Distribution and Risk Assessment of Heavy Metals in Sediment from Bohai Bay, China
Abstract
1. Introduction
2. Materials and Methods
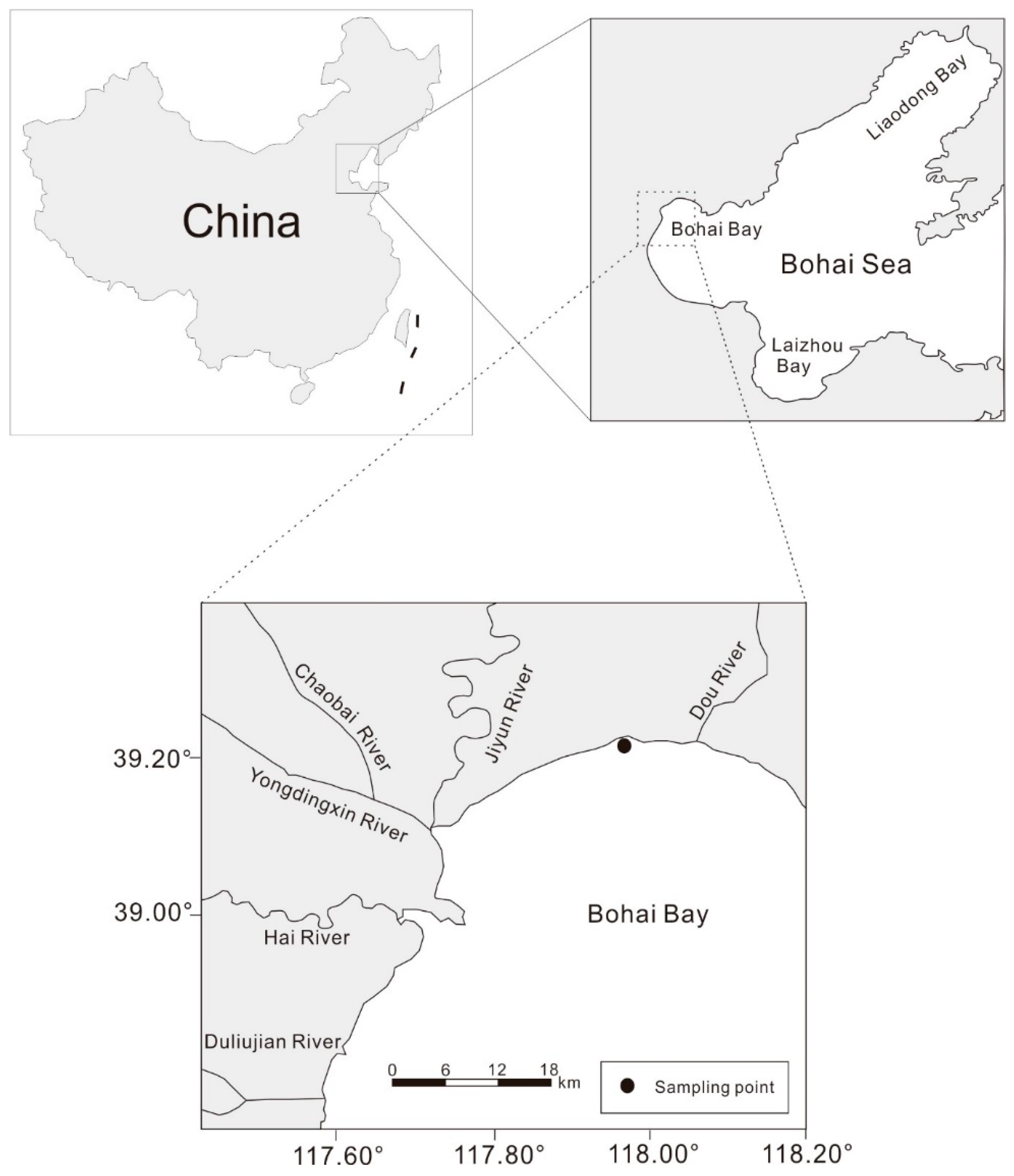
2.1. Sampling and Analysis
2.2. Flux Calculations
2.3. Statistical Analysis
3. Results
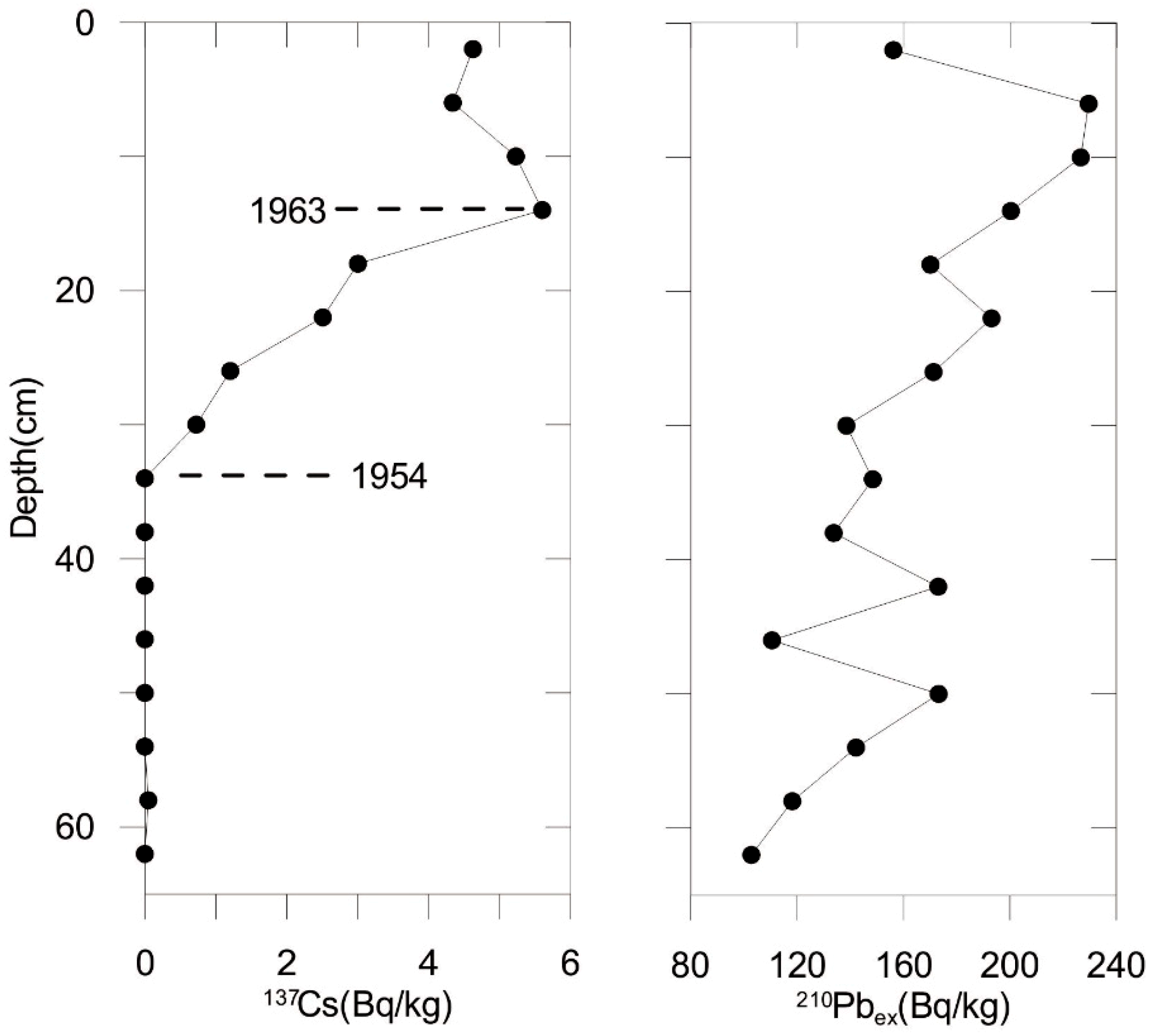
3.1. Sediment Core Dating
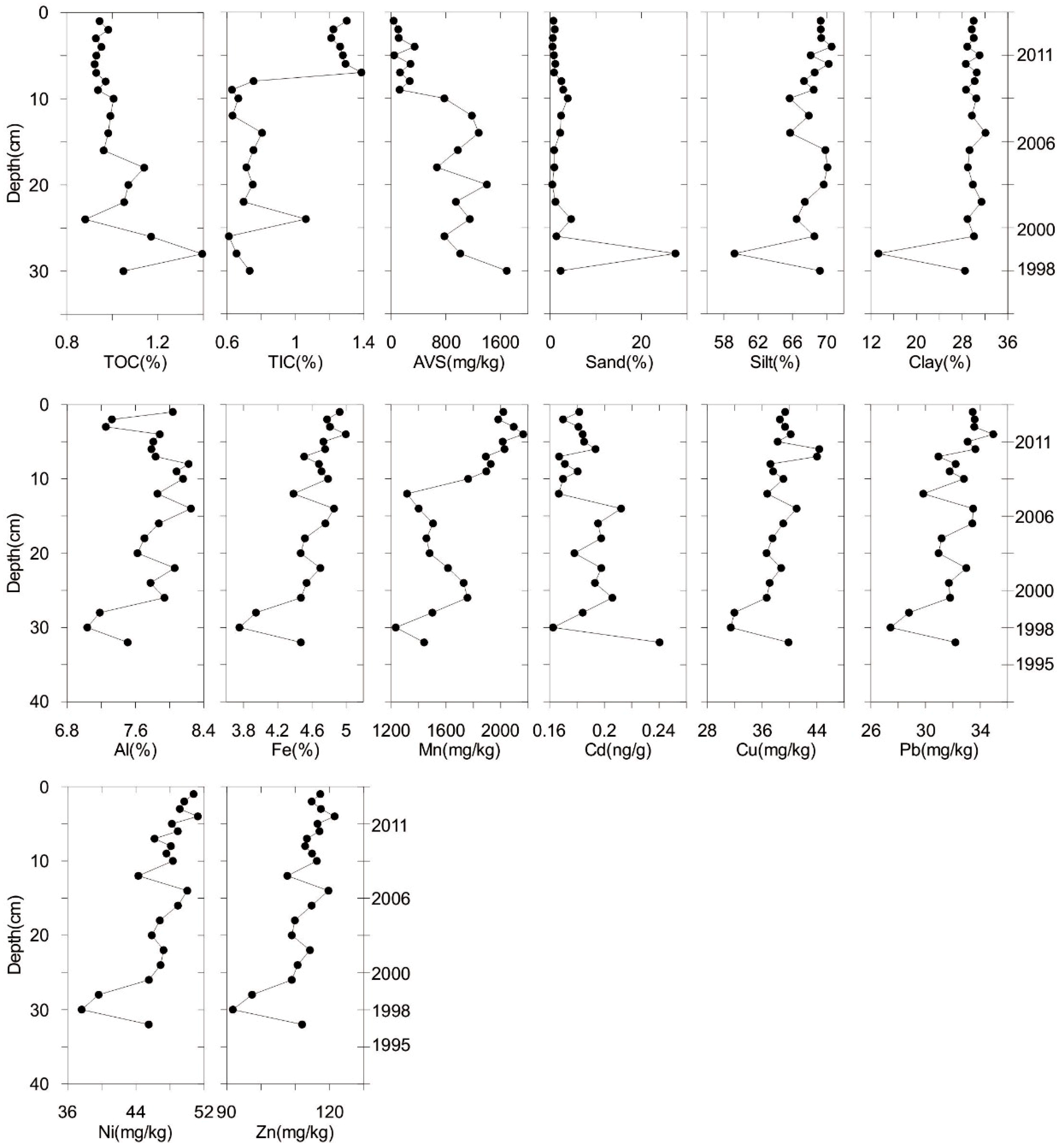
3.2. Sediment Characteristics
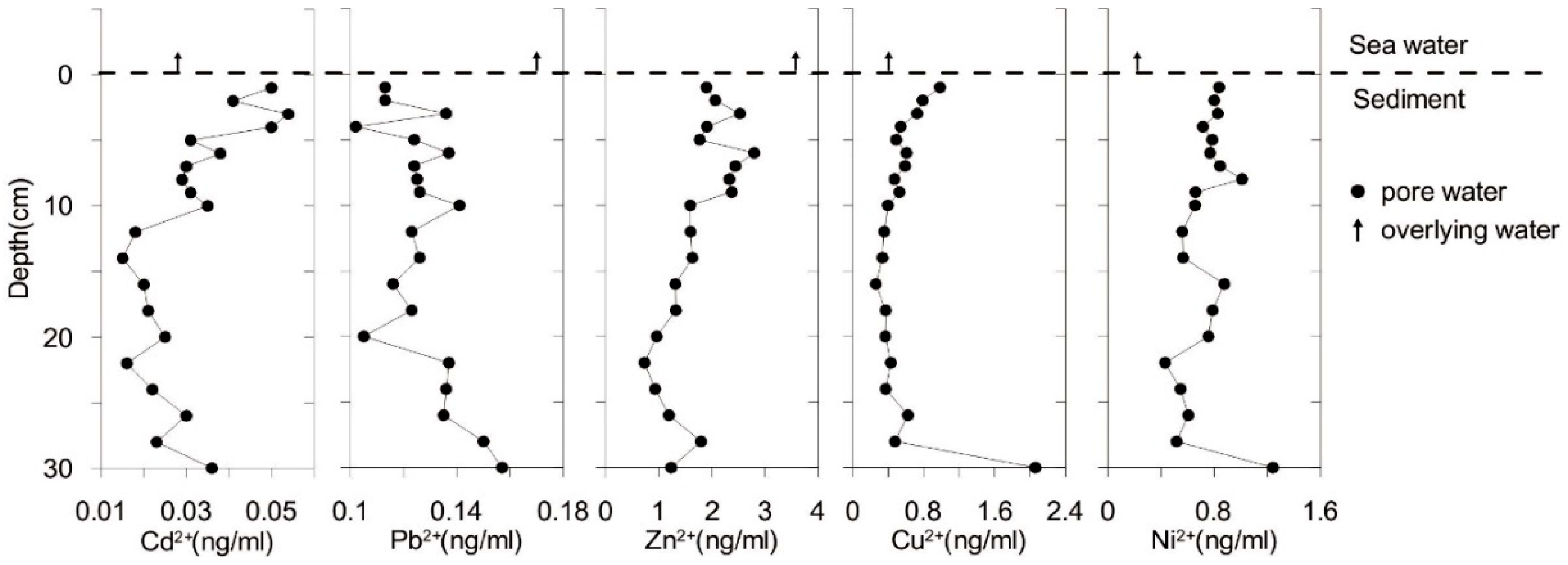
3.3. The Concentrations and Distribution of Metals in the Sediment Core and Pore Water
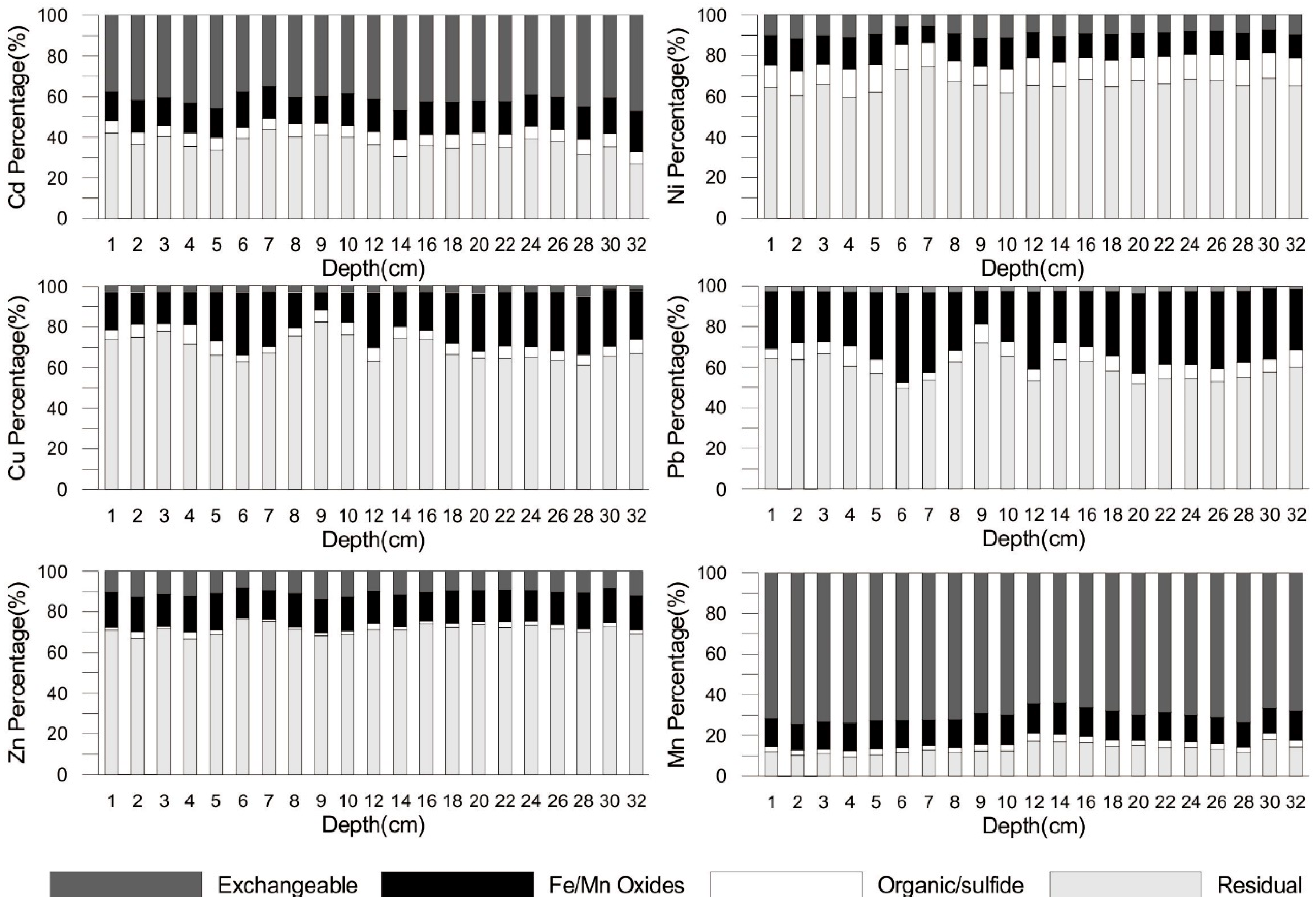
3.4. The Distribution of Metals Fractions along the Sediment Core
3.5. Diffusion of Dissolved Heavy Metals in Pore Water
4. Discussion
4.1. Statistical Analysis
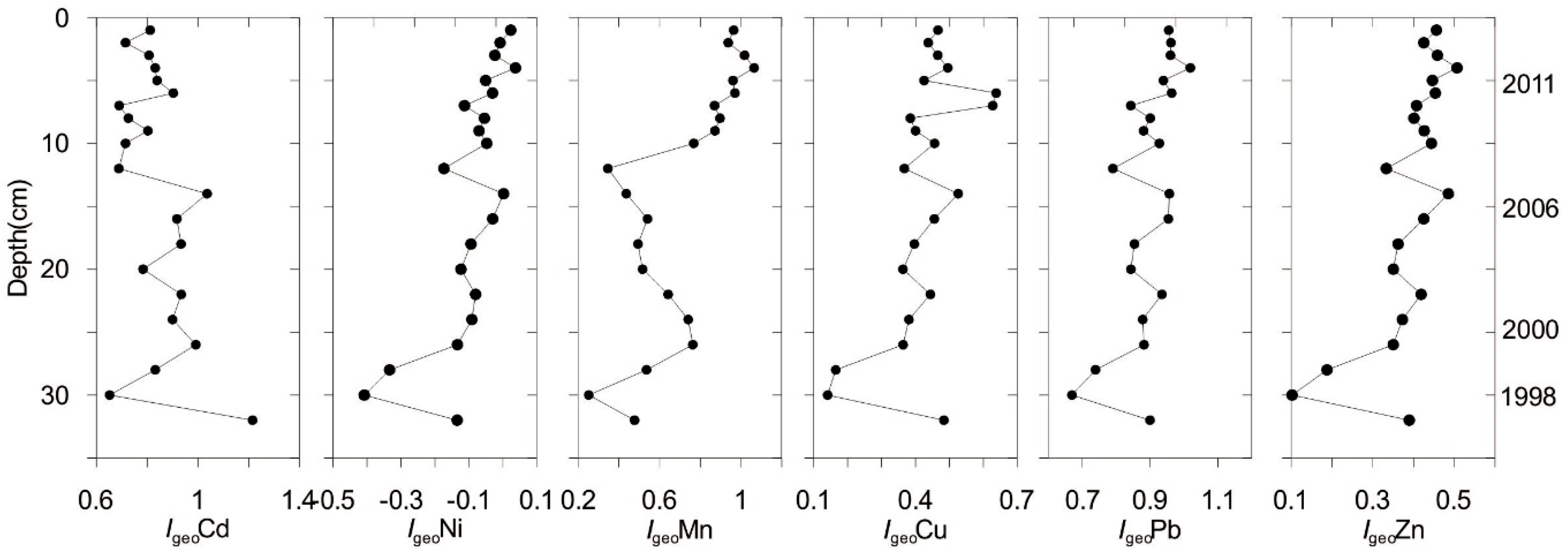
4.2. Risk Assessment and Contamination of Metals in Sediment
4.2.1. Geo-Accumulation Index (Igeo)
4.2.2. Risk Assessment Code (RAC) of Metals
4.3. Source Identification of Metals
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, X.; Zhou, F.; Chen, C.T. Pollution status of the Bohai Sea: An overview of the environmental quality assessment related trace metals. Environ. Int. 2014, 62, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.V.; Cundy, A.B. Heavy metal contamination and mixing processes in sediments from the Humber Estuary, Eastern England. Estuar. Coast. Shelf Sci. 2001, 53, 619–636. [Google Scholar] [CrossRef]
- Arnason, J.G.; Fletcher, B.A. A 40+ year record of Cd, Hg, Pb, and U deposition in sediments of Patroon Reservoir, Albany County, NY, USA. Environ. Pollut. 2003, 123, 383–391. [Google Scholar] [CrossRef]
- Bibi, M.H.; Ahmed, F.; Ishiga, H. Mobility of arsenic and trace element inventories in sediment cores from Masuda City, southwestern Japan. Environ. Geol. 2008, 54, 791–803. [Google Scholar] [CrossRef]
- Hu, C.Y.; Yang, X.L.; Dong, J.Y.; Zhang, X.M. Heavy metal concentrations and chemical fractions in sediment from Swan Lagoon, China: Their relation to the physiochemical properties of sediment. Chemosphere 2018, 209, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Palleiro, L.; Patinha, C.; Rodríguez-Blanco, M.L.; Taboada-Castro, M.M. Metal fractionation in topsoils and bed sediments in the Mero River rural basin: Bioavailability and relationship with soil and sediment properties. Catena 2016, 144, 34–44. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Xiao, R.; Zhao, Q.; Jia, J.; Cui, B.; Liu, X. Heavy metal fractions and ecological risk assessment in sediments from urban, rural and reclamationaffected rivers of the Pearl River Estuary, China. Chemosphere 2017, 184, 278–288. [Google Scholar] [CrossRef]
- Soto-Jiménez, M.; Páez-Osuna, F.; Ruiz-Fernández, A. Geochemical evidences of the anthropogenic alteration of trace metal composition of the sediments of Chiricahueto marsh (SE Gulf of California). Environ. Pollut. 2003, 125, 423–432. [Google Scholar] [CrossRef]
- Ip, C.C.M.; Li, X.D.; Zhang, G.; Farmer, J.G.; Wai, O.W.H.; Li, Y.S. Over one hundred years of trace metal fluxes in the sediments of the Pearl River Estuary, South China. Environ. Pollut. 2004, 132, 157–172. [Google Scholar] [CrossRef]
- Covelli, S.; Fontolan, G.; Faganeli, J.; Ogrinc, N. Anthropogenic markers in the Holocene stratigraphic sequence of the Gulf of Trieste (northern Adriatic Sea). Mar. Geol. 2006, 230, 29–51. [Google Scholar] [CrossRef]
- Vaalgamaa, S.; Conley, D.J. Detecting environmental change in estuaries: Nutrient and heavy metal distributions in sediment cores in estuaries from the Gulf of Finland, Baltic Sea. Estuar. Coast. Shelf Sci. 2008, 76, 45–56. [Google Scholar] [CrossRef]
- Church, T.M.; Sommerfield, C.K.; Velinsky, D.J.; Point, D.; Benoit, C.; Amouroux, D.; Plaa, D.; Donard, O.F.X. Marsh sediments as records of sedimentation, eutrophication and metal pollution in the urban Delaware Estuary. Mar. Chem. 2006, 102, 72–95. [Google Scholar] [CrossRef]
- Kostaschuk, R.; Chen, Z.Y.; Saito, Y.; Wang, Z.Q. Sedimentation rates and heavy metals in a macrotidal salt marsh: Bay of Fundy, Canada. Environ. Geol. 2008, 55, 1291–1298. [Google Scholar] [CrossRef]
- Feng, H.; Jiang, H.; Gao, W.; Weinstein, M.P.; Zhang, Q.; Zhang, W.; Yu, L.; Yuan, D.; Tao, J. Metal contamination in sediments of the western Bohai Bay and adjacent estuaries, China. J. Environ. Manag. 2011, 92, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hu, B.; Li, J.; Yang, J.; Bai, F.; Dou, Y.; Yin, X. One hundred-year sedimentary record of heavy metal accumulation in the southeastern Liaodong Bay of China. Environ. Earth Sci. 2014, 71, 1073–1082. [Google Scholar] [CrossRef]
- Chai, M.; Shi, F.; Li, R.; Shen, X. Heavy metal contamination and ecological risk in Spartina alterniflora marsh in intertidal sediments of Bohai Bay, China. Mar. Pollut. Bull. 2014, 84, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Rashdi, A.S.; Arabi, A.A.; Howari, F.M.; Siad, A. Distribution of heavy metals in the coastal area of Abu Dhabi in the United Arab Emirates. Mar. Pollut. Bull. 2015, 97, 494–498. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, R.; Lu, Y.; Song, S.; Johnson, A.C.; Sweetman, A.; Jones, K. Regional multi-compartment ecological risk assessment: Establishing cadmium pollution risk in the northern Bohai Rim, China. Environ. Int. 2016, 94, 283–291. [Google Scholar] [CrossRef]
- Stephen-Pichaimani, V.; Jonathan, M.P.; Srinivasalu, S.; Rajeshwara-Rao, N.; Mohan, S.P. Enrichment of trace metals in surface sediments from the northern part of Point Calimere, SE coast of India. Environ. Geol. 2008, 55, 1811–1819. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, Y.; Gu, J.; Zhao, J. Distribution and geochemical speciation of heavy metals in sediments from coastal area suffered rapid urbanization, a case study of Shantou Bay, China. Mar. Pollut. Bull. 2013, 68, 140–146. [Google Scholar] [CrossRef]
- Yang, Z.F.; Wang, Y.; Shen, Z.Y.; Niu, J.F.; Tang, Z.W. Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. J. Hazard. Mater. 2009, 166, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.J.; Weindorf, D.C. Heavy metal and trace metal analysis in soil by sequential extraction: A review of procedures. Int. J. Anal. Chem. 2010, 387803. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Qin, Y.W.; Zheng, B.H.; Zhang, L. Heavy metal pollution in Tianjin Bohai Bay, China. Environ. Sci. Pollut. Res. Int. 2008, 20, 814–819. [Google Scholar] [CrossRef]
- Pan, W.Q.; Yao, Y.L.; Ning, X.D. Development of Bohai rim: History, Reality and Future; Enterprise Management Publishing House: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Zhang, Y.; Lu, X.Q.; Shao, X.L.; Liu, H.L.; Xing, M.N.; Zhao, F.; Li, X.J.; Min, Y. Influence of sedimentation rate on the metal contamination in sediments of Bohai Bay, China. Bull. Environ. Contam. Toxicol. 2015, 95, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.H. Numerical simulation of aquatic Eco-environment of Bohai bay. J. Hydrodyn. 2006, 18, 34–42. [Google Scholar] [CrossRef]
- Gao, X.; Chen, C.T. Heavy metal pollution status in surface sediments of the coastal Bohai Bay. Water Res. 2012, 46, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wang, X.L. Spatial distribution of dissolved Pb, Hg, Cd, Cu and As in the Bohai Sea. J. Environ. Sci. 2007, 19, 1061–1066. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Liu, J.; Shi, X.; Ma, D. Assessing metal toxicity in sediments using the equilibrium partitioning model and empirical sediment quality guidelines: A case study in the nearshore zone of the Bohai Sea, China. Mar. Pollut. Bull. 2014, 85, 114–122. [Google Scholar] [CrossRef]
- Wu, G.; Shang, J.; Pan, L.; Wang, Z. Heavy metals in surface sediments from nine estuaries along the coast of Bohai Bay, Northern China. Mar. Pollut. Bull. 2014, 82, 194–200. [Google Scholar] [CrossRef]
- Gao, X.; Li, P. Concentration and fractionation of trace metals in surface sediments of intertidal Bohai Bay, China. Mar. Pollut. Bull. 2012, 64, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, P.; Chen, C.T. Assessment of sediment quality in two important areas of mariculture in the Bohai Sea and the northern Yellow Sea based on acid-volatile sulfide and simultaneously extracted metal results. Mar. Pollut. Bull. 2013, 72, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Gao, X. Acid-volatile sulfide and simultaneously extracted metals in surface sediments of the southwestern coastal Laizhou Bay, Bohai Sea: Concentrations, spatial distributions and the indication of heavy metal pollution status. Mar. Pollut. Bull. 2013, 76, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Hu, K.; Jiang, Z.L.; Qu, F.G.; Su, X. A 50-year sedimentary record of heavy metals and their chemical speciations in the Shuangtaizi River estuary, (China): Implications for pollution and biodegradation. Front. Environ. Sci. Eng. China 2011, 5, 435–444. [Google Scholar] [CrossRef]
- Hsieh, Y.P.; Yang, C.H. Diffusion methods for the determination of reduced inorganic sulfur species in sediments. Limnol. Oceanogr. 1989, 34, 1126–1130. [Google Scholar] [CrossRef]
- Hsieh, Y.P.; Shieh, Y.N. Analysis of reduced inorganic sulfur by diffusion methods: Improved apparatus and evaluation for sulfur isotopic studies. Chem. Geol. 1997, 137, 255–261. [Google Scholar] [CrossRef]
- Cuong, D.T.; Obbard, J.P. Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Appl. Geochem. 2006, 21, 1335–1346. [Google Scholar] [CrossRef]
- Berner, R.A. Early Diagenesis: A Theoretical Approach; Princeton University Press: New York, NY, USA, 1980. [Google Scholar]
- Li, Y.H.; Gregory, S. Diffusion of ions in seawater and in deep sea sediments. Geochim. Cosmochim. Acta 1974, 38, 703–741. [Google Scholar]
- Boudreau, B.P. The diffusive tortuosity of fine-grained unlithified sediments. Geochim. Cosmochim. Acta 1996, 60, 3139–3142. [Google Scholar] [CrossRef]
- Benninger, L.K.; Suayah, I.B.; Stanley, D.J. Manzala lagoon, Nile delta, Egypt: Modern sediment accumulation based on radioactive tracers. Environ. Geol. 1998, 34, 183–193. [Google Scholar] [CrossRef]
- Laforte, L.; Tessier, A.; Gobeil, C.; Carignan, R. Thallium diagenesis in lacustrine sediments. Geochim. Cosmochim. Acta 2005, 69, 5295–5306. [Google Scholar] [CrossRef]
- Xue, B.; Yao, S.C.; Xia, W.L.; Zhu, Y.X. Some sediment-geochemical evidence for the recent environmental changes of the lakes from the middle and lower Yangtze River basin, China. Quat. Int. 2010, 226, 29–37. [Google Scholar] [CrossRef]
- Farmer, J.G.; MacKenzie, A.B.; Eades, L.J.; Kirika, A.; Bailey-Watts, A.E. Influences on the extent and record of heavy metal pollution in sediment cores from Loch Tay in a mineralised area of Scotland. J. Geochem. Explor. 1997, 58, 195–202. [Google Scholar] [CrossRef]
- Ligero, R.A.; Barrera, M.; Casas-Ruiz, M.; Sales, D.; Lopez-Aguayo, F. Dating of marine sediments and time evolution of heavy metal concentrations in the Bay of Cadiz, Spain. Environ. Pollut. 2002, 118, 97–108. [Google Scholar] [CrossRef]
- Xu, B.; Yang, X.; Gu, Z.; Zhang, Y.; Chen, Y.; Lv, Y. The trend and extent of heavy metal accumulation over last one hundred years in the Liaodong Bay, China. Chemosphere 2009, 75, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, H.; Chang, J.; Qu, J.; Xie, H.; Yu, L. Heavy metal contamination in surface sediments of Yangtze River intertidal zone: An assessment from different indexes. Environ. Pollut. 2009, 157, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Choi, M.S.; Lee, J.Y.; Jang, D.J. Regional background concentrations of heavy metals (Cr, Co, Ni, Cu, Zn, Pb) in coastal sediments of the South Sea of Korea. Sci. Total. Environ. 2014, 482–483, 80–91. [Google Scholar] [CrossRef] [PubMed]
- CSBTS (China State Bureau of Quality and Technical Supervision). Marine Sediment Quality; Standards Press of China: Beijing, China, 2002; p. 10. (In Chinese) [Google Scholar]
- Gu, Y.; Yang, Y.; Lin, Q.; Li, Q.; Wang, Z.; Wang, X. Spatial, temporal, and speciation variations of heavy metals in sediments of Nan’ao Island, a representative mariculture base in Guangdong coast, China. J. Environ. Monit. 2012, 14, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Morillo, J.; Usero, J.; Gracia, I. Heavy metal distribution in marine sediments from the southwest coast of Spain. Chemosphere 2004, 55, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Gallon, C.; Tessier, A.; Gobeil, C.; La Torre, M. Modeling diagenesis of lead in sediments of a Canadian Shield lake. Geochim. Cosmochim. Acta 2004, 68, 3531–3545. [Google Scholar] [CrossRef]
- Wang, S.F.; Jia, Y.F.; Wang, S.Y.; Wang, X.; Wang, H.; Zhao, Z.X.; Liu, B.Z. Fractionation of heavy metals in shallow marine sediments from Jinzhou Bay, China. J. Environ. Sci. (China) 2010, 22, 23–31. [Google Scholar] [CrossRef]
- Yuan, C.G.; Shi, J.B.; He, B.; Liu, J.F.; Liang, L.N.; Jiang, G.B. Speciation of heavy metals in marine sediments from the East China Sea by ICP-MS with sequential extraction. Environ. Int. 2004, 30, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Kotoky, P.; Bora, B.J.; Baruah, N.K.; Baruah, J.; Borah, G.C. Chemical fractionation of heavy metals in soils around oil installations, Assam. Chem. Speciat. Bioavail. 2004, 15, 115–126. [Google Scholar] [CrossRef]
- Lasheen, M.R.; Ammar, N.S. Speciation of some heavy metals in River Nile sediments, Cairo, Egypt. Environmentalist 2009, 29, 8–16. [Google Scholar] [CrossRef]
- Li, X.D.; Shen, Z.G.; Wai, O.W.H.; Li, Y.S. Chemical forms of Pb, Zn and Cu in the sediment profiles of the Pearl River Estuary. Mar. Pollut. Bull. 2001, 42, 215–223. [Google Scholar] [CrossRef]
- Guven, D.E.; AkinciI, G. Heavy metals partitioning in the sediments of Izmir Inner Bay. J. Environ. Sci. 2008, 20, 413–418. [Google Scholar] [CrossRef]
- Ramirez, M.; Massolo, S.; Frache, R.; Correa, J.A. Metal speciation and environmental impact on sandy beaches due to El Salvador copper mine, Chile. Mar. Pollut. Bull. 2005, 50, 62–72. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; Taylor & Francis, CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Cottenie, A.; Verloo, M.; Kiekens, L.; Camerlynck, R.; Velghe, G.; Dhaese, A. Essential and Non-Essential Trace Elements in the System Soil-Water-Plant; IWONL: Brussels, Belgium, 1979; p. 75. [Google Scholar]
- Lu, X.Q.; Zhang, Y.; Liu, H.L.; Xing, M.N.; Shao, X.L.; Zhao, F.; Li, X.J.; Liu, Q.Q.; Yu, D.; Yuan, X.Z. Influence of early diagenesis on the vertical distribution of metal forms in sediments of Bohai Bay, China. Mar. Pollut. Bull. 2014, 88, 155–161. [Google Scholar] [CrossRef]
- Lu, C.X.; Cheng, J.M. Speciation of heavy metals in the sediments from different eutrophic lakes of China. Procedia Eng. 2011, 18, 318–323. [Google Scholar]
- González, M.J.; Ramos, L.; Hernández, L.M. Distribution of trace metals in sediments and their relationship with their accumulation in earthworms. Environ. Anal. Chem. 1994, 57, 135–150. [Google Scholar] [CrossRef]
- Audry, S.; Blanc, G.; Schafer, J. Solid state partitioning of trace metals in suspended particulate matter from a river system affected by smelting-waste drainage. Sci. Total. Environ. 2006, 363, 216–236. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.C.; Ma, H.T.; Qian, J.; Zhai, J.B. Distribution of extractable fractions of heavy metals in sludge during the wastewater treatment process. J. Hazard. Mater. 2006, 137, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P. Speciation of Co, Ni and Cu in the coastal and estuarine sediments: Some fundamental characteristics. J. Geochem. Explor. 2012, 115, 13–23. [Google Scholar] [CrossRef]
- Gu, Y.G.; Lin, Q. Trace metals in a sediment core from the largest mariculture base of the eastern Guangdong coast, South China: Vertical distribution, speciation, and biological risk. Mar. Pollut. Bull. 2016, 113, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Rinklebe, J. Vanadium in thirteen different soil profiles originating from Germany and Egypt: Geochemical fractionation and potential mobilization. Appl. Geochem. 2018, 88, 288–301. [Google Scholar] [CrossRef]
- Matong, J.M.; Nyaba, L.; Nomngongo, P.N. Fractionation of trace elements in agricultural soils using ultrasound assisted sequential extraction prior to inductively coupled plasma mass spectrometric determination. Chemosphere 2016, 154, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.M.; Song, J.M.; Yuan, H.M.; Duan, L.Q.; Li, X.G.; Li, N.; Liang, X.M.; Qu, B.X. Speciation of heavy metals in different grain sizes of Jiaozhou Bay sediments: Bioavailability, ecological risk assessment and source analysis on a centennial timescale. Ecotoxicol. Environ. Safe 2017, 143, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Sakan, S.M.; Dordevic, D.S.; Manojlovic, D.D.; Predrag, P. Assessment of heavy metal pollutants accumulation in the Tisza river sediments. J. Environ. Manag. 2009, 90, 3382–3390. [Google Scholar] [CrossRef]
- Li, M.; Zang, S.Y.; Xiao, H.F.; Wu, C.S. Speciation and distribution characteristics of heavy metals and pollution assessments in the sediments of Nashina Lake, Heilongjiang, China. Ecotoxicology 2014, 23, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Sundaray, S.K.; Nayak, B.B.; Lin, S.; Bhatta, D. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments--a case study: Mahanadi basin, India. J. Hazard. Mater. 2011, 186, 1837–1846. [Google Scholar] [CrossRef]
- Helali, M.A.; Oueslati, W.; Zaaboub, N.; Added, A.; Aleya, L. Chemical speciation of Fe, Mn, Pb, Zn, Cd, Cu, Co, Ni and Cr in the suspended particulate matter off the Mejerda River Delta (Gulf of Tunis, Tunisia). J. Afr. Earth Sci. 2016, 118, 35–44. [Google Scholar] [CrossRef]
- Rath, P.; Panda, U.C.; Bhatta, D.; Sahu, K.C. Use of sequential leaching, mineralogy, morphology and multivariate statistical technique for quantifying metal pollution in highly polluted aquatic sediments—A case study: Brahmani and Nandira Rivers, India. J. Hazard. Mater. 2009, 163, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.H.; Cao, S.S.; Chen, S.R.; Cao, F.T. Accumulation and remobilization of metals in superficial sediments in Tianjin, China. Environ. Monit. Assess. 2011, 173, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Norvell, W.A.; Wu, J.; Hopkins, D.G.; Welch, R.M. Association of cadmium in durum wheat grain with soil chloride and chelate-extractable soil cadmium. Soil Sci. Soc. Am. J. 2000, 64, 2162–2168. [Google Scholar] [CrossRef]
- Jain, C.K. Metal fractionation study on bed sediments of River Yamuna, India. Water Res. 2004, 38, 569–578. [Google Scholar] [CrossRef]
- Lan, X.H.; Wang, H.X.; Li, R.H.; Lin, Z.H.; Zhang, Z.X. Major elements composition and provenance analysis in the sediments of the South Yellow Sea. Earth Sci. Front. 2007, 14, 197–203. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Peng, X.T.; Pan, J.M. Distribution, source and enrichment of some chemical elements in sediments of the Pearl River Estuary, China. Cont. Shelf Res. 2004, 24, 1857–1875. [Google Scholar] [CrossRef]
- Ren, J.L.; Liu, S.M.; Zhang, J.; Xie, L.; Li, D.D.; Cheng, Y.; Zhu, D.D. Effects of terrestrial input on the harmful algal bloom area-with aluminum as an example. Chin. J. Appl. Ecol. 2003, 14, 1117–1121. [Google Scholar]
- Brunskill, G.J.; Orpin, A.R.; Zagorskis, I.; Woolfe, K.J.; Ellison, J. Geochemistry and particle size of surface sediments of Exmouth Gulf, Northwest Shelf, Australia. Cont. Shelf Res. 2001, 21, 157–201. [Google Scholar] [CrossRef]
- Tessier, A.; Belzile, N.; Devitre, R.R.; Leppard, G.G.; Fortin, D. Metal sorption to diagenetic iron and manganese oxyhydroxides and associated organic matter: Narrowing the gap between field and laboratory measurements. Geochim. Cosmochim. Acta 1996, 60, 387–404. [Google Scholar] [CrossRef]
- Marin, B.; Giresse, P. Particulate manganese and iron in recent sediments of the Gulf of Lions continental margin (north-western Mediterranean Sea): Deposition and diagenetic process. Mar. Geol. 2001, 172, 147–165. [Google Scholar] [CrossRef]
- Santos, I.R.; Silva-Filho, E.V.; Schaefer, C.E.; Albuquerque-Filho, M.R.; Campos, L.S. Heavy metal contamination in coastal sediments and soils near the Brazilian Antarctic Station, King George Island. Mar. Pollut. Bull. 2005, 50, 185–194. [Google Scholar] [CrossRef]
- Müller, V.G. Schadstoffe in sedimenten—Sedimenteals Schadstoffe. Mitt. Österr. Geol. Ges. 1986, 79, 107–126. (In German) [Google Scholar] [CrossRef]
- Birch, G.F. Determination of sediment metal background concentrations and enrichment in marine environments—A critical review. Sci. Total Environ. 2017, 580, 813–831. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-T.; Lee, C.-W. Application of multiple ecological risk indices for the evaluation of heavy metal contamination in a coastal dredging area. Sci. Total Environ. 1998, 214, 203–210. [Google Scholar] [CrossRef]
- NEPA (National Environmental Protection Agency of China). Background Values of Soil Elements of China; China Environmental Science Press: Beijing, China, 1990. [Google Scholar]
- Li, Y.; Guo, L.; Feng, H. Status and Trends of Sediment Metal Pollution in Bohai Sea, China. Curr. Pollut. Rep. 2015, 1, 191–202. [Google Scholar] [CrossRef]
- Liu, L.; Wang, L.; Yang, Z.; Hu, Y.; Ma, M. Spatial and temporal variations of heavy metals in marine sediments from Liaodong Bay, Bohai Sea in China. Mar. Pollut. Bull. 2017, 124, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Perin, G.; Craboledda, L.; Lucchese, M.; Cirillo, R.; Dotta, L.; Zanette, M.L.; Orio, A.A. Heavy metal speciation in the sediments of Northern Adriatic Sea—A new approach for environmental toxicity determination. In Proceedings of the International Conference “Heavy Metals in the Environment”, Athens, Greece, 10–13 September 1985; Lekkas, T.D., Ed.; 1985; Volume 2, pp. 454–456. [Google Scholar]
- Davutluoglu, O.I.; Seckin, G.; Kalat, D.G.; Yilmaz, T.; Ersu, C.B. Speciation and implications of heavy metal content in surface sediments of Akyatan Lagoon-Turkey. Desalination 2010, 260, 199–210. [Google Scholar] [CrossRef]
- Soares, M.A.R.; Quina, M.J.; Quinta-Ferreira, R.M. Immobilisation of lead and zinc in contaminated soil using compost derived from industrial eggshell. J. Environ. Manag. 2015, 164, 137–145. [Google Scholar] [CrossRef]
- Liang, X.X.; Tian, C.G.; Zong, Z.; Wang, X.P.; Jiang, W.Y.H.; Chen, Y.J.; Ma, J.M.; Luo, Y.M.; Li, J.; Zhang, G. Flux and source-sink relationship of heavy metals and arsenic in the Bohai Sea, China. Environ. Pollut. 2018, 242, 1353–1361. [Google Scholar] [CrossRef]
- Ren, H.; Liu, H.; Qu, J.; Berg, M.; Qi, W.; Xu, W. The influence of colloids on the geochemical behavior of metals in polluted water using as an example Yongdingxin River, Tianjin, China. Chemosphere 2010, 78, 360–367. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, X.Q.; Shao, X.L.; Chen, C.; Li, X.J.; Zhao, F.; Li, G.; Matsumoto, E. Temporal variation of sedimentation rates and potential factors influencing those rates over the last 100 years in Bohai Bay, China. Sci. Total Environ. 2016, 572, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Cochran, J.K.; Hirschberg, D.J.; Dere, J.W.A.C. Atmospheric Deposition of Metals to Coastal Waters (Long Island Sound, New York U.S.A.): Evidence from Saltmarsh Deposits. Estuari. Coast. Shelf Sci. 1998, 46, 503–522. [Google Scholar] [CrossRef]
- Li, X.D.; Li, Y.S.; Coles, B.J.; Ramsey, M.H.; Thornton, I.; Wai, O.W.H. Heavy metal distribution in sediment profiles of the Pearl River estuary, South China. Appl. Geochem. 2000, 15, 567–581. [Google Scholar] [CrossRef]
- Tian, H.Z.; Lu, L.; Cheng, K.; Hao, J.M.; Zhao, D.; Wang, Y.; Jia, W.X.; Qiu, P.P. Anthropogenic atmospheric nickel emissions and its distribution characteristics in China. Sci. Total. Environ. 2012, 417–418, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Kuang, C.P.; Shan, Y.C.; Gu, J.; Shao, H.B.; Zhang, W.L.; Zhang, Y.F.; Zhang, J.B.; Liu, H.X. Assessment of heavy metal contamination in water body and riverbed sediments of the Yanghe River in the Bohai Sea, China. Environ. Earth. Sci. 2016, 75, 1105. [Google Scholar] [CrossRef]
- Ruiz-Fernandez, A.C.; Hillaire-Marcel, C.; Páez-Osuna, F.; Ghaleb, B. Margarita, Caballero-Miranda, 210Pb chronology and trace metal geochemistry at Los Tuxtlas, Mexico, as evidenced by a sedimentary record from the Lago Verde crater lake. Chin. J. Geochem. 2006, 25, 181–192. [Google Scholar] [CrossRef]
- Hu, N.J.; Huang, P.; Zhang, H.; Wang, X.J.; Zhu, A.M.; Liu, J.H.; Shi, X.F. Geochemical source, deposition, and environmental risk assessment of cadmium in surface and core sediments from the Bohai Sea, China. Environ. Sci. Pollut. Res. Int. 2016, 24, 827–843. [Google Scholar]
| Al | Fe | Mn | Ni | Cu | Zn | Cd | Pb | References | |
|---|---|---|---|---|---|---|---|---|---|
| Sediment core from Bohai Bay | |||||||||
| Mean (21) | 7.8 | 4.6 | 0.17 | 47.0 | 38.3 | 112.3 | 0.19 | 32.0 | Present study |
| Minimum | 7.03 | 3.75 | 0.12 | 37.6 | 31.4 | 91.8 | 0.16 | 27.4 | |
| Maximum | 8.25 | 5.0 | 0.21 | 51.3 | 44.4 | 121.5 | 0.24 | 34.9 | |
| Standard Deviation | 0.34 | 0.3 | 0.28 | 3.3 | 3.0 | 7.0 | 0.02 | 1.8 | |
| Haihe River estuary, China | 2.98 | 33.6 | 29.5 | 86.0 | 0.173 | 25.6 | [14] | ||
| Yongding River estuary, China | 3.19 | 31.2 | 31.4 | 92.9 | 0.124 | 20.4 | [14] | ||
| Western Bohai Bay, Bohai Sea | 3.74 | 31.4 | 27.9 | 83.6 | 0.129 | 20.5 | [14] | ||
| Estuaries around Tianjin Bohai Bay | 18.8 | 134.7 | 0.7 | 22.9 | [23] | ||||
| Intertidal Changjiang Estuary | 6.5 | 3.3 | 0.077 | 31.8 | 30.7 | 94.3 | 0.26 | 27.3 | [47] |
| Coast of the South Sea, Korea | 8.8 | 4.0 | 29 | 21.9 | 109 | 31.6 | [48] | ||
| Background value in the Bohai Sea and adjacent estuaries | 19 | 57 | 0.069 | 11.5 | [1] | ||||
| Marine Sediment Quality Grade I | 35 | 150 | 0.5 | 60 | [49] | ||||
| Marine Sediment Quality Grade II | 100 | 350 | 1.5 | 130 | [49] |
| Metal | Interstitial Water | Uppermost Pore Water | Overlying Water | Diffusive | Total Flux | ||
|---|---|---|---|---|---|---|---|
| Jout | Jup | Jdown | J | ||||
| Cd | 0.031 ± 0.011 a | 0.05 | 0.028 | −0.021 | −0.003 | 0.012 | 0.036 |
| Cu | 0.59 ± 0.39 | 0.984 | 0.406 | −0.568 | −0.432 | 0.187 | 1.187 |
| Pb | 0.13 ± 0.014 | 0.113 | 0.17 | 0.072 | −0.017 | 0.011 | 0.1 |
| Zn | 1.72 ± 0.58 | 1.899 | 3.576 | 1.595 | −0.498 | 0.589 | 2.682 |
| Ni | 0.74 ± 0.19 | 0.836 | 0.22 | −0.551 | −0.115 | 0.123 | 0.789 |
| Variable | Mn | Al | Fe | Cd | Cu | Ni | Pb | Zn | Sand | Silt | Clay | TOC | TIC | AVS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn | 1.00 | |||||||||||||
| Al | 0.17 | 1.00 | ||||||||||||
| Fe | 0.66 * | 0.62 * | 1.00 | |||||||||||
| Cd | −0.08 | 0.34 | 0.30 | 1.00 | ||||||||||
| Cu | 0.54 * | 0.49 * | 0.75 * | 0.21 | 1.00 | |||||||||
| Ni | 0.65 * | 0.58 * | 0.99 * | 0.31 | 0.76 * | 1.00 | ||||||||
| Pb | 0.69 * | 0.49 * | 0.96 * | 0.38 ** | 0.71 * | 0.96 * | 1.00 | |||||||
| Zn | 0.66 * | 0.63 * | 0.99 * | 0.31 | 0.82 * | 0.97 * | 0.94 * | 1.00 | ||||||
| Sand | −0.24 | −0.36 | −0.53 * | −0.02 | −0.52 * | −0.55 * | −0.47 * | −0.52 * | 1.00 | |||||
| Silt | 0.29 | 0.08 | 0.40 ** | −0.05 | 0.42 ** | 0.43 ** | 0.37 | 0.37 | −0.89 * | 1.00 | ||||
| Clay | 0.18 | 0.50 * | 0.56 * | 0.06 | 0.52 * | 0.56 * | 0.47 * | 0.56 * | −0.95 * | 0.72 * | 1.00 | |||
| TOC | −0.47 * | −0.36 | −0.61 * | 0.15 | −0.62 * | −0.62 * | −0.53 * | −0.63 * | 0.74 * | −0.60 * | −0.74 * | 1.00 | ||
| TIC | 0.72 * | −0.13 | 0.44 ** | −0.13 | 0.60 * | 0.48 * | 0.49 * | 0.48 * | −0.29 | 0.36 | 0.21 | −0.55 * | 1.00 | |
| AVS | −0.89 * | −0.18 | −0.60 * | 0.13 | −0.53 * | −0.60 * | −0.57 * | −0.61 * | 0.22 | −0.28 | −0.15 | 0.38 ** | −0.64 * | 1.00 |
| Metal | Percentage in the Exchangeable Fraction | Risk | ||
|---|---|---|---|---|
| Mean | Max. | Min. | ||
| Cu | 3.28 | 5.36 | 1.78 | Low |
| Pb | 2.66 | 3.78 | 1.2 | Low |
| Ni | 9.23 | 11.71 | 5.60 | Low to Medium |
| Zn | 10.62 | 13.75 | 8.24 | Low to Medium |
| Cd | 41.4 | 47.3 | 35.1 | High |
| Mn | 69.9 | 74.2 | 63.9 | Very high |
| Risk | Metal in carbonate and exchangeable fractions (%) | |||
| No | <1 | |||
| Low | 1–10 | |||
| Medium | 11–30 | |||
| High | 31–50 | |||
| Very high | >50 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Zhang, W.; Chi, G. Distribution and Risk Assessment of Heavy Metals in Sediment from Bohai Bay, China. Minerals 2019, 9, 111. https://doi.org/10.3390/min9020111
Liu B, Zhang W, Chi G. Distribution and Risk Assessment of Heavy Metals in Sediment from Bohai Bay, China. Minerals. 2019; 9(2):111. https://doi.org/10.3390/min9020111
Chicago/Turabian StyleLiu, Baolin, Wensi Zhang, and Guangxi Chi. 2019. "Distribution and Risk Assessment of Heavy Metals in Sediment from Bohai Bay, China" Minerals 9, no. 2: 111. https://doi.org/10.3390/min9020111
APA StyleLiu, B., Zhang, W., & Chi, G. (2019). Distribution and Risk Assessment of Heavy Metals in Sediment from Bohai Bay, China. Minerals, 9(2), 111. https://doi.org/10.3390/min9020111




