Abstract
In this paper, the effect of the cupric and chloride ions concentrations on copper dissolution from chalcopyrite concentrate was studied in acidified media. Variables included three different concentrations of Cu2+ (0.5, 1.5, and 2.5 g L−1), four different concentrations of Cl− (0, 5, 7, and 10 g L−1), two different pH values of 1 and 2, and a constant temperature of 60 °C. Results indicated that addition of Cl− to the system improves copper extractions, especially at higher concentrations of Cu2+. Initial copper concentrations in the leaching solution did not significantly affect the copper extraction when Cl− was not present. Better copper extractions were obtained at pH 1 as compared with pH 2. As the Cu2+ and Cl− concentrations were increased, higher values of redox potential were obtained. According to the formation constants of the chloro-complexes, the predominant species in the Cu2+/Cl− system in the studied interval were CuCl+ and Cu2+. Using a model of copper speciation in the experimental range predicted for a single copper concentration with increasing Cl− concentration, the Cu2+ concentration decreased significantly while the concentration of the chloro-complex species CuCl+ increased. In the leached residue, evidence of sulfur formation was found using SEM and corroborated by XRD analysis. When chloride is present in the medium, the amounts of copper and iron in the residue decrease, confirming a positive effect of chloride on the extraction of copper from concentrate for the studied conditions.
1. Introduction
Among the copper sulfide ores, chalcopyrite (CuFeS2) is found in greater abundance. It is well known that chalcopyrite is processed via flotation followed by pyrometallurgy. Despite being the most important copper mineral, chalcopyrite has been observed to be refractory under hydrometallurgical conditions. Many researchers have concluded that a passivating layer forms on the surface of the mineral during leaching, which hinders further copper extraction [1,2,3,4,5]. A large number of sulfate-based processes have been developed to overcome the slow rate of chalcopyrite leaching. A review of acidic sulfate, sulfate–chloride, and sulfate–nitrate process options has been provided by Watling [5]. One key approach to the treatment of chalcopyrite has been bioleaching, which is only viable under conditions of sufficiently elevated temperature [6].
Increasing the oxidation kinetics of chalcopyrite has been investigated using high temperature and pressure leaching conditions [7,8,9,10]. Activation of the mineral surfaces using ultrafine grinding (d80 = 5–10 μm) has been employed to accelerate the oxidation kinetics and increase copper extraction from chalcopyrite [11,12]. Other methods to accelerate chalcopyrite dissolution include the addition of activated carbon [13], coal [14], silver [15], nitrate [16], nitrite [17,18], iron powder [19], or by controlling the redox potential [20]. The fundamental mechanisms and kinetics of the leaching of chalcopyrite remain poorly understood. In a review by Li et al. [21], it was concluded that these phenomena are hindered by a lack of understanding at the molecular or atomic scale.
An alternate approach to chalcopyrite leaching involves the use of chloride ions in an acidic sulfate system at ambient temperatures. The use of chloride solutions in chalcopyrite leaching is beneficial due to the aggressive nature of the chloride ion and to the stabilization of cuprous ions as chloro-complex ions. Generally, the leaching of chalcopyrite is more effective in chloride solutions than in sulfate solutions. This is possibly due to kinetic rather than thermodynamic considerations [22]. For example, higher rates of electron transfer occur in chloride solutions than in sulfate solutions, as the chalcopyrite surface passivates more readily in the presence of sulfate ions [22,23,24,25,26,27,28]. Several processing methods have been proposed for the leaching of sulfide ores and copper concentrates in chloride media [7,29,30,31,32,33].
Electrochemical studies on the leaching of chalcopyrite in mixed sulfate–chloride media by Lu et al. [27] indicated that the presence of chloride enhanced chalcopyrite oxidation. Redox potential is a key variable due to the existence of a potential range where the chalcopyrite is not passive. Many researchers have worked sensibly controlling this variable [20,34,35,36,37]. Nicol and co-workers [20,38,39,40,41] conducted an extensive ranging study of the dissolution of chalcopyrite in chloride solutions containing cupric ions and dissolved oxygen.
In chloride-containing solutions, chloride ions displace water molecules in the coordination spheres of many metal ions forming chloro-complexes [42]. In addition, the potential series of metals in chloride solutions are different from the standard ones. Both the Fe3+/Fe2+ and Cu2+/Cu+ redox potentials are higher in chloride solutions than in sulfate solutions. Fe3+ forms strong complexes with the sulfate in sulfate solutions, decreasing the activity of the oxidant. The complexation of Fe3+ in chloride solution is not as strong and the oxidant has a higher activity. In cupric chloride leaching, Cu+ forms very strong complexes with chloride ions, increasing Cu2+ activity. In addition, the redox potential of Cu2+/Cu+ is greater than that of Fe3+/Fe2+ in concentrated chloride solutions. This makes the dissolution more beneficial in cupric chloride solutions compared to ferric chloride solutions [43,44,45]. It has also been revealed that the addition of cupric chloride to ferric chloride leaching helps chalcopyrite dissolution [46]. The capability of chloride ions to form complexes is crucial. Berger et al. [47] proposed the subsequent classification of the strength of Cl acceptors: AgCl > CuCl > PbCl2 > ZnCl2 > CuCl2 > FeCl3 > FeCl2 > NiCl2 > HCl, NaCl, KCl (Cl− donors). Cuprous chloride is sparingly soluble in water, however, in concentrated chloride solutions, higher solubilities can be achieved [44]. The cation of the added chloride salt can also affect the solubility [48,49].
The solubility of Cu(II) declines with increasing Cl− concentration, in contrast to cuprous chloride. It is generally agreed that in aqueous solutions, Cu+ and chloride ions form a series of complexes [CuCln]1−n, where n ≤ 4. There is less agreement about the complexation of cupric ions, since the predominant cupric species has been suggested to be both cupric ion and its chloro-complex, CuCl+ [47,50,51,52].
Various authors have reported that chloride ions play the following key roles in achieving a high leaching rate [20,22,24,26,44,45,46,53]:
- pH is preserved for longer at a desired valued in the presence of excess chloride ions.
- The porosity of the product layer and its surface area are increased by the addition of chloride, reducing the passivation tendency of chalcopyrite.
- The oxidation–reduction potential is lower in a chloride media than in a sulfate media.
In the present study, chalcopyrite concentrate was leached using different chloride and cupric concentrations, and two solution pH setpoints, in order to study a process for leaching copper concentrate in an acid chloride media at moderate temperature. Soluble copper was measured during the leaching experiments. The extraction data were analyzed in respect to the roles of major copper species present in the initial acidified leach media.
2. Materials and Methods
2.1. Chalcopyrite Concentrate Sample
Chalcopyrite concentrate was obtained from a flotation plant in Chile. The chemical composition of the chalcopyrite concentrate was determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES). The mineralogical composition of the concentrate was analyzed using quantitative X-ray diffraction (QXRD) being validated with quantitative evaluation of minerals by scanning electron microscopy (QEMSCAN).
2.2. Modeling Software
HSC version 6.0, Outotec’s chemical reaction and equilibrium software (Espoo, Finland), was used to forecast the distribution of copper chloride species and their thermodynamics of formation in the environment of the leaching solutions. The software is designed for numerous types of chemical reactions and equilibrium calculations in addition to process simulation on a standard computer [54].
2.3. Scanning Electron Microscope and Energy Dispersive Spectrometry
Images from scanning electron microscopy and energy dispersive spectrometry (SEM/EDS) of selected samples were used to study soluble minerals that may have precipitated during leaching experiments. Images were obtained on a Nova Nano field emission gun (FEG) SEM at the Universidad Católica del Norte, Chile. The EDS spectra were collected with an Oxford Instruments X-MAX 20 mm2 silicon drift detector (SDD) at beam energy of 20 keV.
2.4. Quantitative Evaluation of Minerals by Scanning Electron Microscopy
A selection of mineral samples was analyzed using automated mineralogy techniques (QEMSCAN®) housed at CISEM, Universidad Católica del Norte (UCN), Antofagasta, Chile. These samples comprise various rock fragments ranging from 2 to 10 cm in size. The larger samples were cut with a diamond tipped saw and analyzed on the unpolished surface. All samples were mounted in resin, polished, and carbon-coated prior to analysis. The analyses were made using a QEMSCAN® model E430 which is based on a ZEISS EVO 50 scanning electron microscope (SEM) combined with Bruker Series 4 energy dispersive spectrometry (EDS) detectors. Routine analysis was performed with a spot size of less than 1 μm at an operating voltage of 25 kV and a beam current of 5 nA. The standard 1000 counts per point were acquired, and this yields a detection limit of approximately 2 wt. % per element for each mineral classification. Measurements were performed using iMeasure v5.3.2 and data reduction using iDiscover v5.3.2. The samples were analyzed in field scan mode at a field size of 1500 μm (approximate magnification of 50×). Multiple analyses were made at different pixel spacing resolution, most commonly 10, 5, or 2 μm, to observe the fine-scaled textures. The backscattered electron levels were calibrated from 0 to 255, where quartz = 42, copper = 130, and gold = 232.
2.5. Leach Tests
Agitated leaching tests were performed using a double-jacketed glass reactor (2 L) fitted with a lid (this system is not sealed tightly). A simplified schematic of the agitated leaching reactor is shown in Figure 1. The leach reactors were designed and packed in order to minimize any solution losses and changes in the solution level inside the reactor as result of evaporation have been taken into account within the experimental results. A constant temperature of around 60 °C was set using a thermostatic bath. A stirring speed of 450 rpm (calculated from preliminary experiments) was used in all tests, providing a stable suspension of ore particles in the leach reactor.
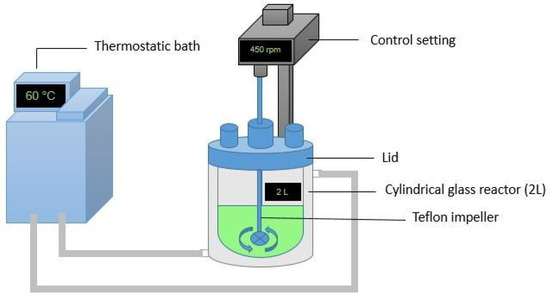
Figure 1.
A schematic diagram of the agitated leaching reactor used in this study.
When the desired temperature was achieved, 2.5 g chalcopyrite concentrate with d80 of 100 µm was added into the reactor containing 500 mL of defined media acidified with H2SO4. The leaching tests continued for five days (optimized in preliminary tests) with variables consisting of different concentrations of Cu2+ (0, 0.5, 1.5, and 2.5 g L−1), and different concentrations of Cl− (0, 5, 7, and 10 g L−1) at two different pH values of 1.0 and 2.0 (Figure 2). Each test was performed in duplicate and the average error was 0.97%. The pH values were monitored during all experiments, and when necessary, sulfuric acid was added to the system in order to keep the pH constant. The chemicals (H2SO4, NaCl, and CuSO4·5H2O) were analytical grade reagents (AR) and all solutions were made up using distilled water. The solution samples were collected at chosen time intervals and copper concentrations in leachates were determined using atomic absorption spectrometry (AAS) using a wavelength of 327.4 nm with a burner angle of 90°, and dilution to below 200 ppm according to protocol of the chemical laboratory. Copper extraction was calculated based on the dissolved copper concentration at a given time t and the initial copper present in the ore sample. pH values and redox potential (versus Ag/AgCl, 3.5 M KCl, reference electrode) were measured periodically using a pH/ORP meter (Hanna HI991001). Buffer solutions of pH 1.00, 1.68, and 4.01 were used to calibrate the pH as the ORP is validated with specific patterns of certain values.
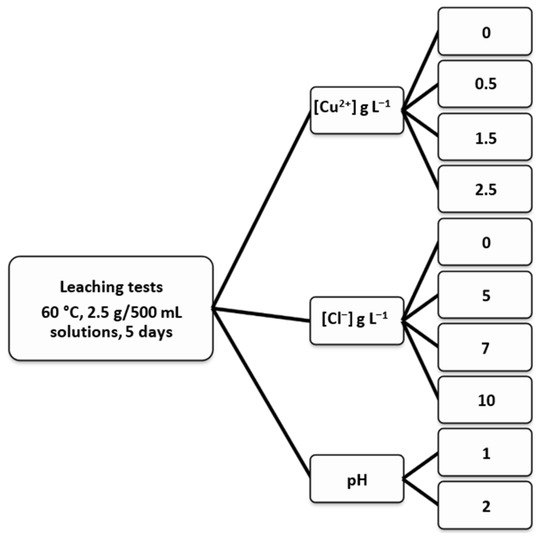
Figure 2.
Summary of the leaching tests and parameters investigated.
3. Results and Discussion
3.1. Chalcopyrite Concentrate Sample Analysis
An inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis was made as shown on Table 1, where copper contents reach a 24.5 wt. %. The quantitative X-ray diffraction (QXRD) analysis indicated that chalcopyrite (CuFeS2) (74 wt. %) is the only copper-bearing mineral. Other minerals were approximately melanterite (FeSO4·7H2O) (5 wt. %), calcite (CaCO3) (4.5 wt. %), quartz (SiO2) (2.5 wt. %), and pyrite (FeS2) (2 wt. %). These results were validated with quantitative evaluation of minerals by scanning electron microscopy (QEMSCAN) (Figure 3), where it was observed that the major mineral is chalcopyrite.

Table 1.
Chemical composition of chalcopyrite concentrate as determined using ICP-AES.
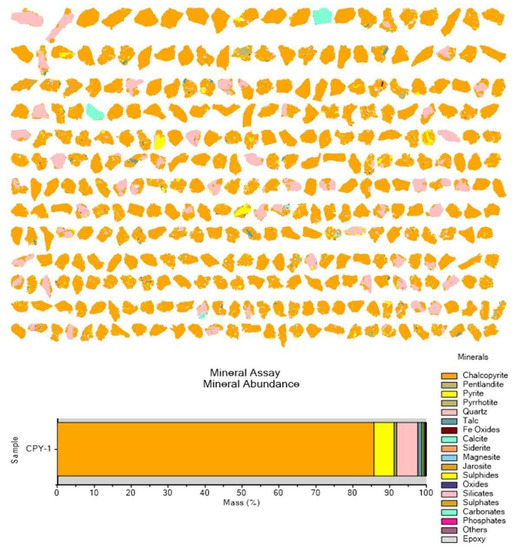
Figure 3.
QEMSCAN output of the chalcopyrite concentrate used as a feed in this study (CPY-1 corresponds to the identity of the feed sample).
3.2. Effect of Cu2+ and Cl− Concentration on Copper Extraction at Different pH
The experimental schematic employing different initial concentrations of Cu2+ (0, 0.5, 1.5, and 2.5 g L−1) and Cl− (0, 5, 7, and 10 g L−1) at an adjusted pH of 1 or 2 that was used to examine copper dissolution from chalcopyrite concentrate is presented in Figure 4.
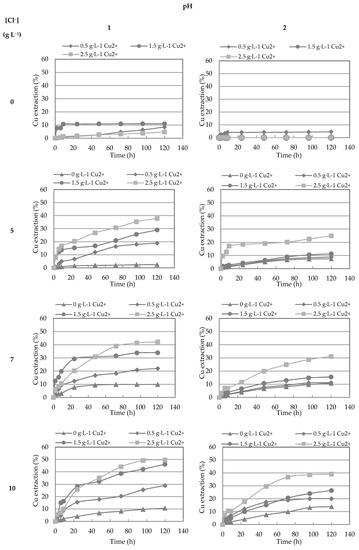
Figure 4.
Cu extraction (%) with different concentration of Cu2+ and Cl− at pH 1 and 2. ▲: 0 g/L Cu2+; ♦: 0.5 g/L Cu2+; ●: 1.5 g/L Cu2+; ■: 2.5 g/L Cu2+.
Figure 4 shows the effect of the Cl− ions on the rate of chalcopyrite dissolution, at pH 1 and 2. When Cl− concentration is increased to 5, 7, and 10 g L−1 in the presence of cupric ions, better extractions were obtained than when no chloride is added. This effect is more noticeable at pH 1 than at pH 2. In addition, the initial copper concentrations in the leaching solution did not significantly affect the copper extraction when Cl− is not present on the medium. This was observed both at pH 1 and 2 with extraction rate ranges of 5–11% and 0–5%, respectively. Accordingly, it is imperative for the presence of cupric ion to achieve a high leaching rate as noted by Velazquez [54] using similar conditions.
At 5 g L−1 Cl−, the maximum copper extraction in the presence of 2.5 g L−1 Cu2+ was 37.9% at pH 1 and 24.9% at pH 2. At pH 2, in presence of 0.5 and 1.5 g L−1 Cu2+, the copper extractions achieved were only 19.0% and 29.0%, respectively. At pH 1, it was observed that with the increase in cupric concentration, copper extraction increases, though this was not noticeable at pH 2. With an increase in chloride concentration to 7 g L−1, higher extractions were achieved. When the Cl− concentration was increased to 10 g L−1, the maximum copper extractions were obtained in the presence of 2.5 g L−1 of Cu2+ of 49.7% and 39.1%, at pH 1 and 2, respectively.
The best extraction was obtained at pH 1 using 2.5 g L−1 Cu2+ and 10 g L−1 Cl−, which could be attributed to a slower formation of the porous iron-rich reaction product layer as compared to a higher pH of 2, where the formation of this layer could impede chalcopyrite dissolution due to high ORP values in the range of 593–642, as suggested by other studies [3,28,37]. Although the presence of cupric ions is necessary for accelerated chalcopyrite dissolution, in the absence of the Cl− ions, the rates of dissolution are not enhanced by increasing the initial copper concentration. Due to this, it is essential to determine which CuCl species are formed at the employed concentrations.
It should be noted that when no cupric ions were added to the system upfront, the obtained copper extraction was greater when there was a high concentration of chloride, but still with a low value reached for 10 g L−1 Cl− (10.7%). This suggests that the copper dissolved during leaching is not of sufficient concentration for the significant formation of chloro-complexes of copper that facilitate higher extents of leaching.
As described above, the presence of chloride in the initial leach medium resulted in increased copper extraction compared with the chloride-free medium. This result is consistent with comparative data mostly obtained from ferric sulfate and ferric chloride leach media [55]. This result is also in agreement with the work by Velásquez et al. [41] and Miki and Nicol [56], who noted that chloride ion plays an important role via complex formation in the autooxidation of ferrous and cuprous ions.
3.3. Reaction Kinetics during Leaching
Based on the redox potential (ORP) values presented in the Table 2, it was observed that as the Cu2+ and Cl− concentrations increase, higher values of the redox potential were obtained. Increasing the solution to pH 2 resulted in the lowering of the ORP. These results are consistent with the lower Cu extractions at pH 2, and the increase in extraction at higher chloride concentrations. At pH 2 and 0 g L−1 Cl−, the measured values of redox potential are lower than 550 mV with low copper extraction (maximum 8.5% Cu) being obtained. According to Velásquez-Yévenez et al. [20], there is an optimal redox potential range that results in an increase in copper extraction (550–620 mV).

Table 2.
Range of redox potential or each test conducted.
3.4. Predicted Distribution of Major Copper Species and Free Chloride in Leach Media
Cuprous and chloride ions form a series of chloro-complex species [CuCln]1−n, where n ≤ 4 [57]. In our work, the chloride concentration used is low and the redox potential is high, therefore, the chloro-cuprous species are present in a low concentration in the studied systems. For that reason, chloro-cuprous complexes species are not considered in the speciation. There is less agreement about the complexation of cupric ions, since the predominant cupric species have been proposed to be both cupric ion and its chloro-complex, CuCl+ [58,59,60]. In this study, the cupric chloro-complex species concentrations were obtained using Excel with Solver, applying the constants of formation (K1–K2–K3–K4) given in Table 3 and obtained using Outotec’s chemical reaction and equilibrium software HSC (version 6.0). The procedure has been previously described by Torres et al. [61].

Table 3.
Formation data for cupric chloride complexes.
Figure 5 shows the predicted copper complexes at different cupric concentrations in a range of 0.1–2.5 g L−1 as a function of chloride concentration (5, 7, and 10 g L−1). In Figure 6, the predicted copper complexes at a different chloride concentration in a range of 0–10 g L−1 as a function of cupric concentration (0.5, 1.5, and 2.5 g L−1) are shown.
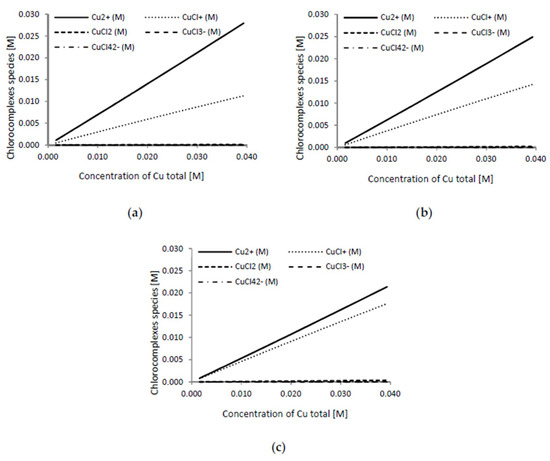
Figure 5.
Predicted copper complexes (M) at a cupric concentration range from 0.1 to 2.5 g L−1, and as a function of chloride concentration: (a) [Cl−] = 5 g L−1; (b) [Cl−] = 7 g L−1; and (c), [Cl−] = 10 g L−1.
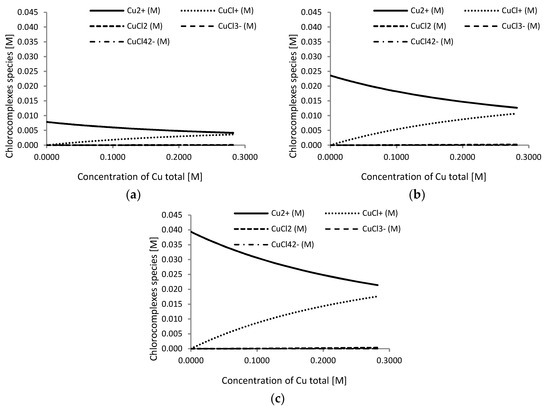
Figure 6.
Predicted copper complex (M) at a chloride concentration range from 0 to 10 g L−1, and as a function of cupric concentration: (a) [Cu2+] = 0.5 g L−1; (b) [Cu2+] = 1.5 g L−1; and (c) [Cu2+] = 2.5 g L−1.
Given the values of the formation constants of the chloro-complexes in Table 3, the main predicted species in the Cu2+/Cl− system in the considered intervals were CuCl+ and Cu2+ [62]. Our previous study indicated that the cupric monochloride complex ion CuCl+ might play a role in copper extraction from copper ore [63]. A model of copper speciation in the experimental range forecasts that for a single copper concentration (Figure 6) with increasing Cl− concentration, Cu2+ concentration diminished significantly while the chloro-complex species CuCl+ concentration increased. Both the cupric ion and the cupric chloride complex CuCl+ are able to dissolve chalcopyrite and the chemical reactions (5) and (6) are shown to be thermodynamically feasible under both standard conditions and at the temperature employed in this study (see Table 4).

Table 4.
Calculated Gibbs free energies for the proposed oxidation reactions.
3.5. Characterization of the Leached Residue
Figure 7 shows two characterized residue samples. Both samples were leached using the same leaching conditions in the initial presence of 2.5 g L−1 Cu2+ and at pH 1 but with different chloride addition (0 and 5 g L−1 Cl−). The SEM–EDS analyses were made in six different locations of the samples. Figure 7 shows two typical EDS spectra for each sample.
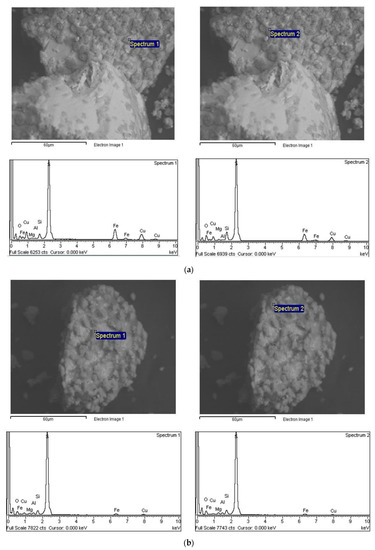
Figure 7.
SEM–EDS analysis of solid residues from leaching at pH 1 and 2.5 g L−1 Cu2+: (a) 0 g L−1 Cl−, and (b) 5 g L−1 Cl−.
In the leached residue, evidence of elemental sulfur formation was found for both samples. SEM images of selected samples (Figure 7a,b) show this phenomenon for different areas on the surface of chalcopyrite leaching residue. This was corroborated by the XRD data (see Figure 8), where sulfur was detected in the residue solid. Figure 8 only shows the pattern for the residue from the test at pH 1, 2.5 g L−1 Cu2+ and 5 g L−1 Cl−1. In addition to elemental sulfur, only trace quartz and unreacted chalcopyrite were detected. The EDS data also indirectly confirm that under the conditions employed, the copper and iron contents of the residue solids decrease. Here, the strong sulfur peak is predominantly associated with the elemental sulfur coating, while the weak iron/copper peaks are due to unreacted located chalcopyrite beneath this coating, which will also make a minor contribution to the sulfur peak from the associated sulfide ions.
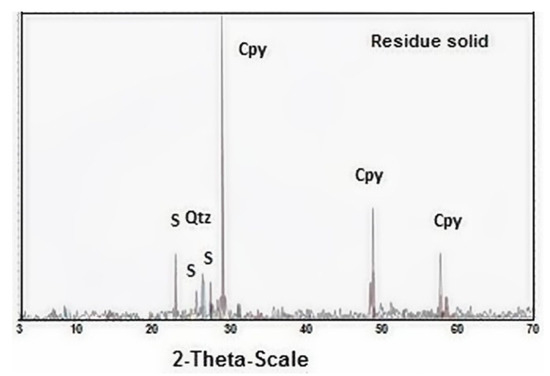
Figure 8.
X-ray diffraction patterns of the solid residue from leaching at pH 1 and 2.5 g L−1 Cu2+ and 5 g L−1 Cl−.
4. Conclusions
In the present paper, copper dissolution from chalcopyrite concentrate was studied using different chloride and cupric concentrations, and two initial solution pH setpoints. The obtained data were analyzed with respect to the roles of major copper species present in initial acidified leach media. The main findings of this study are as follows:
- The addition of Cl− to the system improves copper extractions, mainly in presence of a high concentration of Cu2+. The initial copper concentrations in the leaching solution did not significantly affect the copper extraction when Cl− is not present in the medium.
- In all the performed tests, better copper extractions were obtained at pH 1 compared with those at pH 2.
- As Cu2+ and Cl− concentrations increase, higher values for the redox potential are obtained.
- The cupric chloride complex species concentrations were obtained using Excel with Solver, applying the formation constants (K1–K2–K3–K4) and using HSC software. The predominant species in the Cu2+/Cl− system in the concentration intervals studied were CuCl+ and Cu2+.
- According to the modeling of copper speciation, for each copper concentration with increasing Cl− concentration, the Cu2+ concentration decreased significantly while the chloro-complex species CuCl+ concentration increased.
- Using the information obtained by speciation, the predominant cupric chloride complexes species were Cu2+ and CuCl+. These species contribute to the chalcopyrite leaching mechanism according chemical reactions where the thermodynamic feasibility was indicated by negative Gibbs free energies.
This study provides promising results for the application of chloride-bearing water sources in leaching processes, and more specifically, when potable water is scarce or costly to generate.
Author Contributions
Conceptualization, C.M.T. and P.C.H.; Formal analysis, O.O.H., F.J.J., Y.G. and M.I.A.; Investigation, C.M.T., P.C.H. and M.I.A.; Supervision, C.M.T. and P.C.H.; Validation, O.O.H., F.J.J. and Y.G.; Writing—original draft, C.M.T., F.J.J. and P.C.H.
Funding
This research was supported by FCAC 2018-UCN. The authors also want to thank to Project CORFO ING2030 16EN12-71940 for the financial support given for development of this research.
Acknowledgments
Assistance in ore preparation by the Mining Engineering and Chemical Engineering Departments of UA is acknowledged with thanks. The authors are grateful for the contribution of the Scientific Equipment Unit—MAINI of the Universidad Católica del Norte for aiding in generating data by automated electronic microscopy QEMSCAN® and for facilitating the chemical analysis of the solutions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barriga, F.; Palencia, I.; Carranza, F. The passivation of chalcopyrite subjected to ferric sulfate leaching and its reactivation with metal sulfides. Hydrometallurgy 1987, 19, 159–167. [Google Scholar] [CrossRef]
- Córdoba, E.; Muñoz, J.; Blázquez, M.; González, F.; Ballester, A. Passivation of chalcopyrite during its chemical leaching with ferric ion at 68 °C. Miner. Eng. 2009, 22, 229–235. [Google Scholar] [CrossRef]
- Hackl, R.P.; Dreisinger, D.B.; Peters, E.; King, J.A. Passivation of chalcopyrite during oxidative leaching in sulfate media. Hydrometallurgy 1995, 39, 25–48. [Google Scholar] [CrossRef]
- Harmer, S.L.; Thomas, D.F.; Gerson, A.R. The evolution of surface layers formed during chalcopyrite leaching. Geochim. Cosmochim. Acta 2006, 70, 4392–4402. [Google Scholar] [CrossRef]
- Watling, H. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate–chloride and sulfate–nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- Romero, R.; Mazuelos, I.P.; Carranza, F. Copper recovery from chalcopyrite concentrates by the BRISA process. Hydrometallurgy 2003, 70, 205–215. [Google Scholar] [CrossRef]
- Barr, G.; Defreyne, D.J.; Moore, R. The CESL process–successful refinery of a low grade copper concentrate. In Proceedings of the ALTA Copper 2000, Adelaide, Australia, 2–3 October 2000. [Google Scholar]
- Collins, M.; Buban, F.d.; Xue, T. Copper processing in the Dynatec miniplant. In Proceedings of the ALTA 1998 Copper Sulphides Symposium, Melbourne, Australia, 19 October 1998. [Google Scholar]
- King, J.A.; Dreisinger, D.B. Autoclaving of copper concentrates. In Proceedings of the Copper 95 International Conference: Electrorefining and Hydrometallurgy of Copper, Santiago, Chile, 26–29 November 1995; pp. 511–533. [Google Scholar]
- King, A.J.; Dreisinger, D.B.; Knight, D.A.; King, A.J.; Dreisinger, D.B.; Knight, D.A. The total pressure oxidation of copper concentrates. In Proceedings of the CIMM District 6 Meeting, Vancouver, B.C., Canada, In Proceedings of the CIMM District 6 Meeting, Vancouver, BC, Canada, 1 March 1994. [Google Scholar]
- Corrans, I.J.; Angove, J.E. Activation of a Mineral Species. Australian Patent 663523, 29 April 1993. [Google Scholar]
- Hourn, M.; Halbe, D. The NENATECH process: Results on Frieda River copper gold concentrates. In Proceedings of the International Conference of Randol Copper Hydromet Roundtable’99, Phoenix, AZ, USA, 10–13 October 1999. [Google Scholar]
- Nakazawa, H.; Fujisawa, H.; Sato, H. Effect of activated carbon on the bioleaching of chalcopyrite concentrate. Int. J. Miner. Proc. 1998, 55, 87–94. [Google Scholar] [CrossRef]
- Barta, L.; Buban, J.S.; Collins, M. Pressure leaching of chalcopyrite concentrates by Dynatec. In Proceedings of the Copper 99 Conference, Phoenix, AZ, USA, 10–13 October 1999. [Google Scholar]
- Carranza, F.; Palencia, I.; Romero, R. Silver catalyzed IBES process: Application to a Spanish copper-zinc sulphide concentrate. Hydrometallurgy 1997, 44, 29–42. [Google Scholar] [CrossRef]
- Hernández, P.C.E.; Taboada, M.E.; Herreros, O.O.; Graber, T.A.; Ghorbani, Y. Leaching of Chalcopyrite in Acidified Nitrate Using Seawater-Based Media. Minerals 2018, 8, 238. [Google Scholar]
- Gok, O.; Anderson, C.G. Dissolution of low-grade chalcopyrite concentrate in acidified nitrite electrolyte. Hydrometallurgy 2013, 134, 40–46. [Google Scholar] [CrossRef]
- Sokić, D.M.; Marković, B.; Živković, D. Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid. Hydrometallurgy 2009, 95, 273–279. [Google Scholar] [CrossRef]
- Sanchez, C.E.; Umetsu, Y.; Saito, F. Effect of iron powder on copper extraction by acid leaching of chalcopyrite concentrate. J. Chem. Eng. Jpn. 1996, 29, 720–722. [Google Scholar] [CrossRef][Green Version]
- Velásquez-Yévenes, L.; Nicol, M.; Miki, H. The dissolution of chalcopyrite in chloride solutions: Part 1. The effect of solution potential. Hydrometallurgy 2010, 103, 108–113. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.; Gerson, A. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Watling, H. Chalcopyrite hydrometallurgy at atmospheric pressure: 2. Review of acidic chloride process options. Hydrometallurgy 2014, 146, 96–110. [Google Scholar] [CrossRef]
- Dutrizac, J. The leaching of sulphide minerals in chloride media. Hydrometallurgy 1992, 29, 1–45. [Google Scholar] [CrossRef]
- Hirato, T.H. Majima, and Y. Awakura. The leaching of chalcopyrite with cupric chloride. Metall. Mater. Trans. B 1987, 18, 31–39. [Google Scholar] [CrossRef]
- Lu, J.; Dreisinger, D. Copper chloride leaching from chalcopyrite and bornite concentrates containing high levels of impurities and minor elements. Hydrometallurgy 2013, 138, 40–47. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Jeffrey, M.I.; Lawson, F. The effect of chloride ions on the dissolution of chalcopyrite in acidic solutions. Hydrometallurgy 2000, 56, 189–202. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Jeffrey, M.I.; Lawson, F. An electrochemical study of the effect of chloride ions on the dissolution of chalcopyrite in acidic solutions. Hydrometallurgy 2000, 56, 145–155. [Google Scholar] [CrossRef]
- Parker, A.; Klauber, A.; Kougianos, H.W.; van Bronswijk, W. An X-ray photoelectron spectroscopy study of the mechanism of oxidative dissolution of chalcopyrite. Hydrometallurgy 2003, 71, 265–276. [Google Scholar] [CrossRef]
- Atwood, G.E.; Curtis, H.C. Hydrometallurgical Process for the Production of Copper. U.S. Patent 3785944, 15 January 1974. [Google Scholar]
- Aroca, F.; Backit, A.; Jacob, J. CuproChlor® a hydrometallurgical technology for mineral sulphides leaching. In Hydroprocess 2012, Proceedings of the 4th International Seminar on Process Hydrometallurgy, Santiago, Chile, 12–13 July 2012; Casas, S., Ciminelli, V., Montes-Atenas, G., Stubina, N., Eds.; The Institute of Materials, Minerals and Mining: Santiago, Chile, 2012; pp. 96–180. [Google Scholar]
- Moyes, J.; Houllis, F. Intec base metal processes—Releasing the potential of chloride metallurgy technical update and commercialization status. In Chloride Metallurgy; Peek, E., Van Weert, G., Eds.; Metallurgical Society of the Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2002; pp. 577–593. [Google Scholar]
- Dalton, R.; Diaz, R.P.; Zunkel, A. The Cuprex metal extraction process: Recovering copper from sulfide ores. JOM 1991, 43, 51–56. [Google Scholar] [CrossRef]
- Hyvärinen, O.; Hämäläinen, M. HydroCopper™—A new technology producing copper directly from concentrate. Hydrometallurgy 2005, 77, 61–65. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, M.L.; Blázquez, F.G. Leaching of chalcopyrite with ferric ion. Part II: Effect of redox potential. Hydrometallurgy 2008, 93, 88–96. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, M.L.; Blázquez, F.G.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part III: Effect of redox potential on the silver-catalyzed process. Hydrometallurgy 2008, 93, 97–105. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Kitagawa, H.; Tsunekawa, M. Effect of solution composition on the optimum redox potential for chalcopyrite leaching in sulfuric acid solutions. Hydrometallurgy 2008, 91, 144–149. [Google Scholar] [CrossRef]
- Lundström, M.; Aromaa, J.; Forsén, O. Redox potential characteristics of cupric chloride solutions. Hydrometallurgy 2009, 95, 285–289. [Google Scholar] [CrossRef]
- Miki, H.; Nicol, M. The dissolution of chalcopyrite in chloride solutions. IV. The kinetics of the auto-oxidation of copper (I). Hydrometallurgy 2011, 105, 246–250. [Google Scholar] [CrossRef]
- Miki, H.; Nicol, M.; Velásquez-Yévenes, L. The kinetics of dissolution of synthetic covellite, chalcocite and digenite in dilute chloride solutions at ambient temperatures. Hydrometallurgy 2011, 105, 321–327. [Google Scholar] [CrossRef]
- Nicol, M.; Miki, H.; Velásquez-Yévenes, L. The dissolution of chalcopyrite in chloride solutions: Part 3. Mechanisms. Hydrometallurgy 2010, 103, 86–95. [Google Scholar] [CrossRef]
- Velásquez, L.; Miki, H.; Nicol, M. The dissolution of chalcopyrite in chloride solutions: Part 2, Effect of various parameters on the rate. Hydrometallurgy 2010, 103, 80–85. [Google Scholar] [CrossRef]
- Kekesi, T.; Isshiki, M. Anion Exchange for Ultra-High Purification of Transition Metals. Erzmetall 2003, 56, 59–67. [Google Scholar]
- Wilson, J.; Fisher, W. Cupric chloride leaching of chalcopyrite. JOM 1981, 33, 52–57. [Google Scholar] [CrossRef]
- Winand, R. Chloride hydrometallurgy. Hydrometallurgy 1991, 27, 285–316. [Google Scholar] [CrossRef]
- Muir, D. Basic principles of chloride hydrometallurgy. In Proceedings of the Chloride Metallurgy, 32nd Annual Hydrometallurgy Meeting, Montreal, Canada, 19–23 October 2002. [Google Scholar]
- Dutrizac, J.E. The kinetics of dissolution of chalcopyrite in ferric ion media. Metall. Trans. B 1978, 9, 431–439. [Google Scholar] [CrossRef]
- Berger, M.J.; Winand, R. Solubilities, densities and electrical conductivities of aqueous copper(I) and copper(II) chlorides in solutions containing other chlorides such as iron, zinc, sodium and hydrogen chlorides. Hydrometallurgy 1984, 12, 61–81. [Google Scholar] [CrossRef]
- Benner, B.; Roman, R. The dissolution of copper concentrates. Miner. Sci. Eng. 1973, 5, 3–24. [Google Scholar]
- Stephen, H.; Stephen, T. Solubilities of Inorganic and Organic Compounds; Binary systems: Pt. 1; Elsevier: Amsterdam, The Netherlands; Pergamon Press: Oxford, UK, 1963; Volume 1, pp. 14, 15, 184, 185. [Google Scholar]
- Christov, C. Thermodynamic study of the Na-Cu-Cl-SO4-H2O system at the temperature. J. Chem. Thermodynam. 2000, 32, 285–295. [Google Scholar] [CrossRef]
- Haung, H.-H. Estimation of Pitzer’s ion interaction parameters for electrolytes involved in complex formation using a chemical equilibrium model. J. Solut. Chem. 1989, 18, 1069–1084. [Google Scholar] [CrossRef]
- Lundström, M.; Aromaa, O.F.; Haavanlammi, L. Concentrated cupric chloride solutions: Possibilities offered in copper production. Acta Metallurgica. Slovaca. 2007, 13, 447–459. [Google Scholar]
- Velásquez, L. The Kinetics of Dissolution of Chalcopyrite in Chloride Media; Murdoch University: Perth, Australia, 2008; p. 291. [Google Scholar]
- HSC-Chemistry. Outokumpu Researcher Oy; HSC-Chemistry: Piori, Finland, 2006. [Google Scholar]
- Dutrizac, J. The dissolution of chalcopyrite in ferric sulfate and ferric chloride media. Metall. Trans. B 1981, 12, 371–378. [Google Scholar] [CrossRef]
- Miki, H.; Nicol, M. The kinetics of the copper-catalysed oxidation of iron (II) in chloride solutions. In Hydrometallurgy 2008 Sixth International Symposium; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2008; pp. 971–979. [Google Scholar]
- Ciavatta, L.; Iuliano, M. Copper (I) chloride complexes in aqueous solution. Ann. Chim. 1998, 88, 71–89. [Google Scholar]
- Lobo, M. Handbook of Electrolyte Solutions (Part B): Sodium Chloride-Aqueous Solutions at 25 C; Elsevier: New York, NY, USA, 1989. [Google Scholar]
- Lundström, M.; Aromaa, O.; Forsén, O.H.; Barker, M.H. Cathodic reactions of Cu2+ in cupric chloride solution. Hydrometallurgy 2007, 85, 9–16. [Google Scholar] [CrossRef]
- Lundström, M.; Aromaa, O.; Forsén, O.H.; Barker, M.H. Leaching of chalcopyrite in cupric chloride solution. Hydrometallurgy 2005, 77, 89–95. [Google Scholar] [CrossRef]
- Torres, C.; Taboada, T.; Graber, O.; Herreros, Y.G.; Watling, H. The effect of seawater based media on copper dissolution from low-grade copper ore. Miner. Eng. 2015, 71, 139–145. [Google Scholar] [CrossRef]
- Herreros, O.; Quiroz, H.; Longueira, G.F.; Viñals, J. Leaching of djurleite in Cu2+/Cl− media. Hydrometallurgy 2006, 82, 32–39. [Google Scholar] [CrossRef]
- Torres, C.M.; Taboada, T.A.; Graber, O.O.; Herreros, H.R.; Watling, D.W.S.; Hernández, P.C. Possible role of copper species in enhanced chalcopyrite dissolution. Hydroprocess. 2014. Available online: http://gecamin.com/hydroprocess/. (accessed on 8 October 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).