Microbially Induced Carbonate Precipitation Using Microorganisms Enriched from Calcareous Materials in Marine Environments and Their Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Carbonate Forming Microorganisms (CFMs)
2.2. Ca-Carbonate Precipitation Induced by CFMs
2.2.1. The Effect of Microbial Growth on Ca-Carbonate Precipitation
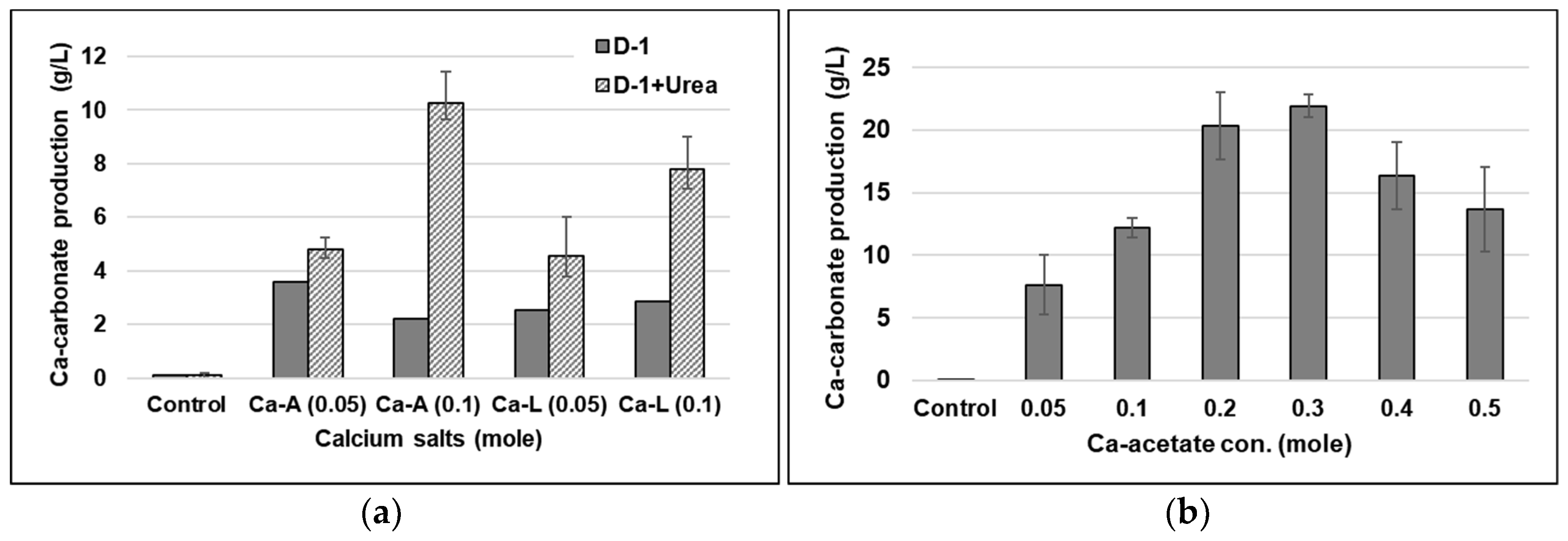
2.2.2. The Effect of Ca-Lactate as a Different Ca-Source
2.2.3. Factors for Maximum Ca-Carbonate Precipitation
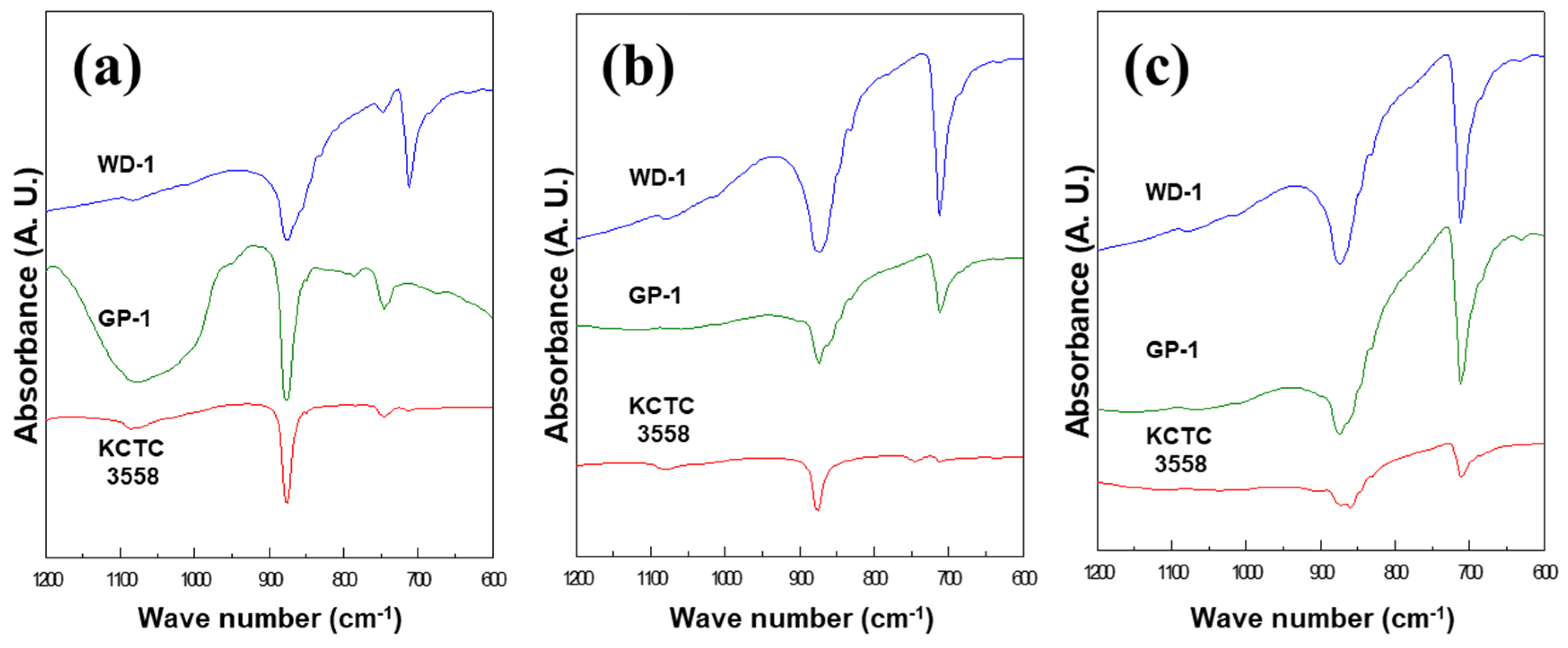
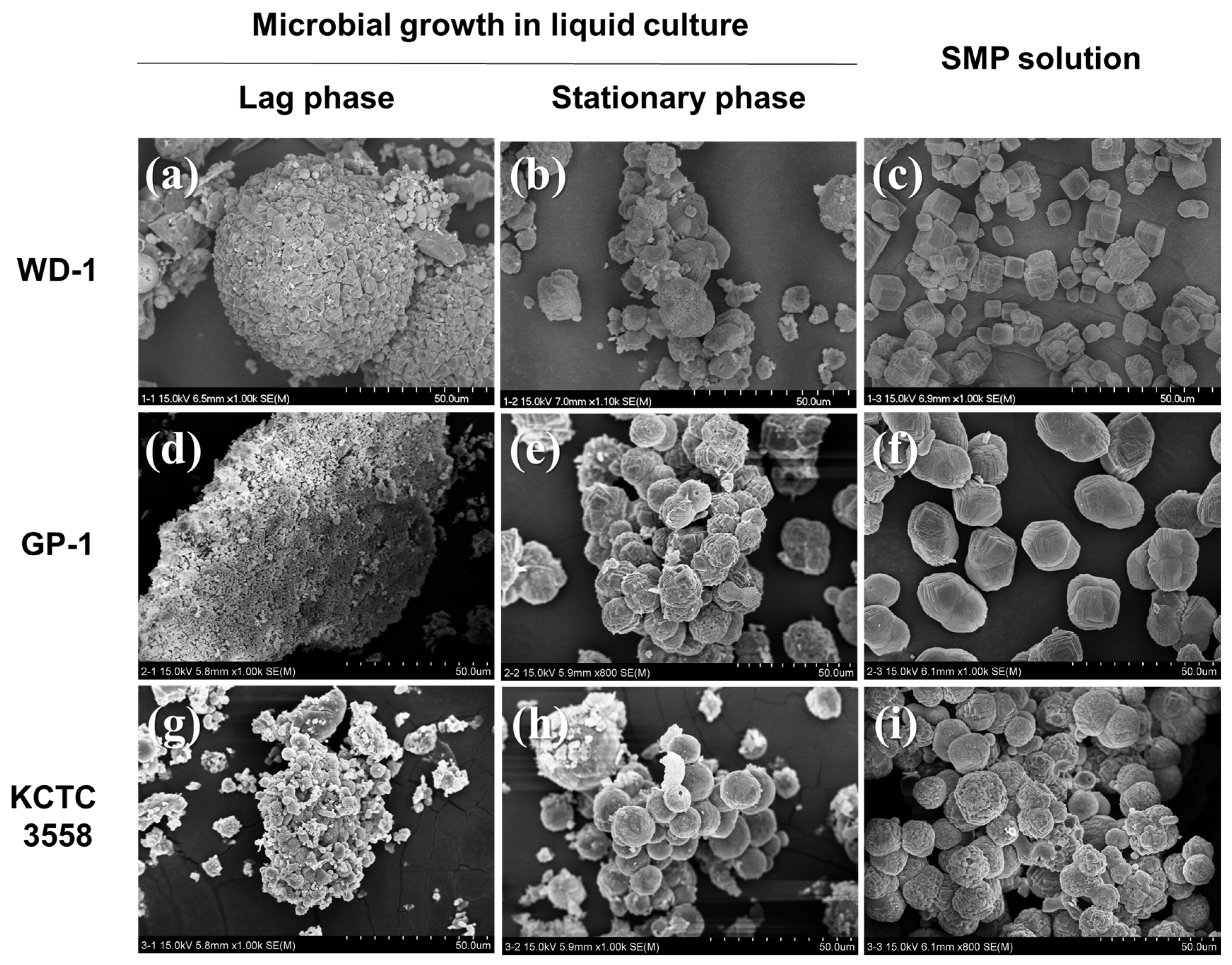
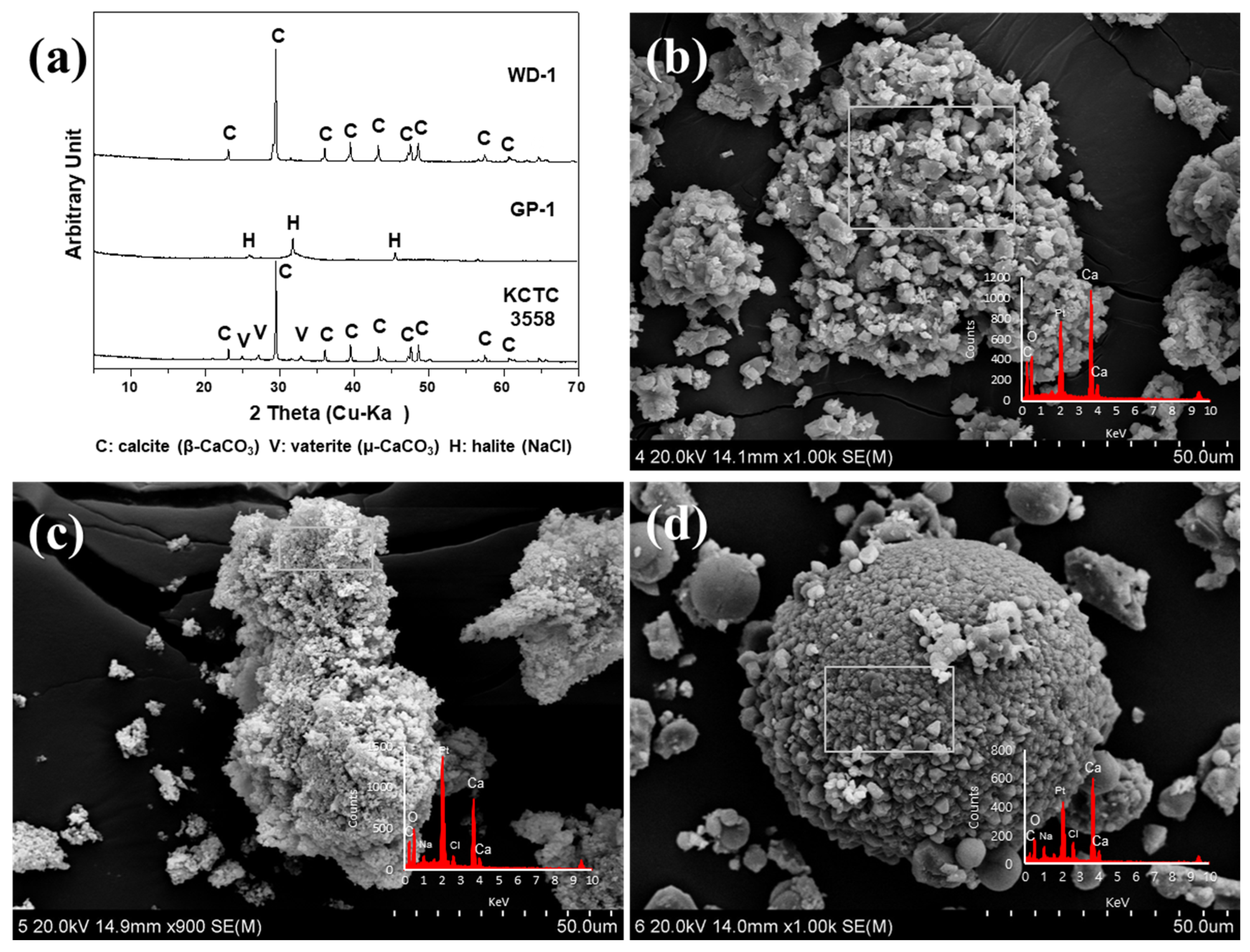
2.3. Analytical Methods
3. Results
3.1. The Effect of Microbial Growth on Ca-Carbonate Precipitation
3.2. The Effect of Ca-Lactate as a Different Ca-Source
3.3. Factors Influencing Maximum Ca-Carbonate Precipitation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chou, C.-W.; Seagren, E.A.; Aydilek, A.H.; Lai, M. Biocalcification of sand through ureolysis. J. Geotech. Geoenviron. Eng. 2011, 137, 1179–1189. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M. Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: A review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, J.-P.; Allemand, D.; Frankignoulle, M. Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: A review on interactions and control by carbonate chemistry. Am. Zool. 1999, 39, 160–183. [Google Scholar] [CrossRef]
- Bibi, S.; Qualha, M.; Ashfaq, M.Y.; Suleiman, M.T.; Zourari, N. Isolation, differentiation and biodiversity of ureolytic bacteria of Qatari soil and their potential in microbially induced calcite precipitation (MICP) for soil stabilization. RSC Adv. 2018, 8, 5854. [Google Scholar] [CrossRef]
- Pham, V.P.; Nakano, A.; van der Star, W.R.L.; Heimovaara, T.J.; van Paassen, L.A. Applying MICP by denitrification in soils: A process analysis. Environ. Geotech. 2018, 5, 79–93. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Rodriguez-Gallego, M.; Ben Chekroun, K.; GonzalezMunoz, M.T. Conservation of ornamental stone by Myxococcus xanthus-induced carbonate biomineralization. Appl. Environ. Microbiol. 2003, 69, 2182–2193. [Google Scholar] [CrossRef]
- Warthmann, R.; van Lith, Y.; Vasconcelos, C.; McKenzie, J.A.; Karpoff, A.M. Bacterially induced dolomite precipitation in anoxic culture experiments. Geology 2000, 28, 1091–1094. [Google Scholar] [CrossRef]
- Phillipsa, A.J.; Troyer, E.; Hiebert, R.; Kirkland, C.; Gerlach, R.; Cunningham, A.B.; Spangler, L.; Kirksey, J.; Rowe, W.; Esposito, R. Enhancing wellbore cement integrity with microbially induced calcite precipitation (MICP): A field scale demonstration. J. Pet. Sci. Eng. 2018, 171, 1141–1148. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Dideriksen, K.; Spangler, L.H.; Cunningham, A.B.; Gerlach, R. Microbially enhanced carbon capture and storage by mineral-trapping and solubility-trapping. Environ. Sci Technol. 2010, 44, 5270–5276. [Google Scholar] [CrossRef]
- Hammes, F.; Verstraete, W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev. Environ. Sci. Biotechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Ercole, C.; Bozzelli, P.; Altieri, F.; Cacchio, P.; Gallo, M.D. Ca-carbonate mineralization: Involvement of extracellular polymeric materials isolated from calcifying bacteria. Microsc. Microanal. 2012, 18, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, L.P.; Zhou, P.-P.; Cao, L.; Yu, L.-J.; Jiang, S.Y. Calcite precipitation induced by bacteria and bacterially produced carbonic anhydrase. Curr. Sci. 2011, 100, 502–508. [Google Scholar]
- Kröger, R. Biomineralization: Ion binding and nucleation. Nat. Mater. 2015, 14, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Jiang, J.; Sun, H.; Huang, Y.; Tao, F.; Lian, B. Carbonate mineral formation under the influence of limestone-colonizing actinobacteria: Morphology and polymorphism. Front. Microbiol. 2016, 7, 366. [Google Scholar] [CrossRef][Green Version]
- Sanchez-Roman, M.; Vasconcelos, C.; Warthmann, R.; Rivadeneyra, M.; McKenzie, J.A. Microbial dolomite precipitation under aerobic conditions: Results from Brejo do Espinho Lagoon (Brazil) and culture experiments. Int. Assoc. Sedimentol. Spec. Publ. 2009, 41, 167–178. [Google Scholar]
- Kang, S.; Roh, Y.M. Biomineralization of Mg-enriched Ca-carbonates by aerobic microorganisms enriched from rhodoliths. J. Nanosci. Nanotechnol. 2017, 17, 2329–2332. [Google Scholar] [CrossRef]
- Gorospe, C.M.; Han, S.H.; Kim, S.G.; Park, J.Y.; Kang, C.H.; Jeong, J.H.; So, J.S. Effects of different calcium salts on Ca-carbonate crystal formation by Sporosarcina pasteurii KCTC 3558. Biotechnol. Bioprocess. Eng. 2013, 18, 903–908. [Google Scholar] [CrossRef]
- Balci, N.; Demirel, C. Formation of carbonate nanoglobules by a mixed natural culture under hypersaline conditions. Minerals 2016, 6, 122. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Jroundi, F.; Schiro, M.; Ruiz-Agudo, E.; González-Muñoz, M.T. Influence of substrate mineralogy on bacterial mineralization of Ca-carbonate: Implications for stone conservation. Appl. Environ. Microbiol. 2012, 78, 4017–4029. [Google Scholar] [CrossRef]
- Tourney, J.; Ngwenya, B.T. Bacterial extracellular polymeric substances (EPS) mediate CaCO3 morphology and polymorphism. Chem. Geol. 2009, 262, 138–146. [Google Scholar] [CrossRef]
- Tourney, J.; Ngwenya, B.T. The role of bacterial extracellular polymeric substances in geomicrobiology. Chem. Geol. 2014, 386, 115–132. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Y.H.; Xie, A.J.; Huang, B.; Jia, R.; Guo, R.Y.; Tang, W.Z. Bacteria-mediated synthesis of metal carbonate minerals with unusual morphologies and structures. Cryst. Growth Des. 2009, 9, 743–754. [Google Scholar] [CrossRef]
- Marvasi, M.; Gallagher, L.K.; Martinez, C.L.; Molina Pagan, C.W.; Rodríguez Santiago, E.R.; Vega, C.G.; Visscher, T.P. Importance of B4 medium in determining organomineralization potential of bacterial environmental isolates. Geomicrobiol. J. 2012, 29, 916–924. [Google Scholar] [CrossRef]
- Stumn, W. Chemistry of the Solid-Water Interface: Processes at the Mineral-Water and Particle-Water Interface in Natural Systems; Wiley: New York, NY, USA, 1992; pp. 211–242. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Roh, Y. Microbially Induced Carbonate Precipitation Using Microorganisms Enriched from Calcareous Materials in Marine Environments and Their Metabolites. Minerals 2019, 9, 722. https://doi.org/10.3390/min9120722
Kim Y, Roh Y. Microbially Induced Carbonate Precipitation Using Microorganisms Enriched from Calcareous Materials in Marine Environments and Their Metabolites. Minerals. 2019; 9(12):722. https://doi.org/10.3390/min9120722
Chicago/Turabian StyleKim, Yumi, and Yul Roh. 2019. "Microbially Induced Carbonate Precipitation Using Microorganisms Enriched from Calcareous Materials in Marine Environments and Their Metabolites" Minerals 9, no. 12: 722. https://doi.org/10.3390/min9120722
APA StyleKim, Y., & Roh, Y. (2019). Microbially Induced Carbonate Precipitation Using Microorganisms Enriched from Calcareous Materials in Marine Environments and Their Metabolites. Minerals, 9(12), 722. https://doi.org/10.3390/min9120722





