Effect of a Small Amount of Iron Impurity in Sphalerite on Xanthate Adsorption and Flotation Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrodes
2.3. Flotation Process
2.4. Analysis Methods
3. Results and Discussion
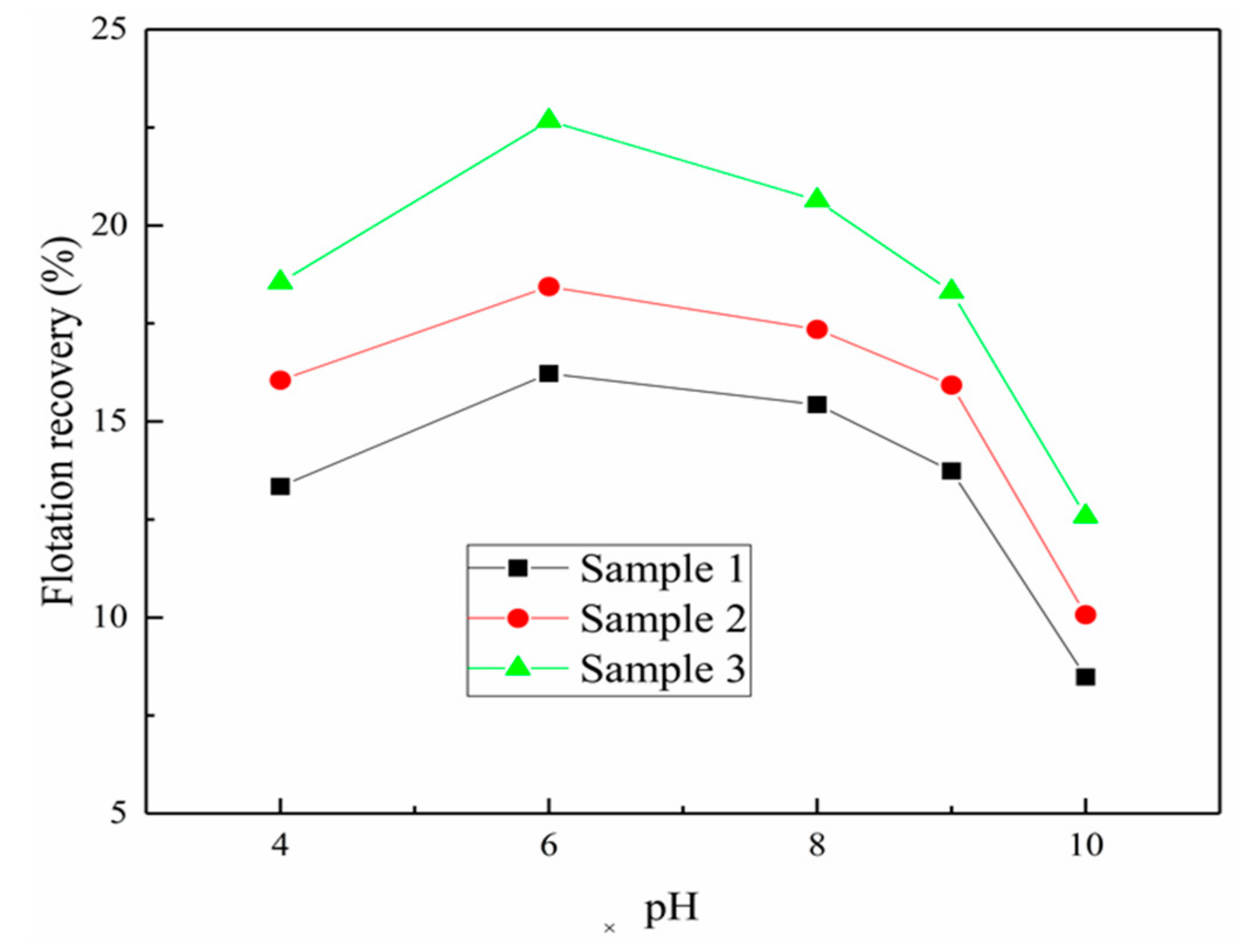
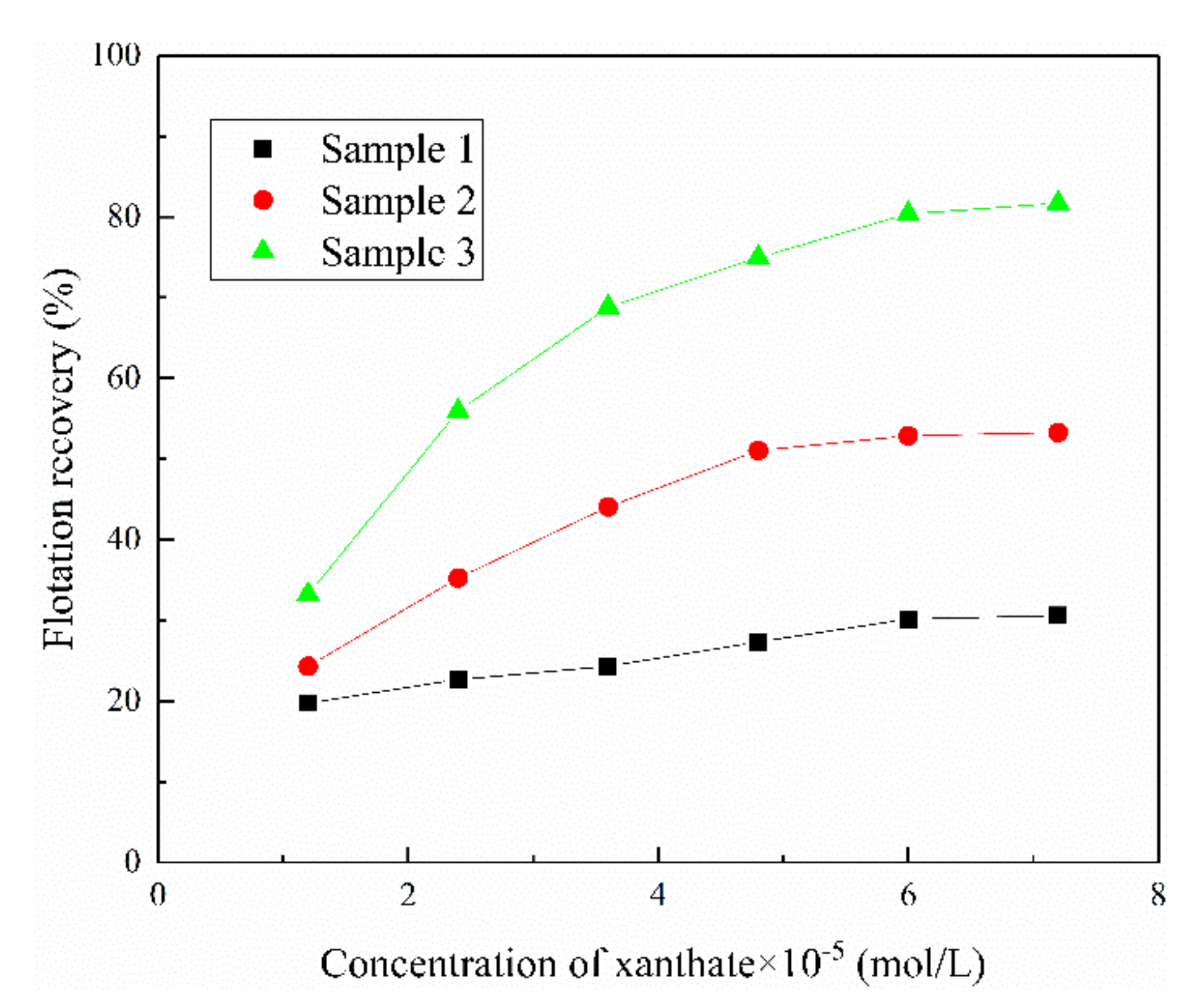
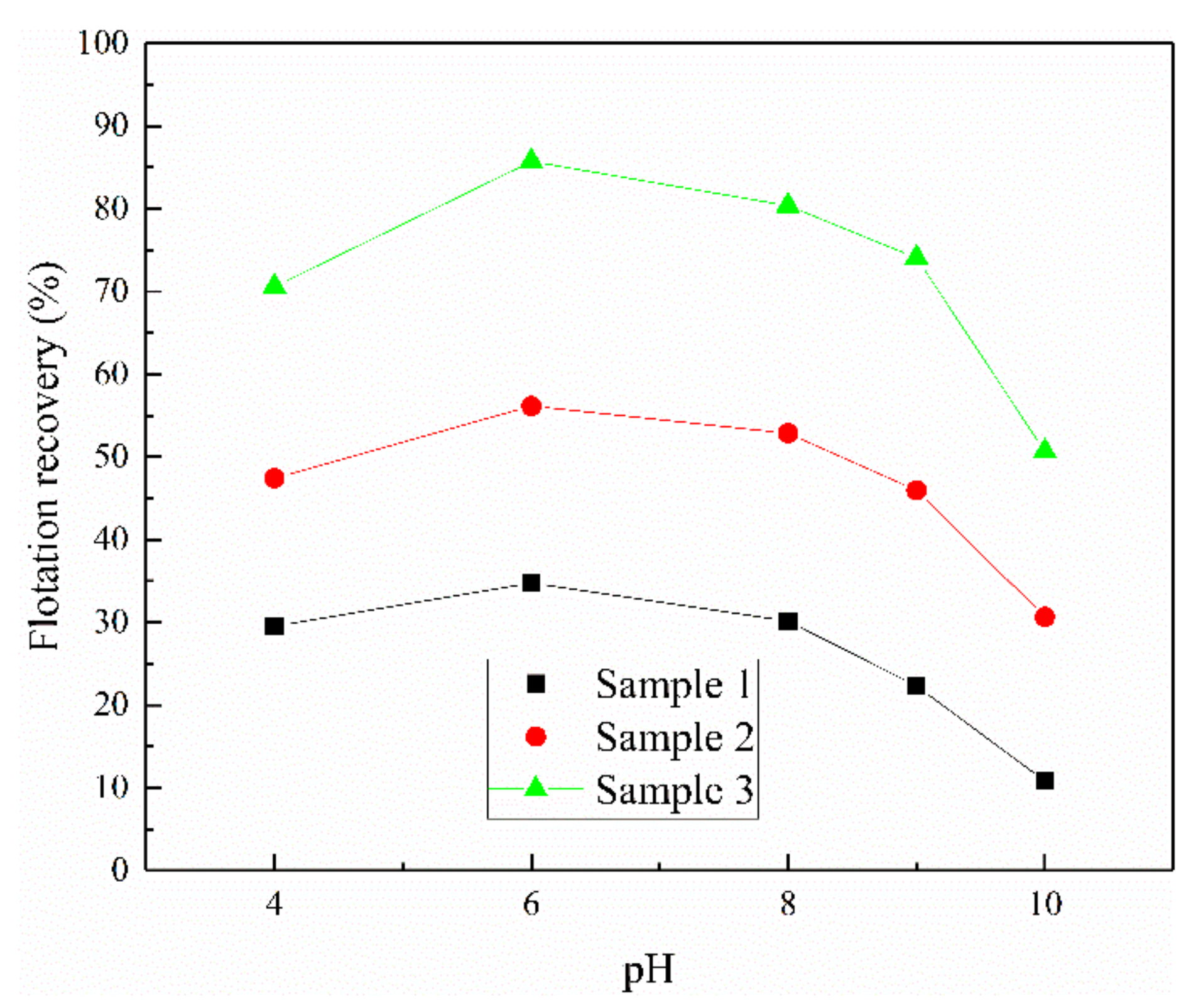
3.1. The Effect of Iron Impurity Content on the Flotation of Sphalerite
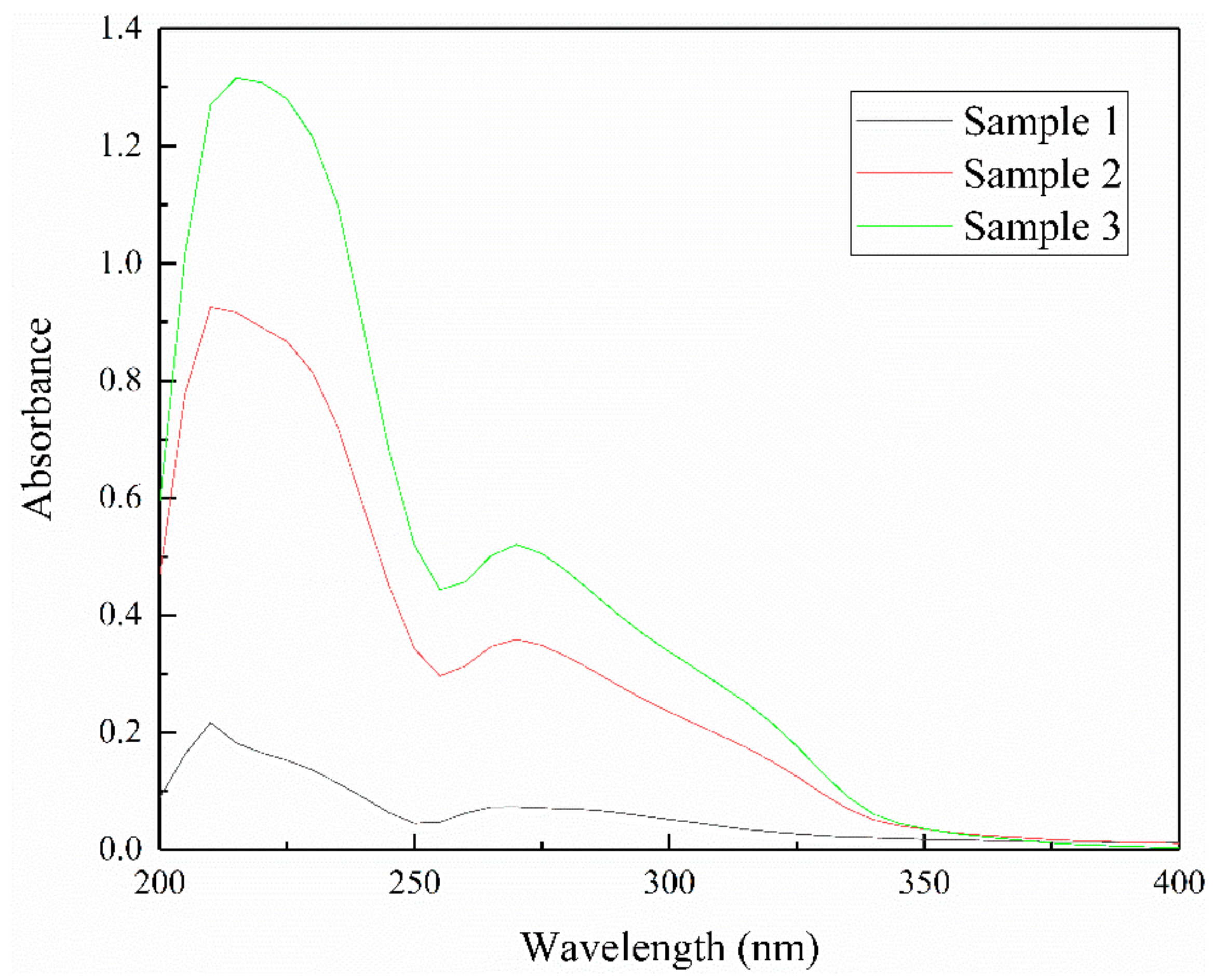
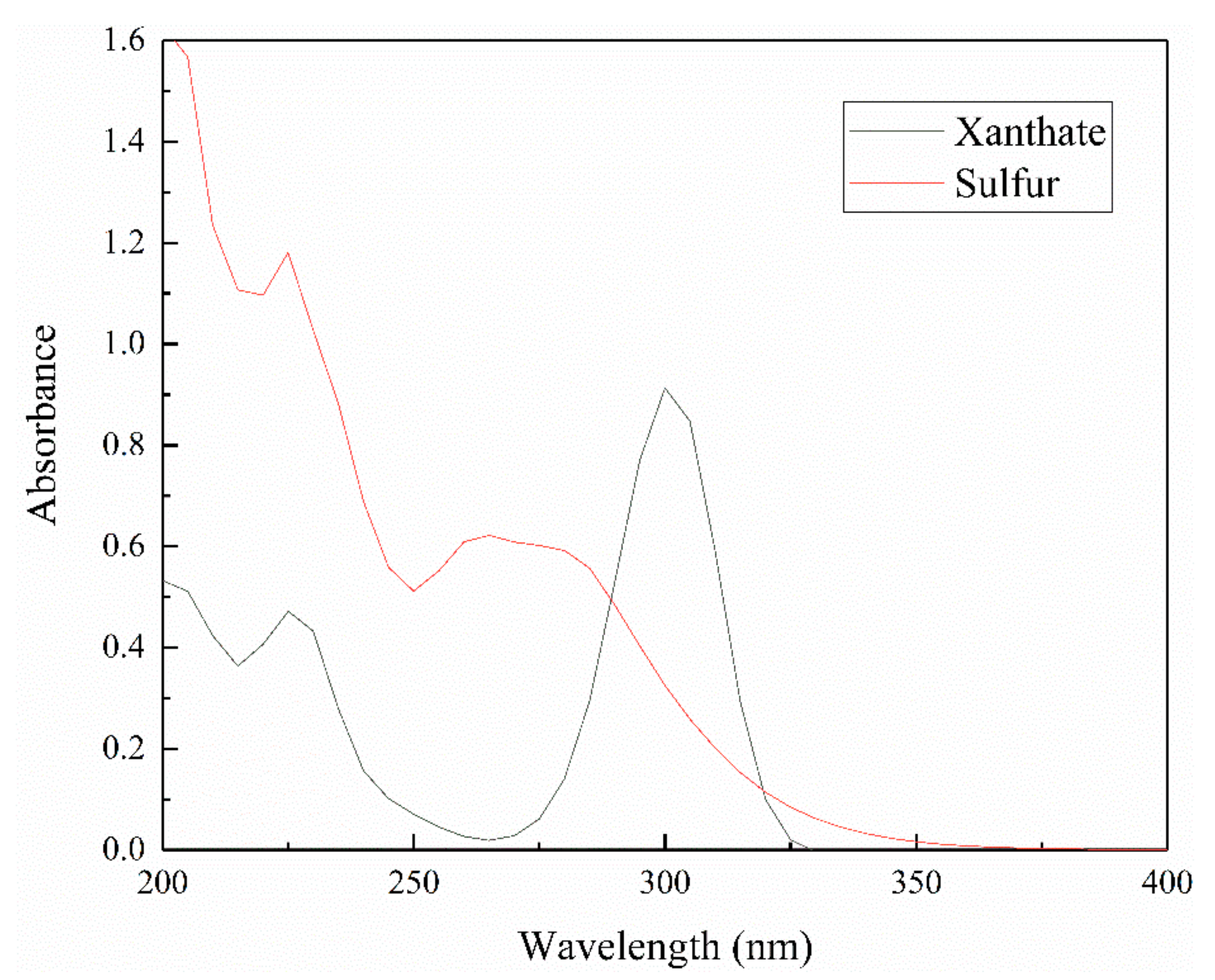
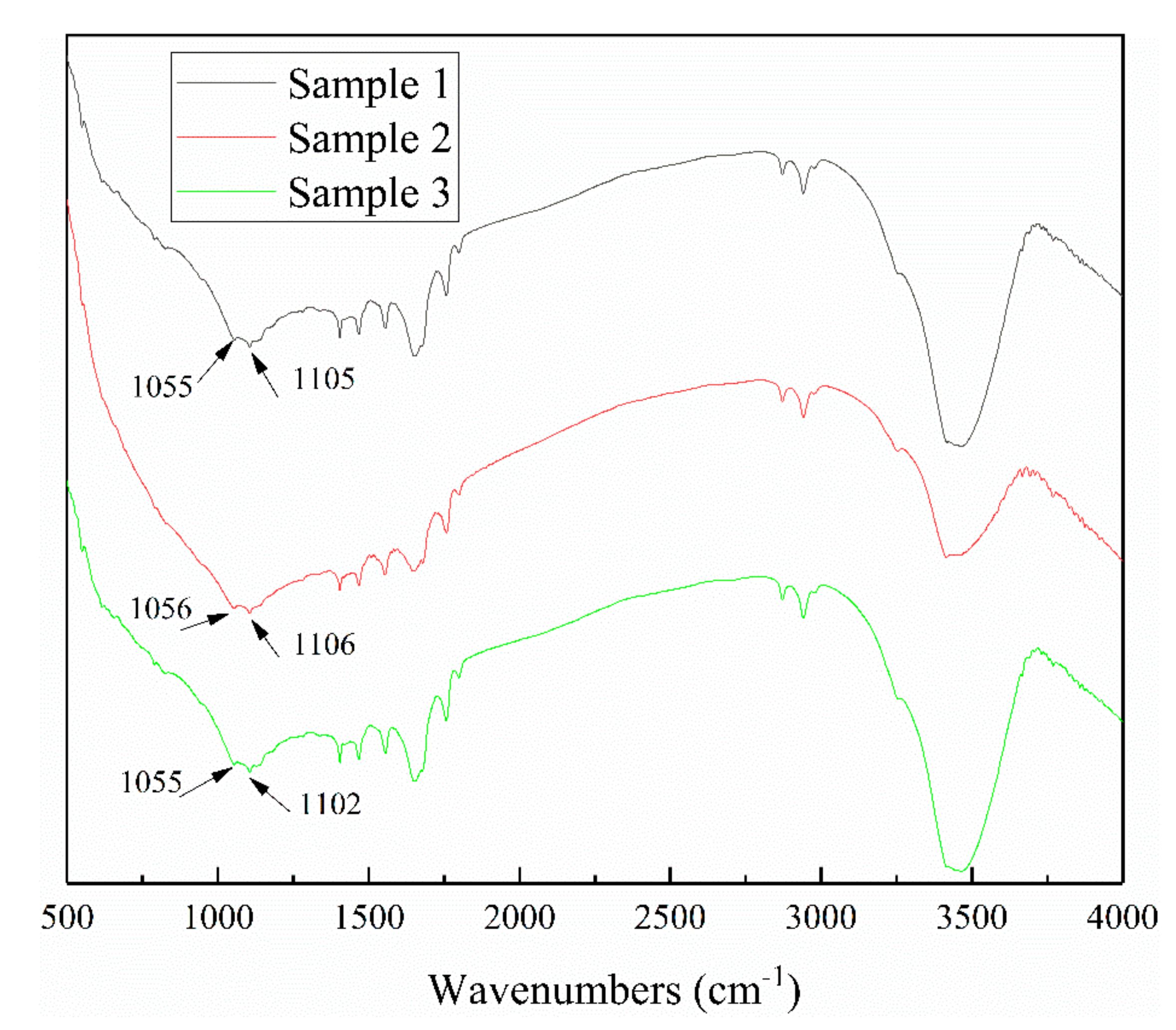
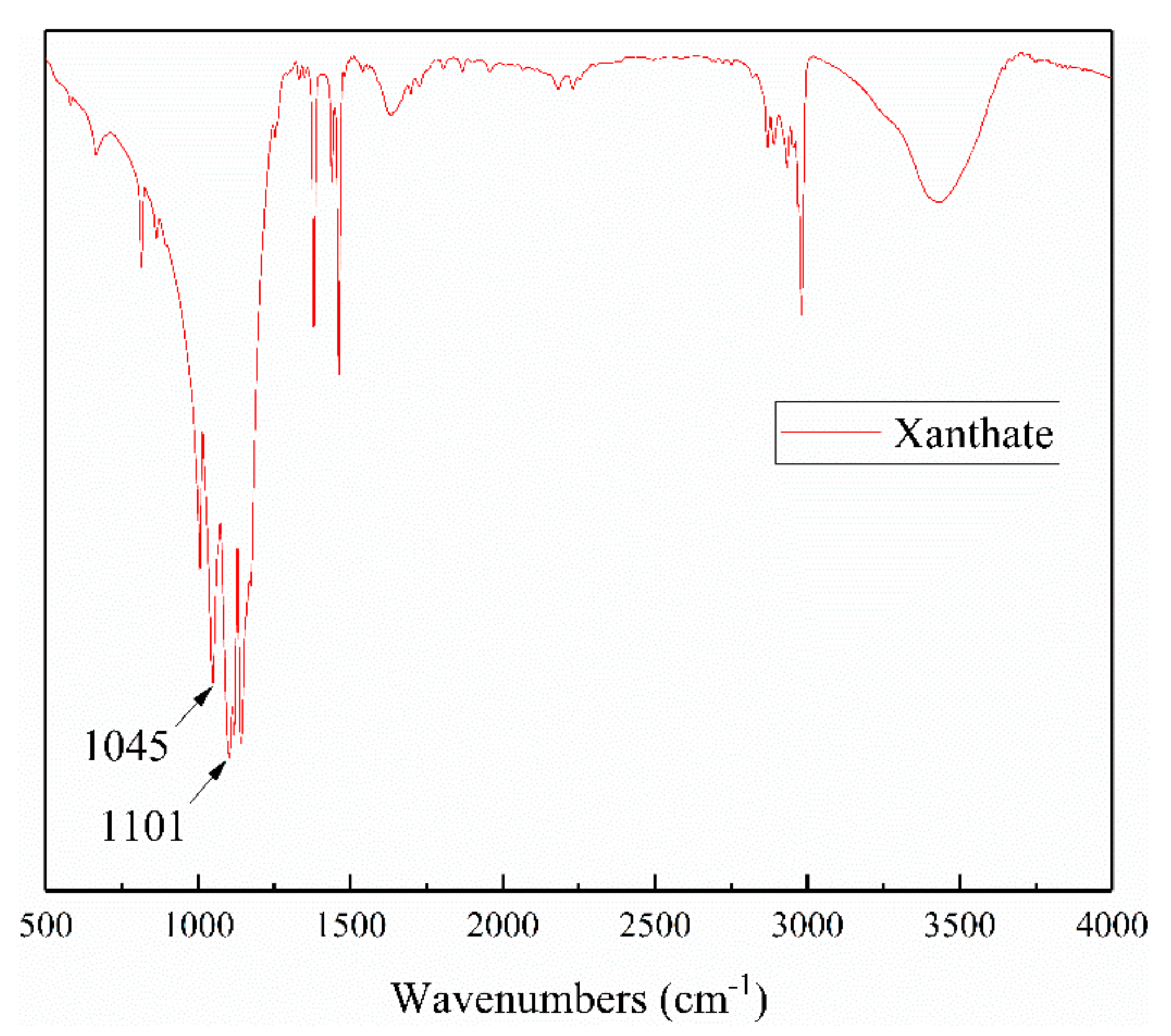
3.2. The Effect of Iron Impurity Content on Adsorption Capacity and Production of Xanthate
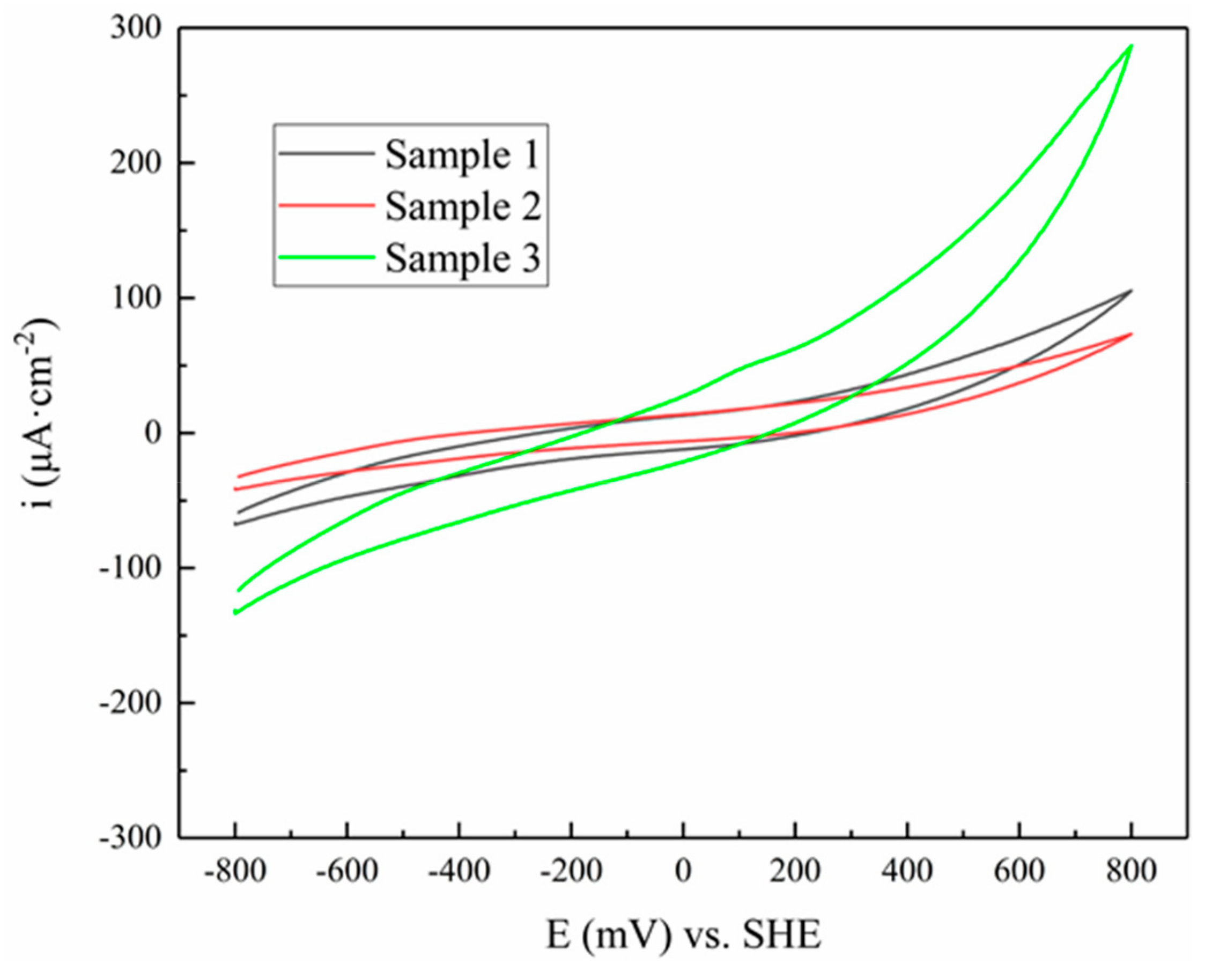
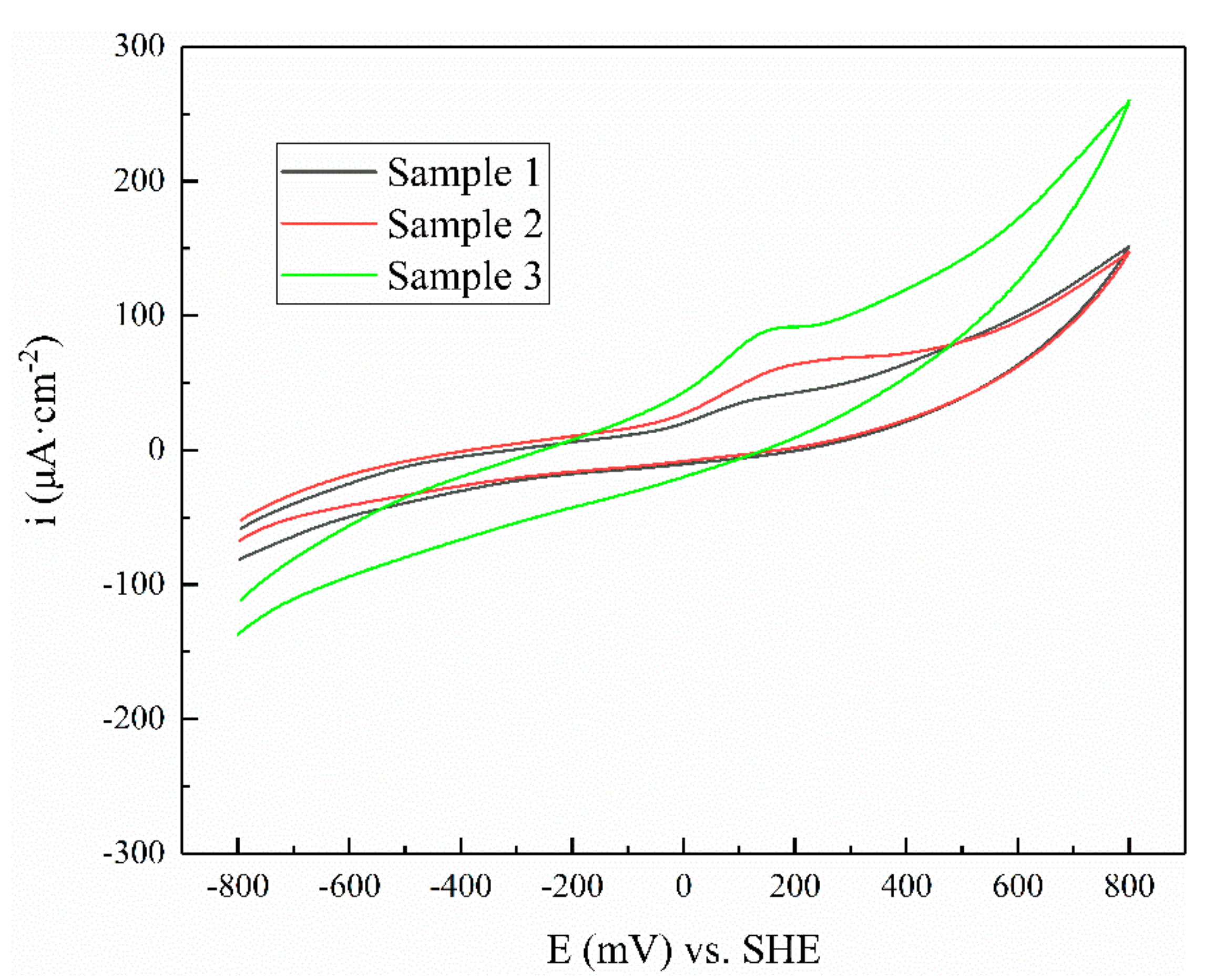
3.3. Cyclic Voltammogram
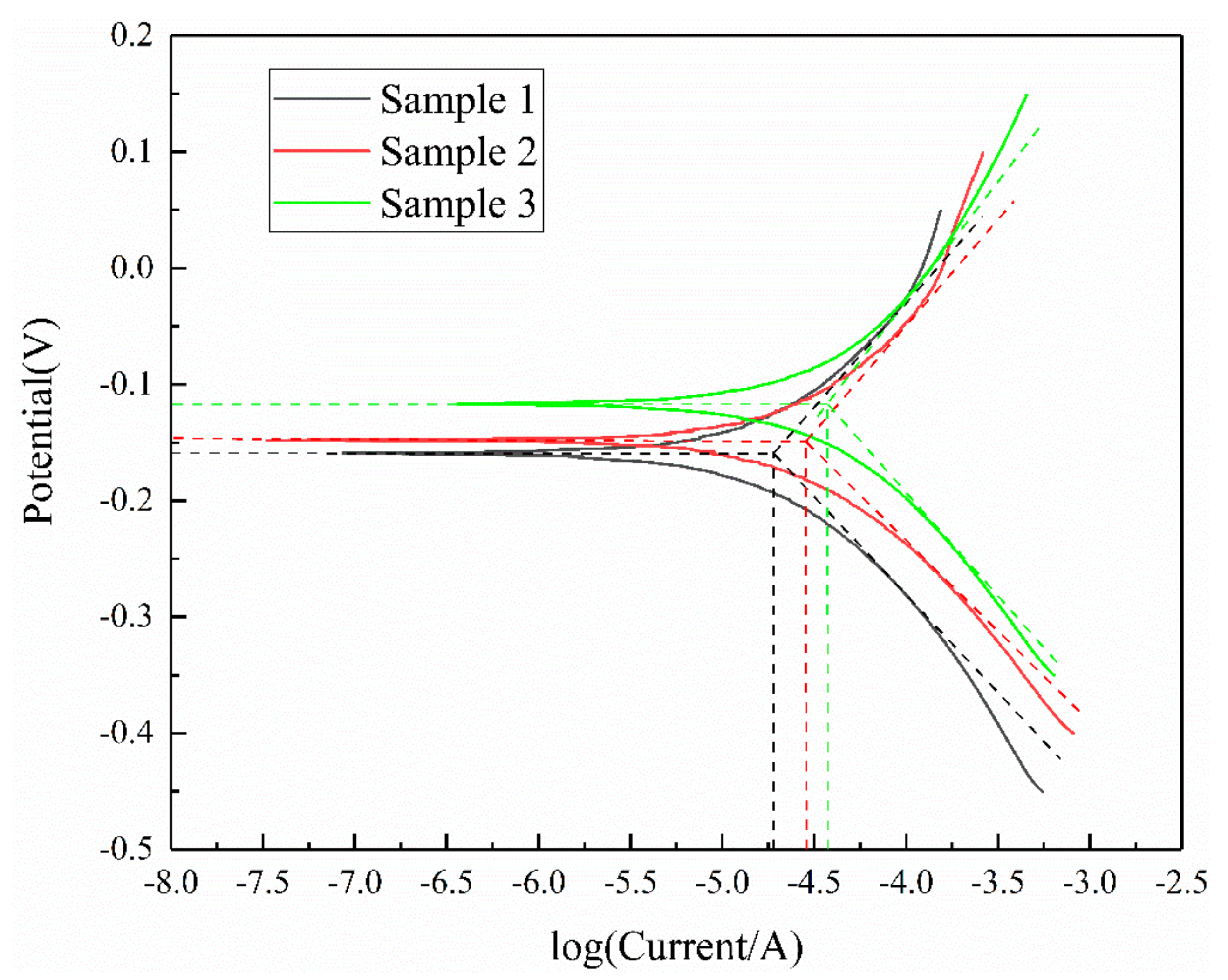
3.4. Tafel Curves
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Osadchii, V.O.; Fedkin, M.V.; Osadchii, E.G. Electrochemical determination of the thermodynamic parameters of sphalerite, ZnS. J. Alloys Compd. 2015, 636, 368–374. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef]
- Lepetit, P.; Bente, K.; Doering, T.; Luckhaus, S. Crystal chemistry of Fe-containing sphalerites. Phys. Chem. Miner. 2003, 30, 185–191. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Wright, K.; Gale, J.D. A first principles study of the distribution of iron in sphalerite. Geochim. Cosmochim. Acta 2010, 74, 3514–3520. [Google Scholar] [CrossRef]
- Xiong, T.; Jian, H.E.; Feng, R.; Si-Qing, L.; Qing-Hua, Z. Experimental Study on Activation of High Iron-bearing Marmatite. Min. Metall. Eng. 2006, 26, 19–22. (In China) [Google Scholar]
- Quast, K.; Hobart, G. Marmatite depression in galena flotation. Miner. Eng. 2006, 19, 860–869. [Google Scholar] [CrossRef]
- Tong, X.; Song, S.; He, J.; Rao, F.; Lopez-Valdivieso, A. Activation of high-iron marmatite in froth flotation by ammoniacal copper(II) solution. Miner. Eng. 2007, 20, 259–263. [Google Scholar] [CrossRef]
- Szczypa, J.; Solecki, J.; Komosa, A. Effect of surface oxidation and iron contents on xanthate ions adsorption of synthetic sphalerites. Int. J. Miner. Process. 1980, 7, 151–157. [Google Scholar] [CrossRef]
- Barzyk, W.; Malysa, K.; Pomianowski, A. The influence of surface oxidation of chalcocite on its floatability and ethyl xanthate sorption. Int. J. Miner. Process. 1981, 8, 17–29. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. Chemisorption—The thermodynamically favoured process in the interaction of thiol collectors with sulphide minerals. Int. J. Miner. Process. 1997, 51, 15–26. [Google Scholar] [CrossRef]
- Janetski, N.D.; Woodburn, S.I.; Woods, R. An electrochemical investigation of pyrite flotation and depression. Int. J. Miner. Process. 1977, 4, 227–239. [Google Scholar] [CrossRef]
- Haung, H.H.; Miller, J.D. Kinetics and thermochemistry of amyl xanthate adsorption by pyrite and marcasite. Int. J. Miner. Process. 1978, 5, 241–266. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Guo, J. A DFT study on the effect of lattice impurities on the electronic structures and floatability of sphalerite. Miner. Eng. 2010, 23, 1120–1130. [Google Scholar] [CrossRef]
- Harmer, S.L.; Mierczynska-Vasilev, A.; Beattie, D.A.; Shapter, J.G. The effect of bulk iron concentration and heterogeneities on the copper activation of sphalerite. Miner. Eng. 2008, 21, 1005–1012. [Google Scholar] [CrossRef]
- Yang, P.; Lü, M.; Xu, D.; Yuan, D.; Song, C. Photoluminescence characteristics of ZnS nanocrystallites co-doped with Cu2+ and Cd2+. J. Phys. Chem. Solids 2003, 64, 155–158. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y. A first-principle study of the effect of vacancy defects and impurities on the adsorption of O2 on sphalerite surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2010, 363, 56–63. [Google Scholar] [CrossRef]
- Deng, J.S.; Lai, H.; Chen, M.; Glen, M.; Wen, S.M.; Zhao, B.; Liu, Z.L.; Yang, H.; Liu, M.S.; Huang, L.Y.; et al. Effect of iron concentration on the crystallization and electronic structure of sphalerite/marmatite: A DFT study. Miner. Eng. 2019, 136, 168–174. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Lan, L.; Yang, M. The influence of the impurities on the flotation behaviors of synthetic ZnS. Miner. Eng. 2012, 27–28, 65–71. [Google Scholar] [CrossRef]
- Solecki, J.; Komosa, A.; Szczypa, J. Copper ion activation of synthetic sphalerites with various iron contents. Int. J. Miner. Process. 1979, 6, 221–228. [Google Scholar] [CrossRef]
- Gigowski, B.; Vogg, A.; Wierer, K.; Dobias, B. Effect of Fe-lattice ions on adsorption, electrokinetic, calorimetric and flotation properties of sphalerite. Int. J. Miner. Process. 1991, 33, 103–120. [Google Scholar] [CrossRef]
- Ahlberg, E.; Asbjornsson, J. Carbon paste electrodes in mineral processing: An electrochemical study of sphalerite. Hydrometallurgy 1994, 36, 19–37. [Google Scholar] [CrossRef]
- Donaldson, E.M. Chloroform extraction of metal ethyl xanthates from hydrochloric acid media. Talanta 1976, 23, 411–416. [Google Scholar] [CrossRef]
- Guo, H.; Yen, W.T. Surface potential and dixanthogen stability on chalcopyrite surface. Int. J. Miner. Process. 2005, 76, 205–213. [Google Scholar] [CrossRef]
- Wang, X.H. Interfacial Electrochemistry of Pyrite Oxidation and Flotation: II. FTIR Studies of Xanthate Adsorption on Pyrite Surfaces in Neutral pH Solutions. J. Colloid Interface Sci. 1995, 171, 413–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Z.; Cao, Y.; Sun, C. FTIR studies of xanthate adsorption on chalcopyrite, pentlandite and pyrite surface. J. Mol. Struct. 2013, 1048, 434–440. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Clifford, K.L.; Kuhn, M.C. The role of zinc-xanthate precipitation in sphalerite flotation. Int. J. Miner. Process. 1974, 1, 307–318. [Google Scholar] [CrossRef]
- Tadie, M.; Corin, K.C.; Wiese, J.G.; Nicol, M.; O’Connor, C.T. An investigation into electrochemical interactions between platinum group minerals and xanthate: Voltammetric study. Miner. Eng. 2015, 70, 148–155. [Google Scholar] [CrossRef]
- Iwasaki, I.; Cooke, S.R.B. The Decomposition of Xanthate in Acid Solution. J. Am. Chem. Soc. 1958, 80, 285–288. [Google Scholar] [CrossRef]
- Jones, M.H.; Woodcock, J.T. Decomposition of alkyl dixanthogens in aqueous solutions. Int. J. Miner. Process. 1983, 10, 1–24. [Google Scholar] [CrossRef]
- Valdivieso, A.L.; López, A.S.; Song, S. On the cathodic reaction coupled with the oxidation of xanthates at the pyrite/aqueous solution interface. Int. J. Miner. Process. 2005, 77, 154–164. [Google Scholar] [CrossRef]
- Wei-Zhong, L.; Wen-Qing, Q.; Wei, S. Electrodeposition of dixanthogen(TETD) on pyrite surface. Trans. Nonferr. Metal. Soc. China 2007, 17, 154–158. [Google Scholar]

| Mineral | Zn/(wt.%) | S/(wt.%) | Fe/(wt.%) |
|---|---|---|---|
| Sample 1 | 65.15 | 32.31 | 2.30 |
| Sample 2 | 63.13 | 32.60 | 3.2 |
| Sample 3 | 61.59 | 33.78 | 4.66 |
| Minerals | Pb/(wt.%) | Bi/(wt.%) | Ca/(wt.%) | Cd/(wt.%) |
|---|---|---|---|---|
| Sample 1 | 0.10 | 0.17 | 0.059 | 0.12 |
| Sample 2 | 0.12 | 0.058 | 0.075 | 0.16 |
| Sample 3 | 0.09 | <0.05 | 0.058 | 0.24 |
| Sphalerite | Adsorption × 10−7 (mol/g) |
|---|---|
| Sample 1 | 5.31 |
| Sample 2 | 7.26 |
| Sample 3 | 8.83 |
| Sphalerite | Adsorption × 10−7 (mol/g) |
|---|---|
| Sample 1 | 5.59 |
| Sample 2 | 7.63 |
| Sample 3 | 9.74 |
| Molecular | Main Functional Groups | Wavenumber (cm−1) |
|---|---|---|
| Xanthate | C=S stretching vibration C–O–C stretching vibration | 1020–1050 1100–1120, 1150–1265 |
| Dixanthogen | C–O | 1240–1265 |
| Xanthate | C–O | 1150–1210 |
| Mineral | Potential/V | Current/×10−5 A |
|---|---|---|
| Sample 1 | −0.156 | 1.90 |
| Sample 2 | −0.148 | 2.88 |
| Sample 3 | −0.119 | 3.81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Wu, X.; Zhao, Z.; Zhu, Y.; Luo, S. Effect of a Small Amount of Iron Impurity in Sphalerite on Xanthate Adsorption and Flotation Behavior. Minerals 2019, 9, 687. https://doi.org/10.3390/min9110687
Yu J, Wu X, Zhao Z, Zhu Y, Luo S. Effect of a Small Amount of Iron Impurity in Sphalerite on Xanthate Adsorption and Flotation Behavior. Minerals. 2019; 9(11):687. https://doi.org/10.3390/min9110687
Chicago/Turabian StyleYu, Jiang, Xiqun Wu, Zhiqiang Zhao, Yangge Zhu, and Sigang Luo. 2019. "Effect of a Small Amount of Iron Impurity in Sphalerite on Xanthate Adsorption and Flotation Behavior" Minerals 9, no. 11: 687. https://doi.org/10.3390/min9110687
APA StyleYu, J., Wu, X., Zhao, Z., Zhu, Y., & Luo, S. (2019). Effect of a Small Amount of Iron Impurity in Sphalerite on Xanthate Adsorption and Flotation Behavior. Minerals, 9(11), 687. https://doi.org/10.3390/min9110687






