Microcrystalline Apatite Minerals: Mechanochemical Activation for Agricultural Application
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials
2.2. Carrot Cultivation for Nutrient Characterizations
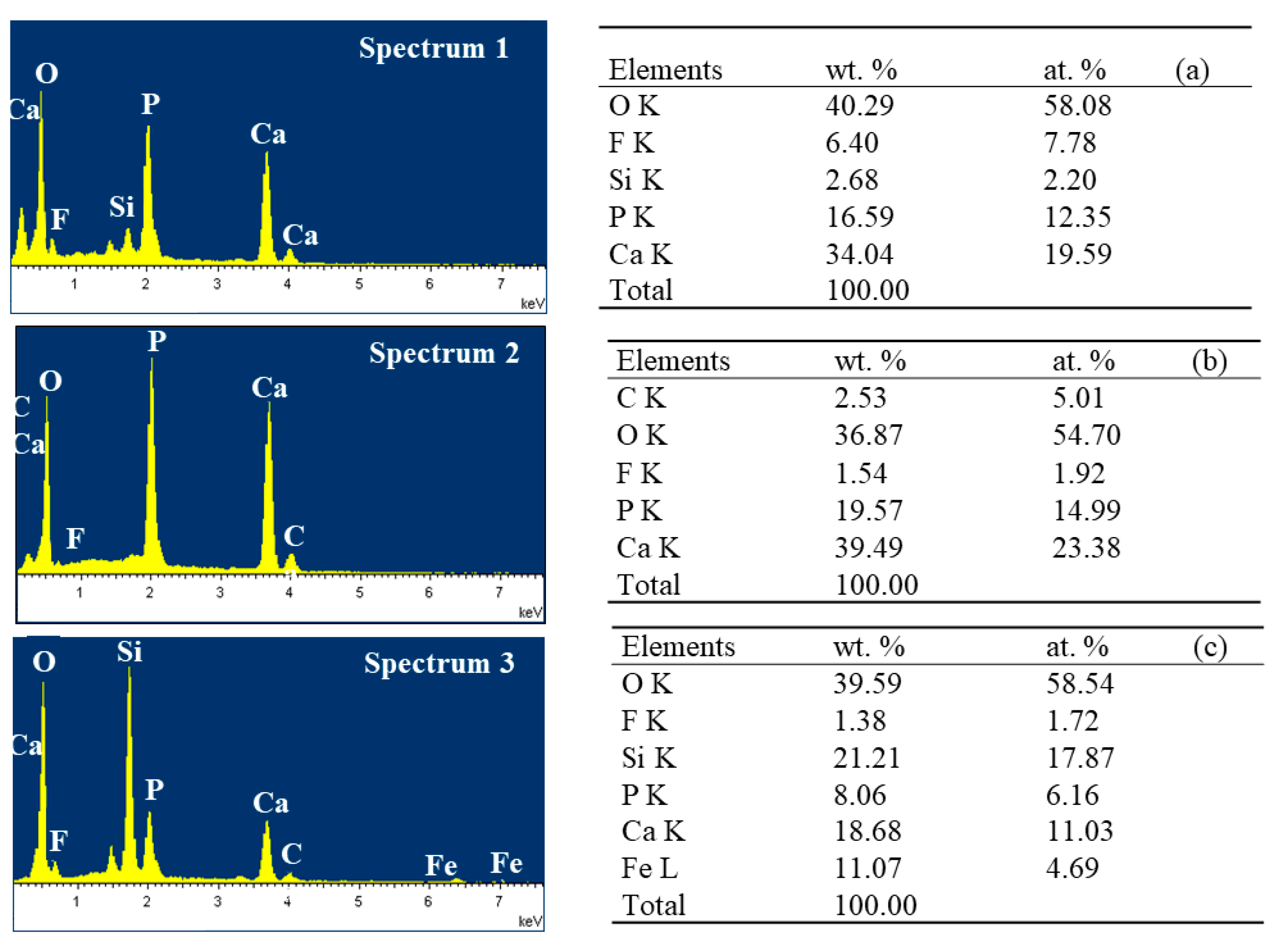
2.3. Characterizations of the Products
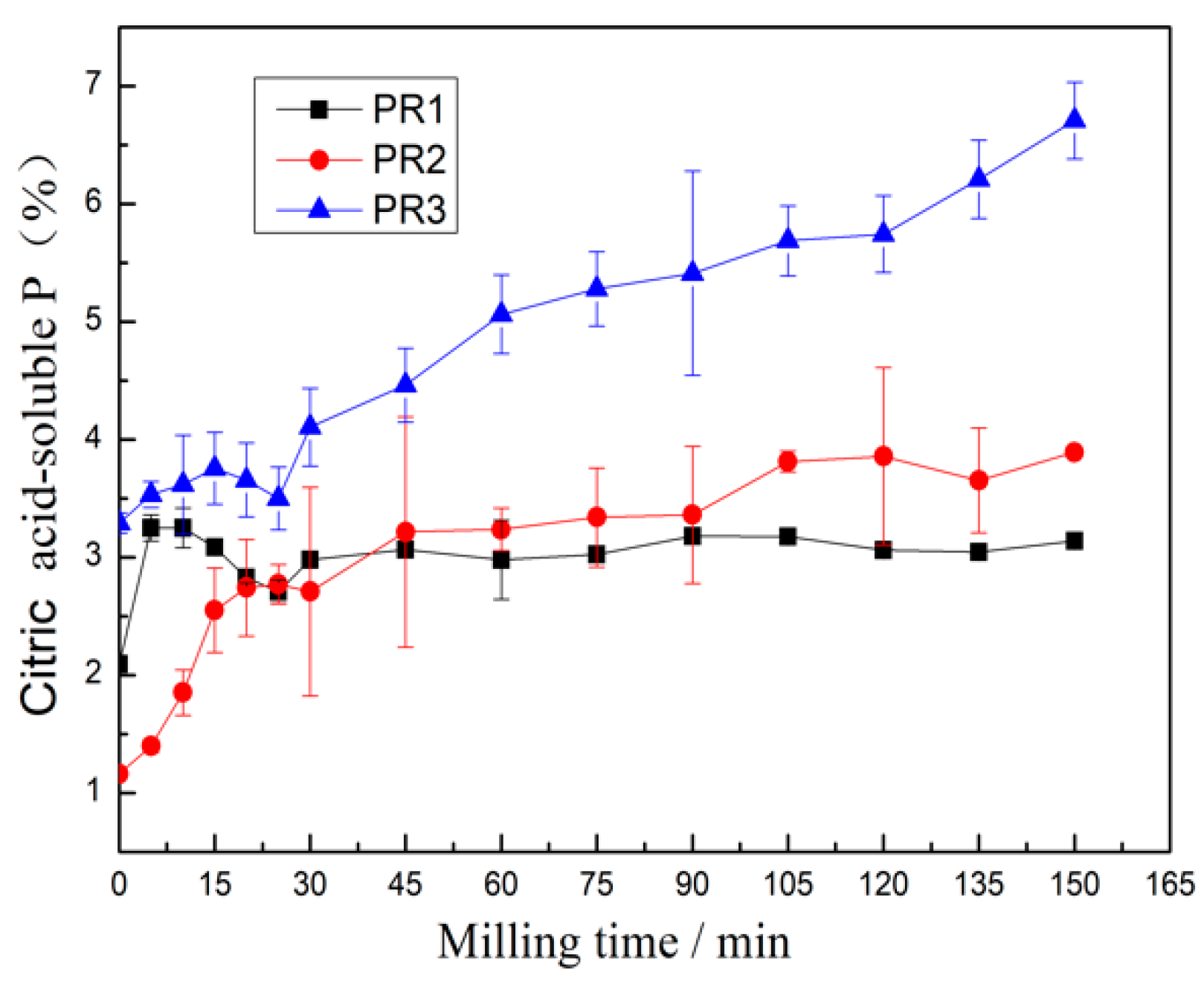
3. Results and Discussion
3.1. Characterization of the Processed PR
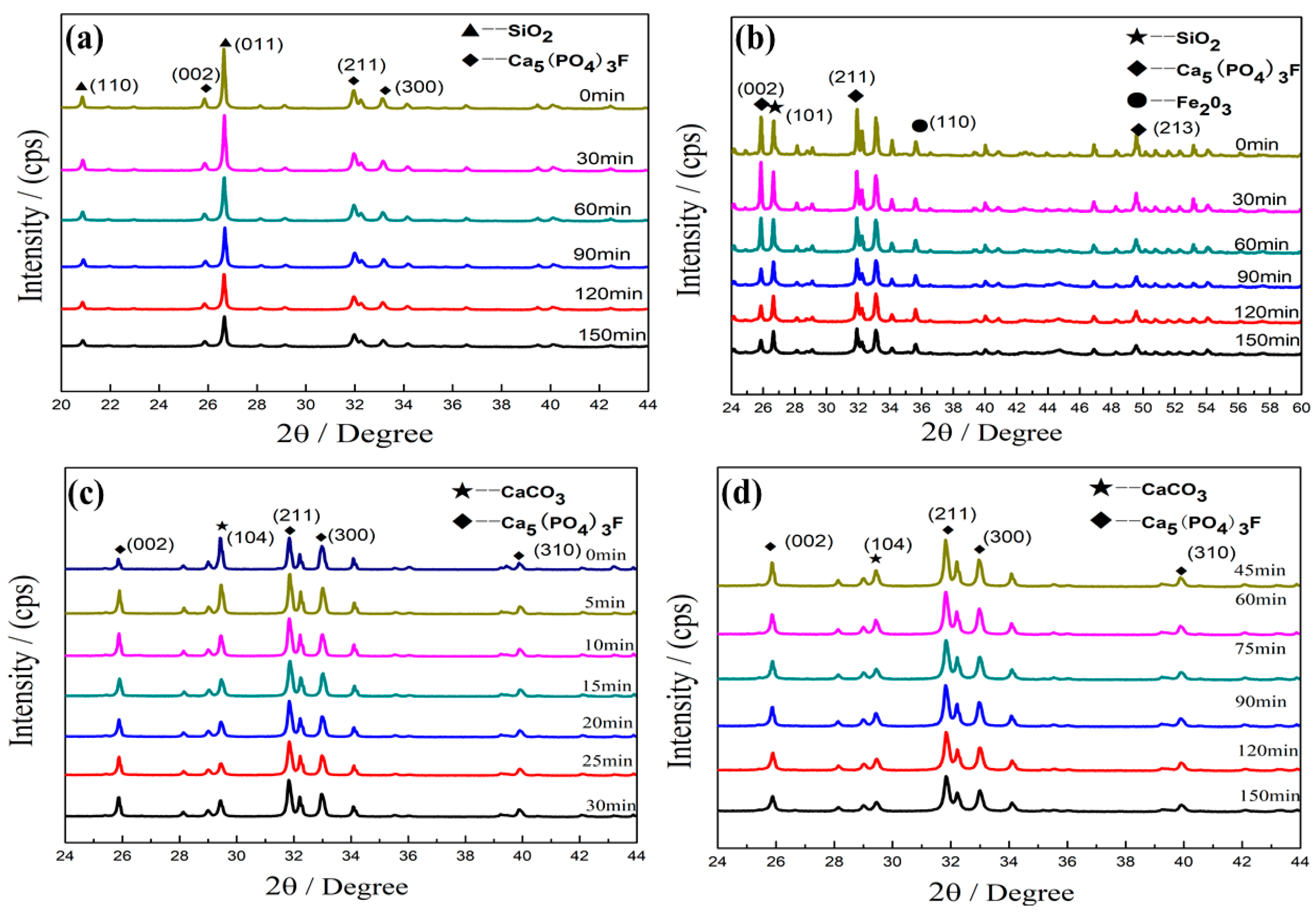
3.1.1. Effects of the Microcrystalline Processing on Diffracted Intensity in PR Powders
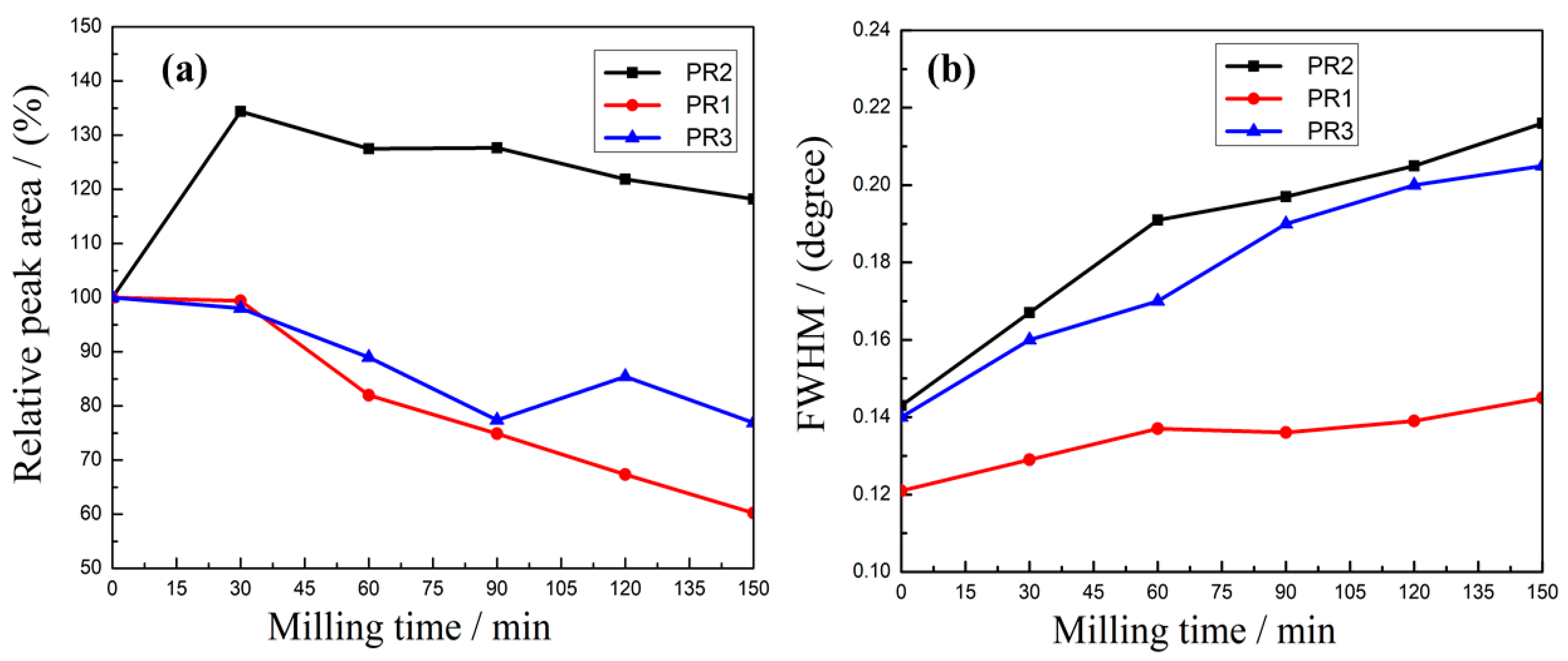
3.1.2. Effects of the Microcrystalline Processing on Relative Peak Area and Half-Width of PR Powders
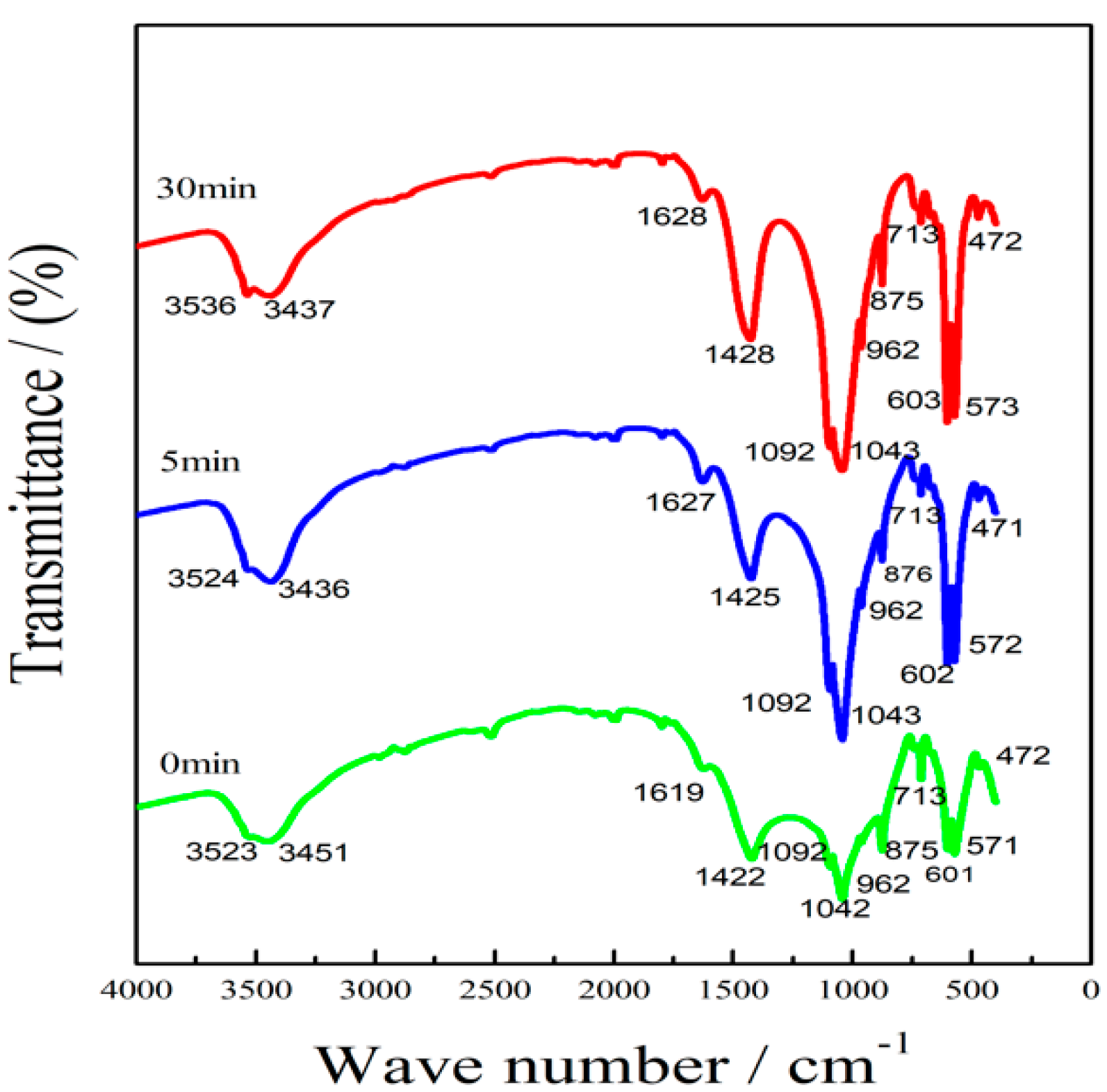
3.1.3. Effects of the Microcrystalline Processing on Infrared Spectrum of PR2
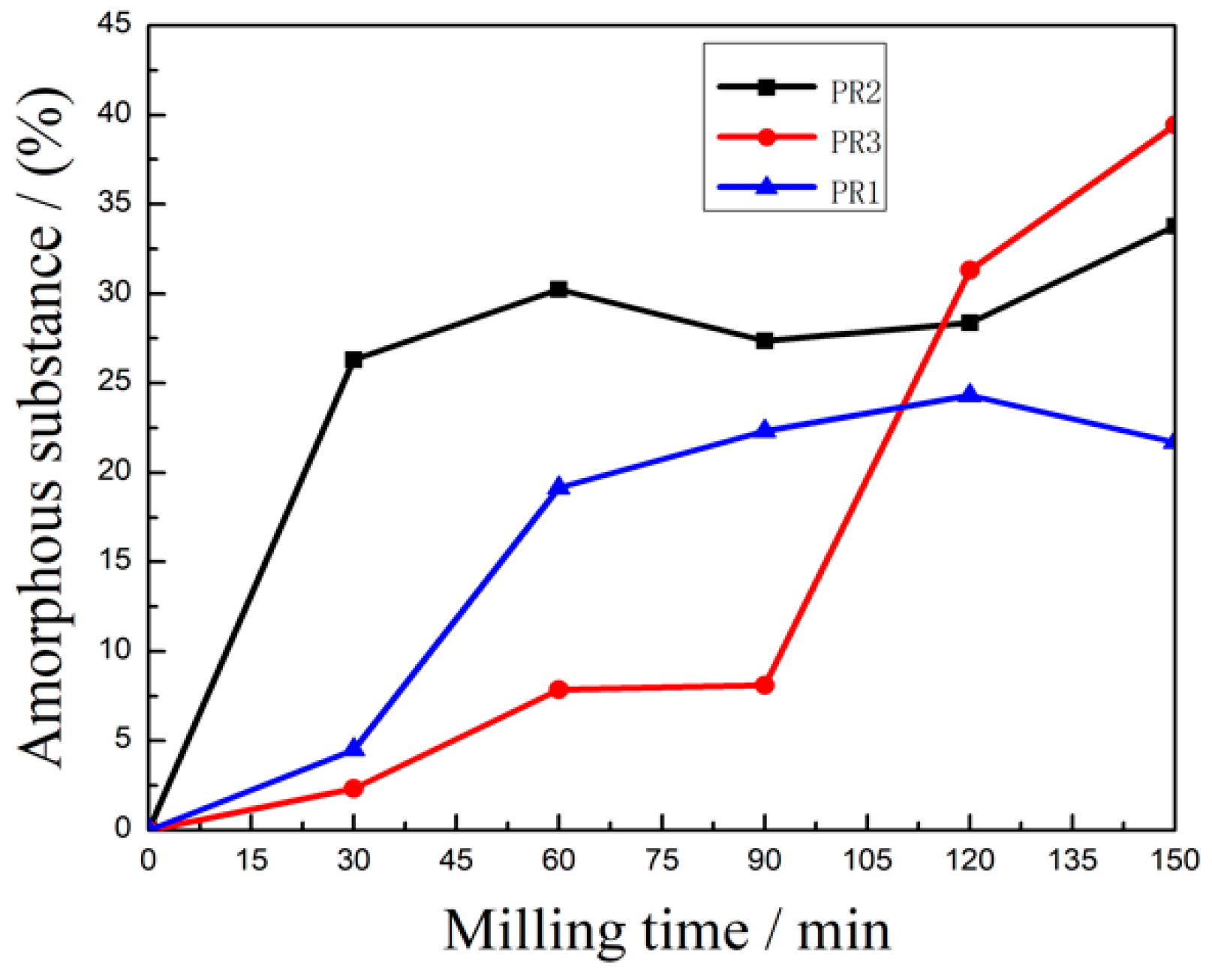
3.1.4. Effects of the Microcrystalline Processing on Amorphous Substance content of PR Powders
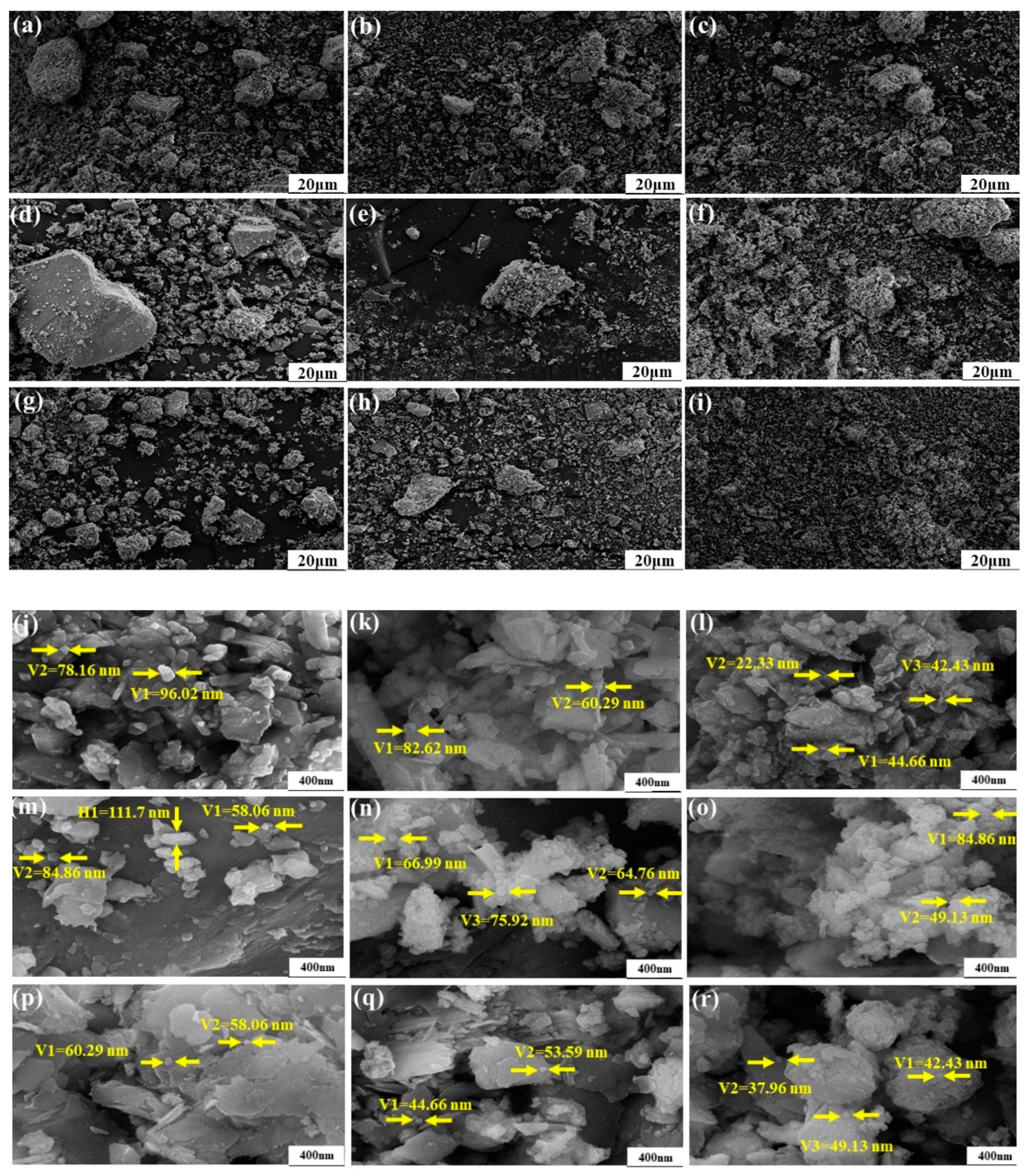
3.1.5. Effects of the Microcrystalline Processing on Granule Morphology of PR Powders
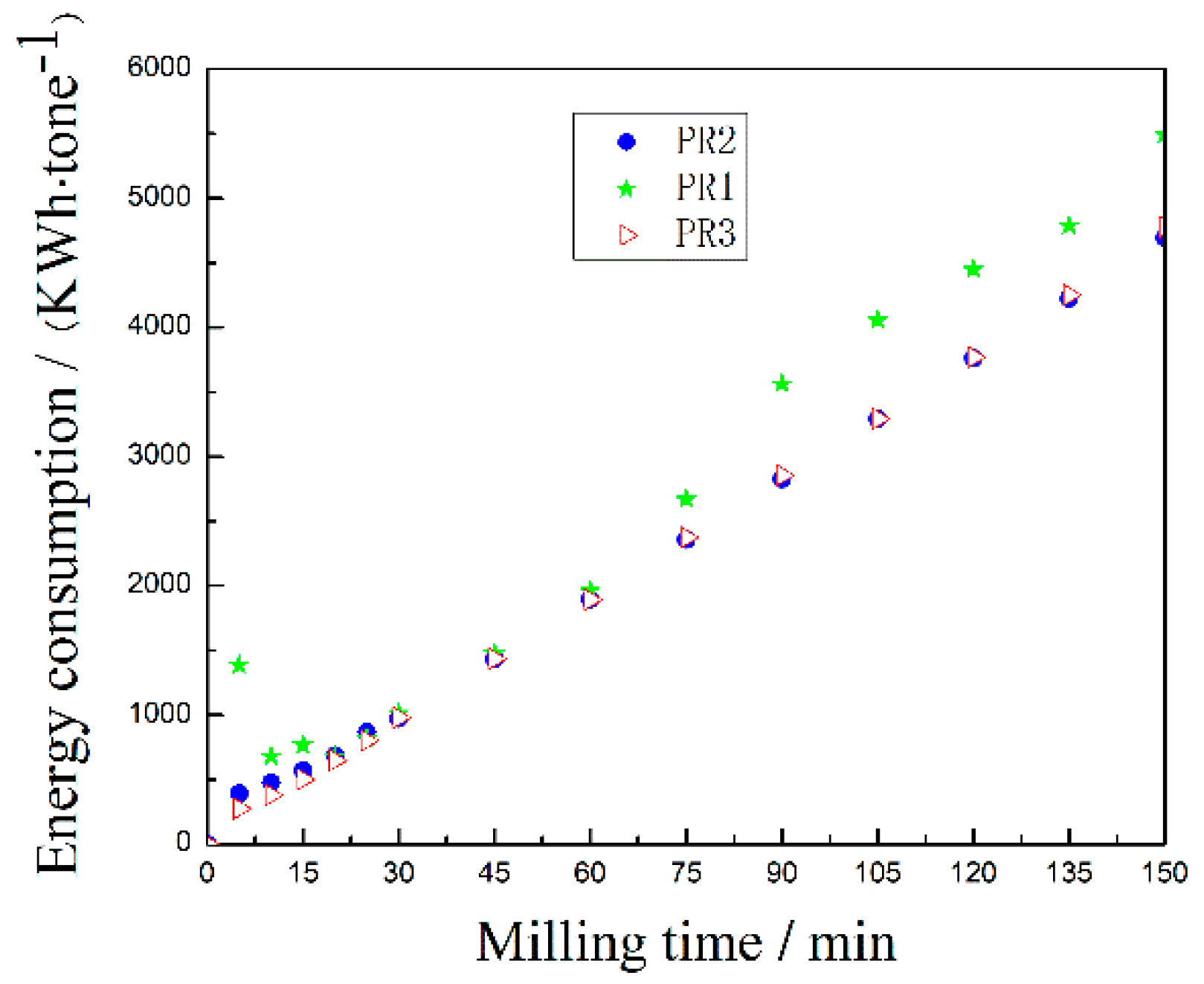
3.2. Analysis of the Energy Consumption of the Microcrystalline PR Powders
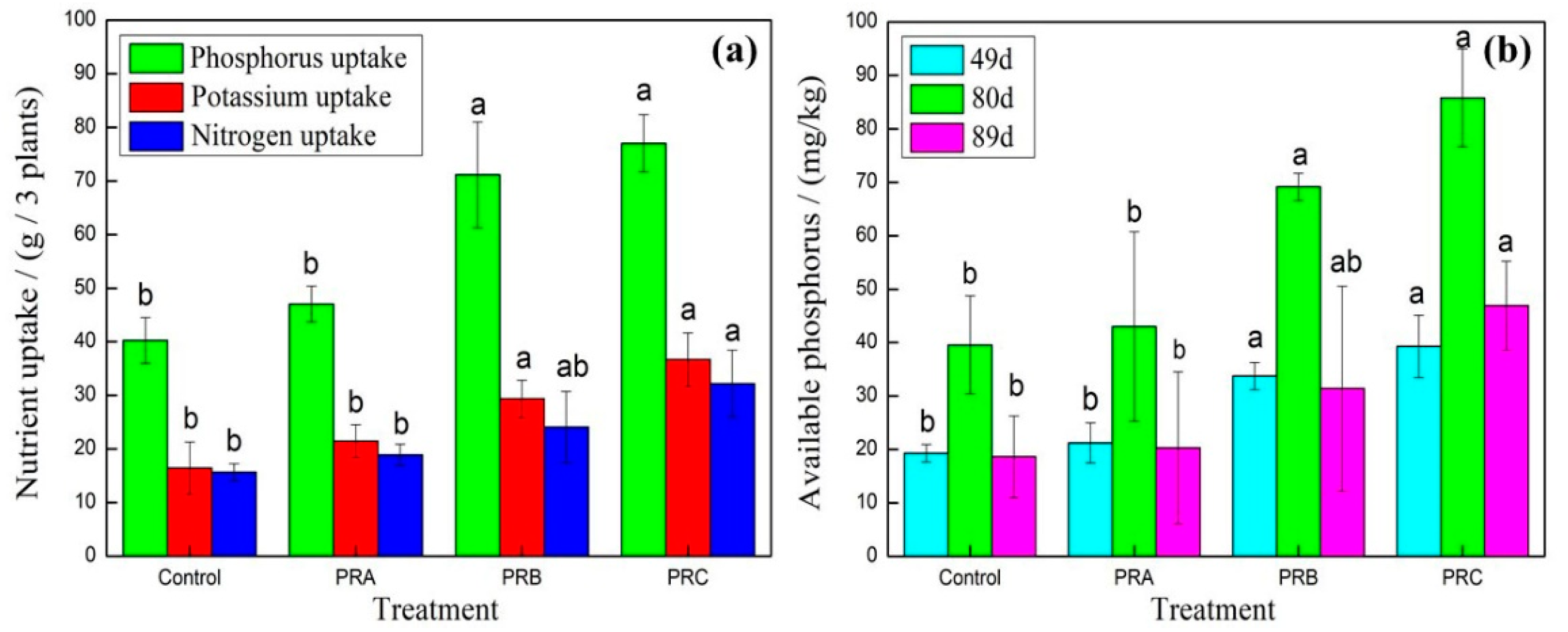
3.3. Evaluation of the Prepared Samples for Agricultural Application
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, S.; Litaker, R.W.; Sunda, W.G. Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton. J. Phycol. 2016, 52, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Kauwenbergh, S.V. World Phosphate Rock Reserves and Resources; The International Fertilizer Development Center: Muscle Shoals, AL, USA, 2010. [Google Scholar]
- Bolan, N.S.; Hedley, M.J.; Loganathan, P. Preparation, forms and properties of controlled-release phosphate fertilizers. Fertil. Res. 1993, 35, 13–24. [Google Scholar] [CrossRef]
- Chien, S.H.; Prochnow, L.I.; Mikkelsen, R. Agronomic use of phosphate rock for direct application. Better Crop. 2010, 94, 21–23. [Google Scholar]
- Han, X.L. The infrared absorption spectra of carbon-fluorapatite. Sci. Geol. Sin. 1980, 2, 15. [Google Scholar]
- Rajan, S.S.S.; Watkinson, J.H.; Sinclair, A.G. Phosphate rocks for direct application to soils. Adv. Agron. 1996, 57, 77–159. [Google Scholar]
- Lv, L.F.; Li, X.Y.; Zhang, H.W.; Dou, X.X.; Gai, G.S. Mechano-chemical effects of phosphorite on superfine grinding in ball-stirring mill in wet. Acta Mineral. Sin. 2016, 36, 382–386. [Google Scholar]
- He, Z.Q.; Liu, C.S.; Gai, G.S.; Yang, Y.F.; Dong, Y.J.; Zhao, F.T. Effect on phosphorus availability of rock phosphate rock powder by grinding it superfine. J. Soil Water Conserv. 2009, 23, 210–212. [Google Scholar]
- Lv, L.F.; Dou, X.X.; Zhang, H.W.; Xie, X.Y.; Gai, G.S. Potassium release characteristics of microcrystalline potassium mineral and its effects on growth of grain amaranth. Acta Prataculturae Sin. 2016, 25, 126–133. [Google Scholar]
- Ying, Y.F.; Chen, M.L.; Gao, H. Effect of dry and wet milling on mechanochemically activated phosphorite. J. Dalian Jiaotong Univ. 2015, 36, 80–83. [Google Scholar]
- Balaz, P.; Pourghahramani, P.; Dutkova, E.; Turianicova, E.; Kova, J.; Satka, A. Mechanochemistry in preparation of nanocrystalline semiconductors. Phys. Status Solidi C 2008, 5, 3756–3758. [Google Scholar] [CrossRef]
- Huang, L.; Mao, X.Y.; Chen, X.; Sun, X.S.; Wang, J.; Liao, Z.W. Phosphorus availability and fertilizer efficiency of rock phosphate as affected by ultrafine activation. Acta Pedol. Sin. 2013, 50, 769–777. [Google Scholar]
- Lv, L.F.; Gai, G.S.; Li, X.H.; Yang, Y.F.; Li, M.Q.; He, Z.Q.; Liu, C.S. Effects of microcrystalline grinding on potassium release in K-feldspar rock. J. Plant Nutr. Fertil. 2012, 18, 1428–1433. [Google Scholar]
- Palaniandy, S.; Azizli, K.A.M.; Hong, E.X.; Hashim, S.F.S.; Hussin, H. Custom made micronized silica for paint industry via fine grinding process. Adv. Technol. Mater. Mater. Process. J. 2006, 8, 109–116. [Google Scholar]
- Liu, J.X. Analysis of domestic phosphate rock resources their exploitation and utilization. Chem. Fertil. Ind. 2009, 36, 27–31. [Google Scholar]
- Zhang, Q.W.; Saito, F. A review on mechanochemical syntheses of functional materials. Adv. Powder Technol. 2012, 23, 523–531. [Google Scholar] [CrossRef]
- Chien, S.H.; Prochnow, L.I.; Cantarella, H. Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. Adv. Agron. 2009, 102, 267–322. [Google Scholar]
- Khan, A.A.; Jilani, G.; Akhtar, M.S.; Naqvi, S.M.S.; Rasheed, M. Phosphorus solubilizing bacteria: Occurrence, mechanisms and their role in crop production. J. Agric. Biol. Sci. 2009, 1, 48–58. [Google Scholar]
- Bao, S.D. Soil Agro-chemistrical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2007; pp. 268–270, 389–391. [Google Scholar]
- Lu, W.D. Statistical Analysis of SPSS for Windows; Electronic Industry Publishing Co.: Beijing, China, 2004; pp. 50–55. [Google Scholar]
- Zeng, G.C. Concise Light Mineralogy, 2nd ed.; China University of Geosciences Press: Beijing, China, 1989; p. 104. [Google Scholar]
- Shi, H.B.; Wang, S.L.; Liang, Y.Z.; Yao, H. Process mineralogy of mid-low-grade silicate-calcareous phosphate rocks in Yunnan province. J. Wuhan Inst. Technol. 2008, 30, 5–8. [Google Scholar]
- Li, L. Metallic Ore Dressing Abroad; BGRIMM Technology Group: Beijing, China, 1991; Volume 28, pp. 36–42. [Google Scholar]
- Lv, L.F. Effects of Microcrystalline Grinding on Potassium Release in Silicate Potassium Rocks and Its Application; Shandong Agricultural University: Taian, China, 2014. [Google Scholar]
- Thompson, G.E.; Wood, G.C. Anodic films on aluminum. Treatise on Materials Science and Technology. In Corrosion: Aqueous Processed and Passive Films; Elsevier: Amsterdam, The Netherlands, 1983; Volume 23, pp. 205–329. [Google Scholar]
- Chow, C.Y. Physico-chemical properties of rock phosphate in relation to the phosphorus availability to plant and its classification. Acta Pedol. Sin. 1966, 14, 23–30. [Google Scholar]
- Zhao, F.T. Study on the Effect of Ultra-Fine Combined Treatment Phosphate Rock Powder; Shandong Agricultural University: Taian, China, 2009. [Google Scholar]
- Ding, H.; Xin, F. Study on mechano-chemical modification of mineral pigment and filler. Compr. Util. Miner. Resour. 2000, 6, 26–31. [Google Scholar]
- Balaz, P.; Achimovicova, M.; Balaz, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutkova, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Balaz, M.; Dutkova, E.; Bujnakova, Z.; Tothova, E.; Kostova, N.G.; Karakirova, Y.; Briancin, J.; Kanuchova, M. Mechanochemistry of copper sulfides: Characterization, surface oxidation and photocatalytic activity. J. Alloys Compd. 2018, 746, 576–582. [Google Scholar] [CrossRef]
- Zhu, P.M.; Yang, X.X.; Xu, Y.C.; Ouyang, H.; Shen, Q.R. High effective phosphate-solubilizing bacteria: Their isolation and promoting effect on corn seedling growth. Chin. J. Appl. Ecol. 2007, 18, 107–112. [Google Scholar]
| Treatment | P2O5 /% | CaO /% | MgO /% | SiO2 /% | Fe2O3 /% | Al2O3 /% | K2O /% | Na2O /% | F /% | CO2 /% | MnO /% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PR1 | 23.6 | 33.0 | 0.4 | 34.1 | 0.9 | 2.0 | 0.3 | 0.2 | 2.7 | 1.2 | 0.3 |
| PR2 | 37.2 | 50.6 | 0.7 | 2.4 | 1.5 | 1.0 | 4.9 | ||||
| PR3 | 25.1 | 33.8 | 1.9 | 19.8 | 3.0 | 2.7 | 2.9 |
| Treatment | Total Yields g/3 Plants * | Carrots Fresh Weight g/3 Plants * | Leaves Dry Weight g/3 Plants * |
|---|---|---|---|
| Control | 105.7 d ± 5.8 | 85.9 d ± 5.1 | 6.0 c ± 0.7 |
| PRA | 169.7 c ± 4.9 | 143.0 c ± 3.8 | 6.9 b,c ± 0.8 |
| PRB | 197.0 b ± 5.4 | 156.8 b ± 6.0 | 8.1b ± 0.4 |
| PRC | 232.3 a ± 11.8 | 180.0 a ± 9.5 | 9.9a ± 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-M.; Hu, C.; He, Z.-Q.; Abbas, Y.; Li, Y.; Lv, L.-F.; Hao, X.-Y.; Gai, G.-S.; Huang, Z.-H.; Yang, Y.-F.; et al. Microcrystalline Apatite Minerals: Mechanochemical Activation for Agricultural Application. Minerals 2019, 9, 211. https://doi.org/10.3390/min9040211
Zhang X-M, Hu C, He Z-Q, Abbas Y, Li Y, Lv L-F, Hao X-Y, Gai G-S, Huang Z-H, Yang Y-F, et al. Microcrystalline Apatite Minerals: Mechanochemical Activation for Agricultural Application. Minerals. 2019; 9(4):211. https://doi.org/10.3390/min9040211
Chicago/Turabian StyleZhang, Xian-Mei, Cheng Hu, Zhen-Quan He, Yasir Abbas, Yi Li, Le-Fu Lv, Xiang-Yang Hao, Guo-Sheng Gai, Zhao-Hui Huang, Yu-Fen Yang, and et al. 2019. "Microcrystalline Apatite Minerals: Mechanochemical Activation for Agricultural Application" Minerals 9, no. 4: 211. https://doi.org/10.3390/min9040211
APA StyleZhang, X.-M., Hu, C., He, Z.-Q., Abbas, Y., Li, Y., Lv, L.-F., Hao, X.-Y., Gai, G.-S., Huang, Z.-H., Yang, Y.-F., & Yun, S.-N. (2019). Microcrystalline Apatite Minerals: Mechanochemical Activation for Agricultural Application. Minerals, 9(4), 211. https://doi.org/10.3390/min9040211





