Process and Mechanism of Gold Mineralization at the Zhengchong Gold Deposit, Jiangnan Orogenic Belt: Evidence from the Arsenopyrite and Chlorite Mineral Thermometers
Abstract
1. Introduction
2. Regional and Local Geology
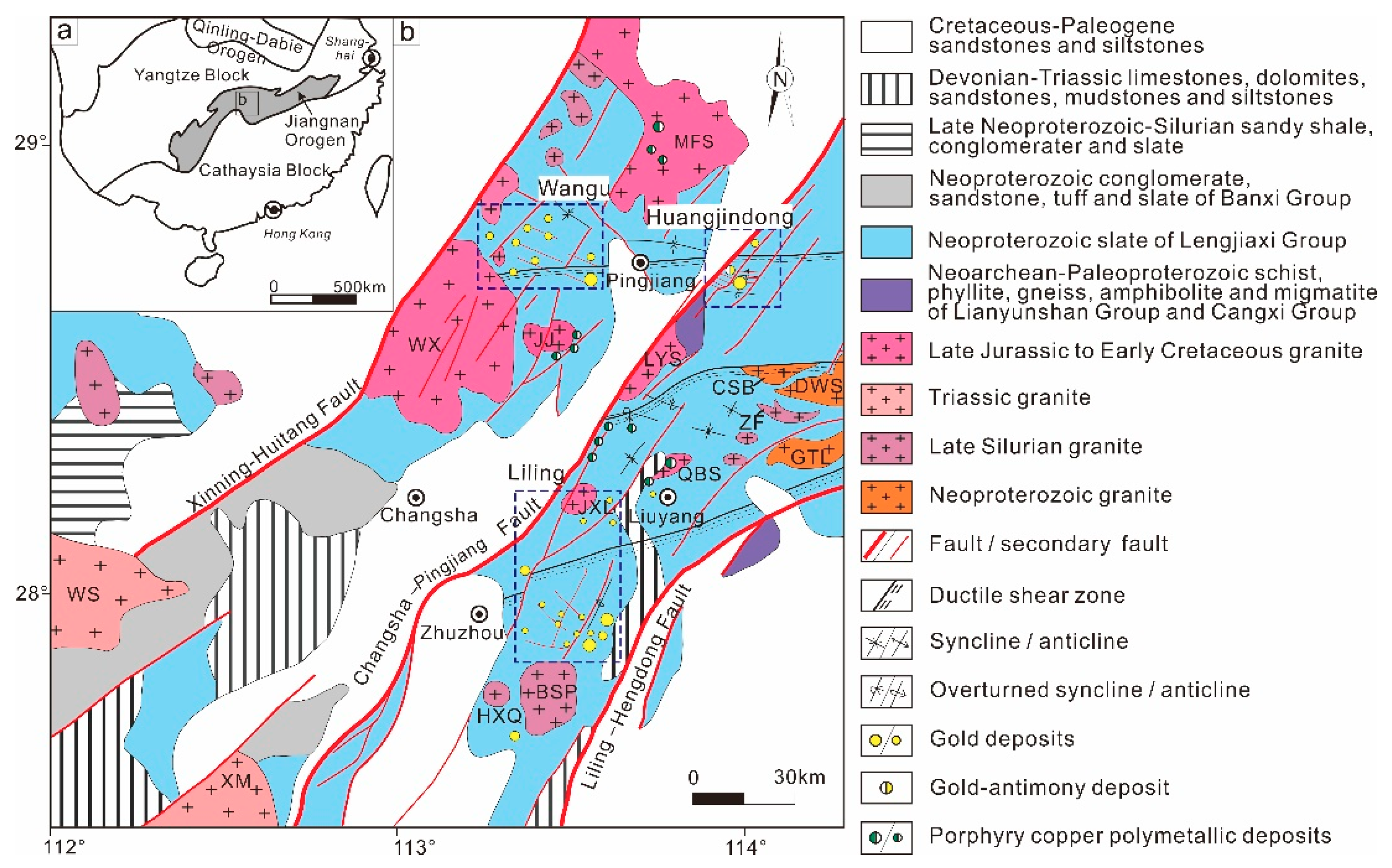
2.1. Regional Geology
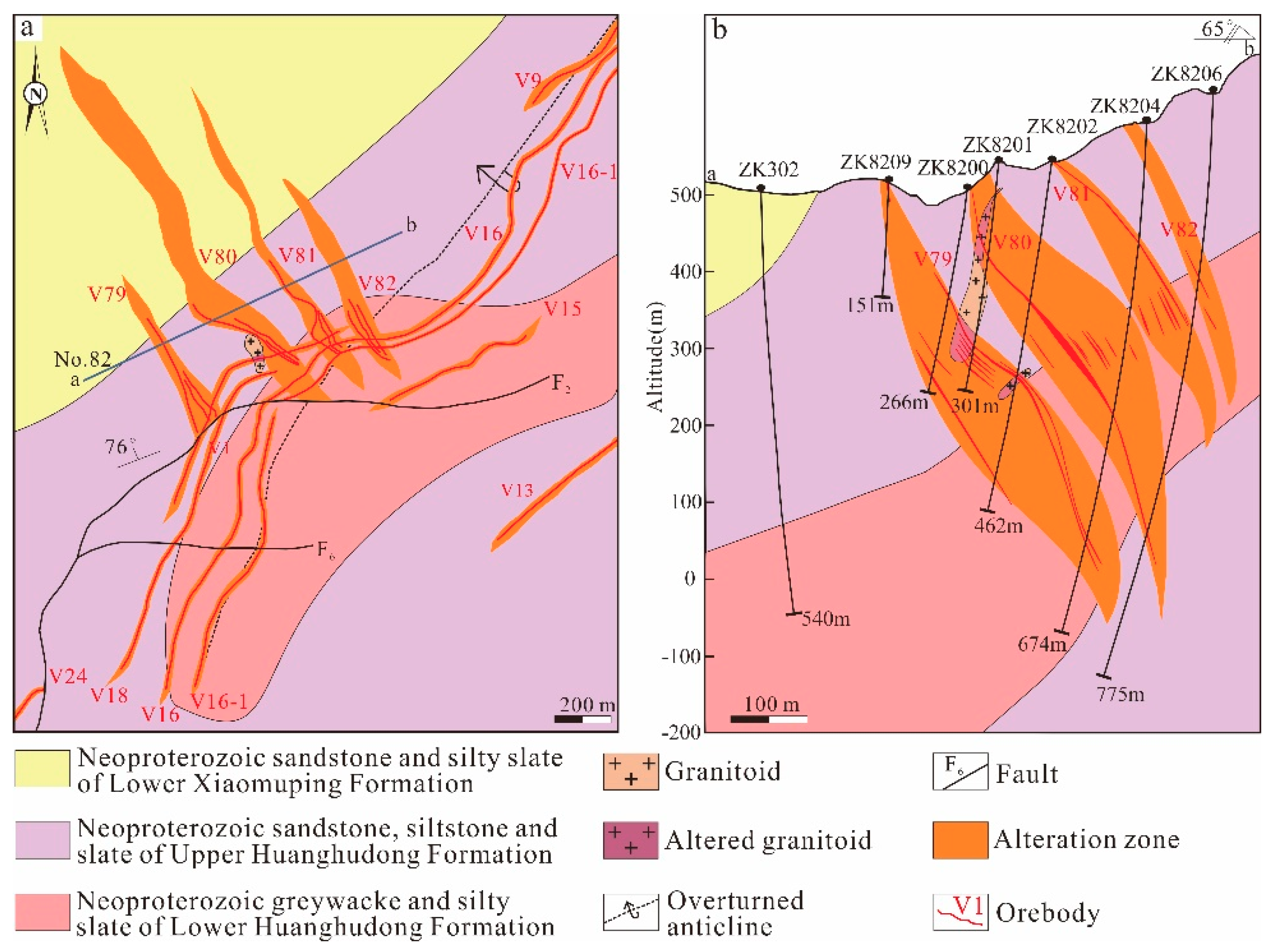
2.2. Deposit Geology
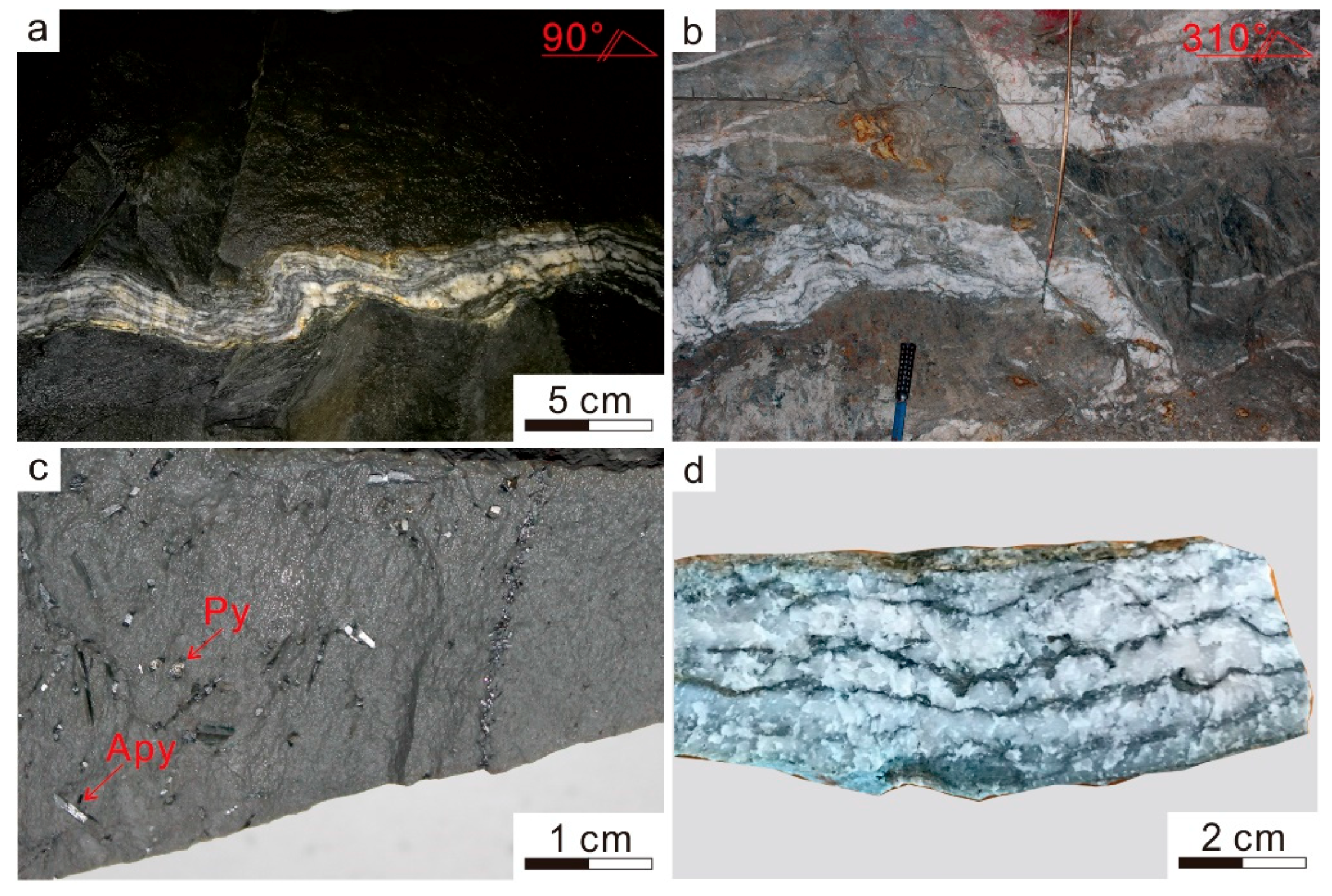
2.3. Mineral Association and Paragenesis
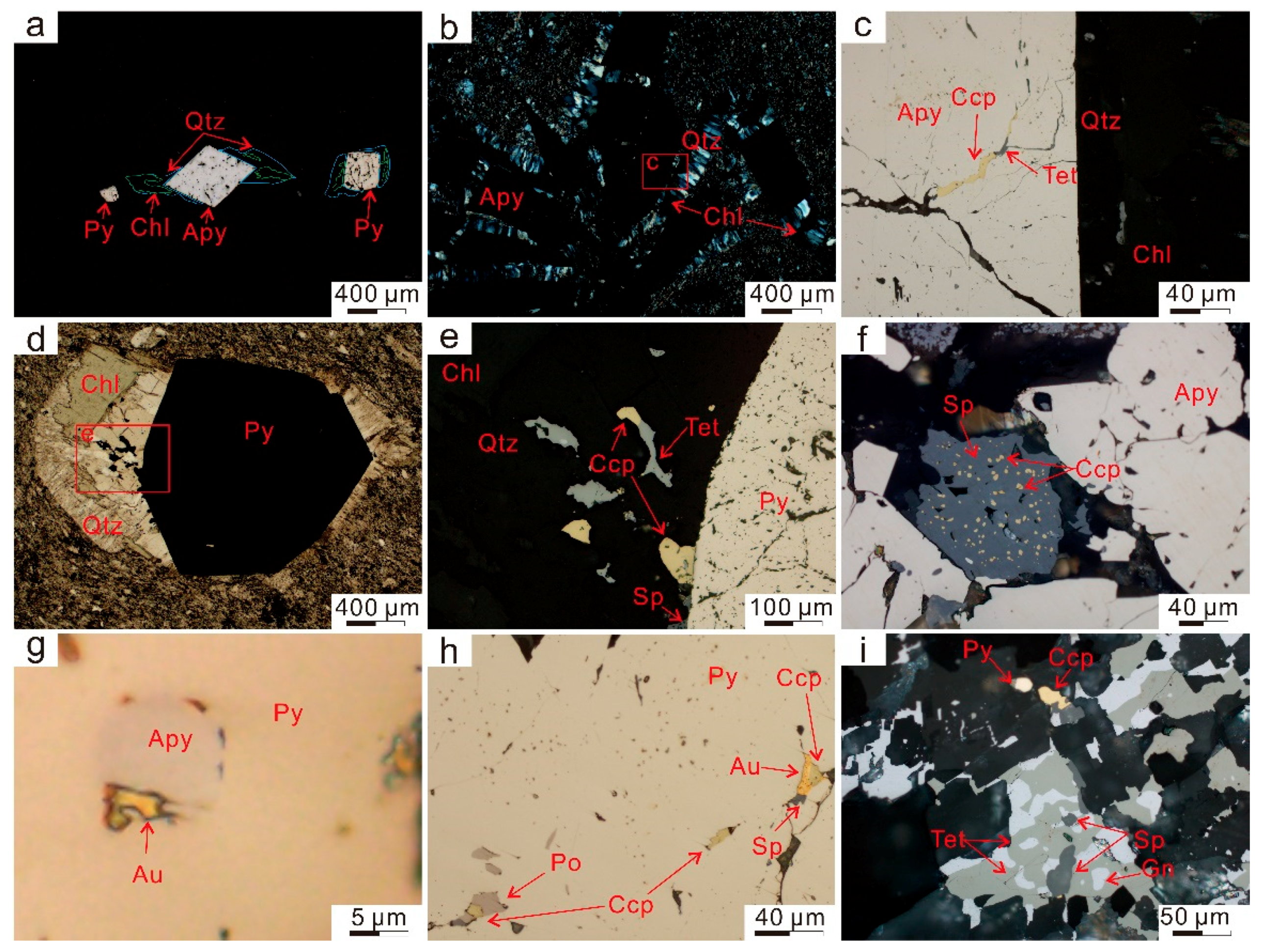
2.3.1. Arsenopyrite
2.3.2. Polymetallic Sulfides
2.3.3. Chlorite
3. Analytical Methods and Results
3.1. Sample Selection and Analytical Methods
3.2. Results
4. Discussion
4.1. The Physicochemical Environment of Mineral Precipitations
4.1.1. Physicochemical Conditions of Arsenopyrite
4.1.2. Physicochemical Conditions of Chlorite
4.2. Process and Mechanism of the Gold Precipitation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, L.Q.; Guo, L.N.; Wang, Z.L.; Zhao, R.X.; Song, M.C.; Zheng, X.L. Timing and mechanism of gold mineralization at the Wang’ershan gold deposit, Jiaodong Peninsula, eastern China. Ore Geol. Rev. 2017, 88, 491–510. [Google Scholar] [CrossRef]
- Deng, J.; Wang, Q.F.; Li, G.J. Tectonic evolution, superimposed orogeny, and composite metallogenic system in China. Gondwana Res. 2017, 50, 216–266. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Groves, D.I. Orogenic gold: Common or evolving fluid and metal sources through time. Lithos 2015, 233, 2–26. [Google Scholar] [CrossRef]
- Qiu, K.F.; Taylor, R.D.; Song, Y.H.; Yu, H.C.; Song, K.R.; Li, N. Geologic and geochemical insights into the formation of the Taiyangshan porphyry copper–molybdenum deposit, Western Qinling Orogenic Belt, China. Gondwana Res. 2016, 35, 40–58. [Google Scholar] [CrossRef]
- Hammond, N.Q.; Robb, L.; Foya, S.; Ishiyama, D. Mineralogical, fluid inclusion and stable isotope characteristics of Birimian orogenic gold mineralization at the Morila Mine, Mali, West Africa. Ore Geol. Rev. 2011, 39, 218–229. [Google Scholar] [CrossRef]
- Wang, Z.L.; Yang, L.Q.; Guo, L.N.; Marsh, E.; Wang, J.P.; Liu, Y.; Zhang, C.; Li, R.H.; Zhang, L.; Zheng, X.L.; et al. Fluid immiscibility and gold deposition in the Xincheng deposit, Jiaodong Peninsula, China: A fluid inclusion study. Ore Geol. Rev. 2015, 65, 701–717. [Google Scholar] [CrossRef]
- Yang, L.Q.; Deng, J.; Guo, L.N.; Wang, Z.L.; Li, X.Z.; Li, J.L. Origin and evolution of ore fluid and gold deposition processes at the giant Taishang gold deposit, Jiaodong Peninsula, eastern China. Ore Geol. Rev. 2016, 72, 585–602. [Google Scholar] [CrossRef]
- Qiu, K.F.; Marsh, E.; Yu, H.C.; Pfaff, K.; Gulbransen, C.; Gou, Z.Y.; Li, N. Fluid and metal sources of the Wenquan porphyry molybdenum deposit, Western Qinling, NW China. Ore Geol. Rev. 2017, 86, 459–473. [Google Scholar] [CrossRef]
- Chinnasamy, S.S.; Uken, R.; Reinhardt, J.; Selby, D.; Johnson, S. Pressure, temperature, and timing of mineralization of the sedimentary rock-hosted orogenic gold deposit at Klipwal, southeastern Kaapvaal Craton, South Africa. Miner. Depos. 2015, 50, 739–766. [Google Scholar] [CrossRef]
- Sun, S.C.; Zhang, L.; Wu, S.G.; Peng, J.S.; Wen, T. Metallogenic mechanism of the Huangjindong gold deposit, Jiangnan Orogenic Belt: Constraints from mineral formation environment and physicochemical conditions of metallogenesis. Acta Petrol. Sin. 2018, 34, 1469–1483, (In Chinese with English Abstract). [Google Scholar]
- Yang, L.Q.; Deng, J.; Wang, Z.L.; Guo, L.N.; Li, R.H.; Groves, D.I.; Danyushevsky, L.V.; Zhang, C.; Zheng, X.L.; Zhao, H. Relationships between gold and pyrite at the Xincheng gold deposit, Jiaodong, Peninsula, China: Implication for gold source and deposition in a brittle epizonal environment. Econ. Geol. 2016, 111, 105–126. [Google Scholar] [CrossRef]
- Large, R.R.; Maslennikov, V.V.; Robert, F.; Danyushevsky, L.V.; Chang, Z.S. Multistage sedimentary and metamorphic origin of pyrite and gold in the giant Sukhoi Log Deposit, Lena gold province, Russia. Econ. Geol. 2007, 102, 1233–1267. [Google Scholar] [CrossRef]
- Finch, E.G.; Tomkins, A.G. Pyrite-Pyrrhotite Stability in a Metamorphic Aureole: Implications for Orogenic Gold Genesis. Econ. Geol. 2017, 112, 661–674. [Google Scholar] [CrossRef]
- Kretschmar, U.; Scott, S.D. Phase relations involving arsenopyrite in the system Fe-As-S and their application. Can. Mineral. 1976, 14, 364–386. [Google Scholar]
- Yang, L.Q.; Deng, J.; Guo, R.P.; Guo, L.N.; Wang, Z.L.; Cheng, B.H.; Wang, B.H.; Wang, X.D. World-class Xincheng gold deposit: An example from the giant Jiaodong Gold Province. Geosci. Front. 2015, 7, 419–430. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Li, R.H.; Huang, T.; Zhang, R.Z.; Chen, B.H.; Li, J.K. Lead isotope geochemistry of Dayingezhuang gold deposit, Jiaodong Peninsula, China. Acta Petrol. Sin. 2014, 30, 2468–2480, (In Chinese with English Abstract). [Google Scholar]
- Zhang, L.; Yang, L.Q.; Wang, Y.; Weinberg, R.F.; An, P.; Chen, B.Y. Thermochronologic constrains on the processes of formation and exhumation of the Xinli orogenic gold deposit, Jiaodong Peninsula, eastern China. Ore Geol. Rev. 2017, 81, 140–153. [Google Scholar] [CrossRef]
- Deng, J.; Yang, L.Q.; Gao, B.F.; Sun, Z.S.; Guo, C.Y.; Wang, Q.F.; Wang, J.P. Fluid evolution and metallogenic dynamics during tectonic regime transition: Example from the Jiapigou gold belt in Northeast China. Resour. Geol. 2009, 59, 140–152. [Google Scholar] [CrossRef]
- Deng, J.; Wang, C.M.; Bagas, L.; Santosh, M.; Yao, E. Crustal architecture and metallogenesis in the south-eastern North China Craton. Earth-Sci. Rev. 2018, 182, 251–272. [Google Scholar] [CrossRef]
- Groves, D.I.; Santosh, M.; Goldfarb, R.J.; Zhang, L. Structural geometry of orogenic gold deposits: Implications for exploration of world-class and giant deposits. Geosci. Front. 2018, 9, 1163–1177. [Google Scholar] [CrossRef]
- Qiu, K.F.; Yu, H.C.; Gou, ZY.; Liang, Z.L.; Zhang, J.L.; Zhu, R. Nature and origin of Triassic igneous activity in the Western Qinling Orogen: The Wenquan composite pluton example. Int. Geol. Rev. 2018, 60, 242–266. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.Q.; Weinberg, R.F.; Groves, D.I.; Wang, Z.L.; Li, G.W.; Liu, Y.; Zhang, C.; Wang, Z.K. Anatomy of a world-class epizonal orogenic-gold system: A holistic thermochronological analysis of the Xincheng gold deposit, Jiaodong Peninsula, eastern China. Gondwana Res. 2019, 70, 50–70. [Google Scholar] [CrossRef]
- Charvet, J.; Shu, L.; Shi, Y.; Guo, L.; Faure, M. The building of South china: Collision of Yangzi and Cathaysia blocks, problems and tentative answers. J. Asian Earth Sci. 1996, 13, 223–235. [Google Scholar] [CrossRef]
- Xue, H.M.; Ma, F.; Song, Y.Q.; Xie, Y.P. Geochronology and geochemisty of the Neoproterozoic granitoid association from eastern segment of the Jiangnan orogeny, China: Constraints on the timing and process of amalgamation between the Yangtze and Cathaysia blocks. Acta Petrol. Sin. 2010, 26, 3215–3244, (In Chinese with English Abstract). [Google Scholar]
- Deng, J.; Wang, Q.F. Gold mineralization in China: Metallogenic provinces, deposit types and tectonic framework. Gondwana Res. 2016, 36, 219–274. [Google Scholar] [CrossRef]
- Xu, D.R.; Deng, T.; Chi, G.X.; Wang, Z.L.; Zou, F.H.; Zhang, J.L.; Zou, S.H. Gold mineralization in the Jiangnan Orogenic Belt of South China: Geological, geochemical and geochronological characteristics, ore deposit-type and geodynamic setting. Ore Geol. Rev. 2017, 88, 565–618. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.Q.; Groves, D.I.; Liu, Y.; Sun, S.C.; Qi, P.; Wu, S.G.; Peng, J.S. Geological and isotopic constraints on ore genesis, Huangjindong gold deposit, Jiangnan Orogen, southern China. Ore Geol. Rev. 2018, 99, 264–281. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Shao, Y.J.; Chen, M.; Algeo, T.J.; Li, H.; Dick, J.M.; Wang, C.; Wang, W.S.; Li, Z.Q.; Liu, Z.F. Insights into the genesis of orogenic gold deposits from the Zhengchong gold field, northeastern Hunan Province, China. Ore Geol. Rev. 2019, 105, 337–355. [Google Scholar] [CrossRef]
- Shao, Y.J.; Wang, W.S.; Liu, Q.Q.; Zhang, Yu. Trace element analysis of pyrite from the Zhengchong gold deposit, Northeast Hunan Province, China: Implications for the ore-forming process. Minerals 2018, 8, 262. [Google Scholar] [CrossRef]
- Wen, Z.L.; Deng, T.; Dong, G.J.; Zou, F.H.; Xu, D.R.; Wang, Z.L.; Lin, G.; Chen, W. Characteristics of ore-controlling structures of Wangu gold deposit in Northeastern Hunan Province. Geotecton. Metallog. 2016, 40, 281–294, (In Chinese with English Abstract). [Google Scholar]
- Peng, Z.H. The Geological Characteristics and Metallogenic Prognosis of Xiaojiashan Gold Deposit in Liling, Hunan Province. Master’s Thesis, Central South University, Changsha, China, 2012; pp. 1–143, (In Chinese with English Abstract). [Google Scholar]
- Cox, S.F.; Sun, S.S.; Etheridge, M.A.; Wall, V.J. Structural and geochemical controls on the development of turbidite-hosted gold quartz vein deposits, Wattle Gully mine, central Victoria, Australia. Econ. Geol. 1995, 90, 1722–1786. [Google Scholar] [CrossRef]
- Sibson, R.H.; Robert, F.; Poulsen, K.H. High-angle reverse faults, fluid-pressure cycling, and mesothermal gold-quartz deposits. Geology 1988, 16, 551–555. [Google Scholar] [CrossRef]
- Wilson, C.J.L.; Schaubs, P.M.; Leader, L.D. Mineral Precipitation in the Quartz Reefs of the Bendigo Gold Deposit, Victoria, Australia. Econ. Geol. 2013, 108, 259–278. [Google Scholar] [CrossRef]
- Zhang, W.L. EPMA of Au occurrence in gold, Huangjindong gold deposit. Geol. J. China Univ. 1997, 3, 256–262. (In Chinese) [Google Scholar]
- Liu, Y.J.; Sun, C.Y.; Cui, W.D.; Ji, J.F. Study on the occurrence of gold in arsenopyrite of Huangjindong gold deposit in Hunan province. Contrib. Geol. Miner. Resour. Res. 1989, 4, 42–49, (In Chinese with English Abstract). [Google Scholar]
- Large, R.; Thomas, H.; Craw, D.; Henne, A.; Henderson, S. Diagenetic pyrite as a source for metals in orogenic gold deposit, Otago schist, New Zealand. N. Z. J. Geol. Geophys. 2012, 55, 137–149. [Google Scholar] [CrossRef]
- Hey, M.H. A new review of the chlorite. Mineral. Mag. 1954, 30, 277–292. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Essene, E.J.; Kelly, W.C. A re-examination of the arsenopyrite geobarometry: Pressure considerations and applications to natural assemblages. Can. Mineral. 1985, 23, 517–534. [Google Scholar]
- Walshe, J.L. A six-component chlorite solid solution model and the conditions of chlorite formation in hydrothermal and geothermal systems. Econ. Geol. 1986, 81, 681–703. [Google Scholar] [CrossRef]
- Dora, M.L.; Randive, K.R. Chloritisation along the Thanewasna shear zone, western Bastar Craton, Central India: Its genetic linkage to Cu–Au mineralization. Ore Geol. Rev. 2015, 70, 151–172. [Google Scholar] [CrossRef]
- Battaglia, S. Applying X-ray geothermometer diffraction to a chlorite. Clays Clay Miner. 1999, 47, 54–63. [Google Scholar] [CrossRef]
- Rausell-Colom, J.A.; Wiewiora, A.; Matesanz, E. Relation between composition and d001 for chlorite. Am. Mineral. 1991, 76, 1373–1379. [Google Scholar]
- Nieto, F. Chemical composition of metapelitic chlorites: X-ray diffraction and optical property approach. Eur. J. Mineral. 1997, 9, 829–841. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Chen, F.R.; Yu, X.Y. Characteristics of chlorite and the mineralization significance of the gold deposit. Acta Mineral. Sin. 1997, 17, 100–106, (In Chinese with English Abstract). [Google Scholar]
- Zhang, W.; Zhang, S.T.; Cao, H.W.; Wu, D.J.; Xiao, C.X.; Chen, H.J.; Tang, L. Mineral characteristics of chlorite and its significance in the tin deposit of Xiaolonghe in western Yunnan Province. J. Chengdu Univ. Technol. (Sci. Technol. Ed.) 2014, 41, 318–328, (In Chinese with English Abstract). [Google Scholar]
- Velásquez, G.; Beziat, D.; Salvi, S.; Siebenaller, L.; Borisova, AY.; Pokrovski, G.S.; Parseval, P.D. Formation and deformation of pyrite and implications for gold mineralization in the El Callao district, Venezuela. Econ. Geol. 2014, 109, 457–486. [Google Scholar] [CrossRef]
- Arehart, G.B. Gold and arsenic in iron sulfides from sediment-hosted disseminated gold deposits: Implications for depositional processes. Econ. Geol. 1993, 88, 171–185. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Kokh, M.A.; Guillaume, D.; Borisova, A.Y.; Gisquet, P.; Hazemann, J.; Lahera, E.; Del Net, W.; Proux, O.; Testemale, D.; et al. Sulfur radical species from gold deposits on Earth. Proc. Natl. Acad. Sci. USA 2015, 112, 13484–13489. [Google Scholar] [CrossRef] [PubMed]
- Bindi, L.; Moelo, Y.; Léone, P.; Suchaud, M. Stoichiometric arsenopyrite, FeAsS, from La Roche-Balue Quarry, Loire-Atlantique, France: Crystal structure and Mössbauer study. Can. Mineral. 2012, 50, 471–479. [Google Scholar] [CrossRef]
- Fougerouse, D.; Micklethwaite, S.; Halfpenny, A.; Reddy, S.M.; Cliff, J.B.; Martin, L.A.J.; Kilburn, M.; Guagliardo, P.; Ultrich, S. The golden ark: Arsenopyrite crystal plasticity and the retention of gold through high strain and metamorphism. Terr. Nova 2016, 28, 181–187. [Google Scholar] [CrossRef]
- Fougerouse, D.; Micklethwaite, S.; Tomkins, A.G.; Mei, Y.; Kilburn, M.; Guagliardo, P.; Fisher, L.A.; Halfpenny, A.; Gee, M.; Paterson, D.; et al. Gold remobilisation and formation of high grade ore shoots driven by dissolution-reprecipitation replacement and Ni substitution into auriferous arsenopyrite. Geochim. Cosmochim. Acta 2016, 178, 143–159. [Google Scholar] [CrossRef]
- Fleck, N.A.; Muller, G.M.; Ashby, M.F.; Hutchinson, J.W. Strain gradient plasticity: Theory and experiment. Acta Metall. Mater. 1994, 42, 475–487. [Google Scholar] [CrossRef]
- Dubosq, R.; Lawley, C.J.M.; Rogowitz, A.; Schneider, D.A.; Jackson, S. Pyrite deformation and connections to gold mobility: Insight from micro-structural analysis and trace element mapping. Lithos 2018, 310–311, 86–104. [Google Scholar] [CrossRef]
- Weatherley, D.K.; Henley, R.W. Flash vaporization during earthquakes evidenced by gold deposits. Nat. Geosci. 2013, 6, 294–298. [Google Scholar] [CrossRef]
- Beaudoin, G.; Chiaradia, M. Fluid mixing in orogenic gold deposits: Evidence from the H-O-Sr isotope composition of the Val-d’Or vein field (Abitibi, Canada). Chem. Geol. 2016, 437, 7–18. [Google Scholar] [CrossRef]
- Pitcairn, I.K.; Olivo, G.R.; Teagle, D.A.H.; Craw, D. Sulfide evolution during prograde metamorphism of the Otago and Alpine schists, New Zealand. Can. Mineral. 2010, 48, 1267–1295. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Bowell, R.J.; Migdisov, A.A. Gold in Solution. Elements 2009, 5, 281–287. [Google Scholar] [CrossRef]

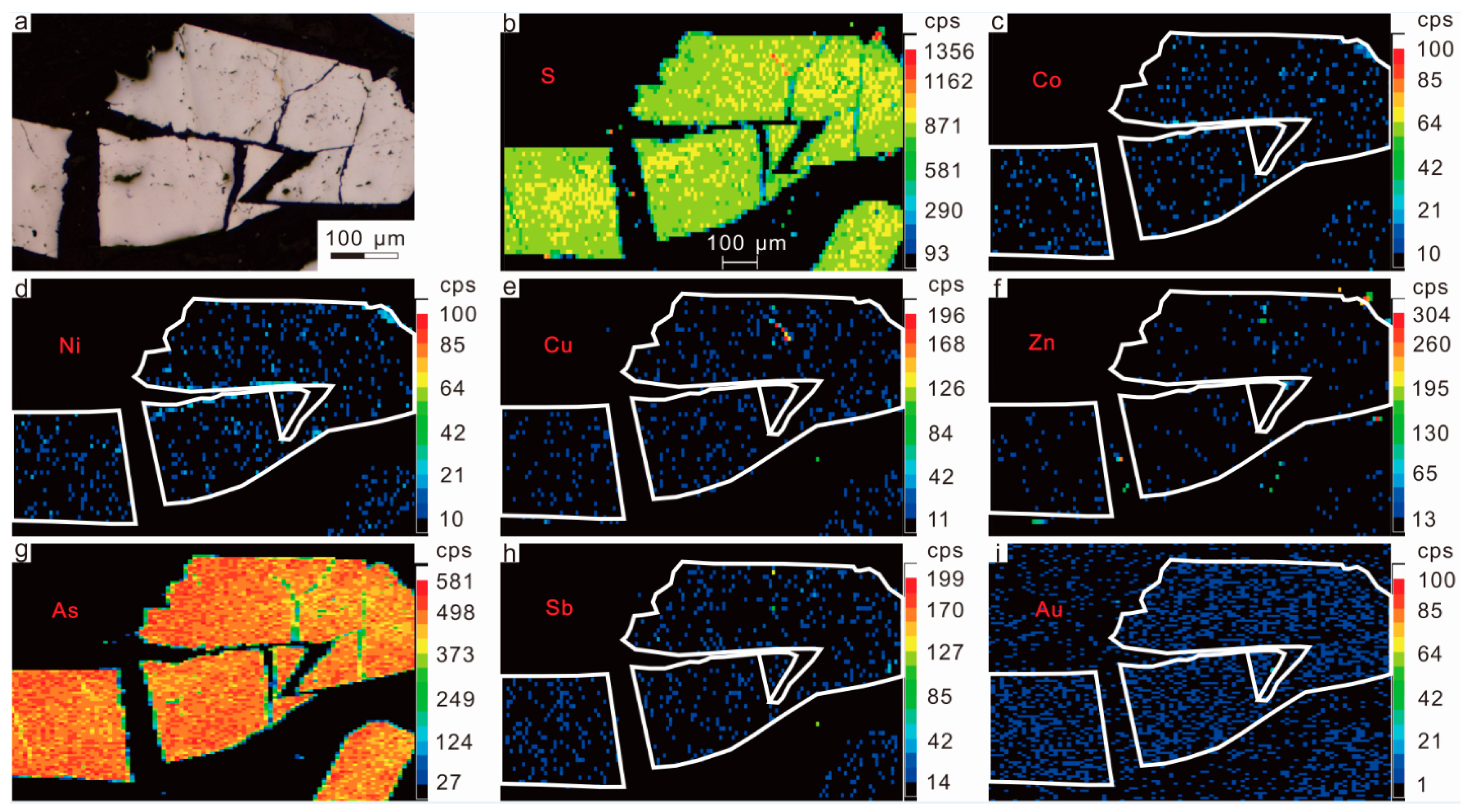

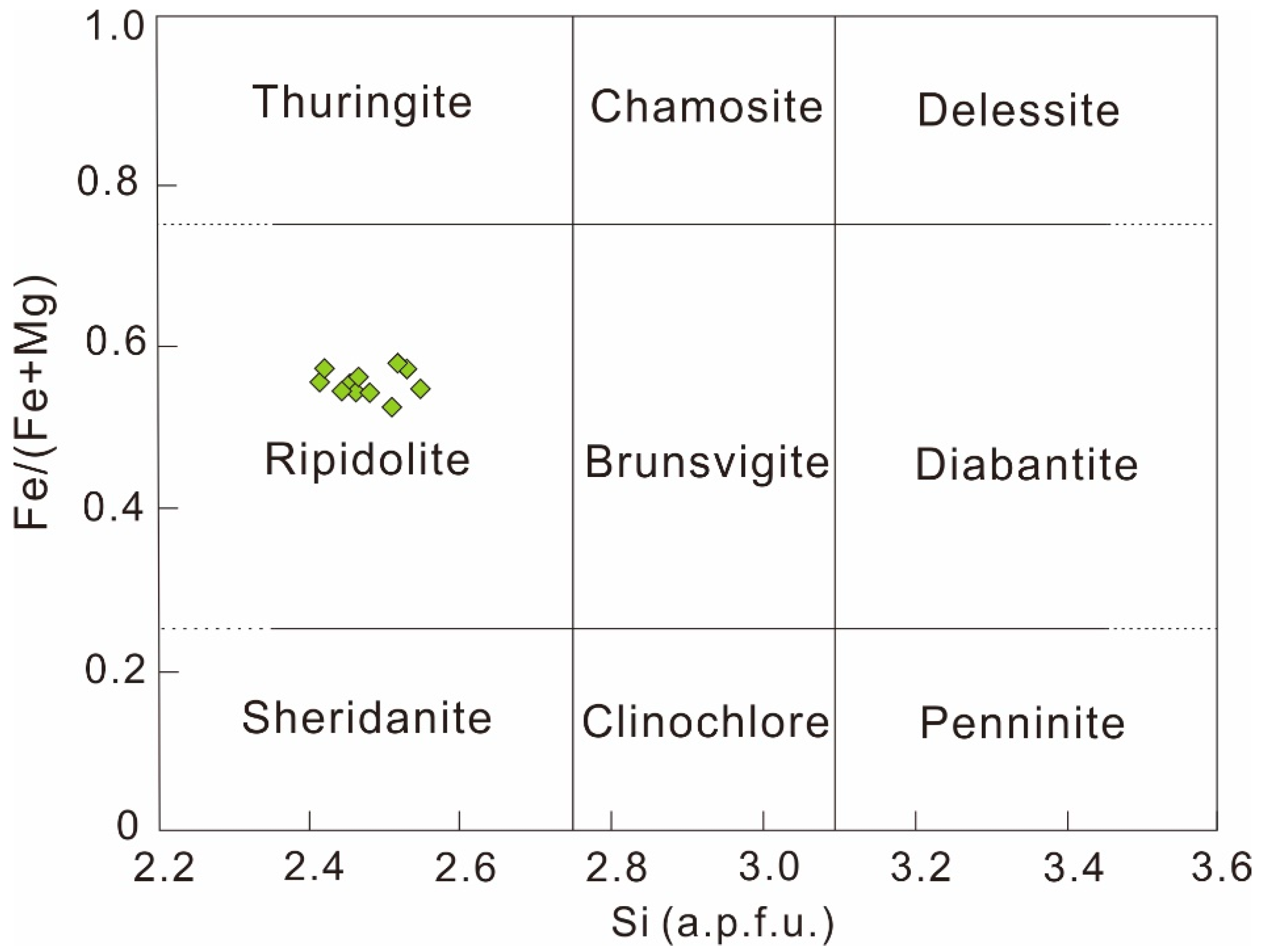
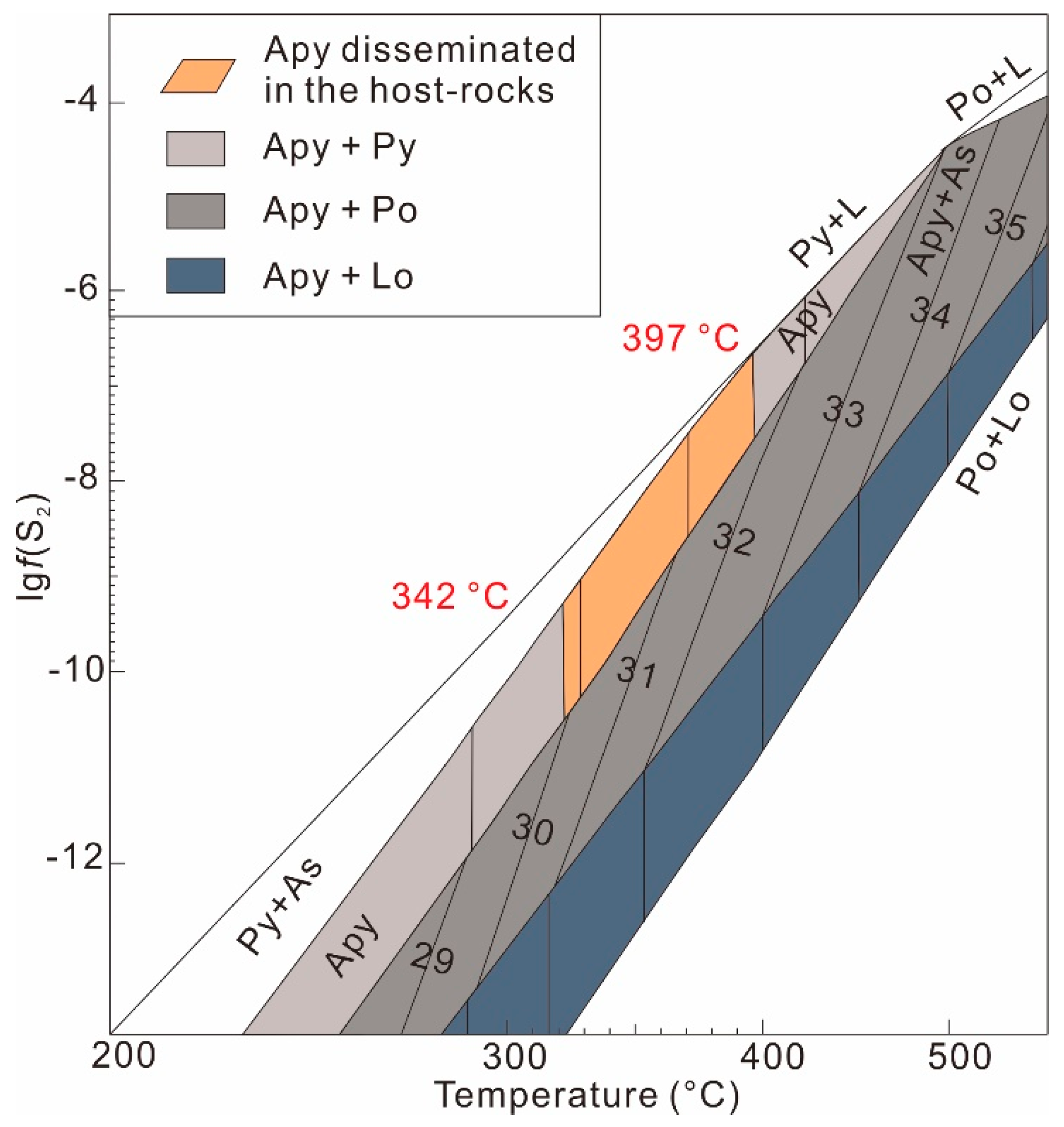
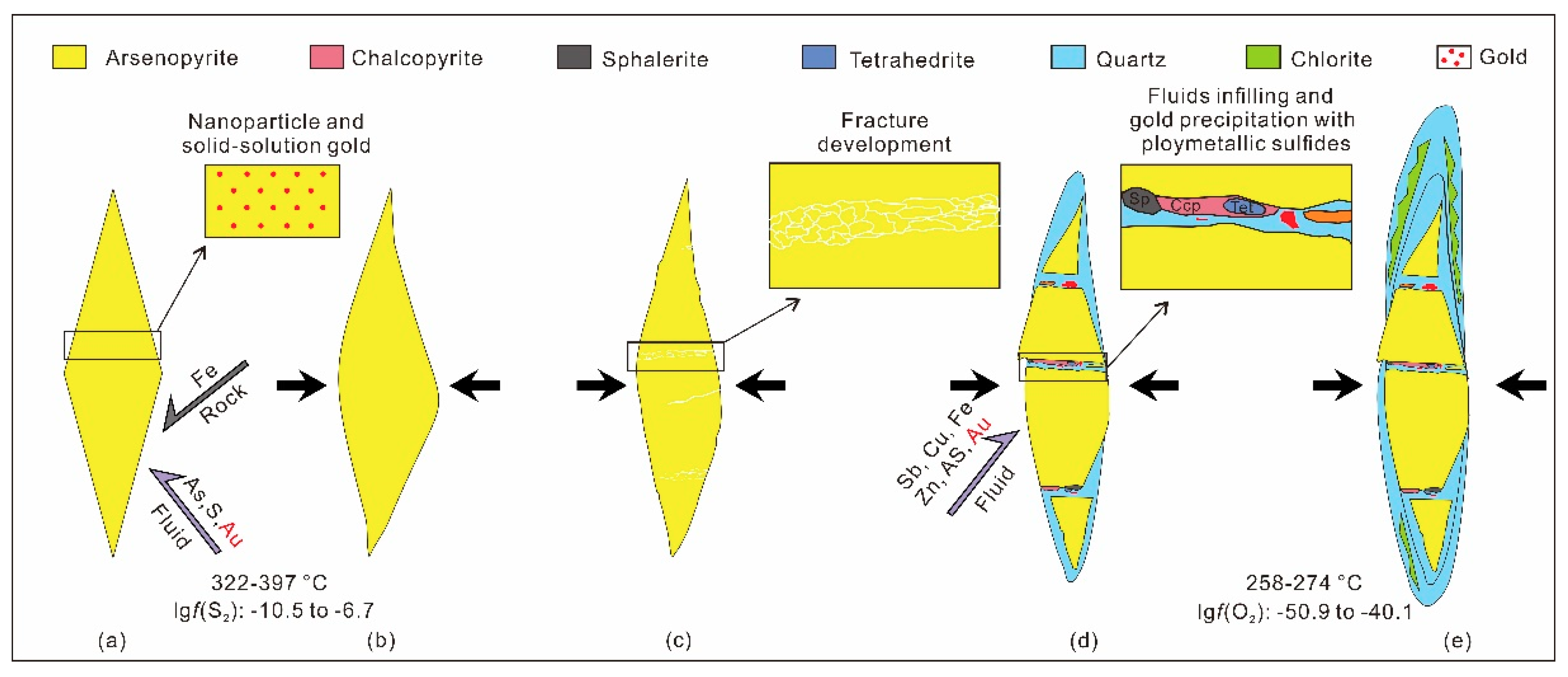
| Spot No. | Elements (wt %) | Chemical Formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Au | S | Ag | Sb | Fe | Co | Ni | Cu | Zn | Total | ||
| ZC17D01B1-1 | 43.07 | 0 | 22.24 | 0 | 0 | 34.80 | 0.05 | 0.02 | 0 | 0 | 100.18 | FeAs0.92S1.11 |
| ZC17D01B1-2 | 42.43 | 0.04 | 22.80 | 0.01 | 0 | 34.64 | 0.01 | 0.07 | 0 | 0 | 99.99 | FeAs0.91S1.15 |
| ZC17D01B1-3 | 41.52 | 0 | 22.24 | 0.01 | 0.04 | 34.37 | 0.07 | 0.01 | 0.02 | 0 | 98.28 | FeAs0.90S1.13 |
| ZC17D01B1-4 | 43.22 | 0 | 22.43 | 0 | 0.05 | 34.45 | 0.02 | 0.01 | 0.01 | 0.12 | 100.30 | FeAs0.94S1.13 |
| ZC17D01B1-5 | 43.39 | 0.08 | 22.05 | 0.01 | 0.01 | 34.84 | 0.07 | 0.01 | 0.01 | 0.06 | 100.54 | FeAs0.93S1.10 |
| ZC17D01B1-6 | 43.85 | 0.01 | 22.02 | 0 | 0 | 35.03 | 0.06 | 0 | 0 | 0.03 | 100.99 | FeAs0.93S1.10 |
| ZC17D01B1-7 | 42.77 | 0.01 | 22.26 | 0 | 0.02 | 34.60 | 0.01 | 0.05 | 0.04 | 0 | 99.75 | FeAs0.92S1.12 |
| ZC17D01B1-8 | 44.72 | 0.07 | 21.37 | 0 | 0 | 34.65 | 0.01 | 0.02 | 0.01 | 0.04 | 100.89 | FeAs0.96S1.07 |
| ZC17D01B1-9 | 43.91 | 0.01 | 21.38 | 0 | 0 | 34.72 | 0.05 | 0 | 0.03 | 0.01 | 100.11 | FeAs0.94S1.07 |
| ZC17D01B1-10 | 44.04 | 0 | 21.66 | 0 | 0 | 34.58 | 0.02 | 0 | 0.07 | 0.01 | 100.37 | FeAs0.95S1.09 |
| ZC17D01B1-11 | 43.89 | 0 | 21.94 | 0.03 | 0 | 34.21 | 0.01 | 0 | 0 | 0 | 100.07 | FeAs0.96S1.12 |
| ZC17D01B1-12 | 44.21 | 0.09 | 21.88 | 0.02 | 0 | 34.71 | 0.01 | 0.05 | 0.06 | 0 | 101.02 | FeAs0.95S1.10 |
| ZC17D01B1-13 | 44.12 | 0 | 21.41 | 0.02 | 0 | 33.84 | 0.03 | 0 | 0.01 | 0 | 99.42 | FeAs0.97S1.10 |
| ZC17D01B1-14 | 43.91 | 0.01 | 21.46 | 0 | 0.01 | 34.29 | 0.02 | 0.03 | 0.08 | 0.02 | 99.82 | FeAs0.96S1.09 |
| ZC17D01B2-15 | 43.22 | 0 | 21.52 | 0 | 0 | 34.09 | 0.03 | 0.04 | 0.03 | 0 | 98.94 | FeAs0.95S1.10 |
| ZC17D01B2-16 | 43.40 | 0.06 | 21.60 | 0.03 | 0.01 | 34.67 | 0.07 | 0.05 | 0.06 | 0.05 | 100.00 | FeAs0.93S1.09 |
| ZC17D01B2-17 | 43.42 | 0.07 | 21.72 | 0 | 0.01 | 34.10 | 0.03 | 0.04 | 0 | 0 | 99.38 | FeAs0.95S1.11 |
| ZC17D01B2-18 | 42.20 | 0.03 | 22.42 | 0 | 0.02 | 34.55 | 0.04 | 0.01 | 0 | 0.04 | 99.29 | FeAs0.91S1.13 |
| ZC17D01B2-19 | 43.11 | 0.02 | 21.56 | 0 | 0.03 | 33.98 | 0.05 | 0.05 | 0.01 | 0.05 | 98.86 | FeAs0.95S1.11 |
| ZC17D01B2-20 | 43.44 | 0.01 | 21.29 | 0 | 0.03 | 34.28 | 0.03 | 9 | 0.07 | 0 | 99.14 | FeAs0.95S1.08 |
| Samples No. | zc17d01b1-1 | zc17d01b1-2 | zc17d01b1-3 | zc17d01b1-4 | zc17d01b1-5 | zc17d01b1-6 | zc17d01b1-7 | zc17d01b1-8 | zc17d01b1-9 | zc17d01b1-10 | zc17d01b1-11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| W(B)/% | |||||||||||
| Na2O | 0.08 | 0.05 | 0.09 | 0.06 | 0.00 | 0.05 | 0.07 | 0.03 | 0.03 | 0.02 | 0.04 |
| MgO | 12.11 | 12.47 | 12.57 | 12.30 | 12.61 | 11.56 | 11.85 | 13.26 | 11.84 | 12.06 | 12.58 |
| Al2O3 | 22.61 | 23.09 | 23.13 | 22.92 | 22.16 | 21.97 | 22.50 | 22.37 | 22.74 | 22.96 | 21.84 |
| SiO2 | 22.22 | 22.07 | 22.39 | 22.15 | 23.16 | 22.57 | 23.02 | 22.79 | 21.74 | 21.51 | 21.96 |
| K2O | 0.03 | 0.02 | 0.03 | 0.02 | 0.02 | 0.03 | 0.04 | 0.02 | 0.01 | 0.00 | 0.02 |
| CaO | 0.00 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.02 | 0.00 | 0.02 | 0.01 | 0.01 |
| P2O5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.03 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 |
| FeO | 27.59 | 26.65 | 26.70 | 25.90 | 27.19 | 28.37 | 28.18 | 26.06 | 28.14 | 26.86 | 27.29 |
| V2O3 | 0.00 | 0.02 | 0.04 | 0.03 | 0.00 | 0.01 | 0.02 | 0.01 | 0.04 | 0.03 | 0.00 |
| MnO | 0.00 | 0.00 | 0.01 | 0.02 | 0.02 | 0.00 | 0.03 | 0.02 | 0.00 | 0.00 | 0.01 |
| Cr2O3 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cations | |||||||||||
| Si | 2.48 | 2.45 | 2.47 | 2.48 | 2.55 | 2.53 | 2.53 | 2.52 | 2.43 | 2.43 | 2.48 |
| AlIV | 1.52 | 1.55 | 1.53 | 1.52 | 1.45 | 1.47 | 1.47 | 1.48 | 1.57 | 1.57 | 1.52 |
| ∑Rt | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | |
| Fe3+ | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.03 | 0.03 | 0.01 | 0.01 | 0.00 | 0.01 |
| Fe2+ | 2.55 | 2.46 | 2.44 | 2.41 | 2.50 | 2.63 | 2.57 | 2.40 | 2.62 | 2.53 | 2.56 |
| AlVI | 1.45 | 1.48 | 1.48 | 1.51 | 1.43 | 1.42 | 1.45 | 1.43 | 1.44 | 1.48 | 1.38 |
| Mg | 2.01 | 2.07 | 2.07 | 2.05 | 2.07 | 1.93 | 1.94 | 2.18 | 1.98 | 2.03 | 2.11 |
| ∑Ro | 6.03 | 6.03 | 6.01 | 5.99 | 6.01 | 6.02 | 6.01 | 6.03 | 6.06 | 6.05 | 6.08 |
| Component Activity | |||||||||||
| Fe/(Fe + Mg) | 0.56 | 0.55 | 0.54 | 0.54 | 0.55 | 0.58 | 0.57 | 0.52 | 0.57 | 0.56 | 0.55 |
| Fe/(Fe + Mg + Mn) | 0.56 | 0.55 | 0.54 | 0.54 | 0.55 | 0.58 | 0.57 | 0.52 | 0.57 | 0.56 | 0.55 |
| d001 | 14.1118 | 14.1109 | 14.1132 | 14.1152 | 14.1215 | 14.1159 | 14.1180 | 14.1195 | 14.1055 | 14.1063 | 14.1115 |
| t/℃ | 267.23 | 268.13 | 265.79 | 263.84 | 257.54 | 263.10 | 261.04 | 259.48 | 273.52 | 272.65 | 267.54 |
| lga3 | −1.34 | −1.40 | −1.42 | −1.43 | −1.39 | −1.29 | −1.32 | −1.48 | −1.29 | −1.35 | −1.37 |
| lga6 | −2.32 | −2.58 | −2.34 | −2.49 | −2.83 | −2.14 | −2.24 | −2.82 | −2.52 | −2.98 | −2.54 |
| lgK1 | 9.77 | 9.74 | 9.81 | 9.87 | 10.05 | 9.89 | 9.95 | 10.00 | 9.58 | 9.61 | 9.76 |
| Lgf(O2) | −43.00 | −43.66 | −42.93 | −43.71 | −45.95 | −42.98 | −43.45 | −45.32 | −43.25 | −44.97 | −43.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.-C.; Zhang, L.; Li, R.-H.; Wen, T.; Xu, H.; Wang, J.-Y.; Li, Z.-Q.; Zhang, F.; Zhang, X.-J.; Guo, H. Process and Mechanism of Gold Mineralization at the Zhengchong Gold Deposit, Jiangnan Orogenic Belt: Evidence from the Arsenopyrite and Chlorite Mineral Thermometers. Minerals 2019, 9, 133. https://doi.org/10.3390/min9020133
Sun S-C, Zhang L, Li R-H, Wen T, Xu H, Wang J-Y, Li Z-Q, Zhang F, Zhang X-J, Guo H. Process and Mechanism of Gold Mineralization at the Zhengchong Gold Deposit, Jiangnan Orogenic Belt: Evidence from the Arsenopyrite and Chlorite Mineral Thermometers. Minerals. 2019; 9(2):133. https://doi.org/10.3390/min9020133
Chicago/Turabian StyleSun, Si-Chen, Liang Zhang, Rong-Hua Li, Ting Wen, Hao Xu, Jiu-Yi Wang, Zhi-Qi Li, Fu Zhang, Xue-Jun Zhang, and Hu Guo. 2019. "Process and Mechanism of Gold Mineralization at the Zhengchong Gold Deposit, Jiangnan Orogenic Belt: Evidence from the Arsenopyrite and Chlorite Mineral Thermometers" Minerals 9, no. 2: 133. https://doi.org/10.3390/min9020133
APA StyleSun, S.-C., Zhang, L., Li, R.-H., Wen, T., Xu, H., Wang, J.-Y., Li, Z.-Q., Zhang, F., Zhang, X.-J., & Guo, H. (2019). Process and Mechanism of Gold Mineralization at the Zhengchong Gold Deposit, Jiangnan Orogenic Belt: Evidence from the Arsenopyrite and Chlorite Mineral Thermometers. Minerals, 9(2), 133. https://doi.org/10.3390/min9020133




