Ultimate Mechanical Properties of Forsterite
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Ground State Properties
3.2. Ideal Strength in Tension and in Shear
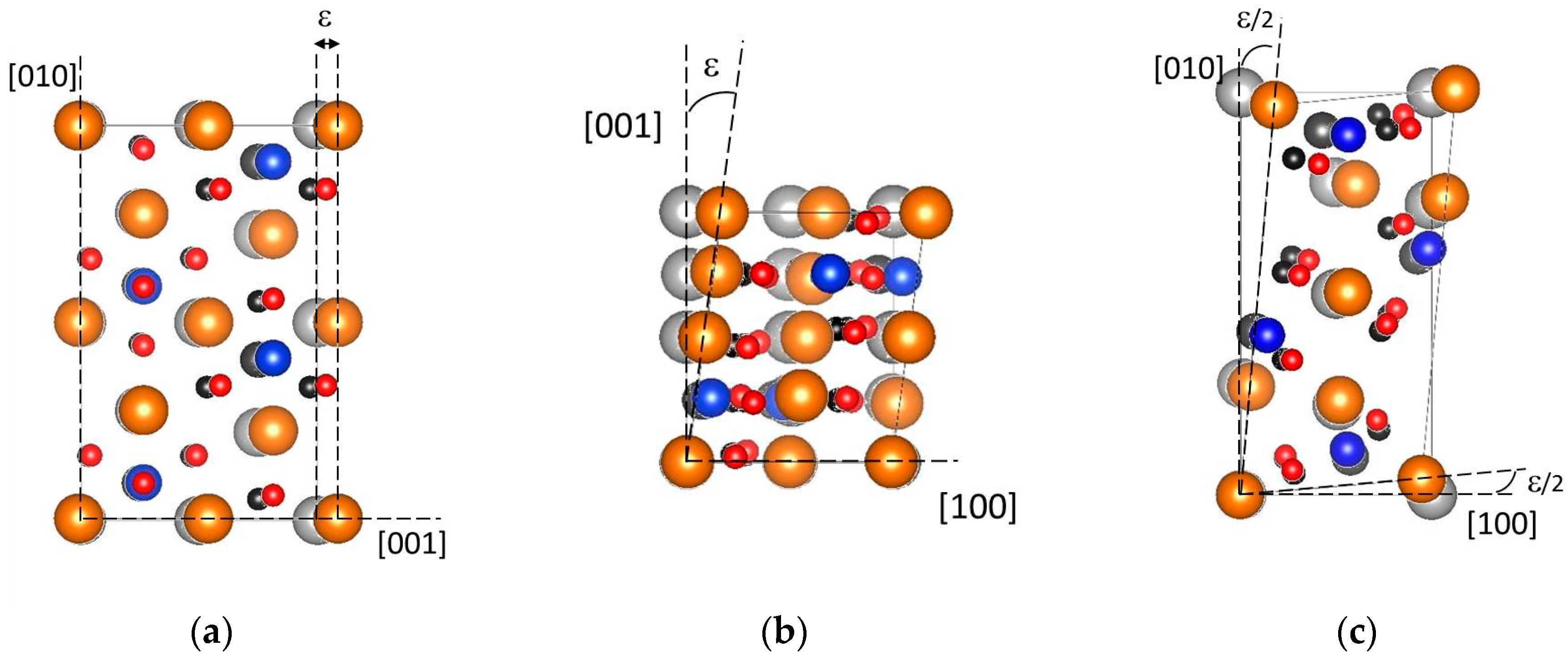
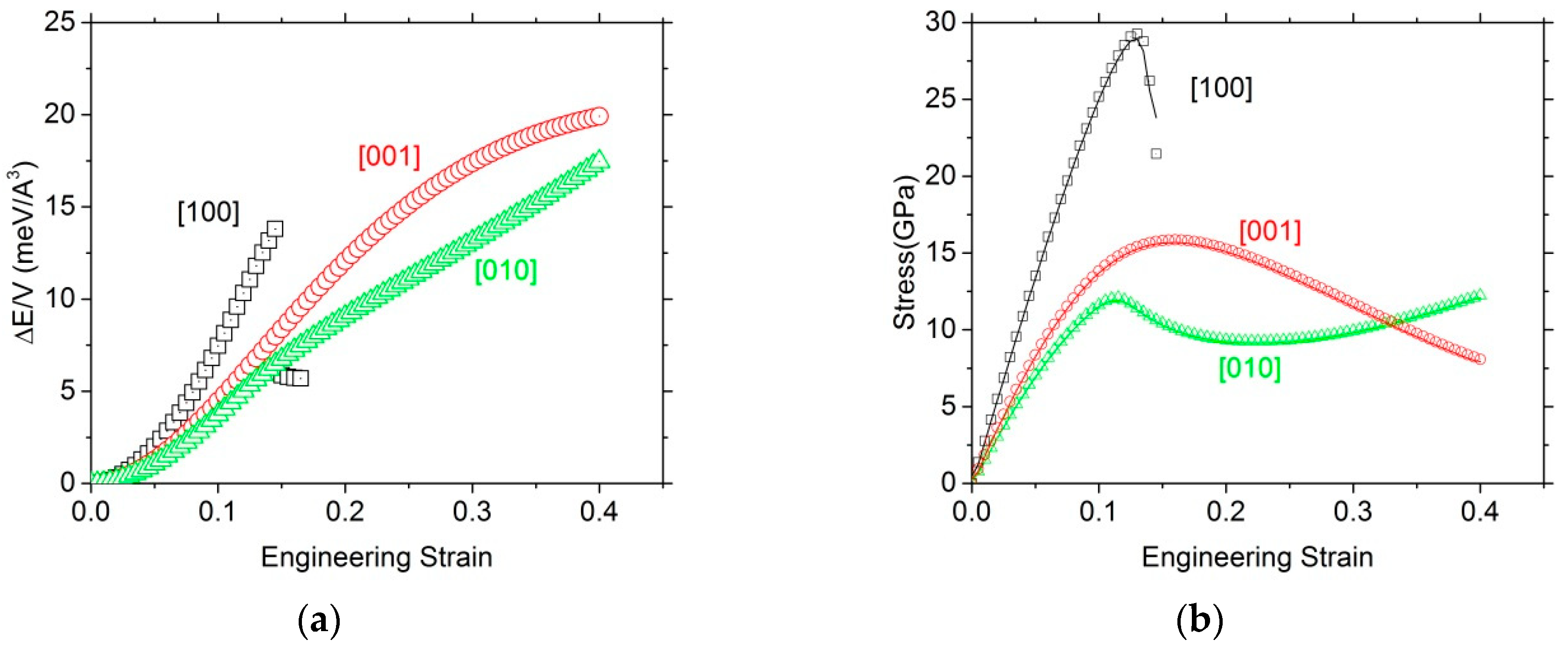
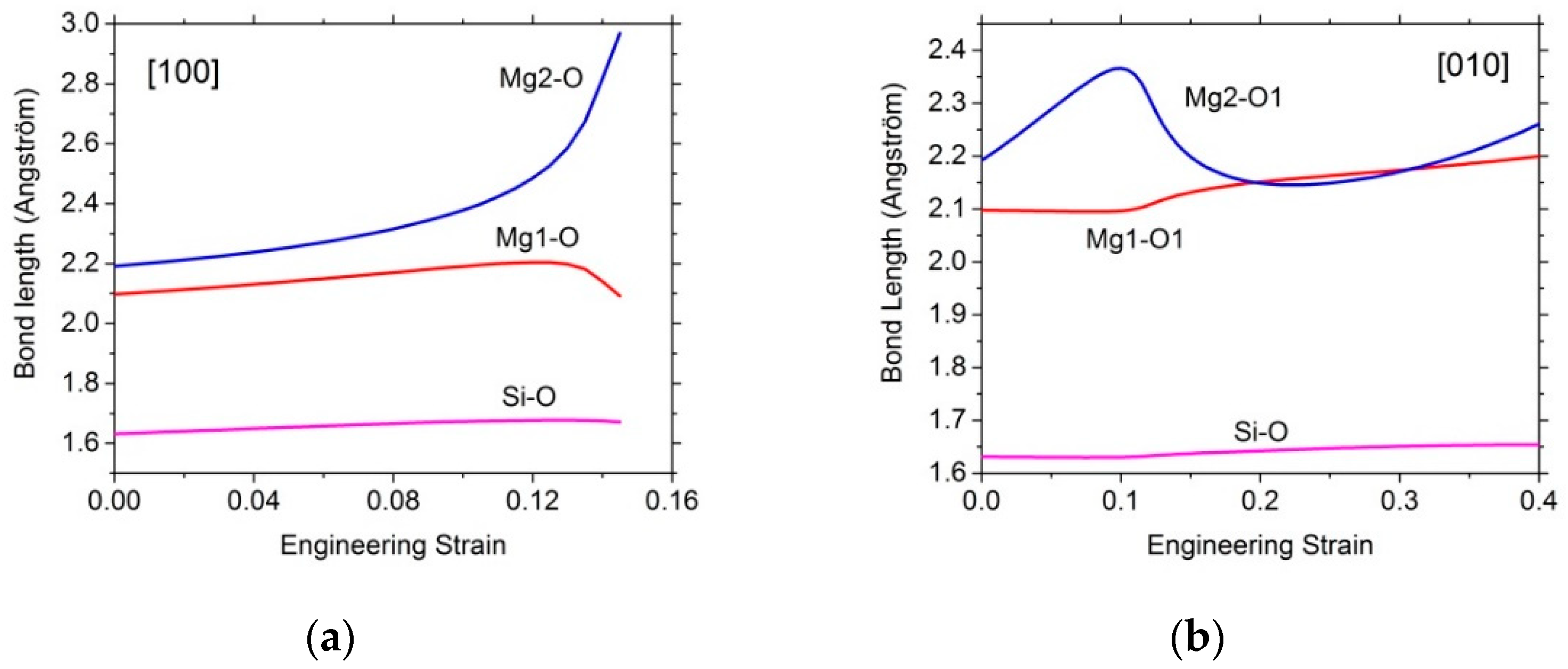
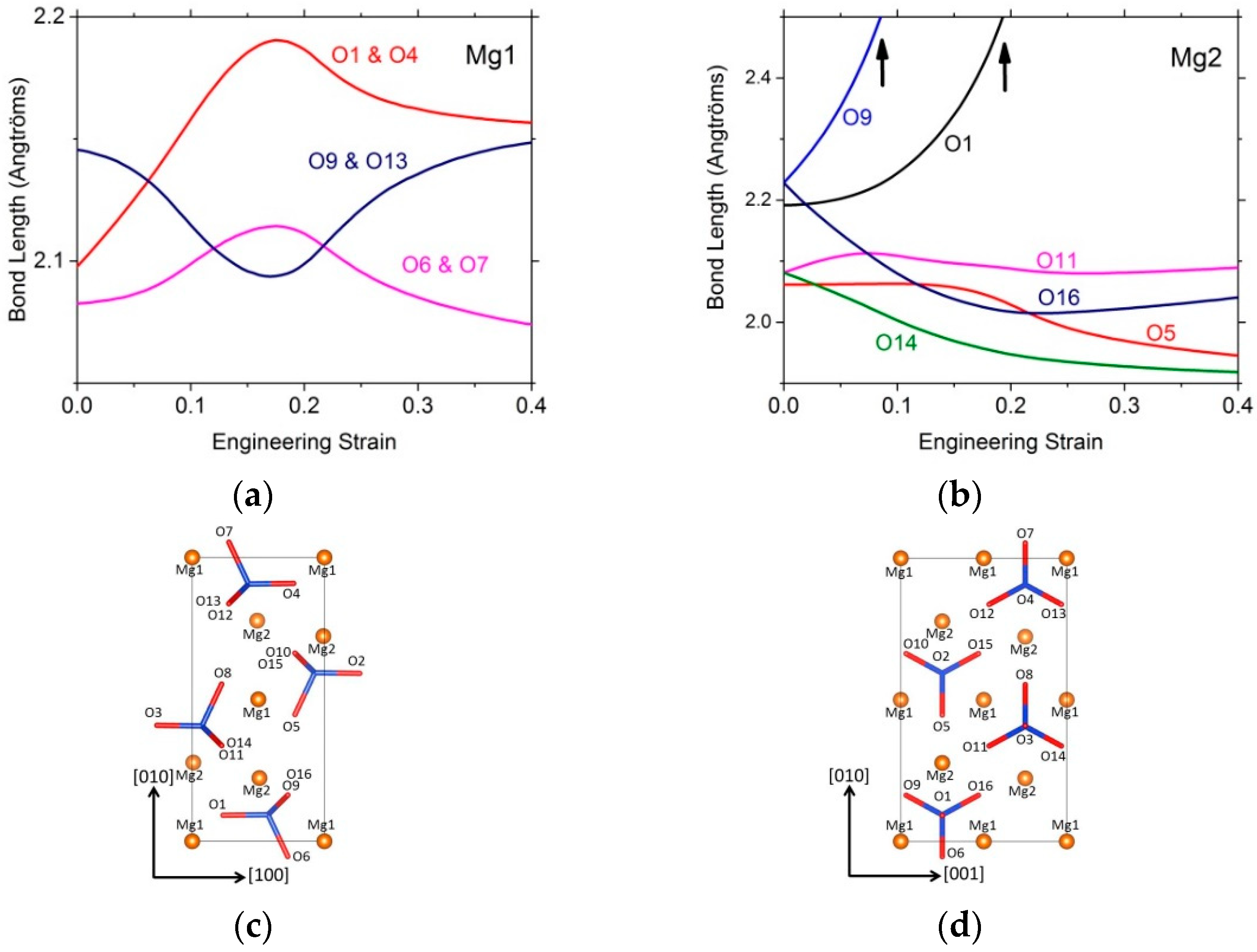
3.2.1. Tensile Tests
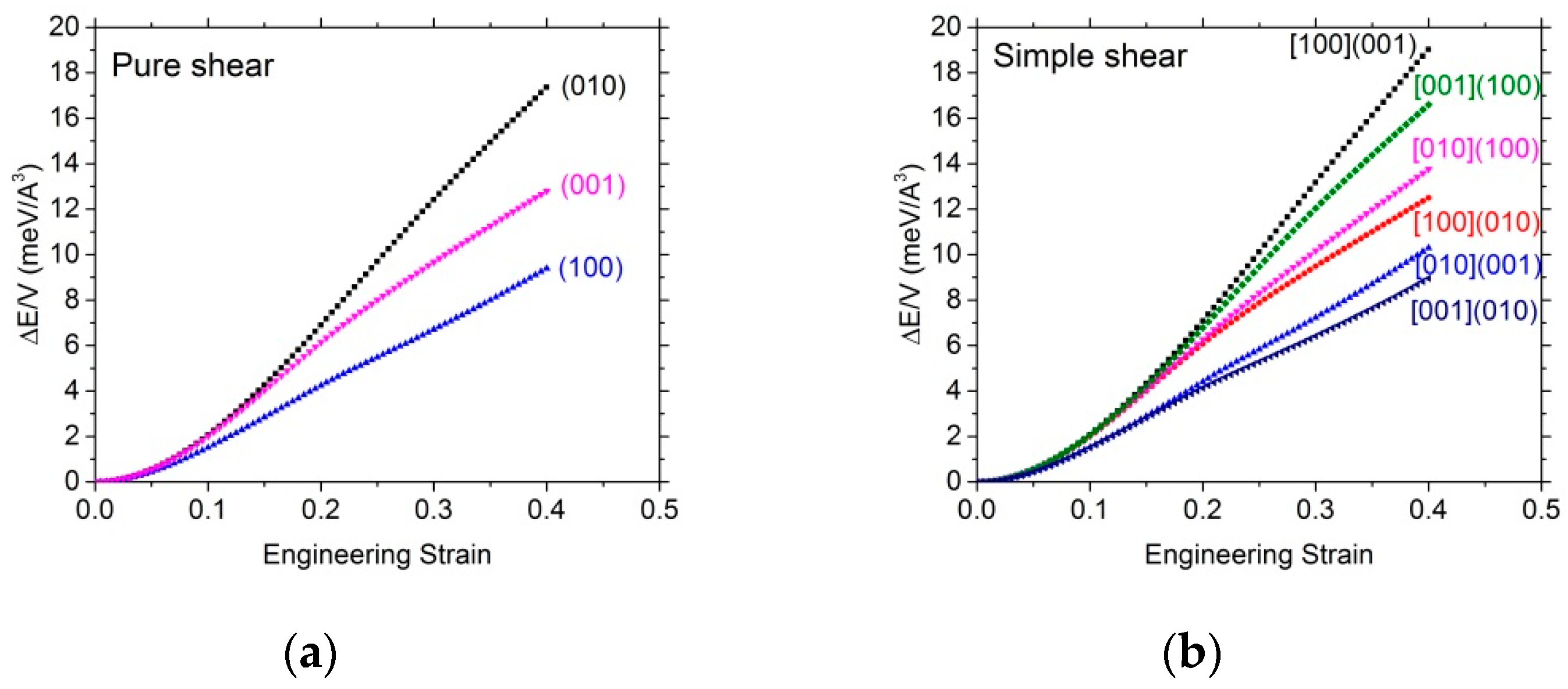
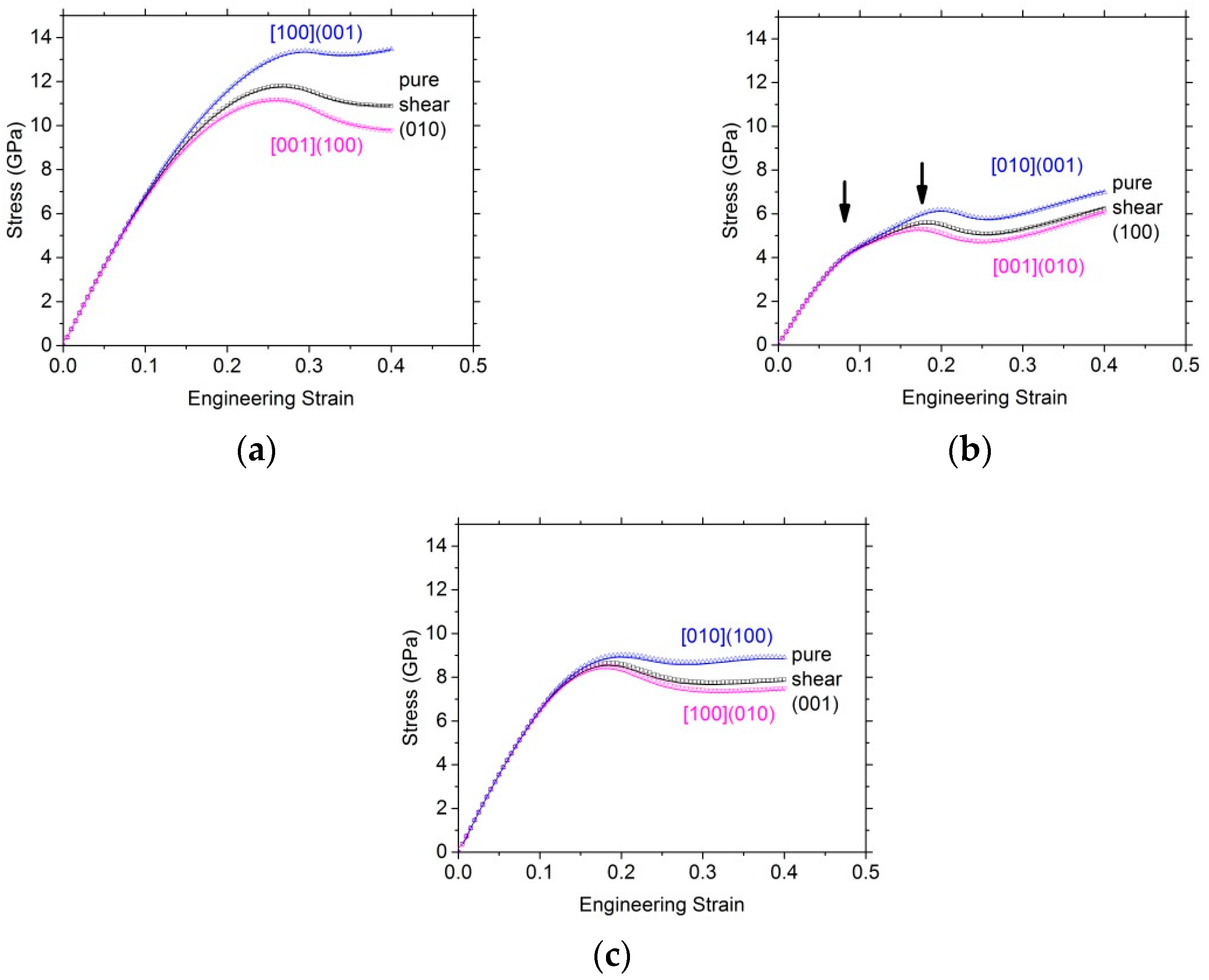
3.2.2. Shear Tests
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fogerty, S.; Forrest, W.; Watson, D.M.; Sargent, B.A.; Koch, I. Silicate Composition of the Interstellar Medium. Astrophys. J. 2016, 830, 71. [Google Scholar] [CrossRef]
- Zolensky, M.E.; Zega, T.J.; Yano, H.; Wirick, S.; Westphal, A.J.; Weisberg, M.K.; Weber, I.; Warren, J.L.; Velbel, M.A.; Tsuchiyama, A. Mineralogy and petrology of comet 81P/Wild 2 nucleus samples. Science 2006, 314, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Brearley, A.J.; Jones, R.H.; Papike, J.J. (Eds.) Chondritic meteorites. Planetary Materials. In Review in Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 1998; Volume 36, p. C1. [Google Scholar]
- Mittlefehldt, D.W.; McCoy Timothy, J.; Goodrich, C.A.; Kracher, A. Non-chondritic meteorites from asteroidal bodies. Rev. Miner. Geochem. 1998, 36, 4.1–4.195. [Google Scholar]
- Ringwood, A.E. Phase transformations and their bearing on the constitution and dynamics of the mantle. Geochim. Cosmochim. Acta 1991, 55, 2083–2110. [Google Scholar] [CrossRef]
- Richet, P.; Leclerc, F.; Benoist, L. Melting of forsterite and spinel, with implications for the glass transition of Mg2SiO4 liquid. Geophys. Res. Lett. 1993, 20, 1675–1678. [Google Scholar] [CrossRef]
- Jeanloz, R.; Ahrens, T.; Lally, J.S.; Nord, G.L.; Christie, J.M.; Heuer, A.H. Shock-Produced Olivine Glass: First Observation. Science 1977, 197, 457–459. [Google Scholar] [CrossRef]
- Lacam, A.; Madon, M.; Poirier, J.P. Olivine glass and spinel formed in a diamond anvil high-pressure cell. Nature 1980, 288, 155–157. [Google Scholar] [CrossRef]
- Williams, Q.; Knittle, E.; Reichlin, R.; Martin, S.; Jeanloz, R. Structural and electronic properties of Fe2SiO4-fayalite at ultrahigh pressures: Amorphization and gap closure. J. Geophys. Res. 1990, 95, 21549–21563. [Google Scholar] [CrossRef]
- Richard, G.; Richet, P. Room-temperature amorphization of fayalite and high-pressure properties of Fe2SiO4 liquid. Geophys. Res. Lett. 1990, 17, 2093–2096. [Google Scholar] [CrossRef]
- Guyot, F.; Reynard, B. Pressure-induced structural modifications and amorphization in olivine compounds. Chem. Geol. 1992, 96, 411–420. [Google Scholar] [CrossRef]
- Andrault, D.; Bouhifd, M.A.; Itie, J.P.; Richet, P. Compression and amorphization of (Mg,Fe)2SiO4 olivines: An X-ray diffraction study up to 70 GPa. Phys. Chem. Min. 1995, 22, 99–107. [Google Scholar] [CrossRef]
- Finkelstein, G.J.; Dera, P.K.; Jahn, S.; Oganov, A.R.; Holl, C.M.; Meng, Y.; Duffy, T.S. Phase transitions and equation of state of forsterite to 90 GPa from single-crystal X-ray diffraction and molecular modeling. Am. Miner. 2014, 99, 35–43. [Google Scholar] [CrossRef]
- Santamaria-Perez, D.; Thomson, A.; Segura, A.; Pellicer-Torres, J.; Manjon, F.J.; Corà, F.; McColl, K.; Wilson, M.; Dobson, D.; McMillan, P.F. Metastable structural transformations and pressure-induced amorphization in natural (Mg,Fe)2SiO4 olivine under static compression: A Raman spectroscopic study. Am. Miner. 2016, 101, 1642–1650. [Google Scholar] [CrossRef]
- Li, W.; Wang, T. Ab initio investigation of the elasticity and stability of aluminium. J. Phys. Condens. Matter. 1998, 10, 9889–9904. [Google Scholar] [CrossRef]
- Roundy, D.; Krenn, C.R.; Cohen Marvin, L.; Morris, J.W., Jr. Ideal Shear Strengths of fcc Aluminum and Copper. Phys. Rev. Lett. 1999, 82, 2713–2716. [Google Scholar] [CrossRef]
- Ogata, S.; Kitagawa, H. Ab initio tensile testing simulation of aluminum and aluminum nitride ceramics based on density functional theory. Comput. Mater. Sci. 1999, 15, 435–440. [Google Scholar] [CrossRef]
- Ogata, S.; Li, J.; Hirosaki, N.; Shibutani, Y.; Yip, S. Ideal shear strain of metals and ceramics. Phys. Rev. B 2004, B70, 104404. [Google Scholar] [CrossRef]
- Yip, S.; Li, J.; Tang, M.; Wang, J. Mechanistic aspects and atomic-level consequences of elastic instabilities in homogeneous crystals. Mater. Sci. Eng. 2001, A317, 236–240. [Google Scholar] [CrossRef]
- Zhang, S.H.; Fu, Z.H.; Zhang, R.F. ADAIS: Automatic Derivation of Anisotropic Ideal Strength via high-throughput first-principles computations. Comput. Phys. Commun. 2019, 238, 244–253. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. 1996, B54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. 1992, B45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Perdew, J.P. Correlation hole of the spin-polarised electron-gas, with exact small-wave-vector and high-density scaling. Phys. Rev. 1991, B44, 13298–13307. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, E.R.; Brodholt, J.; Alfe, D. Structural, vibrational and thermodynamic properties of Mg2 SiO4 and MgSiO3 minerals from first-principles simulations. Phys. Earth Planet. Inter. 2015, 240, 1–24. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. 1976, B13, 5188–5192. [Google Scholar] [CrossRef]
- Durinck, J.; Legris, A.; Cordier, P. Influence of crystal chemistry on ideal plastic shear anisotropy in forsterite: First principle calculations. Am. Miner. 2005, 90, 1072–1077. [Google Scholar] [CrossRef]
- Brodholt, J. Ab initio calculations on point defects in forsterite (Mg2SiO4) and implications for diffusion and creep. Am. Miner. 1997, 82, 1049–1053. [Google Scholar] [CrossRef]
- Brodholt, J.; Patel, A.; Refson, K. An ab initio study of the compressional behavior of forsterite. Am. Miner. 1996, 81, 257–260. [Google Scholar] [CrossRef]
- Fujino, K.; Sasaki, S.; Takeuchi, Y.; Sadanaga, R. X-ray determination of electron distributions in forsterite, fayalite, and tephroite. Acta Cryst. 1981, B37, 513–518. [Google Scholar] [CrossRef]
- Poirier, J.-P. On the slip systems of olivine. J. Geophys. Res. 1975, 80, 4059–4061. [Google Scholar] [CrossRef]
- Webb, S.L. The elasticity of the upper mantle orthosilicates olivine and garnet to 3 GPa. Phys. Chem. Miner. 1989, 16, 684–692. [Google Scholar] [CrossRef]
- Zha, C.-S.; Duffy, T.S.; Downs, R.T.; Mao, H.-K.; Hemley, R.J.; Weidner, D.J. Single-Crystal Elasticity of the α and β of Mg2SiO4 Polymorphs at High Pressure. In Properties of Earth and Planetary Materials at High Pressure and Temperature; Manghnani, M.H., Yagi, T., Eds.; American Geophysical Union: Washington, DC, USA, 1998; Geophysical Monograph; Volume 101, pp. 9–16. [Google Scholar]
- Gibbs, G.V.; Hill, F.C.; Boisen, M.B.; Downs, R.T. Power law relationships between bond length, bond strength and electron density distributions. Phys. Chem. Min. 1998, 25, 585–590. [Google Scholar] [CrossRef]
- Jahnátek, M.; Hafner, J.; Krajčí, M. Shear deformation, ideal strength, and stacking fault formation of fcc metals: A density-functional study of Al and Cu. Phys. Rev. B 2009, B79, 224103. [Google Scholar] [CrossRef]
- Garvik, N.; Carrez, P.; Cordier, P. First-principles study of the ideal strength of Fe3C cementite. Mater. Sci. Eng. 2013, A572, 25–29. [Google Scholar] [CrossRef]
- Jiang, C.; Srinivasan, S.G. Unexpected strain-stiffening in crystalline solids. Nature 2013, 496, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Machon, D.; Meersman, F.; Wilding, M.C.; Wilson, M.; McMillan, P.F. Pressure-induced amorphization and polyamorphism: Inorganic and biochemical systems. Prog. Mater. Sci. 2014, 61, 216–282. [Google Scholar] [CrossRef]
- Saka, H.; Shimatani, A.; Suganuma, M.S. Transmission electron microscopy of amorphization and phase transformation beneath indents in Si. Philos. Mag. A 2002, 82, 1971–1981. [Google Scholar] [CrossRef]
- Ge, D.; Domnich, V.; Juliano, T.; Stach, E.A.; Gogotsi, Y. Structural damage in boron carbide under contact loading. Acta Mater. 2004, 52, 3921–3927. [Google Scholar] [CrossRef]
- Kranjc, K.; Rouse, Z.; Flores, K.M.; Skemer, P. Low temperature plastic rheology of olivine determined by nanoindentation. Geophys. Res. Lett. 2015, 43, 176–184. [Google Scholar] [CrossRef]
- Kumamoto, K.M.; Thom, C.A.; Wallis, D.; Hansen, L.N.; Armstrong, D.E.J.; Warren, J.M.; Goldsby, D.L.; Wilkinson, A.J. Size effects resolve discrepancies in 40 years of work on low-temperature plasticity in olivine. Sci. Adv. 2017, 3, e1701338. [Google Scholar] [CrossRef]
| a (Å) | b (Å) | c (Å) | V (Å3) | |
|---|---|---|---|---|
| This study | 4.79 | 10.27 | 6.03 | 296.63 |
| Calculated GGA [24] | 4.79 | 10.28 | 6.02 | 296.43 |
| Calculated GGA [26] | 4.79 | 10.28 | 6.04 | 297.68 |
| Calculated GGA [27] | 4.71 | 10.15 | 5.96 | 284.92 |
| Calculated LDA [28] | 4.64 | 9.99 | 6.07 | 281.67 |
| Experimental [29] | 4.75 | 10.19 | 5.98 | 289.58 |
| Tensile Tests | [100] | [010] | [001] | |||
|---|---|---|---|---|---|---|
| ITS (GPa) | 29.3 | 12.1 | 15.9 | |||
| Corresponding strain (%) | 13.0 | 11.5 | 16 | |||
| Young’s modulus (GPa) | 274.4 | 153.2 | 170.9 | |||
| Normalized stress | 0.10 | 0.08 | 0.09 | |||
| Pure shear tests | (100) | (010) | (001) | |||
| ISS (GPa) | 5.6 | 11.8 | 8.7 | |||
| Corresponding strain (%) | 18.5 | 26.5 | 18.5 | |||
| Simple shear tests | [010](001) | [001](010) | [100](001) | [001](100) | [010](100) | [100](010) |
| ISS (GPa) | 6.2 | 5.3 | 13.4 | 11.2 | 9.0 | 8.5 |
| Corresponding strain (%) | 20 | 18 | 29.5 | 26 | 20 | 18 |
| Shear modulus (GPa) | 58.7 (i.e., C44) | 58.7 (i.e., C44) | 73.7 (i.e., C55) | 73.7 (i.e., C55) | 73.0 (i.e., C66) | 73.0 (i.e., C66) |
| Normalized stress | 0.10 | 0.09 | 0.18 | 0.15 | 0.12 | 0.11 |
| Tensile Tests | [100] | [010] | [001] |
|---|---|---|---|
| υ[100] | - | 0.13 | 0.14 |
| υ[010] | 0.23 | - | 0.29 |
| υ[001] | 0.20 | 0.24 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouriet, K.; Carrez, P.; Cordier, P. Ultimate Mechanical Properties of Forsterite. Minerals 2019, 9, 787. https://doi.org/10.3390/min9120787
Gouriet K, Carrez P, Cordier P. Ultimate Mechanical Properties of Forsterite. Minerals. 2019; 9(12):787. https://doi.org/10.3390/min9120787
Chicago/Turabian StyleGouriet, Karine, Philippe Carrez, and Patrick Cordier. 2019. "Ultimate Mechanical Properties of Forsterite" Minerals 9, no. 12: 787. https://doi.org/10.3390/min9120787
APA StyleGouriet, K., Carrez, P., & Cordier, P. (2019). Ultimate Mechanical Properties of Forsterite. Minerals, 9(12), 787. https://doi.org/10.3390/min9120787





