Electron Donor Utilization and Secondary Mineral Formation during the Bioreduction of Lepidocrocite by Shewanella putrefaciens CN32
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Analytical Methods
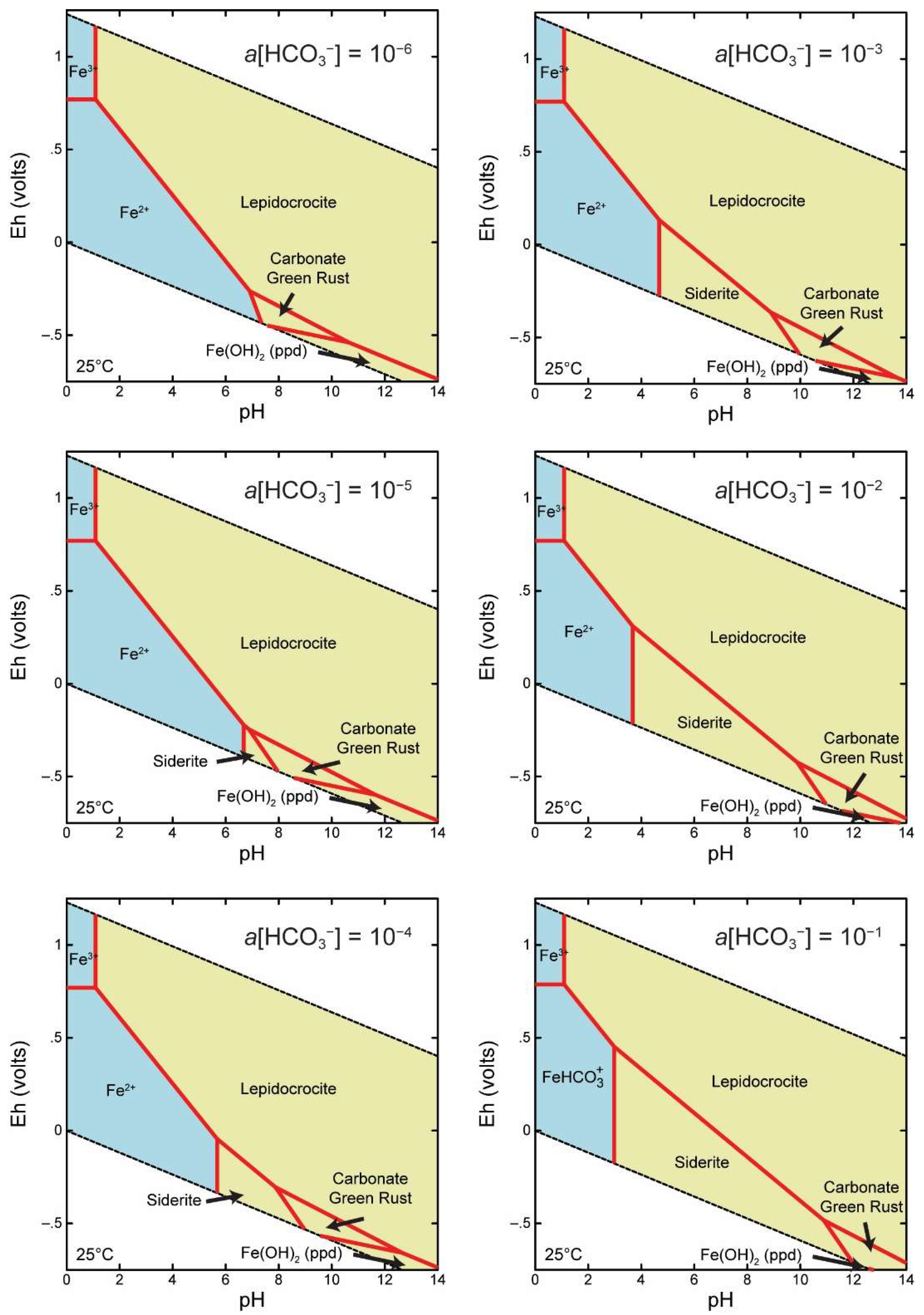
2.3. Thermodynamic Modeling
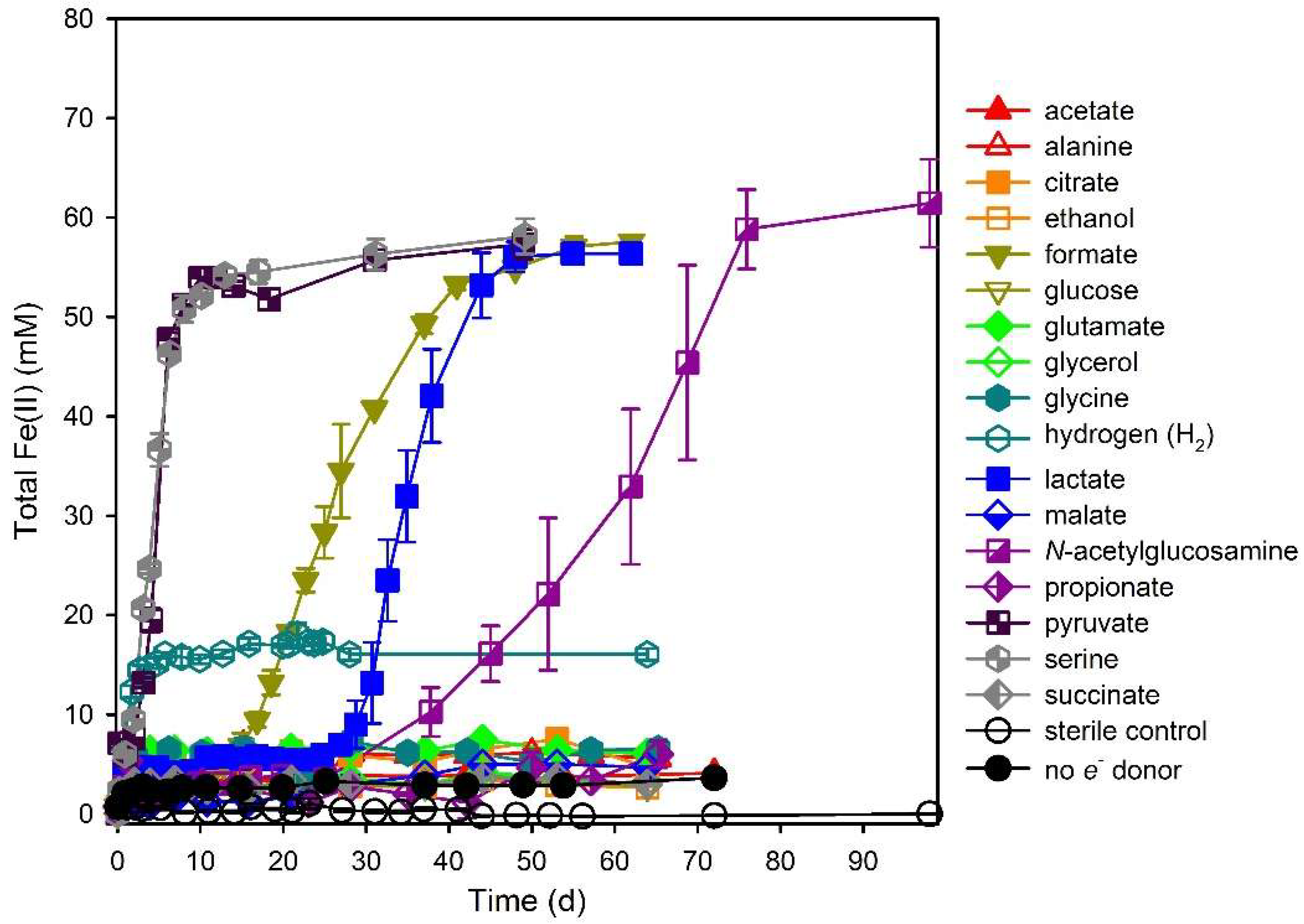
3. Results
3.1. Electron Donor Survey
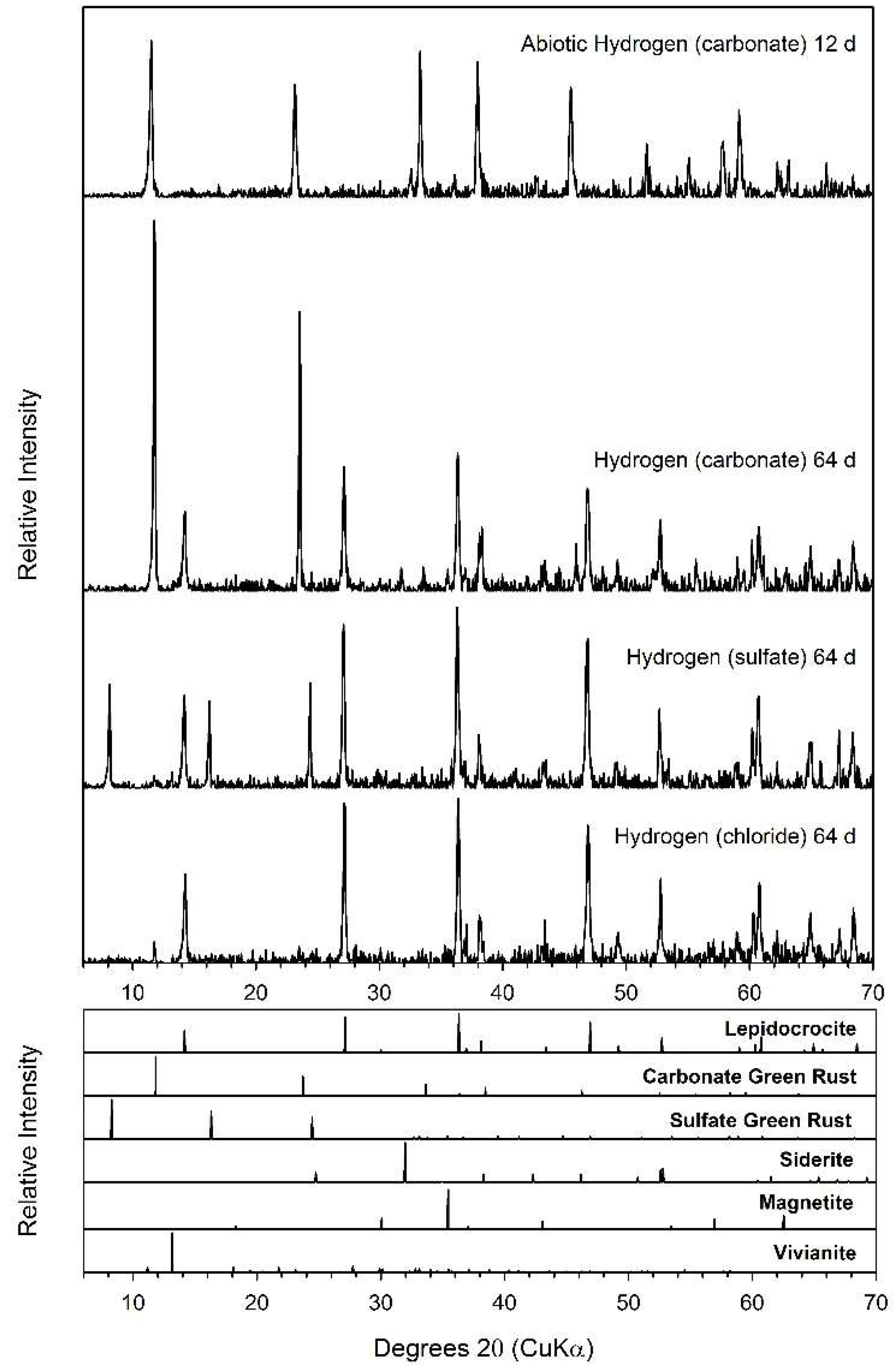
3.2. Hydrogen
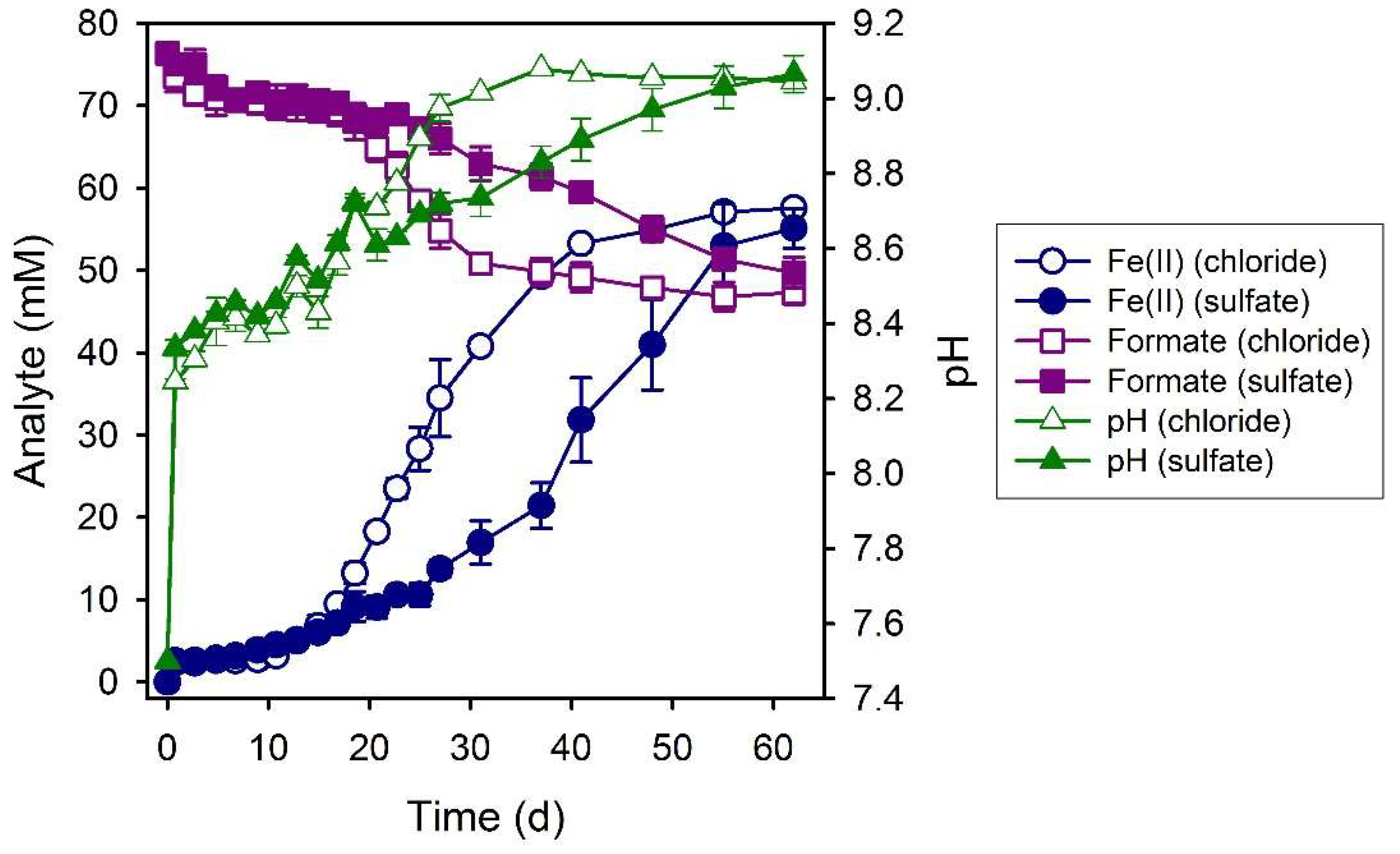
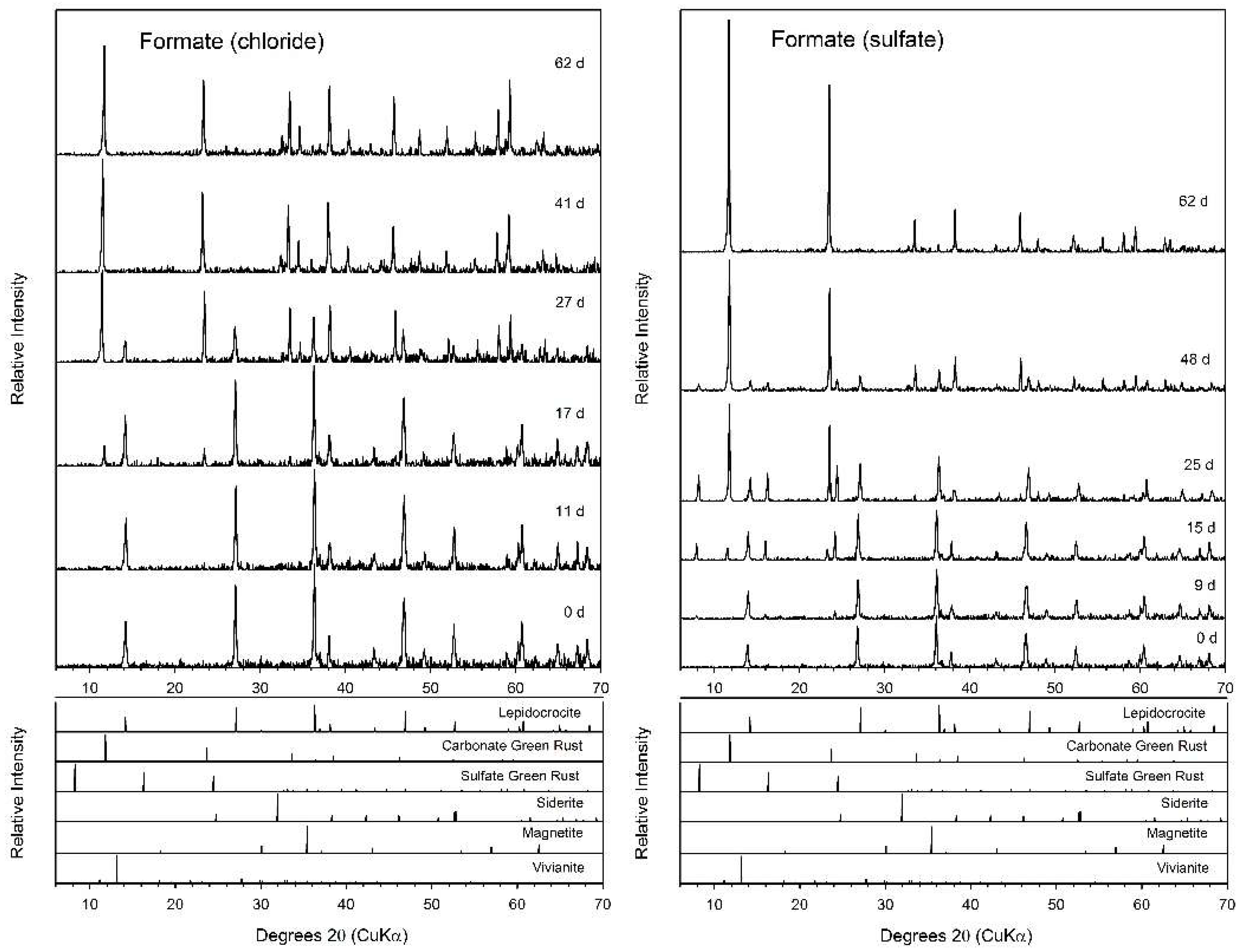
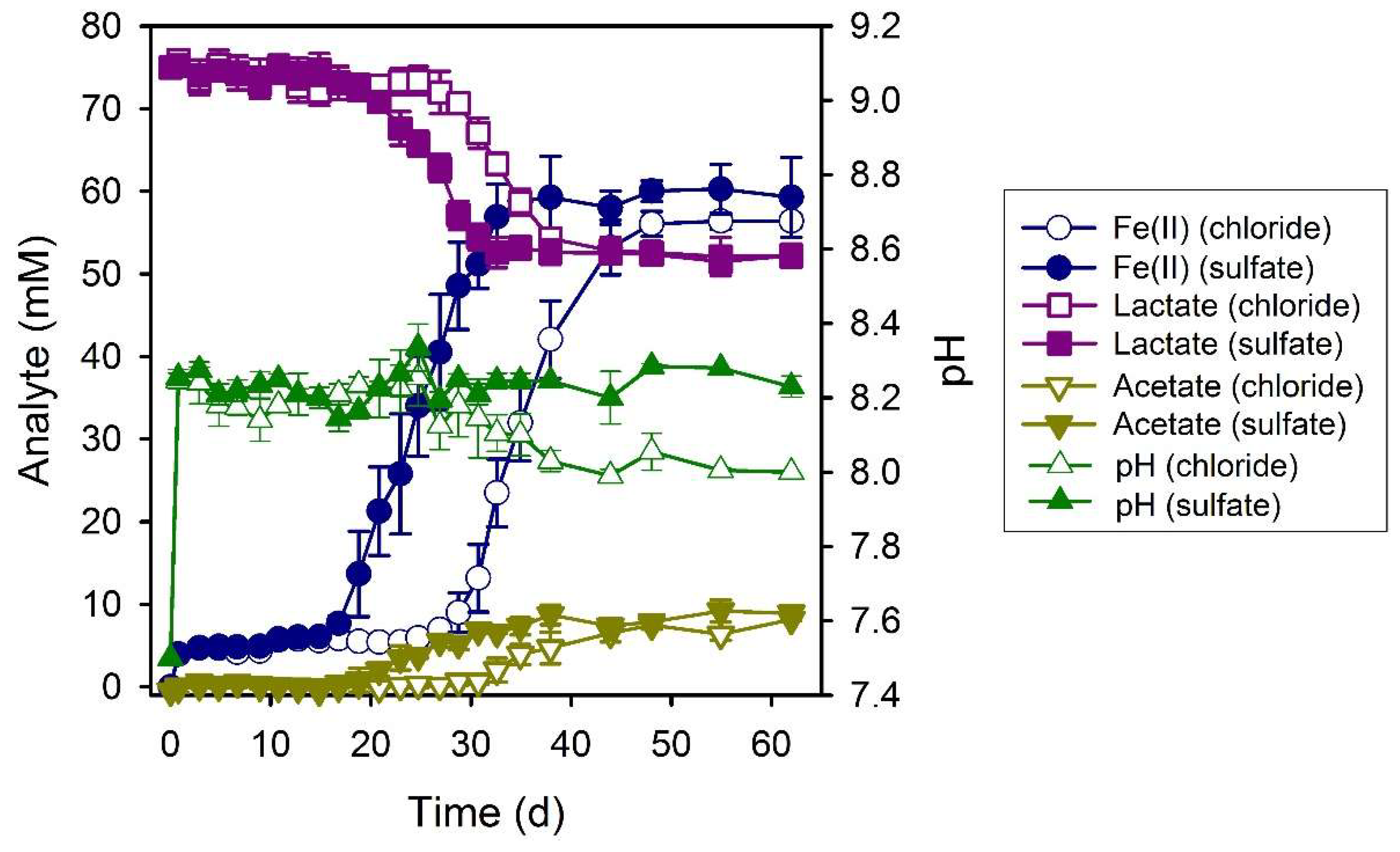
3.3. Formate
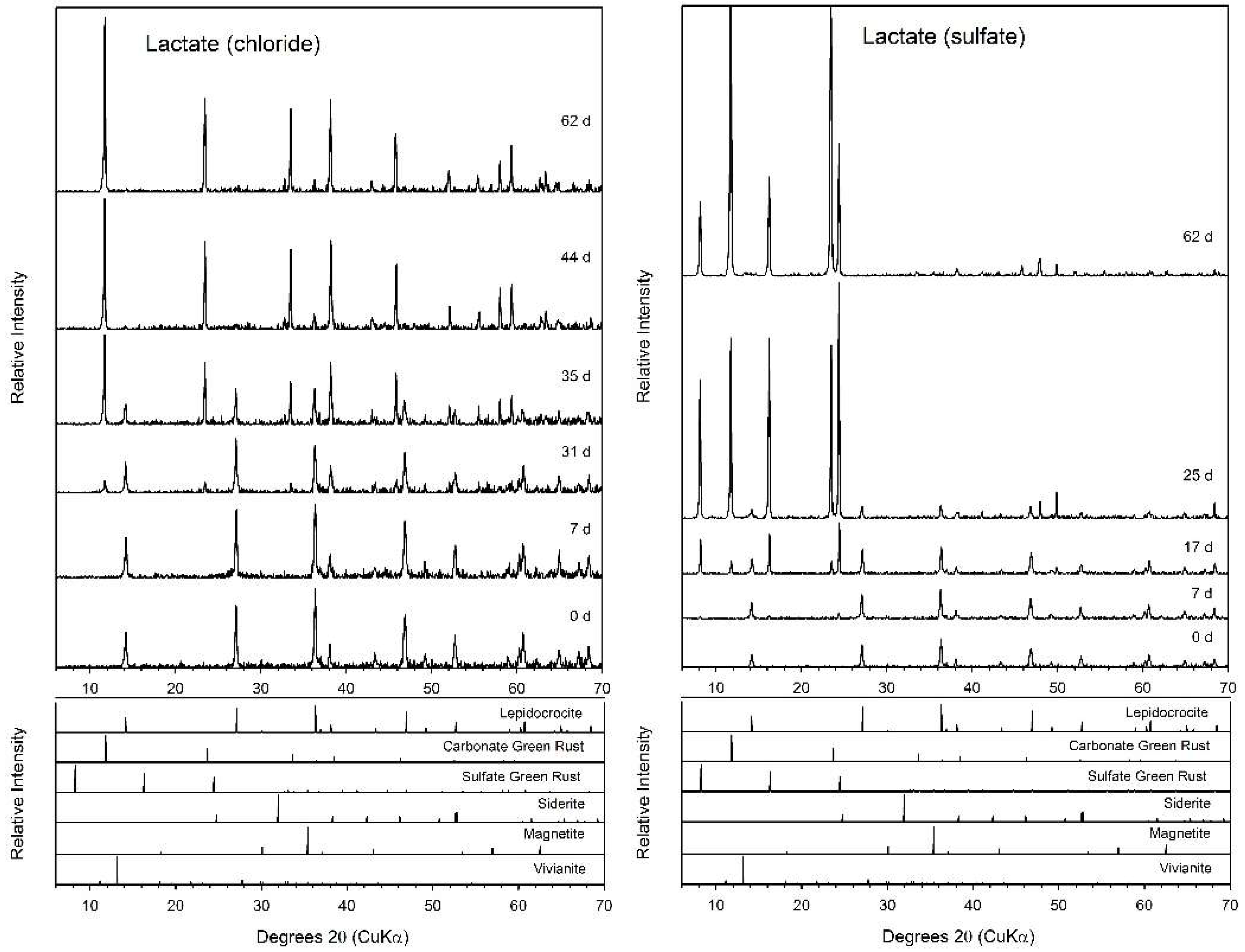
3.4. Lactate
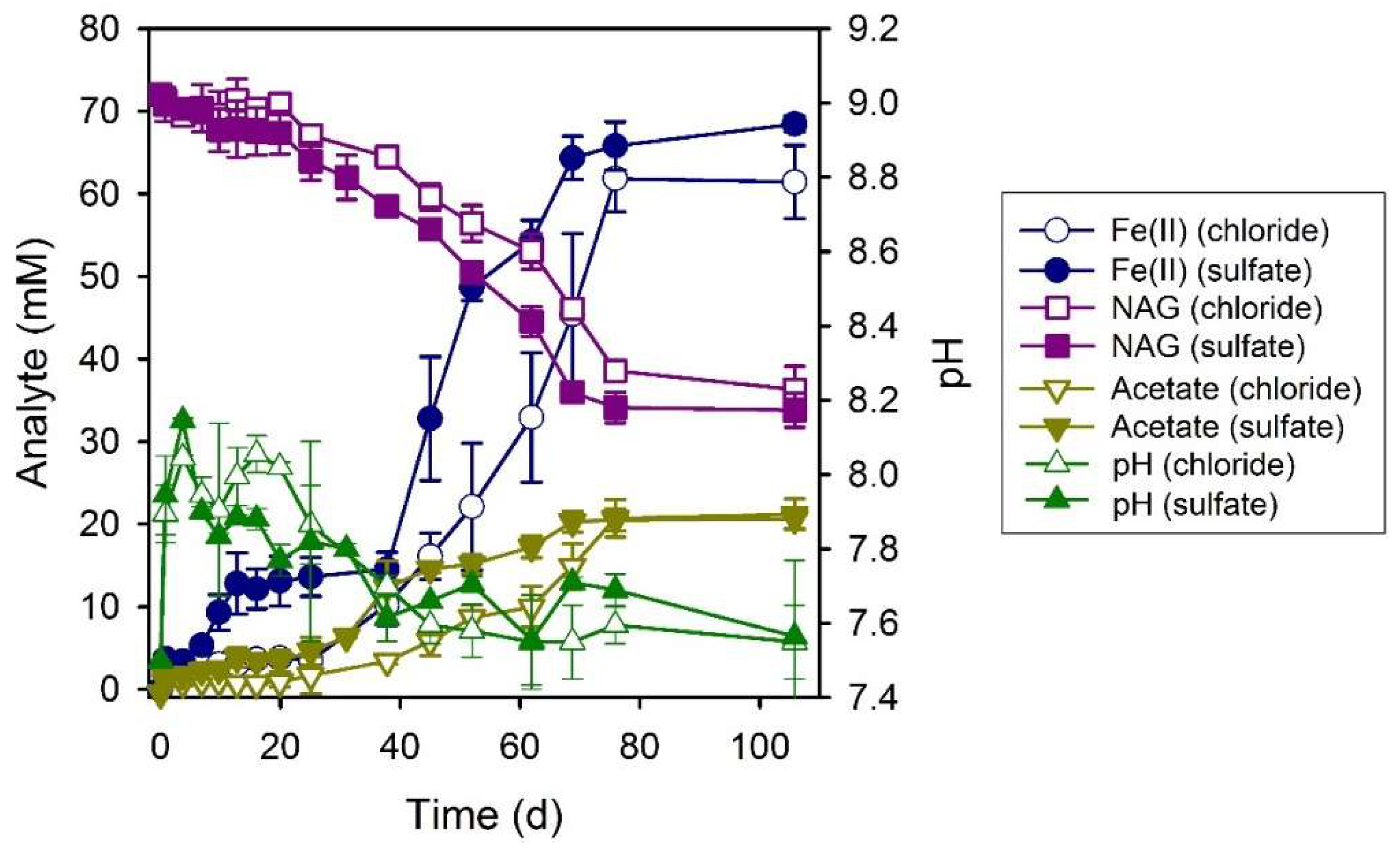
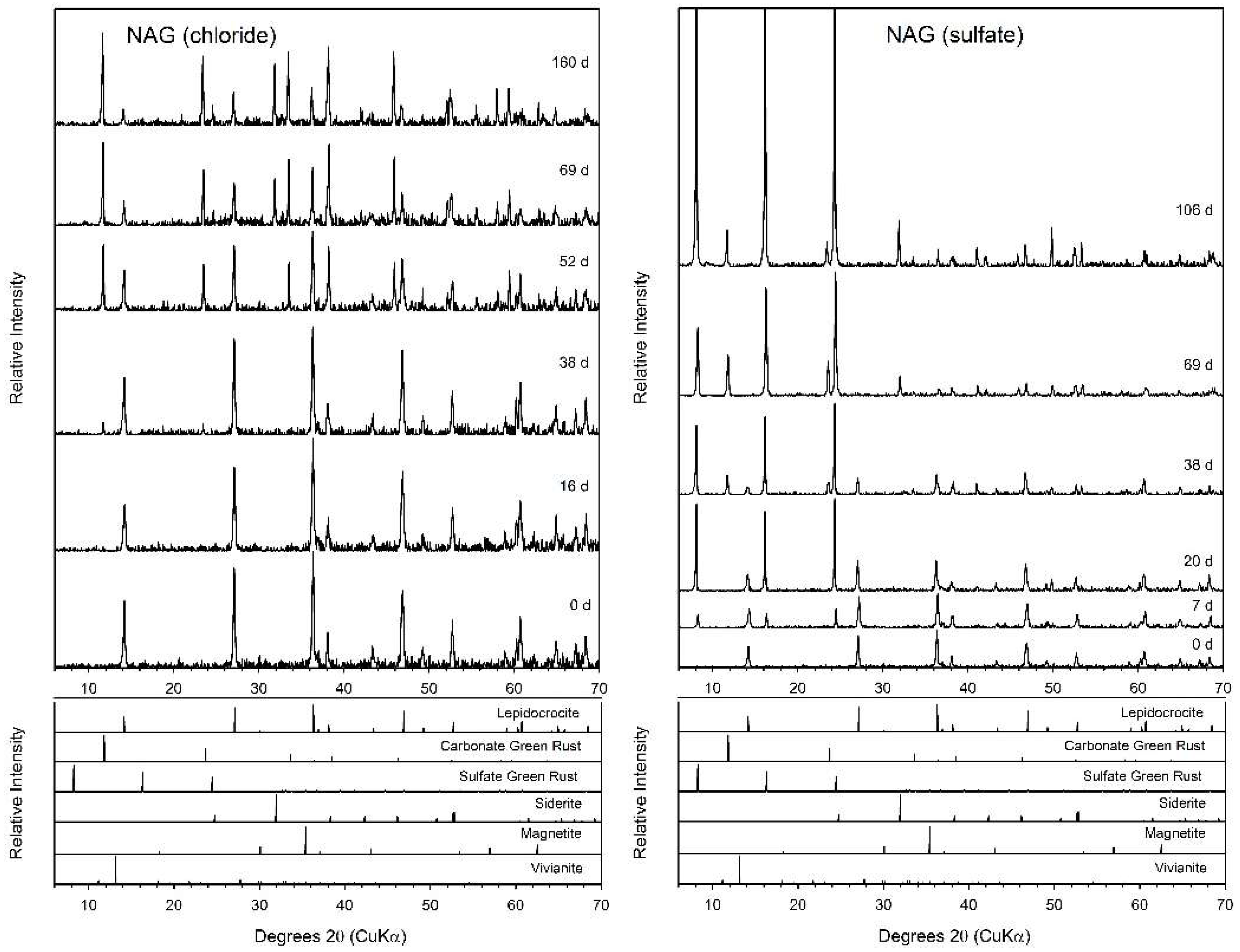
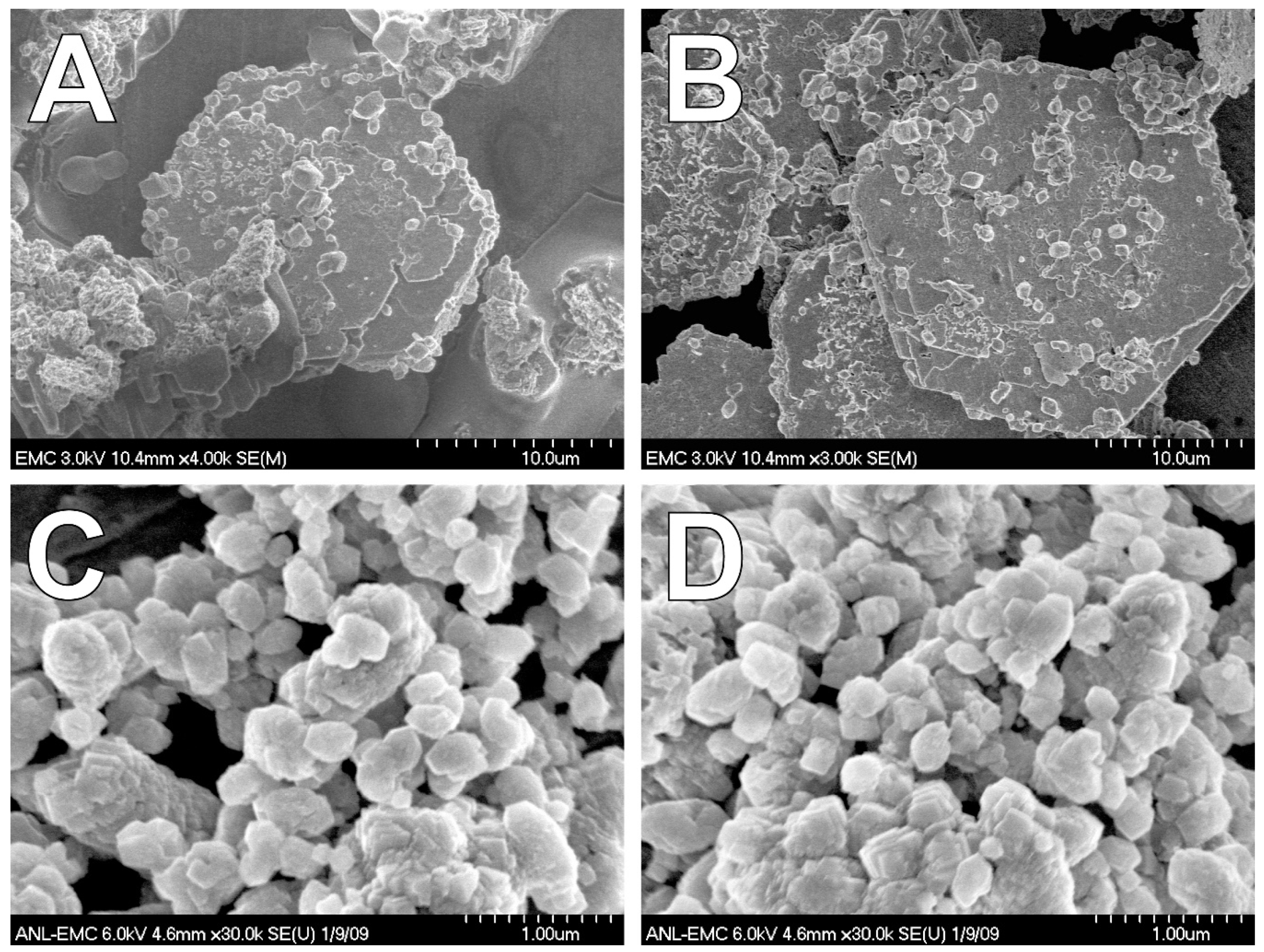
3.5. N-Acetylglucosamine (NAG)
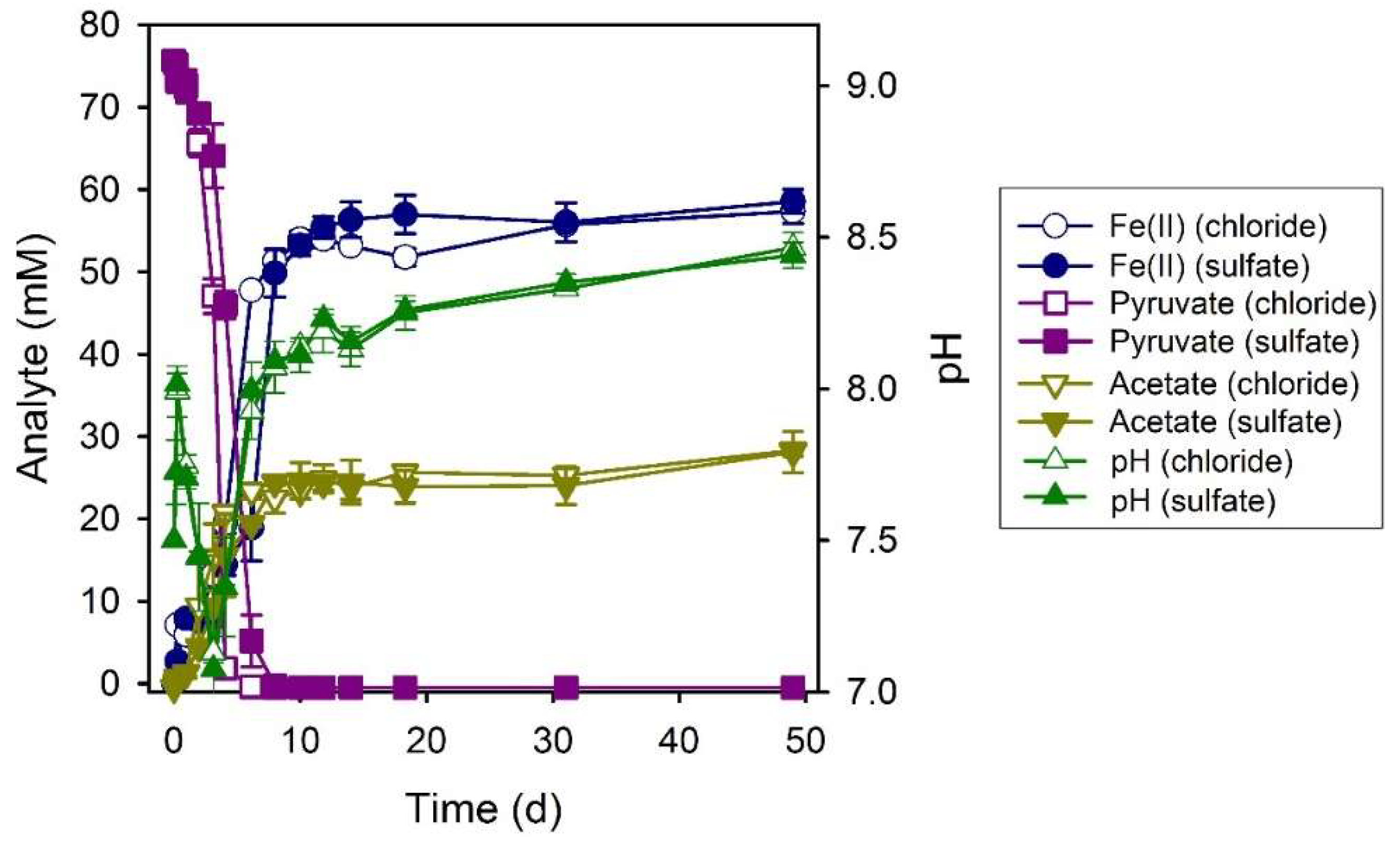
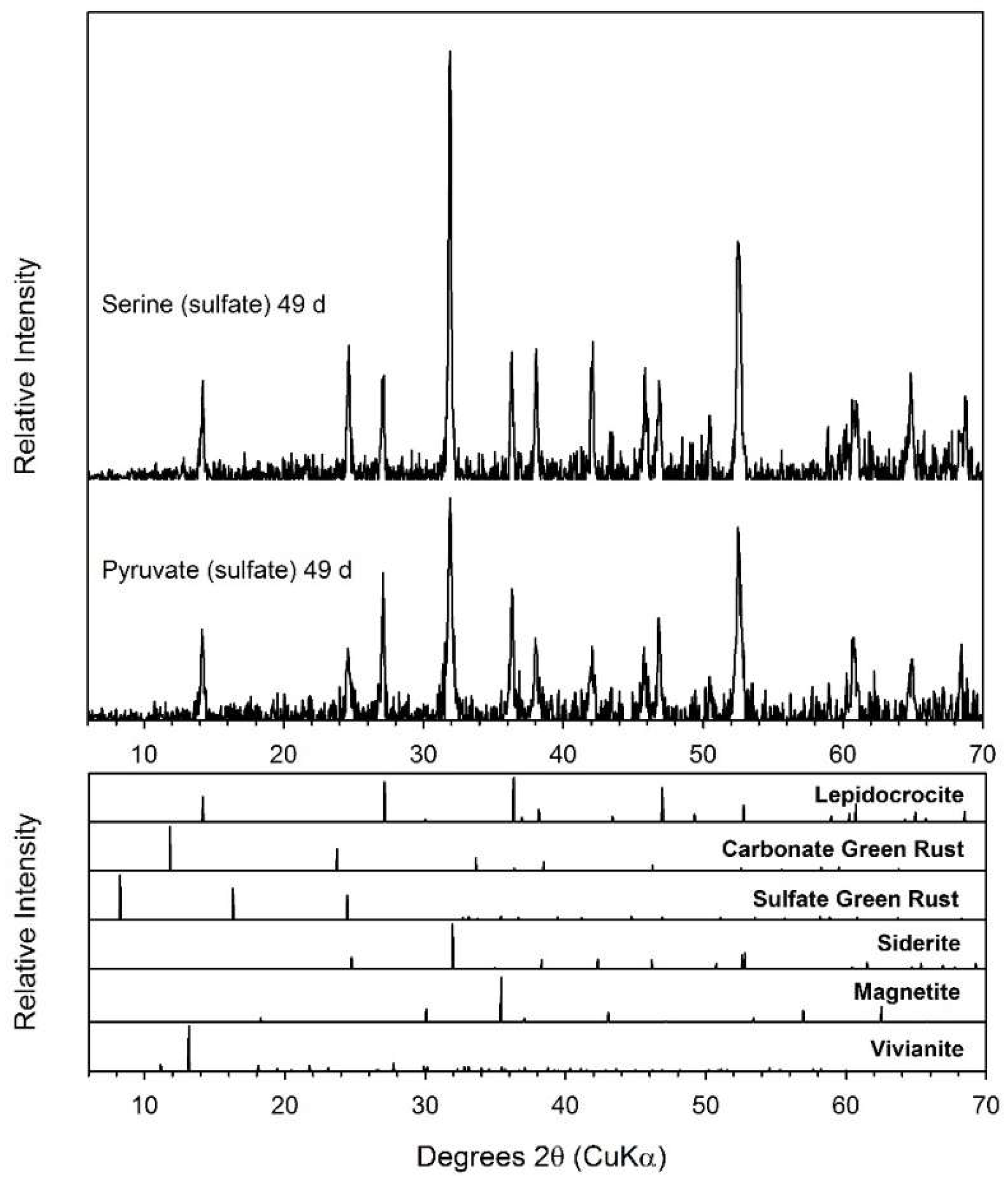
3.6. Pyruvate
3.7. Serine
4. Discussion
4.1. Electron Donor Utilization and Dissimilatory Iron(III) Reduction
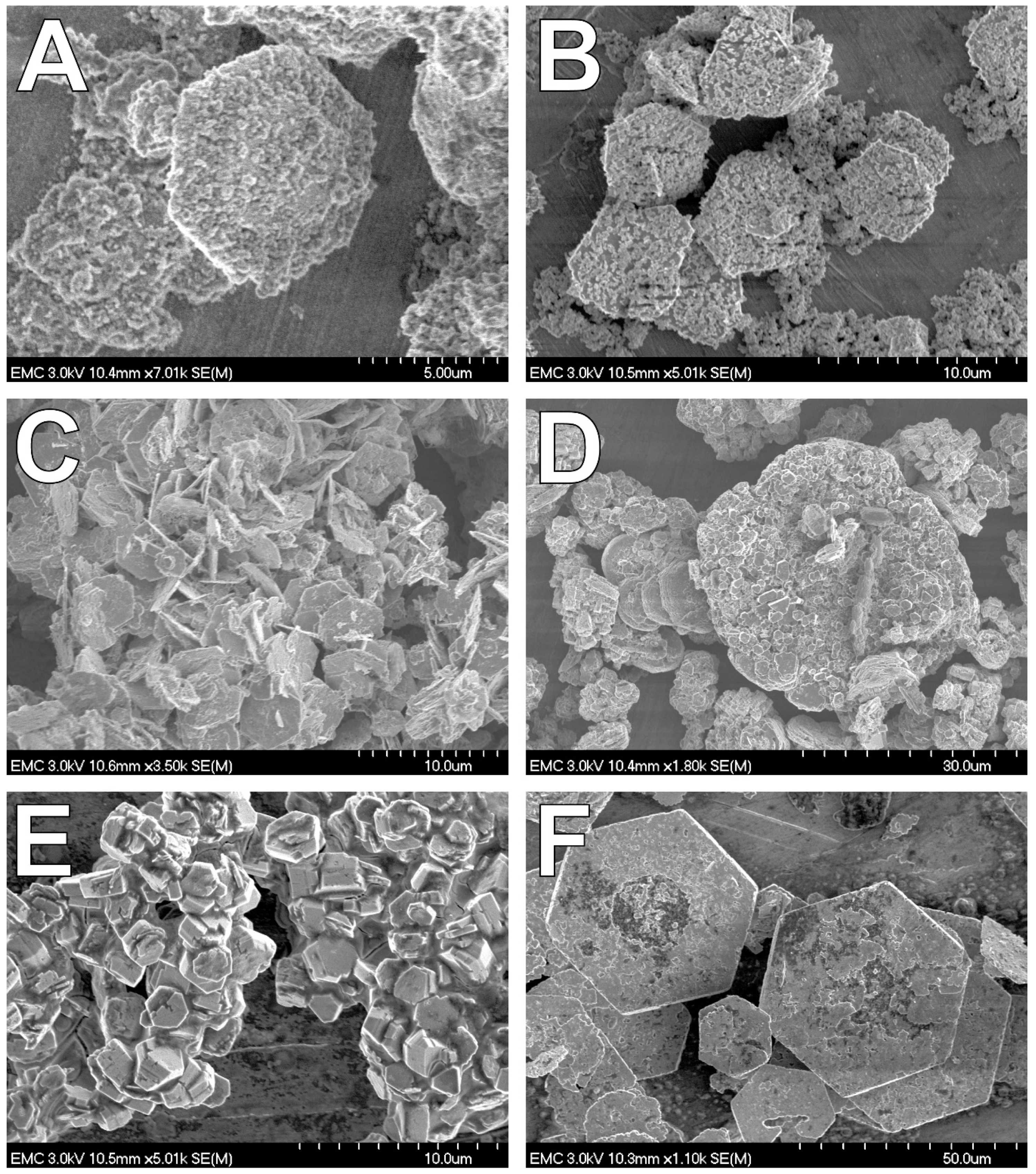
4.2. Fe(II) Secondary Mineral Formation
4.3. Environmental Relevance
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Canfield, D.E.; Thamdrup, B.; Hansen, J.W. The anaerobic degradation of organic matter in Danish coastal sediments: Iron reduction, manganese reduction, and sulfate reduction. Geochim. Cosmochim. Acta 1993, 57, 3867–3883. [Google Scholar] [CrossRef]
- Nealson, K.H.; Saffarini, D.A. Iron and manganese in anaerobic respiration: Environmental significance, physiology, and regulation. Annu. Rev. Microbiol. 1994, 48, 311–343. [Google Scholar] [CrossRef] [PubMed]
- Roden, E.E.; Wetzel, R.G. Organic carbon oxidation and methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol. Oceanogr. 1996, 41, 1733–1748. [Google Scholar] [CrossRef]
- Lovley, D.R. Fe(III) and Mn(IV) reduction. In Environmental Microbe-Metal Interactions; Lovley, D.R., Ed.; American Society for Microbiology Press: Washington, DC, USA, 2000; pp. 3–30. [Google Scholar]
- Thamdrup, B. Bacterial manganese and iron reduction in aquatic sediments. Adv. Microb. Ecol. 2000, 16, 41–84. [Google Scholar]
- Caccavo, F., Jr.; Coates, J.D.; Rossello-Mora, R.A.; Ludwig, W.; Schleifer, K.H.; Lovley, D.R.; McInerney, M.J. Geovibrio ferrireducens, a phylogenetically distinct dissimilatory Fe(III)-reducing bacterium. Arch. Microbiol. 1996, 165, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.D.; Bhupathiraju, V.K.; Achenbach, L.; McInerney, M.J.; Lovley, D.R. Geobacter hydrogenophilus, Geobacter chapelli and Geobacter grbiciae, three new, strictly anaerobic, dissimilatory Fe(III)-reducers. Int. J. Syst. Evol. Microbiol. 2001, 51, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.D.; Ellis, D.J.; Gaw, C.V.; Lovley, D.R. Geothrix fermentans gen. nov., sp. nov., a novel Fe(III)-reducing bacterium from a hydrocarbon-contaminated aquifer. Int. J. Syst. Bacteriol. 1999, 49, 1615–1622. [Google Scholar] [CrossRef]
- Dong, Y.; Sanford, R.A.; Boyanov, M.I.; Kemner, K.M.; Flynn, T.M.; O’Loughlin, E.J.; Chang, Y.J.; Locke, R.A., Jr.; Weber, J.R.; Egan, S.M.; et al. Orenia metallireducens sp. nov. strain Z6, a novel metal-reducing member of the phylum firmicutes from the deep subsurface. Appl. Environ. Microbiol. 2016, 82, 6440–6453. [Google Scholar] [CrossRef]
- Dong, Y.; Sanford, R.A.; Boyanov, M.I.; Kemner, K.M.; Flynn, T.M.; O’Loughlin, E.J.; Locke Ii, R.A.; Weber, J.R.; Egan, S.M.; Fouke, B.W. Tepidibacillus decaturensis sp. nov.: A microaerophilic, moderately thermophilic iron-reducing bacterium isolated from a depth of 1.7 km in the Illinois Basin, USA. Int. J. Syst. Evol. Microbiol. 2016. [Google Scholar] [CrossRef]
- Ettwig, K.F.; Zhu, B.; Speth, D.; Keltjens, J.T.; Jetten, M.S.; Kartal, B. Archaea catalyze iron-dependent anaerobic oxidation of methane. Proc. Natl. Acad. Sci. USA 2016. [Google Scholar] [CrossRef]
- Kashefi, K.; Lovley, D.R. Reduction of Fe(III), Mn(IV), and toxic metals at 100 °C by Pyrobacterium islandicum. Appl. Environ. Microbiol. 2000, 66, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Sanford, R.A.; Cole, J.R.; Tiedje, J.M. Characterization and description of Anaeromyxobacter dehalogens gen. nov., sp. nov., an Aryl-halorespiring facultative anaerobic myxobacterium. Appl. Environ. Microbiol. 2002, 68, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.; Liu, S.V.; Li, G.; Huang, H.; Phelps, T.J.; Zhou, J. Isolation and characterization of metal-reducing Thermoanaerobacter strains from deep subsurface environments of the Piceance Basin, Colorado. Appl. Environ. Microbiol. 2002, 68, 6013–6020. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Giovanoli, S.J.; White, D.C.; Champine, J.E.; Phillips, E.J.P.; Gorby, Y.A.; Goodwin, S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch. Microbiol. 1993, 159, 336–344. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, E.J.; Larese-Casanova, P.; Scherer, M.M.; Cook, R.E. Green rust formation from the bioreduction of g-FeOOH (lepidocrocite): Comparison of several Shewanella species. Geomicrobiol. J. 2007, 24, 211–230. [Google Scholar] [CrossRef]
- Slobodkin, A.; Reysenbach, A.L.; Strutz, N.; Dreier, M.; Wiegel, J. Thermoterrabacterium ferrireducens gen. nov., sp. nov., a thermophilic anaerobic dissimilatory Fe(III)-reducing bacterium from a continental hot spring. Int. J. Syst. Bacteriol. 1997, 47, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.C.; Patel, B.K.; Sheehy, A.J. Deferribacter thermophilus gen. nov., sp. nov., a novel thermophilic manganese- and iron-reducing bacterium isolated from a petroleum reservoir. Int. J. Syst. Bacteriol. 1997, 47, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.; Chon, C.-M.; Moon, J.-W. Metal reduction and biomineralization by an alkaliphilic metal-reducing bacterium, Alkaliphilus metalliredigens (QYMF). Geosci. J. 2007, 11, 415–423. [Google Scholar] [CrossRef]
- Cutting, R.S.; Coker, V.S.; Fellowes, J.W.; Lloyd, J.R.; Vaughan, D.J. Mineralogical and morphological constraints on the reduction of Fe(III) minerals by Geobacter sulfurreducens. Geochim. Cosmochim. Acta 2009, 73, 4004–4022. [Google Scholar] [CrossRef]
- Shelobolina, E.S.; Vanpraagh, C.G.; Lovley, D.R. Use of ferric and ferrous iron containing minerals for respiration by Desulfitobacterium frappieri. Geomicrobiol. J. 2003, 20, 143–156. [Google Scholar] [CrossRef]
- Kostka, J.E.; Haefele, E.; Viehweger, R.; Stucki, J.W. Respiration and dissolution of iron(III)-containing clay minerals by bacteria. Environ. Sci. Technol. 1999, 33, 3127–3133. [Google Scholar] [CrossRef]
- Kostka, J.E.; Nealson, K.H. Dissolution and reduction of magnetite by bacteria. Environ. Sci. Technol. 1995, 29, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, M.M.; Roden, E.E.; Fredrickson, J.K.; Zachara, J.M. Microbial and surface chemistry controls on reduction of synthetic Fe(III) oxide minerals by the dissimilatory iron-reducing bacterium Shewanella alga. Geomicrobiology 1998, 15, 269–291. [Google Scholar] [CrossRef]
- Zachara, J.M.; Fredrickson, J.K.; Li, S.-M.; Kennedy, D.W.; Smith, S.C.; Gassman, P.L. Bacterial reduction of crystalline Fe3+ oxides in single phase suspension and subsurface materials. Am. Mineral. 1998, 83, 1426–1443. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, I.; Roh, Y. Biomineralization of a poorly crystalline Fe(III) oxide, akaganeite, by an anaerobic Fe(III)-reducing bacterium (Shewanella alga) isolated from marine environment. Geosci. J. 2003, 7, 217–226. [Google Scholar] [CrossRef]
- Seabaugh, J.L.; Dong, H.; Kukkadapu, R.K.; Eberl, D.D.; Morton, J.P.; Kim, J. Microbial reduction of Fe(III) in the Fithian and Muloorina illites: Contrasting extents and rates of bioreduction. Clays Clay Miner. 2006, 54, 67–79. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Gorski, C.A.; Scherer, M.M. Effects of phosphate on secondary mineral formation during the bioreduction of akaganeite (b-FeOOH): Green rust versus framboidal magnetite. Curr. Inorg. Chem. 2015, 5, 214–224. [Google Scholar] [CrossRef]
- Thurman, E.M. Organic Geochemistry of Natural Waters; Martinus Nijhoff/Dr. W. Junk Publishers: Dordrecht, The Netherlands, 1985; p. 497. [Google Scholar]
- Megonigal, J.P.; Hines, M.E.; Visscher, P.T. Anaerobic metabolism: Linkages to trace gasses and aerobic processes. In Biogeochemistry; Schlesinger, W.H., Ed.; Elsevier-Pergamon: Oxford, UK, 2004; pp. 317–424. [Google Scholar]
- Lovley, D.R. Microbial Fe(III) reduction in subsurface environments. FEMS Microbiol. Rev. 1997, 20, 305–313. [Google Scholar] [CrossRef]
- Lovley, D.R.; Stolz, J.F.; Nord, G.L., Jr.; Phillips, E.J.P. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature 1987, 330, 252–254. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Zachara, J.M.; Kennedy, D.W.; Dong, H.; Onstott, T.C.; Hinman, N.W.; Li, S.-M. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim. Cosmochim. Acta 1998, 62, 3239–3257. [Google Scholar] [CrossRef]
- Ona-Nguema, G.; Abdelmoula, M.; Jorand, F.; Benali, O.; Géhin, A.; Block, J.-C.; Génin, J.-M.R. Iron(II,III) hydroxycarbonate green rust formation and stabilization from lepidocrocite bioreduction. Environ. Sci. Technol. 2002, 36, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, S.; Weidler, P.G.; Langley, S.; Beveridge, T.J. Controls on Fe reduction and mineral formation by a subsurface bacterium. Geochim. Cosmochim. Acta 2003, 67, 1277–1288. [Google Scholar] [CrossRef]
- Roh, Y.; Zhang, C.-L.; Vali, H.; Lauf, R.J.; Zhou, J.; Phelps, T.J. Biogeochemical and environmental factors in Fe biomineralization: Magnetite and siderite formation. Clays Clay Miner. 2003, 51, 83–95. [Google Scholar] [CrossRef]
- Kukkadapu, R.K.; Zachara, J.M.; Fredrickson, J.K.; Kennedy, D.W.; Dohnalkova, A.C.; Mccready, D.E. Ferrous hydroxy carbonate is a stable transformation product of biogenic magnetite. Am. Mineral. 2005, 90, 510–515. [Google Scholar] [CrossRef][Green Version]
- Behrends, T.; Van Cappellen, P. Transformation of hematite into magnetite during dissimilatory iron reduction-conditions and mechanisms. Geomicrobiol. J. 2007, 24, 403–416. [Google Scholar] [CrossRef]
- Boyanov, M.I.; O’Loughlin, E.J.; Kemner, K.M. Iron phase transformations resulting from the respiration of Shewanella putrefaciens on a mixed mineral phase. J. Phys. Conf. Ser. 2009, 190, 012193. [Google Scholar] [CrossRef]
- Shelobolina, E.; Konishi, H.; Xu, H.; Benzine, J.; Xiong, M.Y.; Wu, T.; Blothe, M.; Roden, E. Isolation of phyllosilicate-iron redox cycling microorganisms from an illite-smectite rich hydromorphic soil. Front. Microbiol. 2012, 3, 134. [Google Scholar] [CrossRef]
- Zegeye, A.; Mustin, C.; Jorand, F. Bacterial and iron oxide aggregates mediate secondary iron mineral formation: Green rust versus magnetite. Geobiology 2010, 8, 209–222. [Google Scholar] [CrossRef]
- Zachara, J.M.; Kukkadapu, R.K.; Frederickson, J.K.; Gorby, Y.A.; Smith, S.C. Biomineralization of poorly crystalline Fe(III) oxides by dissimilatory metal reducing bacteria (DMRB). Geomicrobiol. J. 2002, 19, 179–207. [Google Scholar] [CrossRef]
- Bae, S.; Lee, W. Biotransformation of lepidocrocite in the presence of quinones and flavins. Geochim. Cosmochim. Acta 2013, 10. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Gorski, C.; Scherer, M.M.; Boyanov, M.I.; Kemner, K.M. Effects of oxyanions, natural organic matter, and cell density on the bioreduction of lepidocrocite (g-FeOOH) and secondary mineral formation. Environ. Sci. Technol. 2010, 44, 4570–4576. [Google Scholar] [CrossRef] [PubMed]
- Zegeye, A.; Ruby, C.; Jorand, F. Kinetic and thermodynamic analysis during dissimilatory g-FeOOH reduction: Formation of green rust 1 and magnetite. Geomicrobiol. J. 2007, 24, 51–64. [Google Scholar] [CrossRef]
- Salas, E.C.; Berelson, W.M.; Hammond, D.E.; Kampf, A.R.; Nealson, K.H. The impact of bacterial strain on the products of dissimilatory iron reduction. Geochim. Cosmochim. Acta 2010, 74, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Borch, T.; Masue, Y.; Kukkadapu, R.K.; Fendorf, S. Phosphate imposed limitations on biological reduction and alteration of ferrihydrite. Environ. Sci. Technol. 2007, 41, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Kukkadapu, R.K.; Zachara, J.M.; Fredrickson, J.K.; Kennedy, D.W. Biotransformation of two-line silica-ferrihydrite by a dissimilatory Fe(III)-reducing bacterium: Formation of carbonate green rust in the presence of phosphate. Geochim. Cosmochim. Acta 2004, 68, 2799–2814. [Google Scholar] [CrossRef]
- Sergent, A.-S.; Jorand, F.; Hanna, K. Effects of Si-bearing minerals on the nature of secondary iron mineral products from lepidocrocite bioreduction. Chem. Geol. 2011, 289, 86–97. [Google Scholar] [CrossRef]
- Jorand, F.; Zegeye, A.; Ghanbaja, J.; Abdelmoula, M. The formation of green rust induced by tropical river biofilm components. Sci. Total Envrion. 2011, 409, 2586–2596. [Google Scholar] [CrossRef][Green Version]
- Fredrickson, J.K.; Kota, S.; Kukkadapu, R.K.; Liu, C.; Zachara, J.M. Influence of electron donor/acceptor concentrations on hydrous ferric oxide (HFO) bioreduction. Biodegradation 2003, 14, 91–103. [Google Scholar] [CrossRef]
- Lee, J.-H.; Roh, Y.; Kim, K.-W.; Hur, H.-G. Organic acid-dependent iron mineral formation by a newly isolated iron-reducing bacterium, Shewanella sp. HN-41. Geomicrobiol. J. 2007, 24, 31–41. [Google Scholar] [CrossRef]
- Salas, E.C.; Berelson, W.M.; Hammond, D.E.; Kampf, A.R.; Nealson, K.H. The influence of carbon source on the products of dissimilatory iron reduction. Geomicrobiol. J. 2009, 26, 451–462. [Google Scholar] [CrossRef]
- Lovley, D. Dissimilatory Fe(III)- and Mn(IV)-reducing prokaryotes. In The Prokaryotes—Prokaryotic Physiology and Biochemistry; Dworkin, M., Ed.; Springer: Berlin/Heidelburg, Germany, 2013; pp. 287–308. [Google Scholar]
- O’Loughlin, E.J. Effects of electron transfer mediators on the biodegradation of lepidocrocite (g-FeOOH) by Shewanella putrefaciens CN32. Environ. Sci. Technol. 2008, 42, 6876–6882. [Google Scholar] [CrossRef] [PubMed]
- Stookey, L.L. Ferrozine-A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Sørensen, J. Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Appl. Environ. Microbiol. 1982, 43, 319–324. [Google Scholar] [PubMed]
- Bethke, C.M. Geochemical and Biogeochemical Reaction Modelling, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008; p. 543. [Google Scholar]
- Delany, J.M.; Lundeen, S.R. The LLNL Thermochemical Database; Lawrence Livermore National Laboratory: Livermore, CA, USA, 1989; Report UCRL-21658; p. 150. [Google Scholar]
- Allison, J.D.; Brown, D.S.; Novo-Gradac, K.J. MINTEQA2/PRODEFA2, A Geochemical Assessment Model for Environmental Systems: Version 3.0 User’s Manual; USEPA: Washington, DC, USA, 1991.
- Drissi, S.H.; Refait, P.; Abdelmoula, M.; Génin, J.M.R. The preparation and thermodynamic properties of Fe(II)-Fe(III) hydroxide-carbonate (green rust 1); Pourbaix diagram of iron in carbonate-containing aqueous media. Corros. Sci. 1995, 37, 2025–2041. [Google Scholar] [CrossRef]
- Azoulay, I.; Rémazeilles, C.; Refait, P. Determination of standard Gibbs free energy of formation of chukanovite and Pourbaix diagrams of iron in carbonated media. Corros. Sci. 2012, 58, 229–236. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Boyanov, M.I.; Flynn, T.M.; Gorski, C.; Hofmann, S.M.; McCormick, M.L.; Scherer, M.M.; Kemner, K.M. Effects of bound phosphate on the bioreduction of lepidocrocite (g-FeOOH) and maghemite (g-Fe2O3) and formation of secondary minerals. Environ. Sci. Technol. 2013, 47, 9157–9166. [Google Scholar] [CrossRef]
- Hau, H.H.; Gralnick, J.A. Ecology and biotechnology of the genus Shewanella. Annu. Rev. Microbiol. 2007, 61, 237–258. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, J.K.; Romine, M.F.; Beliaev, A.S.; Auchtung, J.M.; Driscoll, M.E.; Gardner, T.S.; Nealson, K.H.; Osterman, A.L.; Pinchuk, G.; Reed, J.L.; et al. Towards environmental systems biology of Shewanella. Nat. Rev. Microbiol. 2008, 6, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. The genus Shewanella: From the briny depths below to human pathogen. Crit. Rev. Microbiol. 2014, 40, 293–312. [Google Scholar] [CrossRef]
- Serres, M.H.; Riley, M. Genomic analysis of carbon source metabolism of Shewanella oneidensis MR-1: Predictions versus experiments. J. Bacteriol. 2006, 188, 4601–4609. [Google Scholar] [CrossRef]
- Pinchuk, G.; Hill, E.A.; Geydebrekht, O.V.; De Ingeniis, J.; Zhang, X.; Osterman, A.; Scott, J.H.; Reed, S.B.; Romine, M.F.; Konopka, A.E.; et al. Constraint-based model of Shewanella oneidensis MR-1 metabolism: A tool for data analysis and hypothesis generation. PLoS Comput. Biol. 2010, 6, e1000822. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, D.A.; Yang, C.; Li, X.; Rodionova, I.A.; Wang, Y.; Obraztsova, A.Y.; Zagnitko, O.P.; Overbeek, R.; Romine, M.F.; Reed, S.; et al. Genomic encyclopedia of sugar utilization pathways in the Shewanella genus. BMC Genom. 2010, 11, 494. [Google Scholar] [CrossRef]
- Nealson, K.H.; Scott, J. Ecophysiology of the genus Shewanella. In The Prokaryotes: An Evolving Electronic Resource for the Microbiological Community; Dworkin, M., Ed.; Springer: New York, NY, USA, 2006; Volume 6, pp. 848–860. [Google Scholar]
- Caccavo, F., Jr.; Blakemore, R.P.; Lovley, D.R. A hydrogen-oxidizing, Fe(III)-reducing microorganism from the Great Bay Estuary, New Hampshire. Appl. Environ. Microbiol. 1992, 58, 3211–3216. [Google Scholar] [PubMed]
- Lovley, D.R.; Phillips, E.J.P.; Lonergan, D.J. Hydrogen and formate oxidation coupled to dissimilatory reduction of iron or manganese by Alteromonas putrefaciens. Appl. Environ. Microbiol. 1989, 55, 700–706. [Google Scholar] [PubMed]
- Liu, C.; Gorby, Y.A.; Zachara, J.M.; Fredrickson, J.K.; Brown, C.F. Reduction kinetics of Fe(III), Co(III), U(VI), Cr(VI), and Tc(VII) in cultures of dissimilatory metal-reducing bacteria. Biotechnol. Bioeng. 2002, 80, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, R.D.; Sabree, Z.L.; Palumbo, A.V.; Moyer, C.L.; Devol, A.H.; Roh, Y.; Zhou, J. Metal reduction at cold temperatures by Shewanella isolates from various marine environments. Aquat. Microb. Ecol. 2005, 38, 81–91. [Google Scholar] [CrossRef]
- Roh, Y.; Gao, H.; Vali, H.; Kennedy, D.W.; Yang, Z.K.; Gao, W.; Dohnalkova, A.C.; Stapleton, R.D.; Moon, J.-W.; Phelps, T.J.; et al. Metal reduction and iron biomineralization by a psychrotolerant Fe(III)-reducing bacterium, Shewanella sp. strain PV-4. Appl. Environ. Microbiol. 2006, 72, 3236–3244. [Google Scholar] [CrossRef]
- Zegeye, A.; Ona-Nguema, G.; Carteret, C.; Huguet, L.; Abdelmoula, M.; Jorand, F. Formation of hydroxysulfate green rust 2 as a single iron(II-III) mineral in microbial culture. Geomicrobiol. J. 2005, 22, 389–399. [Google Scholar] [CrossRef]
- Jorand, F.P.A.; Sergent, A.S.; Remy, P.P.; Bihannic, I.; Ghanbaja, J.; Lartiges, B.; Hanna, K.; Zegeye, A. Contribution of anionic vs. neutral polymers to the formation of green rust 1 from γ-FeOOH bioreduction. Geomicrobiol. J. 2013, 30, 600–615. [Google Scholar] [CrossRef]
- Pinchuk, G.E.; Geydebrekht, O.V.; Hill, E.A.; Reed, J.L.; Konopka, A.E.; Beliaev, A.S.; Fredrickson, J.K. Pyruvate and lactate metabolism by Shewanella oneidensis MR-1 under fermentation, oxygen limitation, and fumarate respiration conditions. Appl. Environ. Microbiol. 2011, 77, 8234–8240. [Google Scholar] [CrossRef]
- Toffin, L.; Bidault, A.; Pignet, P.; Tindall, B.J.; Slobodkin, A.; Kato, C.; Prieur, D. Shewanella profunda sp. nov., isolated from deep marine sediment of the Nanki Trough. Int. J. Syst. Evol. Microbiol. 2004, 54, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Ringø, E.; Stenberg, E.; Strøm, A.R. Amino acid and lactate catabolism in trimethylamine oxide respiration of Alteromonas putrefaciens NCMB 1735. Appl. Environ. Microbiol. 1984, 46, 1084–1089. [Google Scholar]
- Jørgensen, N.O.G.; Stepanaukas, R.; Pedersen, A.-G.U.; Hansen, M.; Nybroe, O. Occurrence and degradation of peptidoglycan in aquatic environments. FEMS Microbiol. Ecol. 2003, 46, 269–280. [Google Scholar] [CrossRef]
- Beier, S.; Bertilsson, S. Bacterial chitin degradation-mechanisms and ecophysiological strategies. Front. Microbiol. 2013, 4, 149. [Google Scholar] [CrossRef] [PubMed]
- Nedoma, J.; Vrba, J.; Hejzlar, J.; Šimek, K.; Straškrabová, V. N-acetylglucosamine dynamics in freshwater environments: Concentration of amino sugars, extracellular enzyme activities, and microbial uptake. Limnol. Oceanogr. 1994, 39, 1088–1100. [Google Scholar] [CrossRef]
- Riemann, L.; Azam, F. Widespread N-acetyl-D-glucosamine uptake among pelagic marine bacteria and its ecological implications. Appl. Environ. Microbiol. 2002, 68, 5554–5562. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.; Jones, D.L. Microbial and plant uptake of free amino sugars in grassland soils. Soil Biol. Biochem. 2012, 49, 139–149. [Google Scholar] [CrossRef]
- Beier, S.; Bertilsson, S. Uncoupling of chitinase activity and uptake of hydrolysis products in freshwater bacterioplankton. Limnol. Oceanogr. 2011, 56, 1179–1188. [Google Scholar] [CrossRef]
- Tada, Y.; Grossart, H.P. Community shifts of actively growing lake bacteria after N-acetyl-glucosamine addition: Improving the BrdU-FACS method. ISME J. 2014, 8, 441–454. [Google Scholar] [CrossRef]
- Satomi, M.; Oikawa, H.; Yano, Y. Shewanella marinintestina sp. nov., Shewanella schlegeliana sp. nov. and Shewanella sairae sp. nov., novel eicosapentaenoic-acid-producing marine bacteria isolated from sea-animal intestines. Int. J. Syst. Evol. Microbiol. 2003, 53, 491–499. [Google Scholar] [CrossRef]
- Yang, C.; Rodionov, D.A.; Li, X.; Laikova, O.N.; Gelfand, M.S.; Zagnitko, O.P.; Romine, M.F.; Obraztsova, A.Y.; Nealson, K.H.; Osterman, A.L. Comparative genomics and experimental characterization of N-acetylglucosamine utilization pathway of Shewanella oneidensis. J. Biol. Chem. 2006, 281, 29872–29885. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.P.; McCammon, S.; Nichols, D.S.; Skerratt, J.H.; Rea, S.M.; Nichols, P.D.; McMeekin, T.A. Shewanella gelidimarina sp. nov. and Shewanella frigidimarina sp. nov., novel antarctic species with the ability to produce eicosapentaenoic acid (20:5 ω3) and grow anaerobically by dissimilatory Fe(III) reduction. Int. J. Syst. Bacteriol. 1997, 47, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Bozal, N.; Montes, M.J.; Tudela, E.; Jimenez, F.; Guinea, J. Shewanella frigidimarina and Shewanella livingstonensis sp. nov. isolated from Antarctic coastal areas. Int. J. Syst. Evol. Microbiol. 2002, 52, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Zavarzina, D.G.; Sokolova, T.G.; Tourova, T.P.; Chernyh, N.A.; Kostrikina, N.A.; Bonch-Osmolovskaya, E.A. Thermincola ferriacetica sp. nov., a new anaerobic, thermophilic, facultatively chemolithoautotrophic bacterium capable of dissimilatory Fe(III) reduction. Extremeophiles 2007, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Akob, D.M.; Mills, H.J.; Gihring, T.M.; Kerkhof, L.; Stucki, J.W.; Anastacio, A.S.; Chin, K.-J.; Kusel, K.; Palumbo, A.V.; Watson, D.B.; et al. Functional diversity and electron donor dependence of microbial populations capable of U(VI) reduction in radionuclide-contaminated subsurface sediments. Appl. Environ. Microbiol. 2008, 74, 3159–3170. [Google Scholar] [CrossRef]
- Lentini, C.J.; Wankel, S.D.; Hansel, C.M. Enriched iron(III)-reducing bacterial communities are shaped by carbon substrate and iron mineralogy. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Petrie, L.; North, N.N.; Dollhopf, S.L.; Balkwill, D.L.; Kostka, J.E. Enumeration and characterization of iron(III)-reducing microbial communities and acidic subsurface sediments contaminated with uranium(VI). Appl. Environ. Microbiol. 2003, 69, 7467–7479. [Google Scholar] [CrossRef]
- Kwon, M.J.; O’Loughlin, E.J.; Boyanov, M.I.; Brulc, J.M.; Johnston, E.R.; Kemner, K.M.; Antonopoulos, D.A. Impact of organic carbon electron donors on microbial community development under iron- and sulfate-reducing conditions. PLoS ONE 2016, 11, 1–22. [Google Scholar] [CrossRef]
- Jung, J.; Bae, S.; Lee, W. Indirect contact of bio-transformation of lepidocrocite: Role of electron transfer mediator. Sustain. Environ. Res. 2012, 23, 193–198. [Google Scholar]
- Ona-Nguema, G.; Jorand, F.; Benali, O.; Abdelmoula, M.; Génin, J.-M.R.; Block, J.-C. Key role of the kinetics of g-FeOOH bioreduction on the formation of Fe(II-III) minerals. In Hyperfine Interactions (C), Proceedings of the International Conference on the Applications of the Mössbauer Effect (ICAME 2001), Oxford, UK, 2–7 September 2001; Thomas, M.F., Williams, J.M., Gibb, T.C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 415–418. [Google Scholar]
- Ona-Nguema, G.; Morin, G.; Juillot, F.; Calas, G.; Brown, G.E., Jr. EXAFS analysis of arsenite adsorption onto two-line ferrihydrite, hematite, goethite, and lepidocrocite. Environ. Sci. Technol. 2005, 39, 9147–9155. [Google Scholar] [CrossRef]
- Ona-Nguema, G.; Morin, G.; Wang, Y.; Menguy, N.; Juillot, F.; Olovi, L.; Aquilanti, G.; Abdelmoula, M.; Ruby, C.; Bargar, J.R.; et al. Arsenite sequestration at the surface of nano-Fe(OH)2, ferrous-carbonate hydroxide, and green-rust after bioreduction of arsenic-sorbed lepidocrocite by Shewanella putrefaciens. Geochim. Cosmochim. Acta 2009, 73, 1359–1381. [Google Scholar] [CrossRef]
- Christiansen, B.C.; Balic-Zunic, T.; Dideriksen, K.; Stipp, S.L.S. Identification of green rust in groundwater. Environ. Sci. Technol. 2009, 43, 3436–3441. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; Freyer, G.; Fabisch, M.; Caraballo, M.A.; Küsel, K.; Hochella, M.F. Observations and assessment of iron oxide and green rust nanoparticles in metal-polluted mine drainage within a steep redox gradient. Environ. Chem. 2014, 11, 377. [Google Scholar] [CrossRef]
- Zegeye, A.; Bonneville, S.; Benning, L.G.; Sturm, A.; Fowle, D.A.; Jones, C.; Canfield, D.E.; Ruby, C.; MacLean, L.C.; Nomosatryo, S.; et al. Green rust formation controls nutrient availability in a ferruginous water column. Geology 2012, 40, 599–602. [Google Scholar] [CrossRef]
- Feder, F.; Trolard, F.; Klingelhöfer, G.; Bourrié, G. In situ Mössbauer spectroscopy: Evidence for green rust (fougerite) in a gleysol and its mineralogical transformations with time and depth. Geochim. Cosmochim. Acta 2005, 69, 4463–4483. [Google Scholar] [CrossRef]
- Génin, J.-M.R.; Bourrié, G.; Trolard, F.; Abdelmoula, M.; Jaffrezic, A.; Refait, P.; Maitre, V.; Humbert, B.; Herbillon, A. Thermodynamic equilibria in aqueous suspensions of synthetic and natural Fe(II)-Fe(III) green rusts: Occurrences of the mineral in hydromorphic soils. Environ. Sci. Technol. 1998, 32, 1058–1068. [Google Scholar] [CrossRef]
- Refait, P.; Abdelmoula, M.; Trolard, F.; Génin, J.-M.R.; Ehrhardt, J.J.; Bourrié, G. Mössbauer and XAS study of a green rust mineral: The partial substitution of Fe2+ by Mg2+. Am. Mineral. 2001, 86, 731–739. [Google Scholar] [CrossRef]
- Trolard, F.; Génin, J.-M.R.; Abdelmoula, M.; Bourrié, G.; Humbert, B.; Herbillon, A. Identification of a green rust mineral in a reductomorphic soil by Mössbauer and Raman spectroscopies. Geochim. Cosmochim. Acta 1997, 61, 1107–1111. [Google Scholar] [CrossRef]
- Weatherington-Rice, J.; Bigham, J.M. Buried pre-Illinoian-age lacustrine deposits with “green rust” colors in Clermont County, Ohio. Ohio J. Sci. 2006, 106, 35–44. [Google Scholar]
- Bearcock, J.M.; Perkins, W.T.; Dinelli, E.; Wade, S.C. Fe(II)/Fe(III) “green rust” developed within ocherous coal mine drainage sediment in South Wales, UK. Mineral. Mag. 2006, 70, 731–741. [Google Scholar] [CrossRef]
- Bender Koch, C.; Mørup, S. Identification of green rust in an ochre sludge. Clay Miner. 1991, 26, 577–582. [Google Scholar] [CrossRef]
- Root, R.A.; Dixit, S.; Campbell, K.M.; Jew, A.D.; Hering, J.G.; O’Day, P.A. Arsenic sequestration by sorption processes in high-iron sediments. Geochim. Cosmochim. Acta 2007, 71, 5782–5803. [Google Scholar] [CrossRef]
- Trolard, F.; Bourrié, G.; Abdelmoula, M.; Refait, P.; Feder, F. Fougerite, a new mineral of the pyroaurite-iowaite group: Description and crystal structure. Clays Clay Miner. 2007, 55, 323–334. [Google Scholar] [CrossRef]
- Mills, S.J.; Christy, A.G.; Génin, J.M.R.; Kameda, T.; Colombo, F. Nomenclature of the hydrotalcite supergroup: Natural layered double hydroxides. Mineral. Mag. 2012, 76, 1289–1336. [Google Scholar] [CrossRef]
- Génin, J.M.R.; Mills, S.J.; Christy, A.G.; Guérin, O.; Herbillon, A.J.; Kuzmann, E.; Ona-Nguema, G.; Ruby, C.; Upadhyay, C. Mössbauerite, Fe63+O4(OH)8[CO3]·3H2O, the fully oxidized ‘green rust’ mineral from Mont Saint-Michel Bay, France. Mineral. Mag. 2014, 78, 447–465. [Google Scholar] [CrossRef]
- Parmar, N.; Gorby, Y.A.; Beveridge, T.J.; Ferris, F.G. Formation of green rust and immobilization of nickel in response to bacterial reduction of hydrous ferric oxide. Geomicrobiol. J. 2001, 18, 375–385. [Google Scholar] [CrossRef]
- Jorand, F.; Zegeye, A.; Landry, F.; Ruby, C. Reduction of ferric green rust by Shewanella putrefaciens. Lett. Appl. Microbiol. 2007, 45, 515–521. [Google Scholar] [CrossRef]
- Hansel, C.M.; Benner, S.G.; Neiss, J.; Dohnalkova, A.; Kukkadapu, R.K.; Fendorf, S. Secondary mineralization pathways induced by dissimilatory iron reduction of ferrihydrite under advective flow. Geochim. Cosmochim. Acta 2003, 67, 2977–2992. [Google Scholar] [CrossRef]
- Borch, T.; Fendorf, S. Phosphate interactions with iron (hydr)oxides: Mineralization pathways and phosphorous retention upon bioreduction. In Adsorption of Metals by Geomedia II Variables, Mechanisms, and Model Applications; Barnett, M.O., Kent, D., Eds.; Elsevier: New York, NY, USA, 2008; Volume 7, pp. 321–348. [Google Scholar]
- Etique, M.; Jorand, F.P.; Ruby, C. Magnetite as a precursor for green rust through the hydrogenotrophic activity of the iron-reducing bacteria Shewanella putrefaciens. Geobiology 2016, 14, 237–254. [Google Scholar] [CrossRef]
- Refait, P.; Drissi, S.H.; Pytkiewicz, J.; Génin, J.-M.R. The anionic species competition in iron aqueous corrosion: Role of various green rust compounds. Corros. Sci. 1997, 39, 1699–1710. [Google Scholar] [CrossRef]
- Dong, H.; Fredrickson, J.K.; Kennedy, D.W.; Zachara, J.M.; Kukkadapu, R.K.; Onstott, T.C. Mineral transformation associated with the microbial reduction of magnetite. Chem. Geol. 2000, 169, 299–318. [Google Scholar] [CrossRef]
- Liu, C.; Kota, S.; Zachara, J.M.; Fredrickson, J.K.; Brinkman, C.K. Kinetic analysis of the bacterial reduction of goethite. Environ. Sci. Technol. 2001, 35, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Roden, E.E.; Phillips, E.J.P.; Woodward, J.C. Enzymatic iron and uranium reduction by sulfate-reducing bacteria. Mar. Geol. 1993, 113, 41–53. [Google Scholar] [CrossRef]
- Hering, J.G.; Stumm, W. Oxidative and reductive dissolution of minerals. In Mineral-Water Interface Geochemistry; Hochella, M.F.J., White, A.F., Eds.; American Mineralogical Society: Washington, DC, USA, 1990; Volume 23, pp. 427–465. [Google Scholar]
- Heron, G.; Christensen, T.H. Impact of sediment-bound iron on redox buffering in a landfill leachate polluted aquifer (Vejen, Denmark). Environ. Sci. Technol. 1995, 29, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Rügge, K.; Hofstetter, T.B.; Haderlein, S.B.; Bjerg, P.L.; Knudsen, S.; Zraunig, C.; Mosbæk, H.; Christensen, T.H. Characterization of predominant reductants in an anaerobic leachate-contaminated aquifer by nitroaromatic probe compounds. Environ. Sci. Technol. 1998, 32, 23–31. [Google Scholar] [CrossRef]
- Elsner, M.; Schwarzenbach, R.P.; Haderlein, S.B. Reactivity of Fe(II)-bearing minerals toward reductive transformation of organic contaminants. Environ. Sci. Technol. 2004, 38, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Batchelor, B. Reductive capacity of natural reductants. Environ. Sci. Technol. 2003, 37, 535–541. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Charlet, L. Selenite reduction by mackinawite, magnetite and siderite: XAS characterization of nanosized redox products. Environ. Sci. Technol. 2008, 42, 1984–1989. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Kirsch, R.; Banerjee, D.; Fernandez-Martinez, A.; Zaenker, H.; Funke, H.; Charlet, L. X-ray absorption and photoelectron spectroscopy investigation of selenite reduction by FeII-bearing minerals. J. Contam. Hydrol. 2008, 102, 228–245. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Kelly, S.D.; Kemner, K.M. XAFS investigation of the interactions of UVI with secondary mineralization products from the bioreduction of FeIII oxides. Environ. Sci. Technol. 2010, 44, 1656–1661. [Google Scholar] [CrossRef]
- Bond, D.L.; Fendorf, S. Kinetics and structural constraints of chromate reduction by green rusts. Environ. Sci. Technol. 2003, 37, 2750–2757. [Google Scholar] [CrossRef]
- Christiansen, B.C.; Geckeis, H.; Marquardt, C.M.; Bauer, A.; Römer, J.; Wiss, T.; Schild, D.; Stipp, S.L.S. Neptunyl (NpO2+) interaction with green rust, GRNa,SO4. Geochim. Cosmochim. Acta 2011, 75, 1216–1226. [Google Scholar] [CrossRef]
- Erbs, M.; Hansen, H.C.B.; Olsen, C.E. Reductive dechlorination of carbon tetrachloride using iron(II) iron(III) hydroxide sulfate (green rust). Environ. Sci. Technol. 1999, 33, 307–311. [Google Scholar] [CrossRef]
- Hansen, H.C.B.; Bender Koch, C.; Nancke-Krogh, H.; Borggaard, O.K.; Sorensen, J. Abiotic nitrate reduction to ammonium: Key role of green rust. Environ. Sci. Technol. 1996, 30, 2053–2056. [Google Scholar] [CrossRef]
- Hansen, H.C.B.; Guldberg, S.; Erbs, M.; Bender Koch, C. Kinetics of nitrate reduction by green rusts—Effects of interlayer anion and Fe(II):Fe(III) ratio. Appl. Clay Sci. 2001, 18, 81–91. [Google Scholar] [CrossRef]
- Heasman, D.M.; Sherman, D.M.; Ragnarsdottir, K.V. The reduction of aqueous Au3+ by sulfide minerals and green rust phases. Am. Mineral. 2003, 88, 725–738. [Google Scholar] [CrossRef]
- Kone, T.; Hanna, K.; Abdelmoula, M.; Ruby, C.; Carteret, C. Reductive transformation and mineralization of an azo dye by hydroxysulphate green rust preceding oxidation using H2O2 at neutral pH. Chemoshpere 2009, 75, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Larese-Casanova, P.; Scherer, M.M. Abiotic transformation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by green rusts. Environ. Sci. Technol. 2008, 42, 3975–3981. [Google Scholar] [CrossRef]
- Lee, W.; Batchelor, B. Abiotic reductive dechlorination of chlorinated ethylenes by iron-bearing soil minerals. 2. Green rust. Environ. Sci. Technol. 2002, 36, 5348–5354. [Google Scholar] [CrossRef]
- Legrand, L.; El Figuigui, A.; Mercier, F.; Chausse, A. Reduction of aqueous chromate by Fe(II)/Fe(III) carbonate green rust: Kinetic and mechanistic studies. Environ. Sci. Technol. 2004, 38, 4587–4595. [Google Scholar] [CrossRef]
- Loyaux-Lawniczak, S.; Refait, P.; Lecomte, P.; Ehrhardt, J.-J.; Génin, J.-M.R. The reduction of chromate ions by Fe(II) layered hydroxides. Hydrol. Earth Syst. Sci. 1999, 3, 593–599. [Google Scholar] [CrossRef]
- Mitsunobu, S.; Takahashi, Y.; Sakai, Y. Abiotic reduction of antimony(V) by green rust (Fe4(II)Fe2(III)(OH)12SO4∙3H2O). Chemosphere 2008, 70, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Myneni, S.C.B.; Tokunaga, T.K.; Brown, G.E., Jr. Abiotic selenium redox transformations in the presence of Fe(II,III) oxides. Science 1997, 278, 1106–1109. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Burris, D.R. Reduction of halogenated ethanes by green rust. Environ. Toxicol. Chem. 2004, 23, 41–48. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Kelly, S.D.; Csencsits, R.; Cook, R.E.; Kemner, K.M. Reduction of uranium(VI) by mixed iron(II)/iron(III) hydroxide (green rust): Formation of UO2 nanoparticles. Environ. Sci. Technol. 2003, 37, 721–727. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, E.J.; Kelly, S.D.; Kemner, K.M.; Csencsits, R.; Cook, R.E. Reduction of AgI, AuIII, CuII, and HgII by FeII/FeIII hydroxysulfate green rust. Chemoshpere 2003, 53, 437–446. [Google Scholar] [CrossRef]
- Pepper, S.E.; Bunker, D.J.; Bryan, N.D.; Livens, F.R.; Charnock, J.M.; Pattrick, R.A.D.; Collison, D. Treatment of radioactive wastes: An X-ray adsorption spectroscopy study of the treatment of technetium with green rust. J. Colloid Interface Sci. 2003, 268, 408–412. [Google Scholar] [CrossRef]
- Refait, P.; Simon, L.; Génin, J.-M.R. Reduction of SeO42− anions and anoxic formation of iron(II)-iron(III) hydroxy-selenate green rust. Environ. Sci. Technol. 2000, 34, 819–825. [Google Scholar] [CrossRef]
- Skovbjerg, L.L.; Stipp, S.L.S.; Utsunomiya, S.; Ewing, R.C. The mechanisms of reduction of hexavalent chromium by green rust sodium sulphate: Formation of Cr-goethite. Geochim. Cosmochim. Acta 2006, 70, 3582–3592. [Google Scholar] [CrossRef]
- Williams, A.G.B.; Scherer, M.M. Kinetics of Cr(VI) reduction by carbonate green rust. Environ. Sci. Technol. 2001, 35, 3488–3494. [Google Scholar] [CrossRef]
- Yan, S.; Boyanov, M.I.; Mishra, B.; Kemner, K.M.; O’Loughlin, E.J. U(VI) reduction by biogenic and abiotic hydroxycarbonate green rusts: Impacts on U(IV) speciation and stability over time. Environ. Sci. Technol. 2018, 52, 4601–4609. [Google Scholar] [CrossRef]
- Etique, M.; Zegeye, A.; Gregoire, B.; Carteret, C.; Ruby, C. Nitrate reduction by mixed iron(II-III) hydroxycarbonate green rust in the presence of phosphate anions: The key parameters influencing the ammonium selectivity. Water Res. 2014, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]


| System | Final a pH | Final Fe(II)aq (mM) | Final Fe(II)tot (mM) | Fe(II)tot Production during Bioreduction c (mmol d−1) | e− Donor Consumed (mM) | Fe(II)tot:e− Donor Consumed |
|---|---|---|---|---|---|---|
| Formate (chloride) | 9.05 ± 0.01 | 0.8 ± 0.1 | 57.6 ± 0.2 | 2.32 ± 0.09 | 27.7 ± 1.4 | 2.1 |
| Formate (sulfate) | 9.06± 0.05 | 0.7 ± 0.1 | 55.1 ± 2.4 | 1.39 ± 0.09 | 25.3 ± 1.9 | 2.2 |
| H2 abiotic | 9.30 ± 0.01 | ND b | 63.8 ± 1.3 | 16.36 ± 0.52 | ND | ND |
| H2 (carbonate) | 8.83 ± 0.13 | 0.8 ± 0.1 | 16.0 ± 0.6 | 7.07 ± 1.31 | ND | ND |
| H2 (sulfate) | 8.59 ± 0.09 | 0.9 ± 0.1 | 12.6 ± 2.3 | 7.09 ± 0.97 | ND | ND |
| H2 (chloride) | 8.36 ± 0.11 | 1.2 ± 0.3 | 8.5 ± 0.6 | 5.64 ± 0.95 | ND | ND |
| Lactate (chloride) | 8.01 ± 0.01 | 4.7 ± 0.1 | 56.4 ± 0.8 | 3.76 ± 0.23 | 22.6 ± 1.3 | 2.5 |
| Lactate (sulfate) | 8.23 ± 0.03 | 2.9 ± 0.1 | 59.3 ± 4.8 | 3.18 ± 0.12 | 22.6 ± 1.4 | 2.6 |
| NAG (chloride) | 7.55 ± 0.10 | 6.8 ± 2.2 | 61.4 ± 4.4 | 1.31 ± 0.14 | 38.4 ± 2.8 | 1.6 |
| NAG (sulfate) | 7.69 ± 0.04 | 6.8 ± 0.7 | 70.6 ± 3.7 | 1.57 ± 0.24 | 40.9 ± 2.1 | 1.7 |
| Pyruvate (chloride) | 8.47 ± 0.05 | 2.0 ± 0.1 | 57.3 ± 1.5 | 9.84 ± 1.63 | 75 | 0.8 |
| Pyruvate (sulfate) | 8.44 ± 0.04 | 1.8 ± 0.2 | 59.3 ± 5.7 | 10.20 ± 1.91 | 75 | 0.8 |
| Serine (chloride) | 8.17 ± 0.02 | 2.3 ± 0.1 | 58.1 ± 1.8 | 7.25 ± 0.34 | 75 | 0.8 |
| Serine (sulfate) | 8.46 ± 0.29 | 1.4 ± 1.0 | 61.0 ± 7.0 | 8.38 ± 0.28 | 75 | 0.8 |
| Sample | Temp (K) | CS (mm s−1) | QS (mm s−1) | H (T) | Mineral | RA (%) |
|---|---|---|---|---|---|---|
| Formate (chloride) | 77 | 1.26 | 2.83 | - | Green Rust Fe(II) | 70.5 |
| - | 0.48 | 0.38 | - | Green Rust Fe(III) | 29.5 | |
| 13 | 1.27 | 2.80 | - | Green Rust Fe(II) | 69.9 | |
| - | 0.49 | 0.37 | - | Green Rust Fe(III) | 30.1 | |
| Formate (sulfate) | 77 | 1.26 | 2.85 | - | Green Rust Fe(II) | 68.1 |
| - | 0.47 | 0.40 | - | Green Rust Fe(III) | 31.9 | |
| 13 | 1.26 | 2.82 | - | Green Rust Fe(II) | 67.6 | |
| 13 | 0.47 | 0.41 | - | Green Rust Fe(III) | 32.4 | |
| Lactate (chloride) | 77 | 1.27 | 2.85 | - | Green Rust Fe(II) | 66.7 |
| - | 0.47 | 0.41 | - | Green Rust Fe(III) | 33.3 | |
| 13 | 1.27 | 2.82 | - | Green Rust Fe(II) | 69.4 | |
| - | 0.48 | 0.38 | - | Green Rust Fe(III) | 30.6 | |
| Lactate (sulfate) | 77 | 1.26 | 2.89 | - | Green Rust Fe(II) | 61.9 |
| - | 0.45 | 0.46 | - | Green Rust Fe(III) | 38.1 | |
| 13 | 1.26 | 2.88 | - | Green Rust Fe(II) | 58.8 | |
| - | 0.41 | 0.51 | - | Green Rust Fe(III) | 41.2 | |
| NAG (chloride) | 77 | 1.36 | 2.05 | - | Siderite | 17.7 |
| - | 1.27 | 2.88 | - | Green Rust Fe(II) | 47.4 | |
| - | 0.48 | 0.42 | - | Green Rust Fe(III) | 13.2 | |
| - | 0.49 | 0.6 | - | Lepidocrocite | 21.4 | |
| 13 | 1.33 | 2.09 | 16.8 | Siderite | 14.7 | |
| - | 1.28 | 2.84 | - | Green Rust Fe(II) | 46.4 | |
| - | 0.5 | 0.4 | - | Green Rust Fe(III) | 18.3 | |
| - | 0.49 | 0.03 | 45.3 | Lepidocrocite | 20.6 | |
| NAG (sulfate) | 77 | 1.35 | 2.04 | - | Siderite | 20.9 |
| - | 1.27 | 2.91 | - | Green Rust Fe(II) | 50 | |
| - | 0.42 | 0.55 | - | Green Rust Fe(III) | 29.1 | |
| 13 | 1.35 | 2.1 | 16.8 | Siderite | 29.4 | |
| - | 1.28 | 2.91 | - | Green Rust Fe(II) | 41.5 | |
| - | 0.43 | 0.56 | - | Green Rust Fe(III) | 29.1 | |
| Pyruvate (chloride) | 77 | 1.36 | 2.11 | - | Siderite | 38.7 |
| - | 1.33 | 2.76 | - | Siderite 2 | 33.8 | |
| - | 0.48 | 0.56 | - | Lepidocrocite | 27.5 | |
| 13 | - | - | - | - | - | |
| No quantitation can be made—siderite and lepidocrocite present | ||||||
| Pyruvate (sulfate) | 77 | 1.36 | 2.09 | - | Siderite | 38.7 |
| - | 1.33 | 2.79 | - | Siderite 2 | 30.6 | |
| - | 0.48 | 0.57 | - | Lepidocrocite | 30.7 | |
| 13 | - | - | - | - | - | |
| No quantitation can be made—siderite and lepidocrocite present | ||||||
| Serine (chloride) | 77 | 1.36 | 2.08 | - | Siderite | 43 |
| - | 1.33 | 2.77 | - | Siderite 2 | 33.3 | |
| - | 0.48 | 0.56 | - | Lepidocrocite | 23.6 | |
| 13 | - | - | - | - | - | |
| No quantitation can be made—siderite and lepidocrocite present | ||||||
| Serine (sulfate) | 77 | 1.36 | 2.08 | - | Siderite | 43 |
| - | 1.33 | 2.77 | - | Siderite 2 | 33.3 | |
| - | 0.48 | 0.56 | - | Lepidocrocite | 23.6 | |
| 13 | - | - | - | - | - | |
| No quantitation can be made—siderite and lepidocrocite present | ||||||
| H2 (chloride) | 77 | 1.23 | 2.87 | - | Green Rust Fe(II) | 19.9 |
| - | 0.43 | 0.53 | - | Green Rust Fe(III) | 7.7 | |
| - | 0.5 | 0.6 | - | Lepidocrocite | 72.4 | |
| 13 | - | |||||
| No quantitation can be made—green rust and lepidocrocite present | ||||||
| H2 (carbonate) | 77 | 1.25 | 2.78 | - | Green Rust Fe(II) | 32.2 |
| - | 0.5 | 0.37 | - | Green Rust Fe(III) | 18.7 | |
| - | 0.49 | 0.62 | - | Lepidocrocite | 49.1 | |
| 13 | 1.27 | 2.85 | - | Green Rust Fe(II) | 8.5 | |
| - | 0.48 | 0.38 | - | Green Rust Fe(III) | 3.3 | |
| - | 0.50 | 0.01 | 45.3 | Lepidocrocite | 88.2 | |
| H2 (sulfate) | 77 | 1.28 | 2.88 | - | Green Rust Fe(II) | 16.1 |
| - | 0.49 | 0.41 | - | Green Rust Fe(III) | 19.0 | |
| - | 0.49 | 0.59 | - | Lepidocrocite | 65.0 | |
| 13 | - | - | - | - | - | |
| No quantitation can be made—predominantly lepidocrocite | ||||||
| System | pXRD | Mössbauer | SEM |
|---|---|---|---|
| Formate (chloride) | GRC a | GR | GR |
| Formate (sulfate) | GRC | GR | GR |
| H2 abiotic | GRC | ND | ND |
| H2 (carbonate) | GRC | GR | GR |
| H2 (sulfate) | GRS a | GR | GR |
| H2 (chloride) | GRC | GR | GR |
| Lactate (chloride) | GRC | GR | GR |
| Lactate (sulfate) | GRC, GRS | GR | GR |
| NAG (chloride) | GRC, Sid b | GR, Sid | GR, Sid |
| NAG (sulfate) | GRC, GRS, Sid | GR, Sid | GR, Sid |
| Pyruvate (chloride) | Sid | Sid | Sid |
| Pyruvate (sulfate) | Sid | Sid | Sid |
| Serine (chloride) | Sid | Sid | Sid |
| Serine (sulfate) | Sid | Sid | Sid |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Loughlin, E.J.; Gorski, C.A.; Flynn, T.M.; Scherer, M.M. Electron Donor Utilization and Secondary Mineral Formation during the Bioreduction of Lepidocrocite by Shewanella putrefaciens CN32. Minerals 2019, 9, 434. https://doi.org/10.3390/min9070434
O’Loughlin EJ, Gorski CA, Flynn TM, Scherer MM. Electron Donor Utilization and Secondary Mineral Formation during the Bioreduction of Lepidocrocite by Shewanella putrefaciens CN32. Minerals. 2019; 9(7):434. https://doi.org/10.3390/min9070434
Chicago/Turabian StyleO’Loughlin, Edward J., Christopher A. Gorski, Theodore M. Flynn, and Michelle M. Scherer. 2019. "Electron Donor Utilization and Secondary Mineral Formation during the Bioreduction of Lepidocrocite by Shewanella putrefaciens CN32" Minerals 9, no. 7: 434. https://doi.org/10.3390/min9070434
APA StyleO’Loughlin, E. J., Gorski, C. A., Flynn, T. M., & Scherer, M. M. (2019). Electron Donor Utilization and Secondary Mineral Formation during the Bioreduction of Lepidocrocite by Shewanella putrefaciens CN32. Minerals, 9(7), 434. https://doi.org/10.3390/min9070434






