microprobe study of luminescence centers in cassiterite. Am. Miner. 1971, 56, 31–45. [Google Scholar]
- Farmer, C.B.; Searl, A.; Halls, C. Cathodoluminescence and growth of cassiterite in the composite lodes at South Crofty Mine, Cornwall, England. Mineral. Mag. 1991, 55, 447–458. [Google Scholar] [CrossRef]
- Ferrari, S.; Pampillo, L.G.; Saccone, F.D. Magnetic properties and environment sites in Fe doped SnO2 nanoparticles. Mater. Chem. Phys. 2016, 177, 206–212. [Google Scholar] [CrossRef]
- Ohmoto, H. Stable isotope geochemistry of ore deposits. Rev. Min. 1986, 16, 491–559. [Google Scholar]
- Sheppard, S.M.F. Characterization and isotopic variations in natural waters. Rev. Min. 1986, 16, 165–183. [Google Scholar] [CrossRef]
- Huang, F.S.; Mu, Z.G.; Chen, C.Y.; Wang, Z.F. The study of isotopic composition of oxygen, Hydrogen and carbon in granite of tin deposits, Gejiu. Acta Petrol. Sin. 1983, 2, 241–247. (In Chinese) [Google Scholar]
- Liu, M. Research on the Metallogenia of the Granite Contach-Depression Zones in GeJiu Tin-Copper Polymetallic Deposit. Ph.D. Thesis, Central South University, Hunan, China, 2007. [Google Scholar]
- Möller, P.; Dulski, P.; Szacki, W.; Malow, G.; Riedel, E. Substitution of tin in cassiterite by tantalum, niobium, tungsten, iron and manganese. Geochim. Cosmochim. Acta 1988, 52, 1497–1503. [Google Scholar] [CrossRef]
- Schneider, H.J.; Dulski, P.; Luck, J.; Möller, P.; Villalpando, A. Correlation of trace element distribution in cassiterites and geotectonic position of their deposits in Bolivia. Miner. Depos. 1978, 13, 119–122. [Google Scholar] [CrossRef]
- Tindle, A.G.; Breaks, F.W. Oxide minerals of the separation rapids rare-element granitic pegmatite group, northwestern Ontario. Can. Mineral. 1998, 36, 609–635. [Google Scholar]
- Yu, J.M.; Jiang, S.Y. Trace element geochemistry of cassiterites from the Neves Corvo massive sulfide deposit, Portugal. Geochimica 2001, 30, 140–146. (In Chinese) [Google Scholar]
- Cheng, Y.B. Spatial-Temperal Evolution of the Magmatism and Mineralization in the Gejiu Supergiant Sn Polymetaliic District and Insights into Several Key Problems. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2012. [Google Scholar]
- Zaraisky, G.P.; Korzhinskaya, V.; Kotova, N. Experimental studies of Ta2O5 and columbite–tantalite solubility in fluoride solutions from 300 to 550 °C and 50 to 100 MPa. Miner. Petrol. 2010, 99, 287–300. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Lu, J.; Fan, W. Geochemical mechanism of Nb-, Ta-mineralization during the late stage of granite crystallization. Geochemistry 1982, 1, 175–185. [Google Scholar] [CrossRef]
- Rapp, J.F.; Klemme, S.; Butler, I.B.; Harley, S.L. Extremely high solubility of rutile in chloride and fluoride-bearing metamorphic fluids: An experimental investigation. Geology 2010, 38, 323–326. [Google Scholar] [CrossRef]
- Jackson, J.K.; Helgeson, H.C. Chemical and thermodynamic constraints on the hydrothermal transport and deposition of tin: I. Calculation of the solubility of cassiterite at high pressures and temperatures. Geochim. Cosmochim. Acta 1985, 49, 1–22. [Google Scholar] [CrossRef]
- Chen, J. Experiment on solubility of cassiterite in the presence of charcoal. Geol. Rev. 1986, 32, 287–294. (In Chinese) [Google Scholar]
- Naitza, S.; Conte, A.M.; Cuccuru, S.; Oggiano, G.; Secchi, F.; Tecce, F. A Late Variscan tin province associated to the ilmenite-series granites of the Sardinian Batholith (Italy): The Sn and Mo mineralisation around the Monte Linas ferroan granite. Ore Geol. Rev. 2017, 80, 1259–1278. [Google Scholar] [CrossRef]
- Villemant, B.; Boudon, G. H2O and halogen (F, Cl, Br) behaviour during shallow magma degassing processes. Earth Planet. Sci. Lett. 1999, 168, 271–286. [Google Scholar] [CrossRef]
- Audétat, A.; Güther, D.; Heinrich, C.A. Magmatic-hydrothermal evolution in a fractionating granite: A microchemical study of the Sn-W-F-mineralized Mole Granite (Australia). Geochim. Cosmochim. Acta 2000, 64, 3373–3393. [Google Scholar] [CrossRef]
- Hu, X.Y.; Shang, L.B.; Bi, X.W.; Hu, R.Z.; Fan, W.L.; Chen, Y.W. Experimental study on the tin partition between granitic silicate melt and coexisting aqueous fluid. Bull. Min. Pet. Geochem. 2007, 26, 359–365. (In Chinese) [Google Scholar] [CrossRef]
- Webster, J.D.; Holloway, J.R. Partitioning of F and Cl between magmatic hydrothermal fluids and highly evolved granitic magmas. Geol. Soc. Am. Spec. Pap. 1990, 246, 21–34. [Google Scholar]
- Heinrich, C.A. Thermodynamic predictions of hydrothermal chemistry of arsenic and their significance for the paragenic sequence of some cassiterite-arsenopyrite-base metal sulfide deposits. Econ. Geol. 1986, 81, 511–529. [Google Scholar] [CrossRef]
- Robb, L. Introduction to Ore-Forming Processes; Blackwell Publishing: Oxford, UK, 2005; pp. 1–374. [Google Scholar]
- Taylor, R.G. Geology of Tin Deposits; Elsevier: Amsterdam, The Netherland, 1979; pp. 1–543. [Google Scholar]
- Oliveira, J.T.; Pacheco, N.; Carvalho, P.; Ferreira, A. The Neves Corvo mine and the Paleozoic geology of southwest Portugal. Geology and VMS Deposits of the Iberian Pyrite belt. In SEG Neves Corvo Field Conference, Abstracts and Program; University of Lisbon: Lisbon, Portugal, 1997; pp. 21–71. [Google Scholar]
- Relvas, J.M.; Tassinari, C.C.; Munhá, J.; Barriga, F.J. Multiple sources for ore-forming fluids in the Neves Corvo VHMS Deposit of the Iberian Pyrite Belt (Portugal): Strontium, neodymium and lead isotope evidence. Miner. Depos. 2001, 36, 416–427. [Google Scholar] [CrossRef]
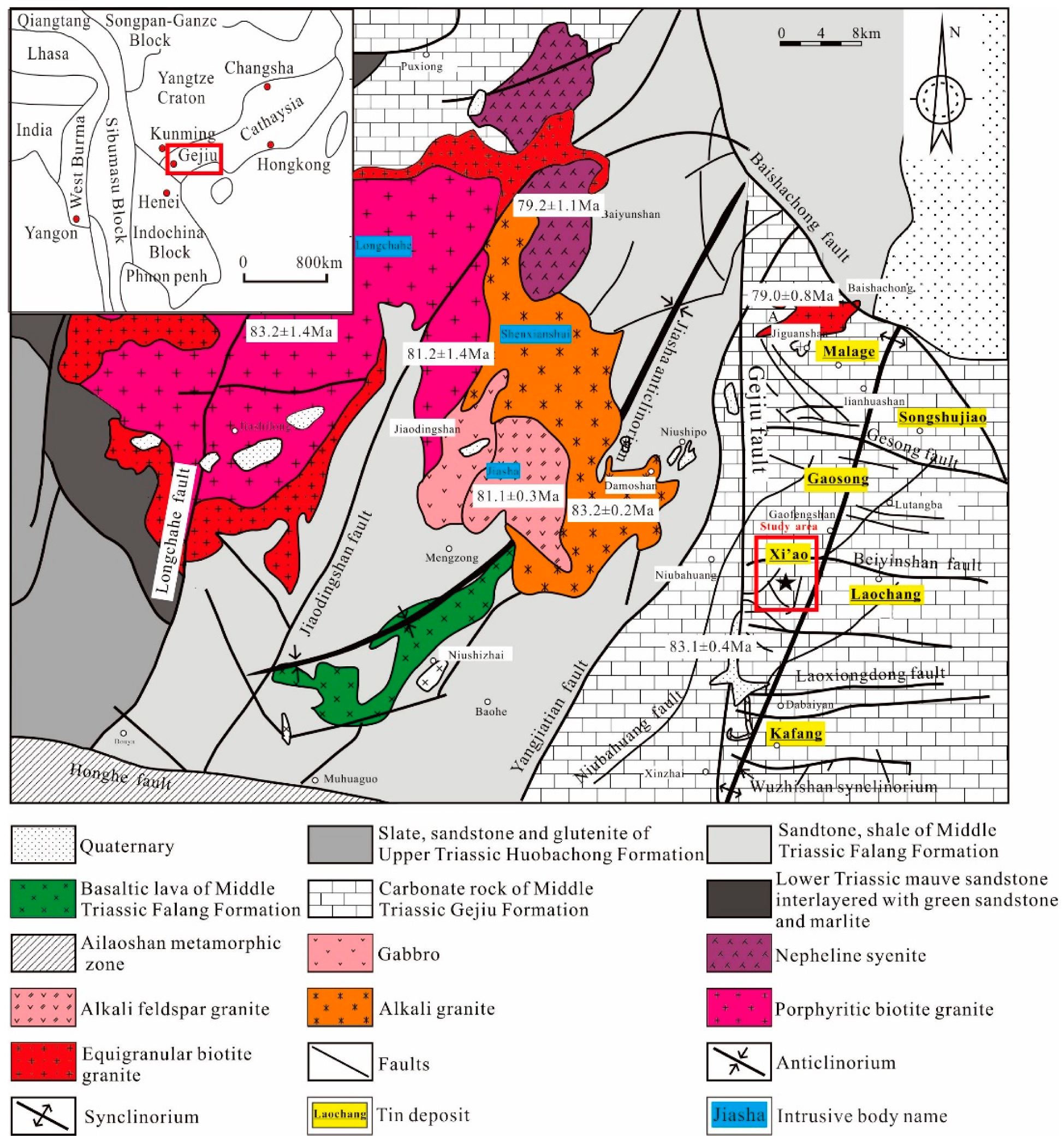
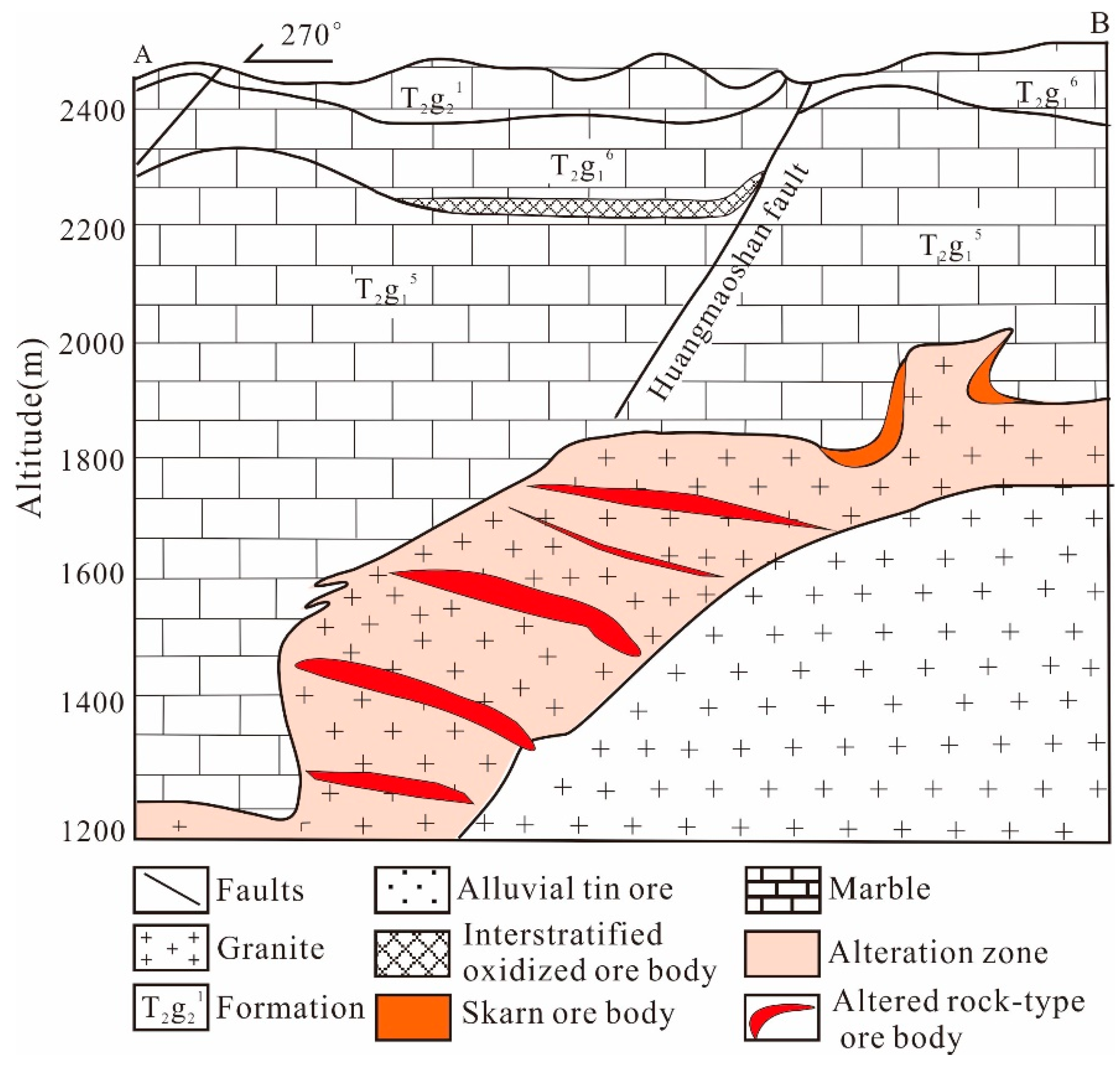
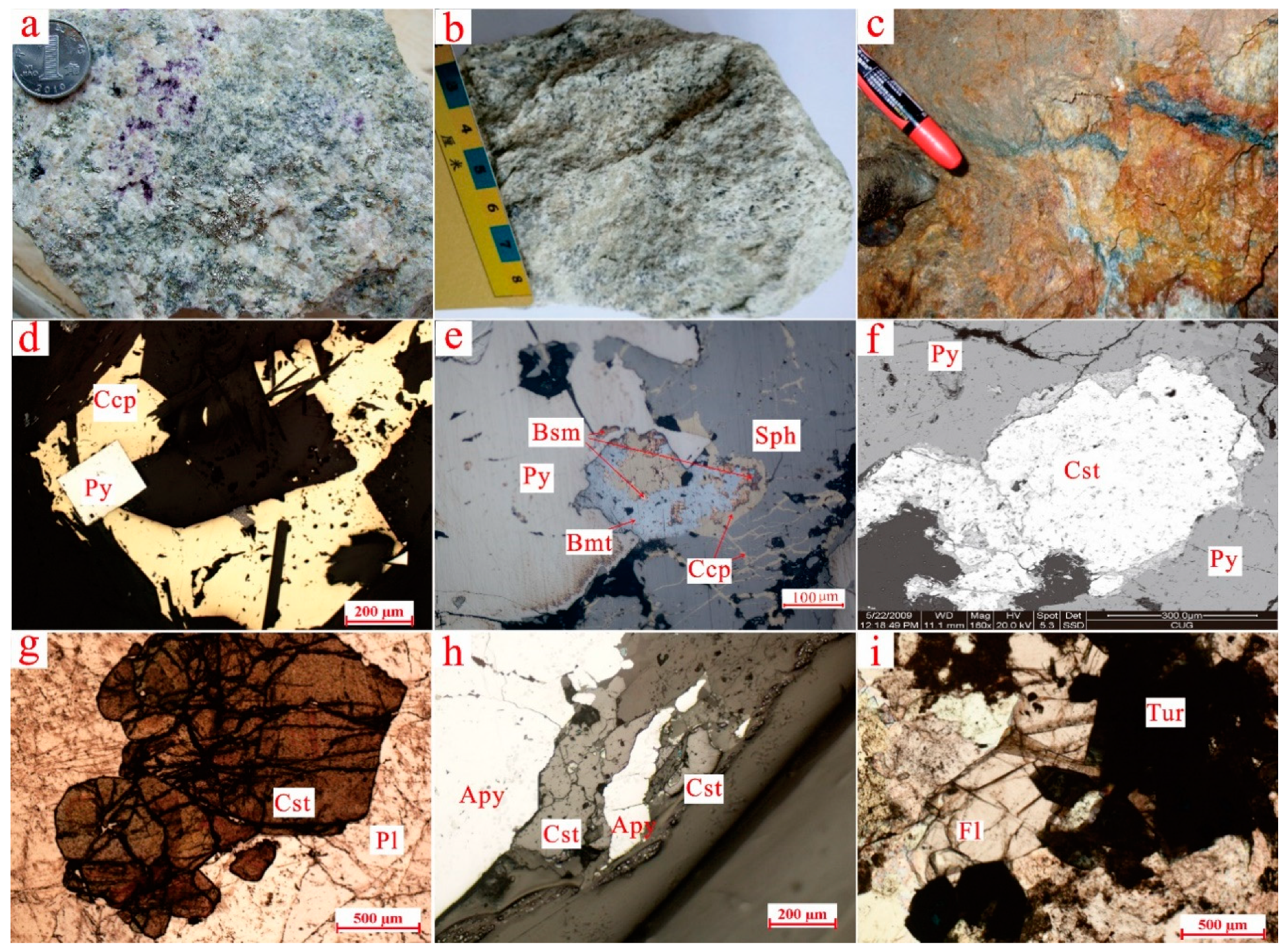
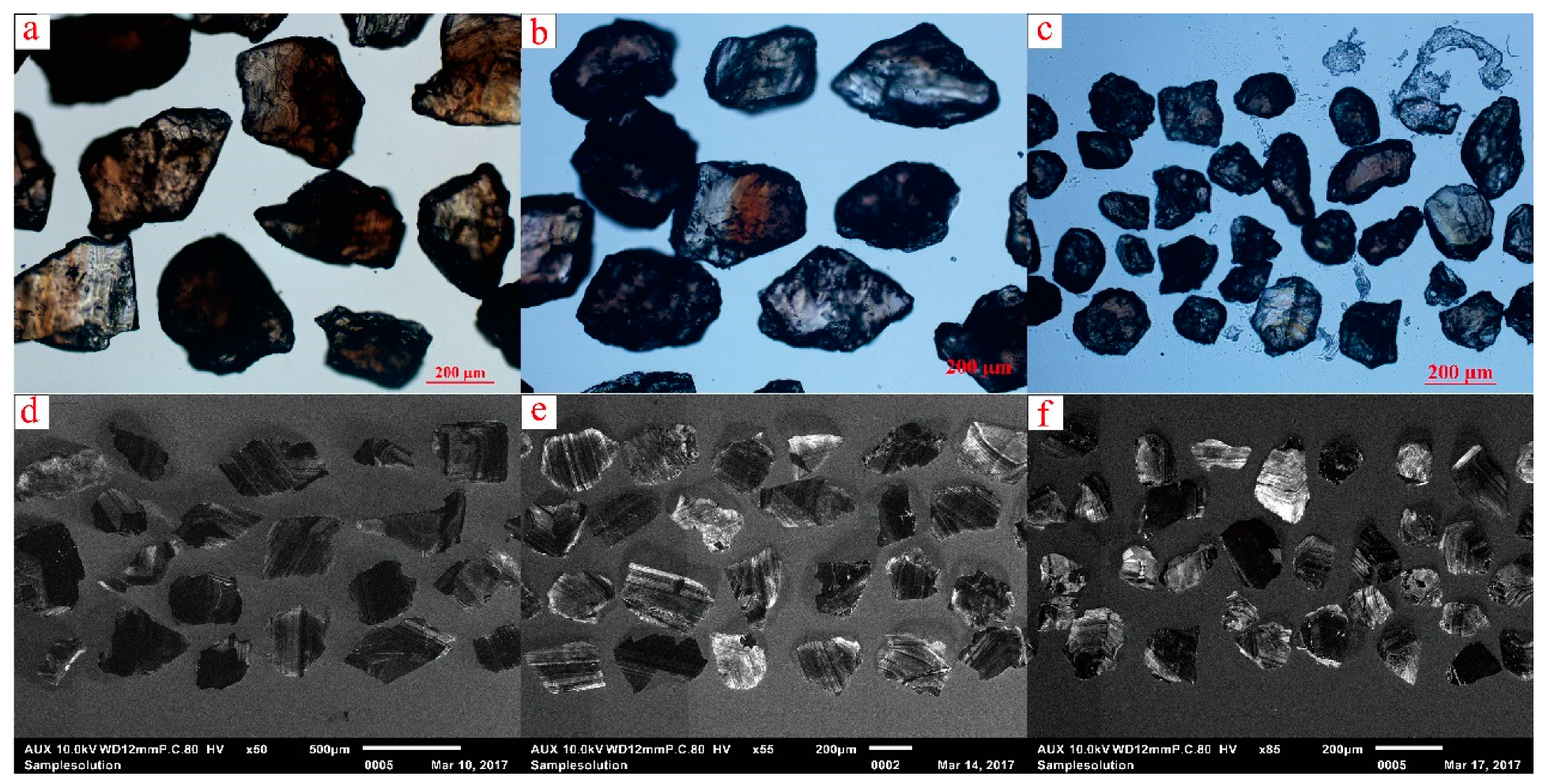

| Pluton Name | Lithology | Research Object | Dating Method | Age | Petrogenesis | Reference |
|---|---|---|---|---|---|---|
| Longchahe | Porphyritic biotite granite | biotite | K-Ar | 100~115 Ma | A-type; peraluminous, alkaline granite | [51] |
| whole rock | Rb-Sr | 147 ± 3 Ma | ||||
| zircon | U-Pb | 82.0 ± 0.3 Ma~83.2 ± 1.4 Ma | [43] | |||
| Masong | Porphyritic biotite granite | biotite | K-Ar | 100~102 Ma | A-type and S-type; metaluminous—peraluminous, calc-alkaline granite | [51] |
| K-feldspar | K-Ar | 91.5~116 Ma | ||||
| whole rock | Rb-Sr | 90.4 ± 6.3Ma | ||||
| zircon | U-Pb | 82.8 ± 1.7 Ma | [43] | |||
| Shenxianshui | Equigranular granite | biotite | K-Ar | 72~87 Ma | A-type; peraluminous, alkaline granite | [51] |
| whole rock | Rb-Sr | 84.4 ± 1.1 Ma | ||||
| zircon | U-Pb | 81.0 ± 0.52 Ma ~81.4 ± 0.4 Ma | [43] | |||
| Baishachong | Equigranular granite | biotite | K-Ar | 53 Ma | Peraluminous, calc-alkaline granite | [52] |
| whole rock | Rb-Sr | 81.0 ± 2 Ma | ||||
| zircon | U-Pb | 77.4 ± 2.5 Ma | [43] | |||
| Laoka | Equigranular granite | biotite | K-Ar | 64 Ma~80 Ma | S-type; peraluminous, calc-alkaline | [52] |
| whole rock | Rb-Sr | 81.0 ± 4.9 Ma | ||||
| zircon | U-Pb | 85.0 ± 0.85 Ma | [41] | |||
| Porphyritic granite | zircon | U-Pb | 83.3 ± 1.6 Ma | [43] | ||
| Baiyunshan | Alkali feldspar granite | biotite | K-Ar | 59.5 Ma~62 Ma | / | [52] |
| whole rock | Rb-Sr | 94.3 ± 2.4 Ma | ||||
| zircon | U-Pb | 76.6 ± 3.6 Ma | [53] | |||
| Jiasha | Gabbro | zircon | U-Pb | 84.0 ± 0.6 Ma | / | [54] |
| Lamprophyre | zircon | U-Pb | 77.2 ± 2.4 Ma | / | [53] |
| Mineral Assemblage Type | Minerals | Ore Structure | Mineralization Significance |
|---|---|---|---|
| Potassium-fluoride-sulfide | K-feldspar, pyrite, chalcopyrite, and less fluorite, quartz, tourmaline, mica, etc. | Block, crumb, veinlet and disseminated | Big |
| Epidote and chlorite-pyritization-fluoride | Quartz, plagioclase, k-feldspar, biotite, epidote, chlorite, pyrite, fluorite, tourmaline, etc. | Veinlet and disseminated. | Small |
| XA-3 Altered Rock-Type Ore | ||||||||||||||||||
| XA-3-1 | XA-3-2 | XA-3-4 | XA-5-6 | XA-3-7 | XA-3-8 | XA-3-9 | XA-3-10 | XA-3-11 | XA-3-12 | XA-3-13 | XA-3-15 | XA-3-17 | XA-3-18 | XA-3-20 | ||||
| H | H | O | O | O | O | O | O | O | O | O | O | O | H | H | ||||
| SnO2 | 100.416 | 98.882 | 99.667 | 99.445 | 99.716 | 98.754 | 99.966 | 99.775 | 96.631 | 98.772 | 98.278 | 98.957 | 99.815 | 100.087 | 98.392 | |||
| WO3 | 0.000 | 0.667 | 0.047 | 0.487 | 0.387 | 0.017 | 0.161 | 0.080 | 0.082 | 0.031 | 0.030 | 0.029 | 0.000 | 0.000 | 0.037 | |||
| Nb2O5 | 0.000 | 0.058 | 0.192 | 0.000 | 0.024 | 0.038 | 0.010 | 0.039 | 0.558 | 0.019 | 0.005 | 0.063 | 0.039 | 0.029 | 0.592 | |||
| Ta2O5 | 0.000 | 0.014 | 0.255 | 0.000 | 0.000 | 0.277 | 0.000 | 0.213 | 0.000 | 0.000 | 0.000 | 0.047 | 0.000 | 0.000 | 0.110 | |||
| FeO | 0.625 | 0.782 | 0.626 | 0.744 | 0.678 | 0.501 | 0.498 | 0.530 | 0.712 | 1.168 | 0.952 | 0.721 | 0.876 | 0.653 | 0.621 | |||
| MnO | 0.023 | 0.032 | 0.015 | 0.024 | 0.000 | 0.016 | 0.000 | 0.006 | 0.002 | 0.005 | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | |||
| TiO2 | 0.000 | 0.018 | 0.211 | 0.150 | 0.240 | 0.000 | 0.000 | 0.145 | 0.810 | 0.209 | 1.052 | 0.772 | 0.235 | 0.219 | 0.638 | |||
| SiO2 | 0.012 | 0.005 | 0.080 | 0.024 | 0.011 | 0.041 | 0.004 | 0.047 | 0.054 | 0.000 | 0.000 | 0.026 | 0.000 | 0.031 | 0.000 | |||
| Total | 101.076 | 100.458 | 101.093 | 100.874 | 101.056 | 99.644 | 100.639 | 100.835 | 98.849 | 100.204 | 100.317 | 100.615 | 100.965 | 101.021 | 100.390 | |||
| Cation Formula Based on Four Atoms of Oxygen | ||||||||||||||||||
| Sn4+ | 1.9795 | 1.9593 | 1.9580 | 1.9603 | 1.9615 | 1.9798 | 1.9849 | 1.9737 | 1.9327 | 1.9639 | 1.9383 | 1.9504 | 1.9700 | 1.9745 | 1.9428 | |||
| W6+ | 0.0000 | 0.0086 | 0.0006 | 0.0062 | 0.0049 | 0.0002 | 0.0021 | 0.0010 | 0.0011 | 0.0004 | 0.0004 | 0.0004 | 0.0000 | 0.0000 | 0.0005 | |||
| Nb5+ | 0.0000 | 0.0013 | 0.0043 | 0.0000 | 0.0005 | 0.0009 | 0.0002 | 0.0009 | 0.0126 | 0.0004 | 0.0001 | 0.0014 | 0.0009 | 0.0006 | 0.0133 | |||
| Ta5+ | 0.0000 | 0.0002 | 0.0034 | 0.0000 | 0.0000 | 0.0038 | 0.0000 | 0.0029 | 0.0000 | 0.0000 | 0.0000 | 0.0006 | 0.0000 | 0.0000 | 0.0015 | |||
| Fe2+ | 0.0388 | 0.0488 | 0.0387 | 0.0462 | 0.0420 | 0.0351 | 0.0346 | 0.0367 | 0.0498 | 0.0812 | 0.0657 | 0.0497 | 0.0605 | 0.0451 | 0.0429 | |||
| Mn2+ | 0.0010 | 0.0013 | 0.0006 | 0.0010 | 0.0000 | 0.0007 | 0.0000 | 0.0003 | 0.0001 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0000 | |||
| Ti4+ | 0.0000 | 0.0007 | 0.0078 | 0.0056 | 0.0089 | 0.0000 | 0.0000 | 0.0054 | 0.0306 | 0.0078 | 0.0391 | 0.0287 | 0.0087 | 0.0081 | 0.0238 | |||
| Si4+ | 0.0006 | 0.0003 | 0.0039 | 0.0012 | 0.0005 | 0.0020 | 0.0002 | 0.0023 | 0.0027 | 0.0000 | 0.0000 | 0.0013 | 0.0000 | 0.0015 | 0.0000 | |||
| Total | 2.0199 | 2.0204 | 2.0175 | 2.0205 | 2.0184 | 2.0225 | 2.0219 | 2.0232 | 2.0295 | 2.0540 | 2.0436 | 2.0324 | 2.0401 | 2.0299 | 2.0247 | |||
| Nb + Ta | 0.0000 | 0.0015 | 0.0077 | 0.0000 | 0.0005 | 0.0047 | 0.0002 | 0.0037 | 0.0126 | 0.0004 | 0.0001 | 0.0020 | 0.0009 | 0.0006 | 0.0147 | |||
| Fe + Mn | 0.0398 | 0.0501 | 0.0393 | 0.0472 | 0.0420 | 0.0358 | 0.0346 | 0.0370 | 0.0499 | 0.0814 | 0.0657 | 0.0497 | 0.0605 | 0.0452 | 0.0429 | |||
| XA-4 Altered Rock-Type Ore | ||||||||||||||||||
| XA-4-1 | XA-4-2 | XA-4-3 | XA-4-4 | XA-4-6 | XA-4-7 | XA-4-8 | XA-4-9 | XA-4-5 | XA-4-10 | XA-4-11 | XA-4-12 | XA-4-13 | XA-4-14 | XA-4-15 | XA-4-16 | XA-4-17 | ||
| O | O | O | H | H | H | H | H | H | ||||||||||
| SnO2 | 98.483 | 99.626 | 99.369 | 99.397 | 99.949 | 99.423 | 98.811 | 99.598 | 96.516 | 98.760 | 99.027 | 98.777 | 99.634 | 99.005 | 98.994 | 98.236 | 100.022 | |
| WO3 | 0.062 | 0.065 | 0.000 | 0.052 | 0.011 | 0.000 | 0.037 | 0.000 | 0.087 | 0.143 | 0.000 | 0.000 | 0.042 | 0.000 | 0.000 | 0.547 | 0.012 | |
| Nb2O5 | 0.029 | 0.000 | 0.039 | 0.000 | 0.106 | 0.067 | 0.000 | 0.082 | 0.513 | 0.250 | 0.005 | 0.000 | 0.000 | 0.086 | 0.000 | 0.077 | 0.038 | |
| Ta2O5 | 0.047 | 0.077 | 0.000 | 0.000 | 0.000 | 0.019 | 0.000 | 0.049 | 0.339 | 0.184 | 0.000 | 0.025 | 0.000 | 0.000 | 0.118 | 0.093 | 0.000 | |
| FeO | 0.614 | 0.680 | 0.965 | 0.597 | 0.521 | 0.800 | 0.795 | 0.553 | 0.959 | 0.588 | 0.587 | 0.751 | 0.656 | 0.606 | 0.802 | 0.606 | 0.590 | |
| MnO | 0.028 | 0.000 | 0.028 | 0.000 | 0.007 | 0.000 | 0.000 | 0.000 | 0.000 | 0.019 | 0.011 | 0.000 | 0.015 | 0.000 | 0.014 | 0.000 | 0.000 | |
| TiO2 | 0.400 | 0.381 | 0.308 | 0.066 | 0.282 | 0.014 | 0.036 | 0.000 | 1.448 | 0.225 | 0.754 | 0.392 | 0.179 | 0.628 | 0.632 | 0.849 | 0.201 | |
| SiO2 | 0.048 | 0.019 | 0.032 | 0.033 | 0.030 | 0.057 | 0.064 | 0.003 | 0.064 | 0.028 | 0.021 | 0.024 | 0.043 | 0.021 | 0.000 | 0.016 | 0.048 | |
| Total | 99.711 | 100.848 | 100.741 | 100.145 | 100.906 | 100.380 | 99.743 | 100.285 | 99.926 | 100.197 | 100.405 | 99.969 | 100.569 | 100.346 | 100.560 | 100.424 | 100.911 | |
| Cation Formula Based on Four Atoms of Oxygen | ||||||||||||||||||
| Sn4+ | 1.9648 | 1.9667 | 1.9631 | 1.9810 | 1.9727 | 1.9759 | 1.9762 | 1.9838 | 1.8733 | 1.9632 | 1.9570 | 1.9662 | 1.9749 | 1.9591 | 1.9557 | 1.9394 | 1.9753 | |
| W6+ | 0.0008 | 0.0008 | 0.0000 | 0.0007 | 0.0001 | 0.0000 | 0.0005 | 0.0000 | 0.0011 | 0.0018 | 0.0000 | 0.0000 | 0.0005 | 0.0000 | 0.0000 | 0.0070 | 0.0002 | |
| Nb5+ | 0.0007 | 0.0000 | 0.0009 | 0.0000 | 0.0024 | 0.0015 | 0.0000 | 0.0018 | 0.0121 | 0.0056 | 0.0001 | 0.0000 | 0.0000 | 0.0019 | 0.0000 | 0.0017 | 0.0009 | |
| Ta5+ | 0.0006 | 0.0010 | 0.0000 | 0.0000 | 0.0000 | 0.0003 | 0.0000 | 0.0007 | 0.0045 | 0.0025 | 0.0000 | 0.0003 | 0.0000 | 0.0000 | 0.0016 | 0.0013 | 0.0000 | |
| Fe2+ | 0.0428 | 0.0470 | 0.0667 | 0.0416 | 0.0360 | 0.0556 | 0.0556 | 0.0386 | 0.0651 | 0.0409 | 0.0405 | 0.0523 | 0.0455 | 0.0419 | 0.0554 | 0.0418 | 0.0408 | |
| Mn2+ | 0.0012 | 0.0000 | 0.0012 | 0.0000 | 0.0003 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0008 | 0.0005 | 0.0000 | 0.0006 | 0.0000 | 0.0006 | 0.0000 | 0.0000 | |
| Ti4+ | 0.0151 | 0.0142 | 0.0115 | 0.0025 | 0.0105 | 0.0005 | 0.0013 | 0.0000 | 0.0530 | 0.0084 | 0.0281 | 0.0147 | 0.0067 | 0.0234 | 0.0235 | 0.0316 | 0.0075 | |
| Si4+ | 0.0024 | 0.0009 | 0.0016 | 0.0017 | 0.0015 | 0.0028 | 0.0032 | 0.0002 | 0.0031 | 0.0014 | 0.0010 | 0.0012 | 0.0021 | 0.0010 | 0.0000 | 0.0008 | 0.0024 | |
| Total | 2.0284 | 2.0306 | 2.0448 | 2.0274 | 2.0234 | 2.0366 | 2.0368 | 2.0251 | 2.0122 | 2.0247 | 2.0272 | 2.0348 | 2.0303 | 2.0274 | 2.0368 | 2.0236 | 2.0269 | |
| Nb + Ta | 0.0013 | 0.0010 | 0.0009 | 0.0000 | 0.0024 | 0.0018 | 0.0000 | 0.0025 | 0.0166 | 0.0081 | 0.0001 | 0.0003 | 0.0000 | 0.0019 | 0.0016 | 0.0030 | 0.0009 | |
| Fe + Mn | 0.0440 | 0.0470 | 0.0678 | 0.0416 | 0.0362 | 0.0556 | 0.0556 | 0.0386 | 0.0651 | 0.0417 | 0.0410 | 0.0523 | 0.0461 | 0.0419 | 0.0560 | 0.0418 | 0.0408 | |
| XA-5 Tourmaline Vein-Type Ore | ||||||||||||||||||
| XA-5-6 | XA-5-8 | XA-5-9 | XA-5-10 | XA-5-11 | XA-5-12 | XA-5-13 | XA-5-15 | XA-5-16 | XA-5-17 | XA-5-18 | XA-5-19 | XA-5-20 | XA-5-21 | XA-5-22 | XA-5-23 | |||
| O | O | O | O | H | O | O | O | O | O | O | O | O | O | O | O | |||
| SnO2 | 99.885 | 99.022 | 99.149 | 99.757 | 99.161 | 99.584 | 99.124 | 98.823 | 99.568 | 98.975 | 99.168 | 99.867 | 99.452 | 98.699 | 99.362 | 99.816 | ||
| WO3 | 0.000 | 0.031 | 0.128 | 0.000 | 0.033 | 0.000 | 0.016 | 0.066 | 0.000 | 0.070 | 0.062 | 0.000 | 0.046 | 0.000 | 0.035 | 0.000 | ||
| Nb2O5 | 0.082 | 0.000 | 0.067 | 0.048 | 0.048 | 0.043 | 0.149 | 0.000 | 0.000 | 0.000 | 0.034 | 0.000 | 0.111 | 0.000 | 0.115 | 0.024 | ||
| Ta2O5 | 0.000 | 0.000 | 0.066 | 0.000 | 0.376 | 0.003 | 0.334 | 0.123 | 0.060 | 0.189 | 0.104 | 0.000 | 0.000 | 0.044 | 0.305 | 0.071 | ||
| FeO | 0.655 | 0.666 | 0.723 | 0.543 | 1.081 | 0.645 | 0.643 | 1.040 | 0.705 | 0.991 | 1.082 | 1.181 | 0.588 | 1.065 | 0.857 | 0.649 | ||
| MnO | 0.034 | 0.000 | 0.011 | 0.000 | 0.000 | 0.009 | 0.000 | 0.000 | 0.009 | 0.012 | 0.000 | 0.006 | 0.035 | 0.017 | 0.000 | 0.010 | ||
| TiO2 | 0.000 | 0.216 | 0.070 | 0.098 | 0.135 | 0.154 | 0.237 | 0.188 | 0.177 | 0.005 | 0.021 | 0.000 | 0.095 | 0.085 | 0.202 | 0.154 | ||
| SiO2 | 0.028 | 0.000 | 0.040 | 0.000 | 0.012 | 0.041 | 0.037 | 0.030 | 0.077 | 0.068 | 0.033 | 0.070 | 0.053 | 0.025 | 0.025 | 0.011 | ||
| Total | 100.684 | 99.935 | 100.254 | 100.446 | 100.846 | 100.479 | 100.540 | 100.270 | 100.596 | 100.310 | 100.504 | 101.124 | 100.380 | 99.935 | 100.901 | 100.735 | ||
| Cation Formula Based on Four Atoms of Oxygen | ||||||||||||||||||
| Sn4+ | 1.9752 | 1.9704 | 1.9671 | 1.9780 | 1.9526 | 1.9707 | 1.9592 | 1.9558 | 1.9665 | 1.9608 | 1.9600 | 1.9584 | 1.9707 | 1.9612 | 1.9557 | 1.9715 | ||
| W6+ | 0.0000 | 0.0004 | 0.0016 | 0.0000 | 0.0004 | 0.0000 | 0.0002 | 0.0008 | 0.0000 | 0.0009 | 0.0008 | 0.0000 | 0.0006 | 0.0000 | 0.0004 | 0.0000 | ||
| Nb5+ | 0.0018 | 0.0000 | 0.0015 | 0.0011 | 0.0011 | 0.0010 | 0.0033 | 0.0000 | 0.0000 | 0.0000 | 0.0008 | 0.0000 | 0.0025 | 0.0000 | 0.0026 | 0.0005 | ||
| Ta5+ | 0.0000 | 0.0000 | 0.0009 | 0.0000 | 0.0050 | 0.0000 | 0.0045 | 0.0017 | 0.0008 | 0.0026 | 0.0014 | 0.0000 | 0.0000 | 0.0006 | 0.0041 | 0.0010 | ||
| Fe2+ | 0.0408 | 0.0417 | 0.0452 | 0.0339 | 0.0670 | 0.0402 | 0.0401 | 0.0648 | 0.0439 | 0.0618 | 0.0673 | 0.0751 | 0.0367 | 0.0666 | 0.0531 | 0.0403 | ||
| Mn2+ | 0.0014 | 0.0000 | 0.0005 | 0.0000 | 0.0000 | 0.0004 | 0.0000 | 0.0000 | 0.0004 | 0.0005 | 0.0000 | 0.0003 | 0.0015 | 0.0007 | 0.0000 | 0.0004 | ||
| Ti4+ | 0.0000 | 0.0081 | 0.0026 | 0.0037 | 0.0050 | 0.0057 | 0.0088 | 0.0070 | 0.0066 | 0.0002 | 0.0008 | 0.0000 | 0.0036 | 0.0032 | 0.0075 | 0.0057 | ||
| Si4+ | 0.0014 | 0.0000 | 0.0020 | 0.0000 | 0.0006 | 0.0021 | 0.0018 | 0.0015 | 0.0038 | 0.0034 | 0.0017 | 0.0037 | 0.0026 | 0.0013 | 0.0013 | 0.0006 | ||
| Total | 2.0207 | 2.0206 | 2.0214 | 2.0167 | 2.0318 | 2.0200 | 2.0180 | 2.0315 | 2.0219 | 2.0301 | 2.0327 | 2.0374 | 2.0182 | 2.0335 | 2.0246 | 2.0200 | ||
| Nb + Ta | 0.0018 | 0.0000 | 0.0024 | 0.0011 | 0.0061 | 0.0010 | 0.0078 | 0.0017 | 0.0008 | 0.0026 | 0.0022 | 0.0000 | 0.0025 | 0.0006 | 0.0067 | 0.0015 | ||
| Fe + Mn | 0.0422 | 0.0417 | 0.0456 | 0.0339 | 0.0670 | 0.0406 | 0.0401 | 0.0648 | 0.0442 | 0.0623 | 0.0673 | 0.0754 | 0.0382 | 0.0673 | 0.0531 | 0.0407 | ||
| Sample | SnO2 | WO3 | Nb2O5 | Ta2O5 | FeO | MnO | TiO2 | SiO2 |
|---|---|---|---|---|---|---|---|---|
| XA-3 | 99.170 ± 0.95 a | 0.137 ± 0.21 | 0.111 ± 0.19 | 0.061 ± 0.1 | 0.713 ± 0.18 | 0.008 ± 0.01 | 0.313 ± 0.34 | 0.022 ± 0.02 |
| 96.631~100.416 b | 0–0.667 | 0–0.592 | 0–0.277 | 0.498–1.168 | 0–0.032 | 0–1.052 | 0–0.080 | |
| XA-4 | 99.037 ± 0.82 | 0.062 ± 0.13 | 0.076 ± 0.13 | 0.056 ± 0.09 | 0.686 ± 0.14 | 0.007 ± 0.01 | 0.400 ± 0.37 | 0.032 ± 0.02 |
| 96.516–100.022 | 0–0.547 | 0–0.513 | 0–0.339 | 0.521–0.964 | 0–0.028 | 0–1.448 | 0–0.064 | |
| XA-5 | 99.338 ± 0.38 | 0.030 ± 0.04 | 0.045 ± 0.05 | 0.105 ± 0.13 | 0.820 ± 0.22 | 0.009 ± 0.01 | 0.115 ± 0.08 | 0.034 ± 0.02 |
| 98.699–99.885 | 0–0.128 | 0–0.149 | 0–0.376 | 0.543–1.181 | 0–0.035 | 0–0.237 | 0–0.077 |
| Samples | Minerals | δ18OV-PDB (‰) | δ18OV-SMOW (‰) | Temperature (°C) a | δ18OH2O-SMOW (‰) |
|---|---|---|---|---|---|
| XA-3 | Cassiterite | −25.40 | 4.7 | 374.10 | 8.25 |
| XA-4 | −25.70 | 4.4 | 374.10 | 7.95 | |
| XA-5 | −26.20 | 3.9 | 353.82 | 7.16 |
| XA-3 Altered Rock-Type Ore | ||||||||||||||||
| Spots | Th | U | Isotopic Ratios | Age (Ma) | ||||||||||||
| 238U/206Pb | 1σ | 207Pb/206Pb | 1σ | 206Pb/238U | 1σ | 207Pb/235U | 1σ | 207Pb/206Pb | 1σ | 206Pb/238U | 1σ | 207Pb/235U | 1σ | |||
| XA16-3-3 | 0 | 20,627 | 75.1314801 | 3.217501 | 0.03543 | 0.00497 | 0.01331 | 0.00057 | 0.06532 | 0.00877 | 0.1 | 0 | 85.3 | 3.62 | 64.2 | 8.36 |
| XA16-3-4 | 8 | 64,216 | 77.5193798 | 2.223424 | 0.02693 | 0.00255 | 0.0129 | 0.00037 | 0.04806 | 0.00438 | 0.1 | 0 | 82.6 | 2.33 | 47.7 | 4.24 |
| XA16-3-5 | 0 | 38,135 | 77.4593338 | 2.699977 | 0.03421 | 0.00376 | 0.01291 | 0.00045 | 0.06101 | 0.00639 | 0.1 | 0 | 82.7 | 2.86 | 60.1 | 6.11 |
| XA16-3-6 | 0 | 32,544 | 79.491256 | 3.033053 | 0.031 | 0.00423 | 0.01258 | 0.00048 | 0.05385 | 0.00708 | 0.1 | 0 | 80.6 | 3.07 | 53.3 | 6.82 |
| XA16-3-8 | 0 | 23,724 | 79.4281176 | 3.532942 | 0.06637 | 0.00797 | 0.01259 | 0.00056 | 0.11525 | 0.01289 | 818.2 | 232.61 | 80.7 | 3.55 | 110.8 | 11.74 |
| XA16-3-11 | 0 | 46,501 | 75.8150114 | 2.759 | 0.0378 | 0.00616 | 0.01319 | 0.00048 | 0.06922 | 0.01094 | 0.1 | 0 | 84.5 | 3.08 | 68 | 10.39 |
| XA16-3-12 | 0 | 51,406 | 80.1282051 | 6.869966 | 0.05307 | 0.01635 | 0.01248 | 0.00107 | 0.09228 | 0.02726 | 331.9 | 579.52 | 80 | 6.84 | 89.6 | 25.34 |
| XA16-3-13 | 0 | 64,041 | 75.5857899 | 2.742342 | 0.05508 | 0.00849 | 0.01323 | 0.00048 | 0.102 | 0.01516 | 415.3 | 312.01 | 84.7 | 3.08 | 98.6 | 13.97 |
| XA16-3-14 | 0 | 23,742 | 75.5287009 | 3.650934 | 0.07457 | 0.01498 | 0.01324 | 0.00064 | 0.13904 | 0.02702 | 1056.5 | 359.19 | 84.8 | 4.08 | 132.2 | 24.09 |
| XA16-3-16 | 0 | 32,855 | 75.8725341 | 3.511551 | 0.07766 | 0.01786 | 0.01318 | 0.00061 | 0.14698 | 0.0334 | 1138.3 | 400.23 | 84.4 | 3.86 | 139.2 | 29.57 |
| XA-4 Altered Rock-Type Ore | ||||||||||||||||
| Spots | Th | U | Isotopic Ratios | Ages (Ma) | ||||||||||||
| 238U/206Pb | 1σ | 207Pb/206Pb | 1σ | 206Pb/238U | 1σ | 207Pb/235U | 1σ | 207Pb/206Pb | 1σ | 206Pb/238U | 1σ | 207Pb/235U | 1σ | |||
| XA-4-1 | 0 | 38,164 | 70.52186 | 2.5364 | 0.07462 | 0.00723 | 0.01418 | 0.00051 | 0.14623 | 0.0133 | 1058 | 183.86 | 90.8 | 3.26 | 138.6 | 11.78 |
| XA-4-2 | 1 | 36,083 | 69.39625 | 3.178454 | 0.09839 | 0.01033 | 0.01441 | 0.00066 | 0.19591 | 0.01873 | 1593.9 | 184.1 | 92.2 | 4.18 | 181.7 | 15.9 |
| XA-4-3 | 3 | 91,084 | 74.46016 | 1.940511 | 0.04016 | 0.00318 | 0.01343 | 0.00035 | 0.07453 | 0.00565 | 0.1 | 0 | 86 | 2.26 | 73 | 5.34 |
| XA-4-4 | 0 | 31,407 | 72.83321 | 2.811479 | 0.0469 | 0.00583 | 0.01373 | 0.00053 | 0.08894 | 0.01058 | 44 | 273.39 | 87.9 | 3.4 | 86.5 | 9.86 |
| XA-4-5 | 0 | 58,372 | 85.6898 | 3.597944 | −0.00366 | 0.00568 | 0.01167 | 0.00049 | −0.0059 | 0.00916 | 0.1 | 0 | 74.8 | 3.13 | -6 | 9.35 |
| XA-4-6 | 0 | 72,882 | 74.51565 | 2.276559 | 0.04511 | 0.00408 | 0.01342 | 0.00041 | 0.08358 | 0.00721 | 0.1 | 157.16 | 85.9 | 2.62 | 81.5 | 6.75 |
| XA-4-7 | 0 | 18,842 | 74.46016 | 3.492919 | 0.04477 | 0.00769 | 0.01343 | 0.00063 | 0.08302 | 0.01377 | 0.1 | 305.34 | 86 | 3.99 | 81 | 12.91 |
| XA-4-8 | 0 | 6665 | 63.77551 | 4.392701 | 0.08595 | 0.01659 | 0.01568 | 0.00108 | 0.18605 | 0.03366 | 1336.9 | 333.6 | 100.3 | 6.88 | 173.3 | 28.81 |
| XA-4-9 | 0 | 11,272 | 63.85696 | 3.466055 | 0.12024 | 0.01501 | 0.01566 | 0.00085 | 0.25995 | 0.02943 | 1959.8 | 207.41 | 100.2 | 5.42 | 234.6 | 23.72 |
| XA-4-10 | 0 | 7160 | 58.34306 | 3.676225 | 0.12216 | 0.01768 | 0.01714 | 0.00108 | 0.28893 | 0.03792 | 1988 | 237.23 | 109.6 | 6.82 | 257.7 | 29.88 |
| XA-4-11 | 0 | 126,487 | 80.84074 | 2.156625 | 0.03191 | 0.00289 | 0.01237 | 0.00033 | 0.05443 | 0.00475 | 0.1 | 0 | 79.2 | 2.1 | 53.8 | 4.58 |
| XA-4-12 | 0 | 5181 | 74.23905 | 6.448381 | 0.12237 | 0.02421 | 0.01347 | 0.00117 | 0.22732 | 0.04059 | 1991.1 | 315.34 | 86.2 | 7.43 | 208 | 33.58 |
| XA-4-13 | 0 | 58,639 | 78.125 | 2.563477 | 0.05157 | 0.00471 | 0.0128 | 0.00042 | 0.09105 | 0.00785 | 266.4 | 196.22 | 82 | 2.65 | 88.5 | 7.31 |
| XA-4-14 | 0 | 1704 | 68.3527 | 9.1573 | 0.11805 | 0.04805 | 0.01463 | 0.00196 | 0.23816 | 0.09165 | 1926.9 | 591.79 | 93.6 | 12.46 | 216.9 | 75.16 |
| XA-4-15 | 0 | 9525 | 80.97166 | 6.294153 | 0.05115 | 0.01484 | 0.01235 | 0.00096 | 0.08714 | 0.02439 | 247.4 | 558.56 | 79.2 | 6.11 | 84.8 | 22.78 |
| XA-4-16 | 0 | 6612 | 74.34944 | 5.970067 | 0.09207 | 0.01935 | 0.01345 | 0.00108 | 0.17076 | 0.03324 | 1468.7 | 354.03 | 86.1 | 6.9 | 160.1 | 28.83 |
| XA-4-17 | 0 | 97,931 | 75.18797 | 2.20476 | 0.04487 | 0.00372 | 0.0133 | 0.00039 | 0.08227 | 0.00645 | 0.1 | 128.04 | 85.2 | 2.48 | 80.3 | 6.06 |
| XA-4-18 | 0 | 26,046 | 77.27975 | 3.404131 | 0.03189 | 0.0063 | 0.01294 | 0.00057 | 0.0569 | 0.01097 | 0.1 | 0 | 82.9 | 3.64 | 56.2 | 10.54 |
| XA-5 Tourmaline Vein-Type Ore | ||||||||||||||||
| Spots | Th | U | Isotopic Ratios | Age (Ma) | ||||||||||||
| 238U/206Pb | 1σ | 207Pb/206Pb | 1σ | 206Pb/238U | 1σ | 207Pb/235U | 1σ | 207Pb/206Pb | 1σ | 206Pb/238U | 1σ | 207Pb/235U | 1σ | |||
| XA-5-1 | 57 | 33,209 | 10.9769484 | 0.277135 | 0.70382 | 0.02819 | 0.0911 | 0.0023 | 8.88783 | 0.30736 | 4738.5 | 56.34 | 562 | 13.59 | 2326.6 | 31.56 |
| XA-5-2 | 1 | 20,863 | 70.2247191 | 2.958907 | 0.07856 | 0.00878 | 0.01424 | 0.0006 | 0.155 | 0.01621 | 1161 | 206.92 | 91.1 | 3.8 | 146.3 | 14.25 |
| XA-5-4 | 35 | 30,390 | 30.0390508 | 0.776016 | 0.46305 | 0.01974 | 0.03329 | 0.00086 | 2.13586 | 0.0787 | 4127.7 | 61.84 | 211.1 | 5.33 | 1160.5 | 25.48 |
| XA-5-5 | 8 | 25,787 | 49.4071146 | 4.027754 | 0.23846 | 0.03313 | 0.02024 | 0.00165 | 0.66883 | 0.07615 | 3109.7 | 205.5 | 129.2 | 10.44 | 520 | 46.33 |
| XA-5-6 | 133 | 143,761 | 59.2417062 | 1.228353 | 0.19086 | 0.00774 | 0.01688 | 0.00035 | 0.44634 | 0.01674 | 2749.6 | 65.1 | 107.9 | 2.2 | 374.7 | 11.75 |
| XA-5-7 | 60 | 27,856 | 17.6584849 | 0.763964 | 0.53733 | 0.03368 | 0.05663 | 0.00245 | 4.21513 | 0.20401 | 4347 | 88.87 | 355.1 | 14.93 | 1677 | 39.72 |
| XA-5-8 | 0 | 125,483 | 75.4716981 | 1.822713 | 0.04799 | 0.00317 | 0.01325 | 0.00032 | 0.0881 | 0.00555 | 97.6 | 150.41 | 84.9 | 2.04 | 85.7 | 5.18 |
| XA-5-9 | 23 | 8254 | 66.0938533 | 4.106294 | 0.15994 | 0.01956 | 0.01513 | 0.00094 | 0.33533 | 0.03576 | 2455.1 | 193.27 | 96.8 | 5.94 | 293.6 | 27.19 |
| XA-5-10 | 74 | 112,650 | 78.1860829 | 3.239924 | 0.11092 | 0.00998 | 0.01279 | 0.00053 | 0.19659 | 0.01594 | 1814.6 | 155.02 | 81.9 | 3.38 | 182.2 | 13.52 |
| XA-5-11 | 44 | 47,919 | 47.3484848 | 1.546897 | 0.25907 | 0.01524 | 0.02112 | 0.00069 | 0.75849 | 0.03861 | 3241 | 89.79 | 134.8 | 4.35 | 573.1 | 22.29 |
| XA-5-12 | 55 | 32,069 | 52.6315789 | 1.717452 | 0.3831 | 0.02155 | 0.019 | 0.00062 | 1.00882 | 0.04815 | 3844.1 | 82.39 | 121.3 | 3.95 | 708.3 | 24.34 |
| XA-5-13 | 40 | 6225 | 2.12417954 | 0.049588 | 0.67373 | 0.03003 | 0.47077 | 0.01099 | 43.97262 | 1.81795 | 4675.7 | 62.73 | 2486.9 | 48.17 | 3864.6 | 41.05 |
| XA-5-14 | 31 | 5892 | 16.2839928 | 0.654966 | 0.5602 | 0.035 | 0.06141 | 0.00247 | 4.77057 | 0.24063 | 4408 | 88.3 | 384.2 | 15.03 | 1779.7 | 42.34 |
| XA-5-15 | 0 | 75,871 | 70.5716302 | 1.942338 | 0.04468 | 0.00343 | 0.01417 | 0.00039 | 0.08782 | 0.00641 | 0.1 | 104.97 | 90.7 | 2.51 | 85.5 | 5.98 |
| XA-5-16 | 0 | 151,920 | 76.5110941 | 1.873263 | 0.03887 | 0.00258 | 0.01307 | 0.00032 | 0.0705 | 0.00446 | 0.1 | 0 | 83.7 | 2.04 | 69.2 | 4.23 |
| Samples | Minerals | δ18Omineral (‰) | Reference |
|---|---|---|---|
| Laoka granite | Whole rock (4) | 11.85 | [88] |
| Biotite (2) | 8.58 | ||
| Quartz | 12.40 | [57] | |
| Ore-bearing quartz vein | Quartz | 12.60 | [88] |
| Gejiu Formation limestone | Limestone | 27.31 | [89] |
| Gejiu Formation marble | Marble (2) | 22.25 | |
| Gejiu Formation dolomite | Dolomite | 18.70 | |
| Basalt amygdala | calcite | 19.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Chen, S.; Huang, Y.; Zhao, J.; Tong, X.; Chen, X. U-Pb Ages, O Isotope Compositions, Raman Spectrum, and Geochemistry of Cassiterites from the Xi’ao Copper-Tin Polymetallic Deposit in Gejiu District, Yunnan Province. Minerals 2019, 9, 212. https://doi.org/10.3390/min9040212
Zhao Y, Chen S, Huang Y, Zhao J, Tong X, Chen X. U-Pb Ages, O Isotope Compositions, Raman Spectrum, and Geochemistry of Cassiterites from the Xi’ao Copper-Tin Polymetallic Deposit in Gejiu District, Yunnan Province. Minerals. 2019; 9(4):212. https://doi.org/10.3390/min9040212
Chicago/Turabian StyleZhao, Yuehua, Shouyu Chen, Yuqiang Huang, Jiangnan Zhao, Xiang Tong, and Xingshou Chen. 2019. "U-Pb Ages, O Isotope Compositions, Raman Spectrum, and Geochemistry of Cassiterites from the Xi’ao Copper-Tin Polymetallic Deposit in Gejiu District, Yunnan Province" Minerals 9, no. 4: 212. https://doi.org/10.3390/min9040212
APA StyleZhao, Y., Chen, S., Huang, Y., Zhao, J., Tong, X., & Chen, X. (2019). U-Pb Ages, O Isotope Compositions, Raman Spectrum, and Geochemistry of Cassiterites from the Xi’ao Copper-Tin Polymetallic Deposit in Gejiu District, Yunnan Province. Minerals, 9(4), 212. https://doi.org/10.3390/min9040212




