Improving the Quality of Ferruginous Chromite Concentrates Via Physical Separation Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chromite Samples
2.2. Particle Characterisation
2.3. Separation Processes
2.3.1. Spiral Concentrator Tests
2.3.2. Wet High Intensity Magnetic Concentrator Tests
2.3.3. Experimental Analysis
3. Result and Discussion
3.1. Characterisation Studies
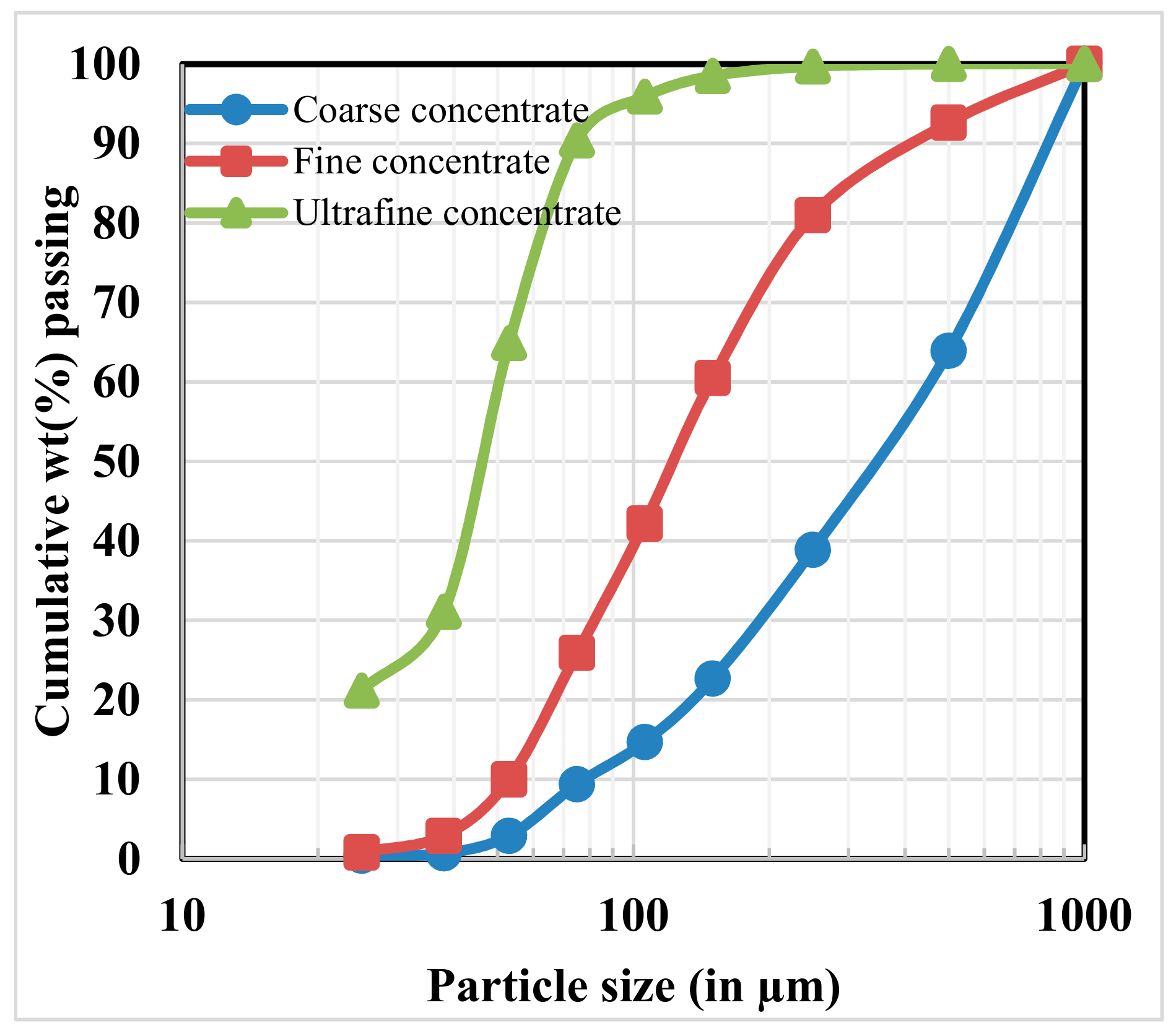
3.1.1. Particle Size and Chemical Analysis
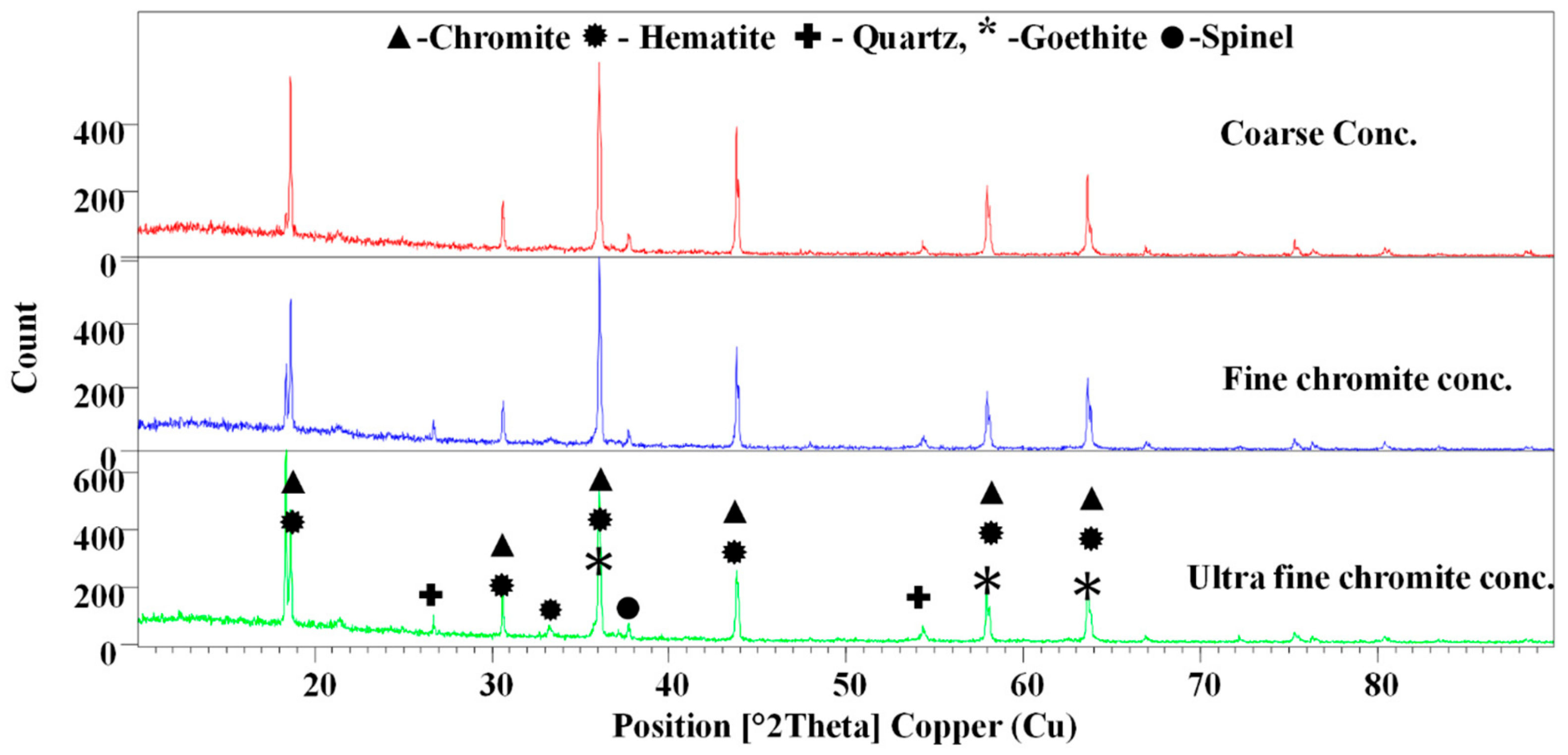
3.1.2. X-ray Diffraction Analysis
3.1.3. Heavy Liquid Separation Tests
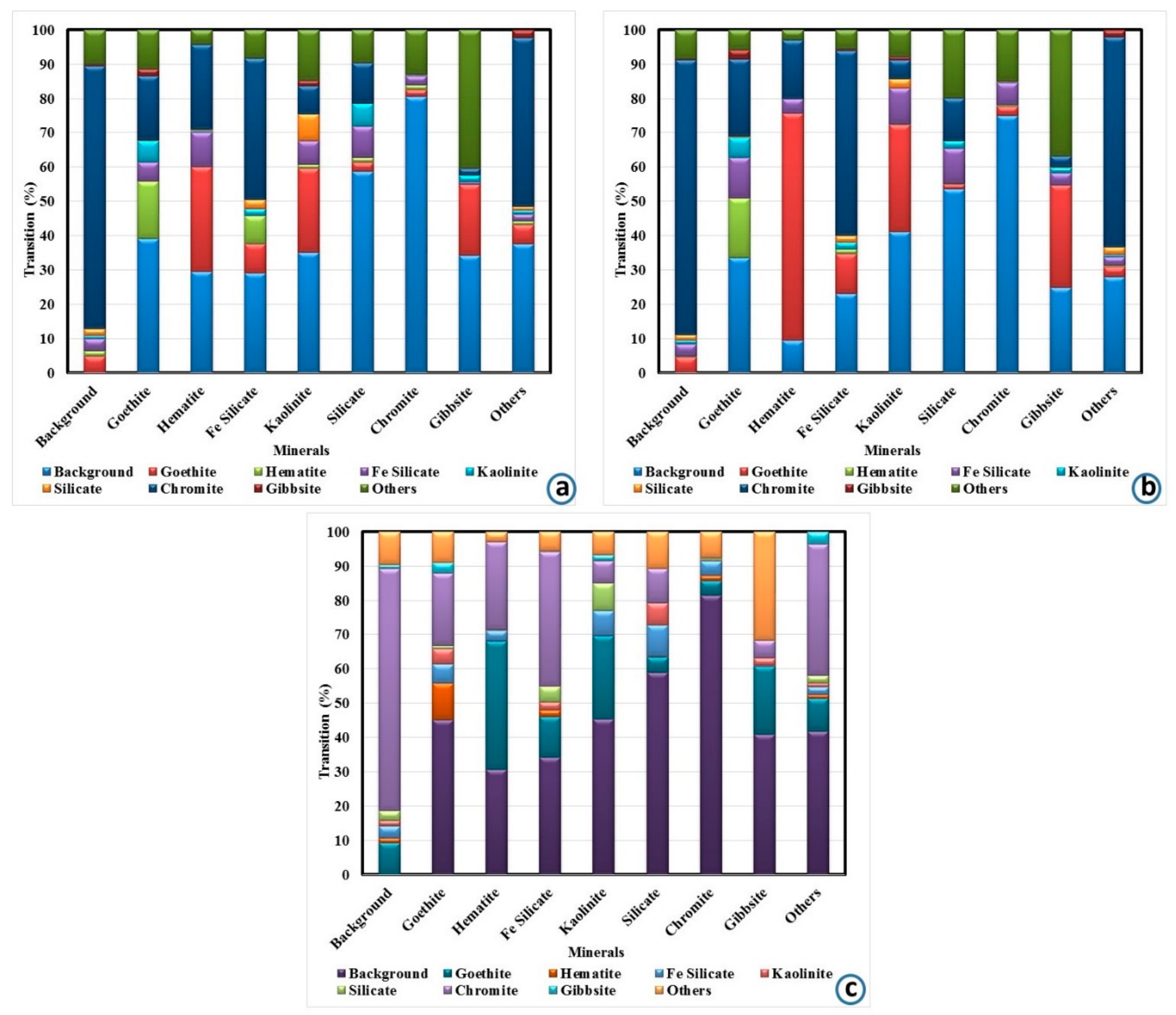
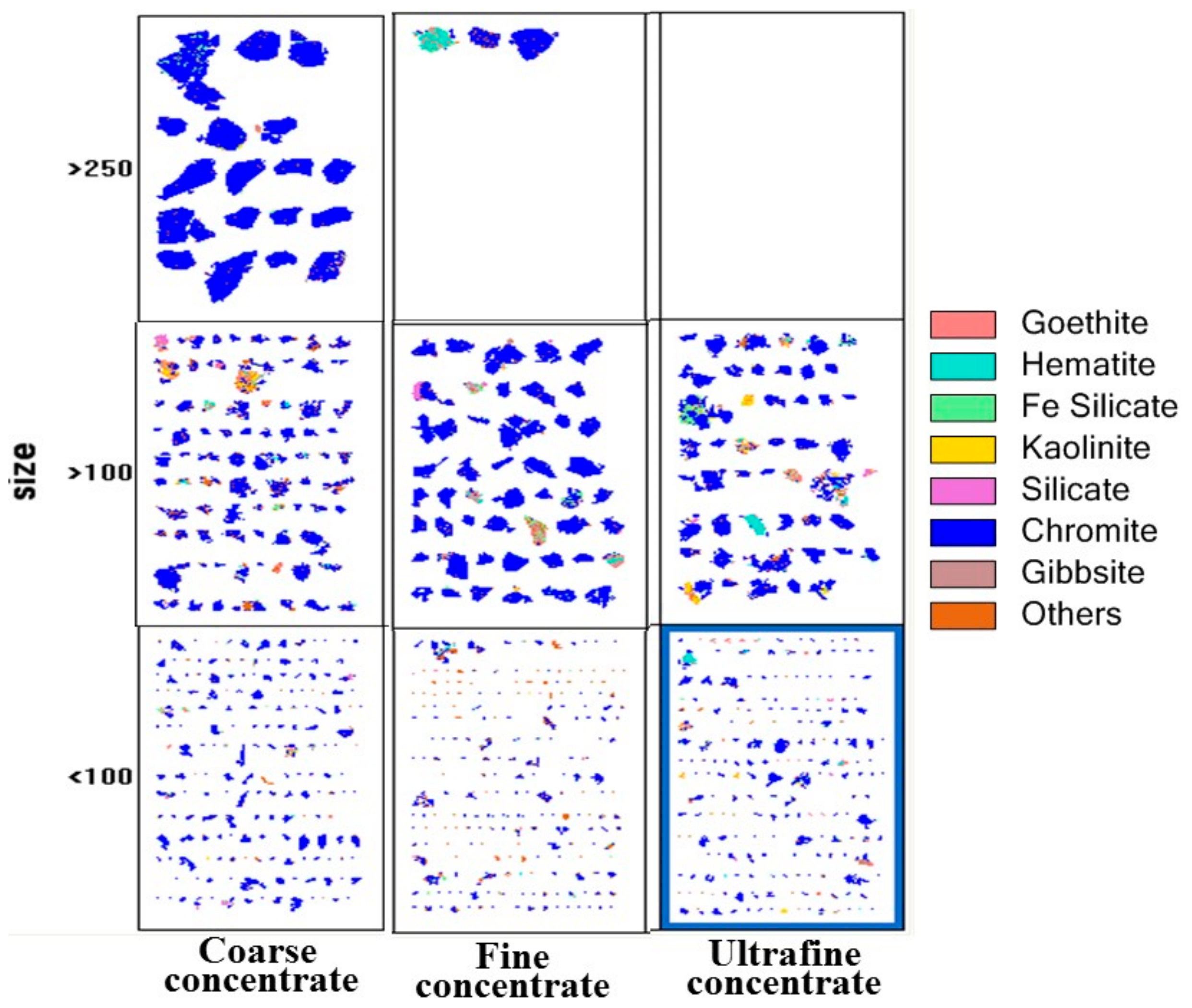
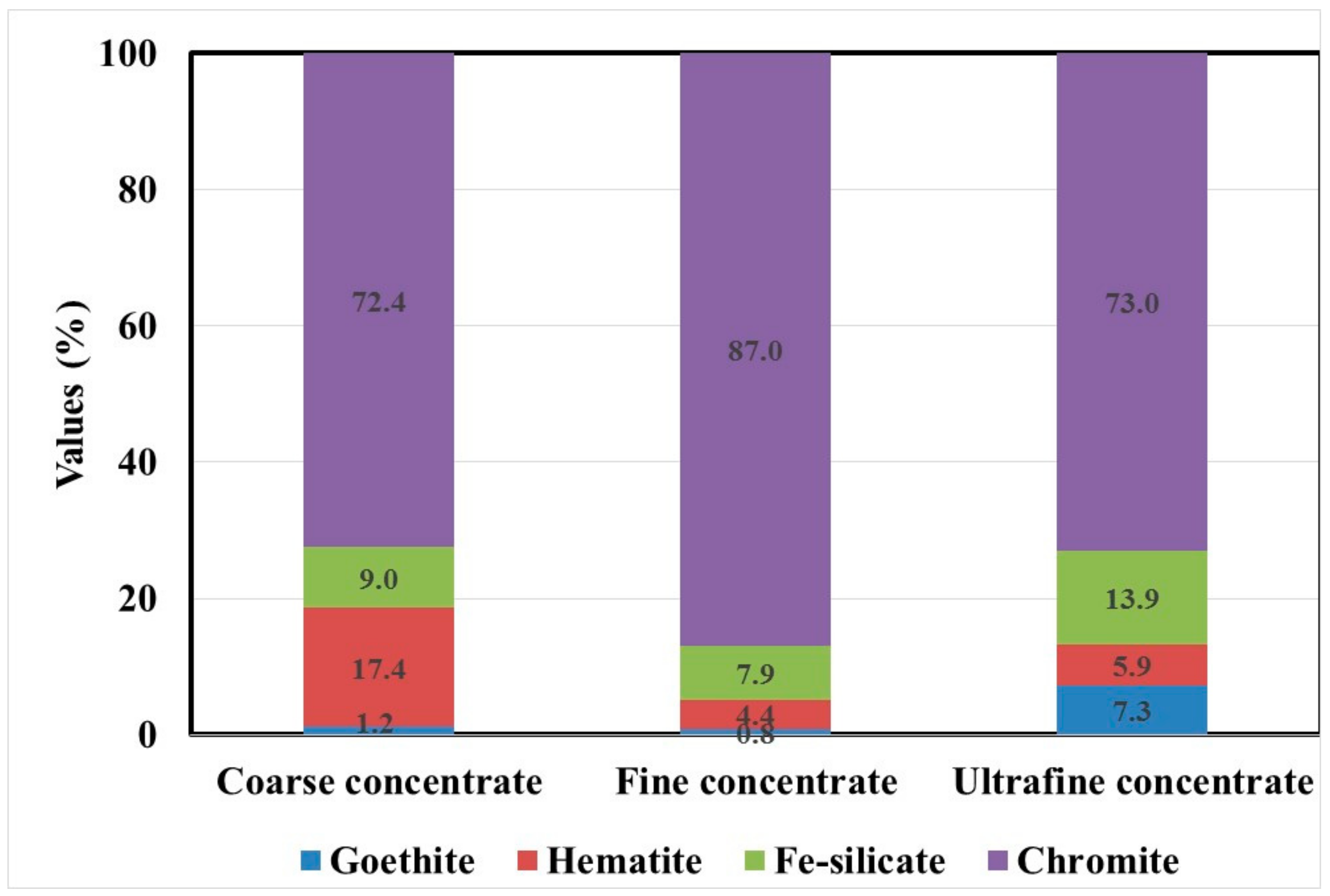
3.1.4. Mineralogical Studies
3.2. Separation Studies
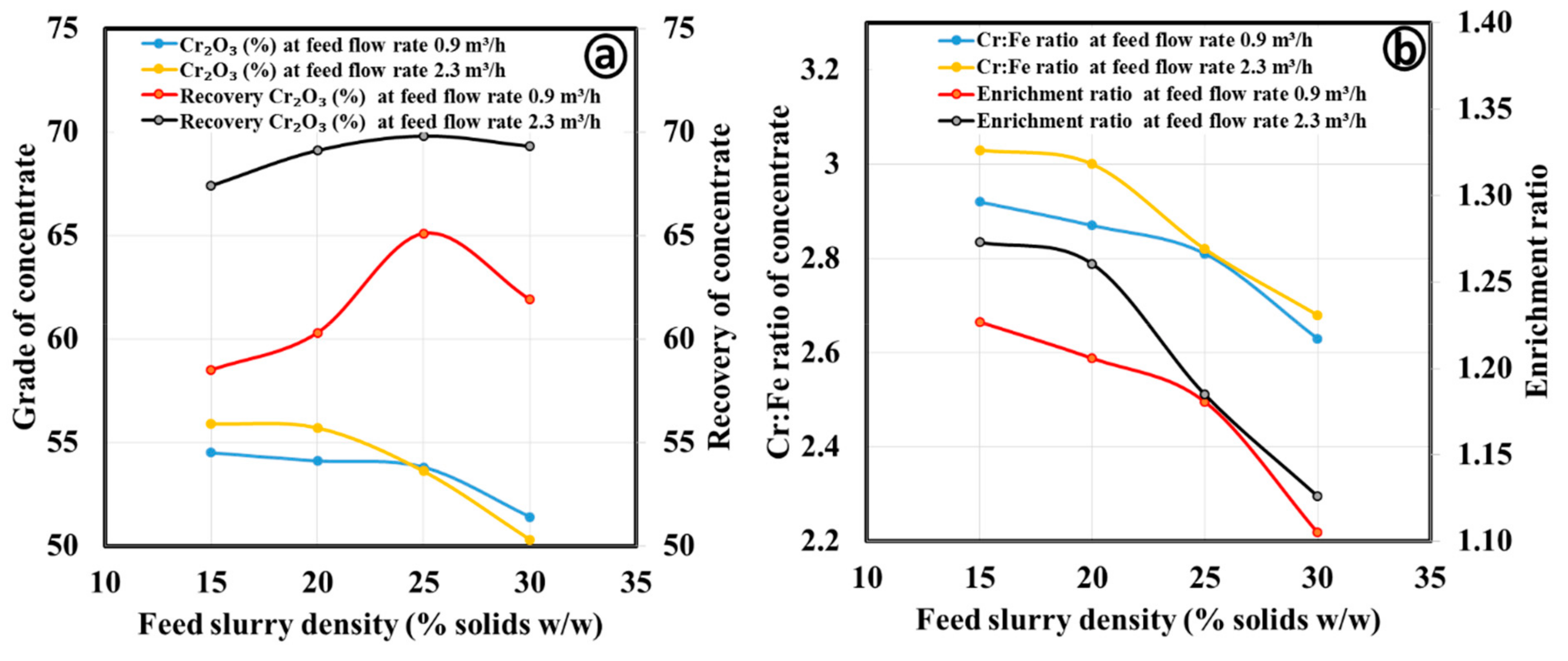
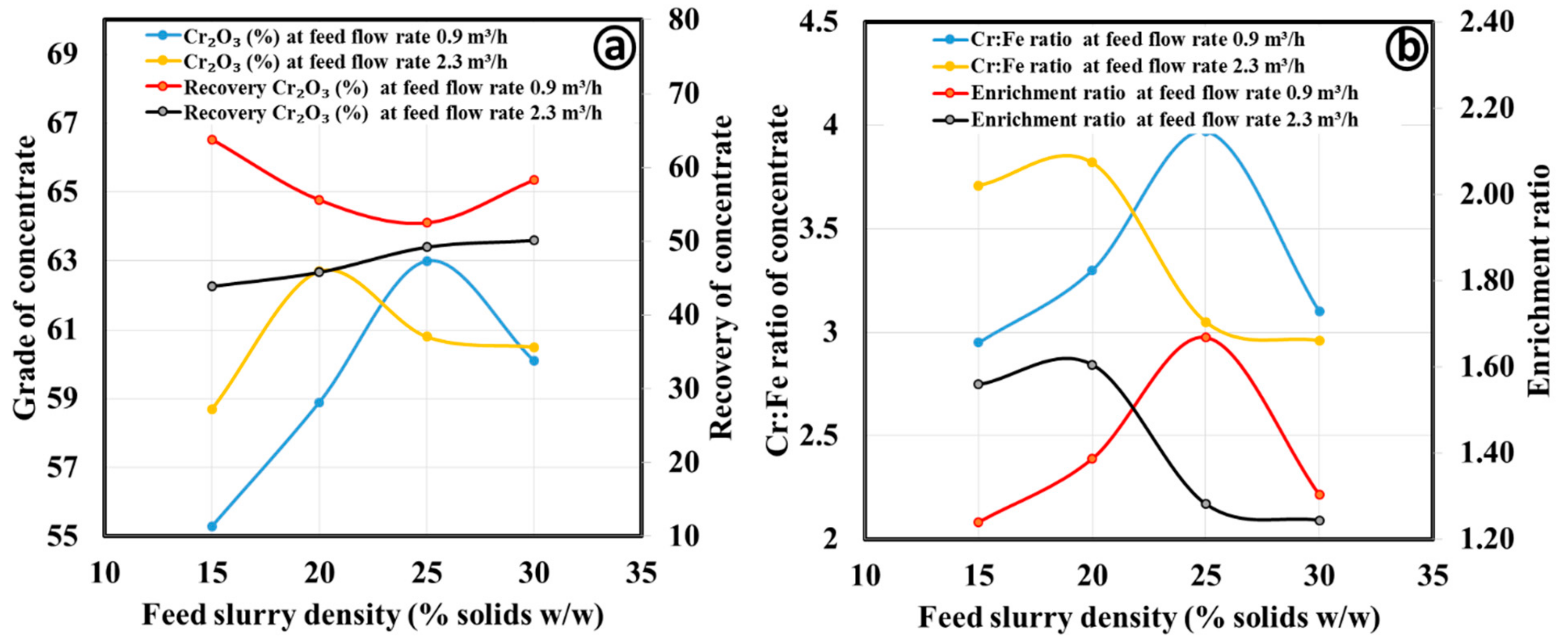
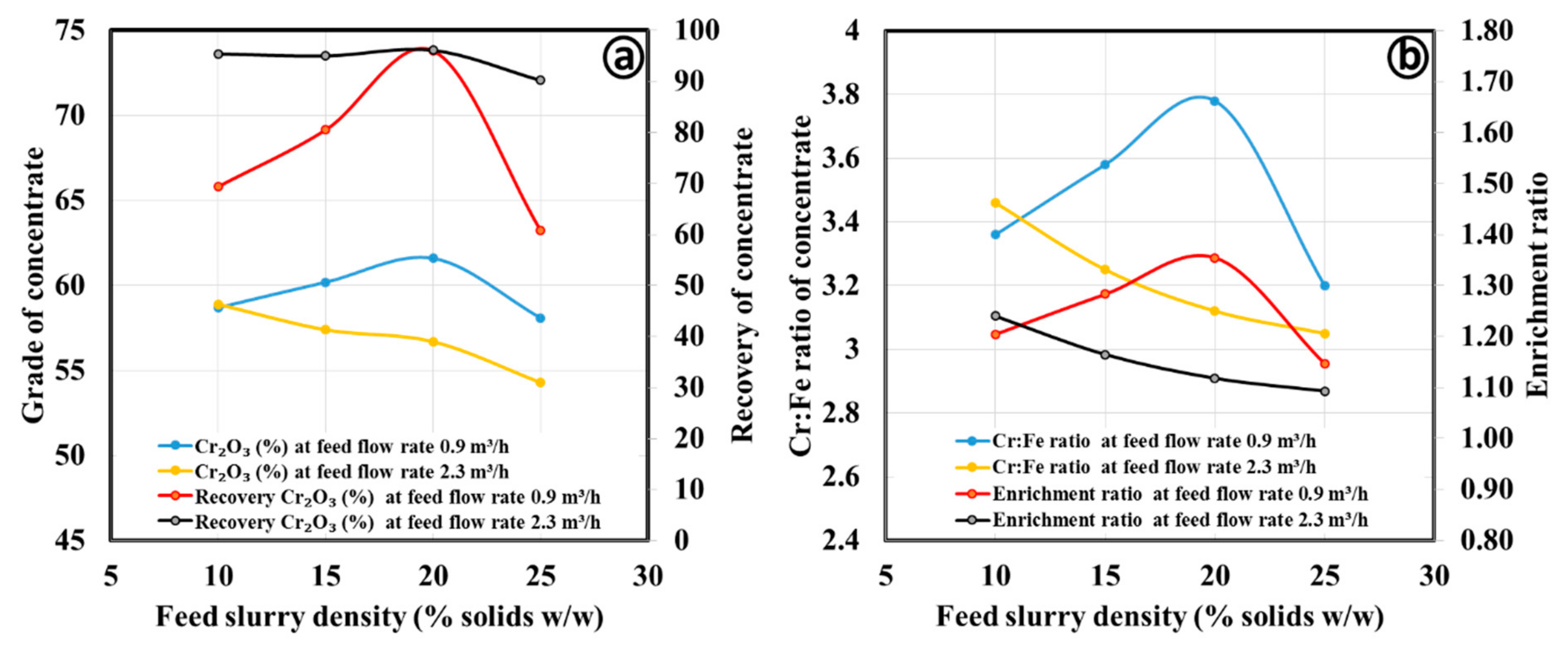
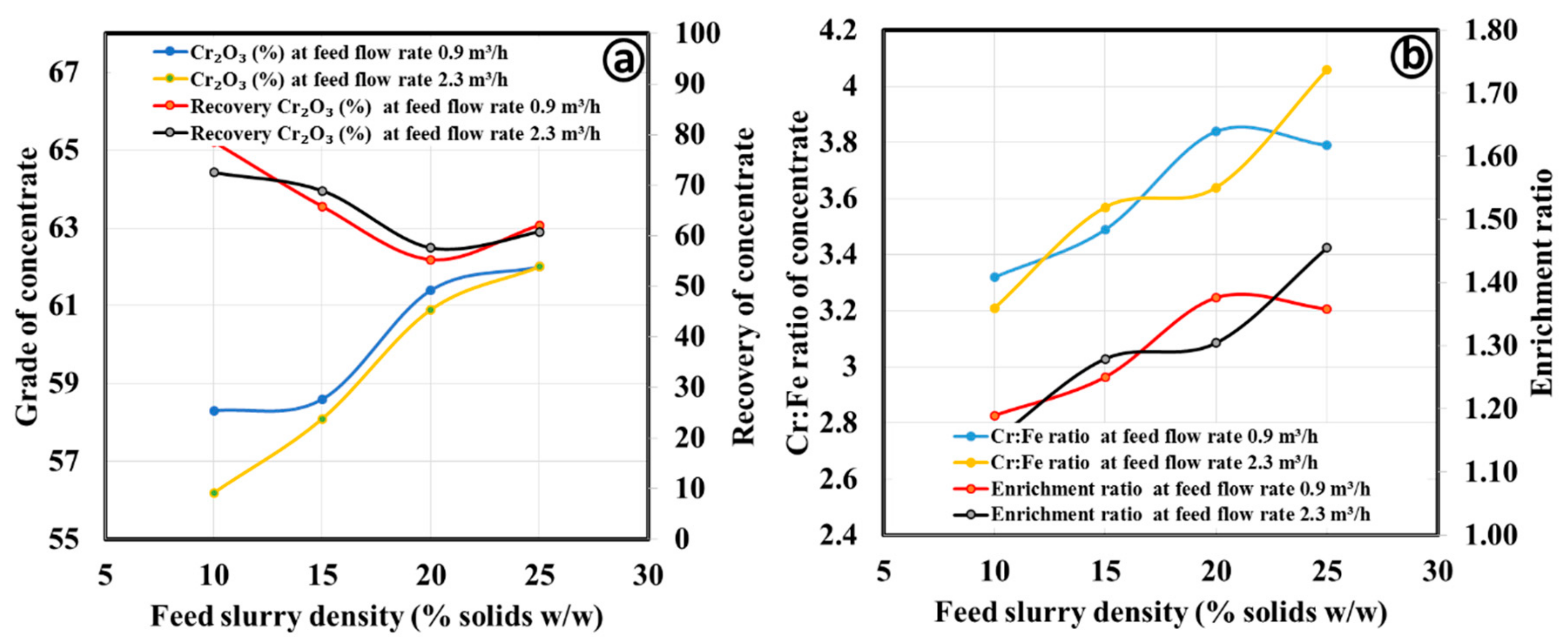
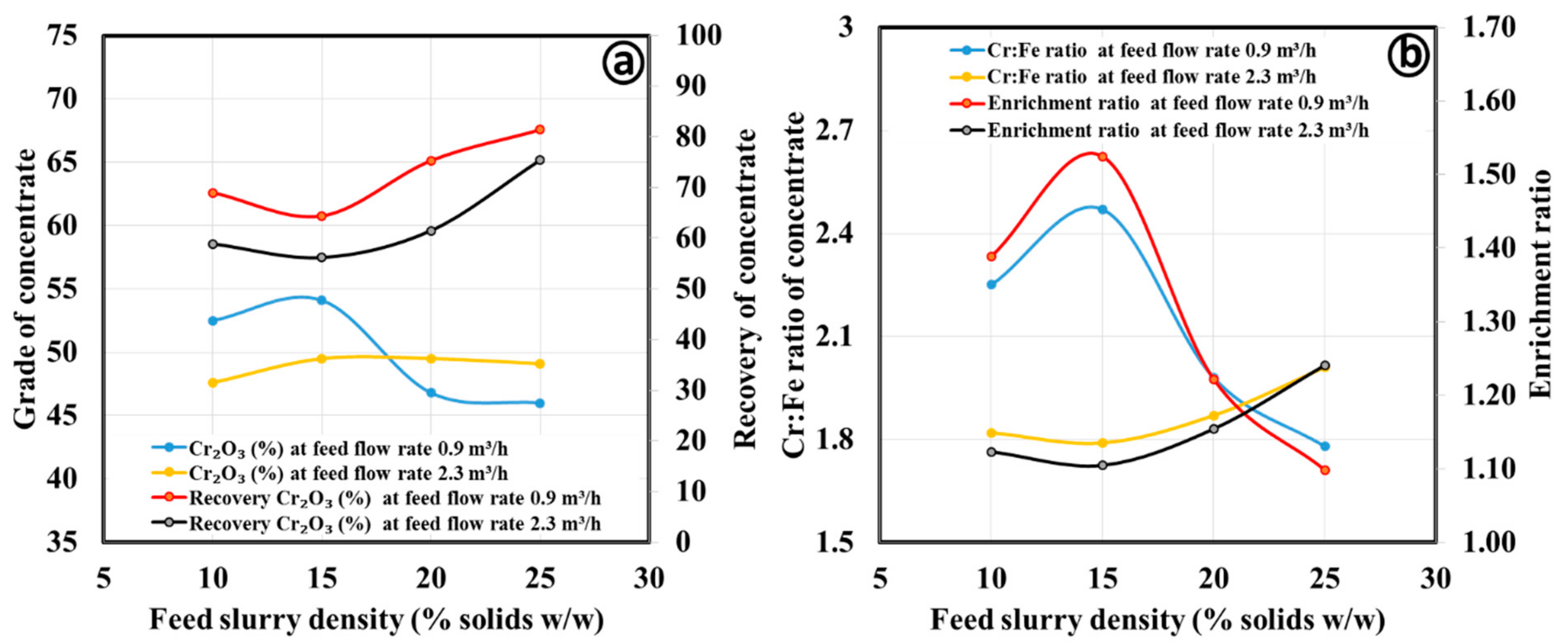
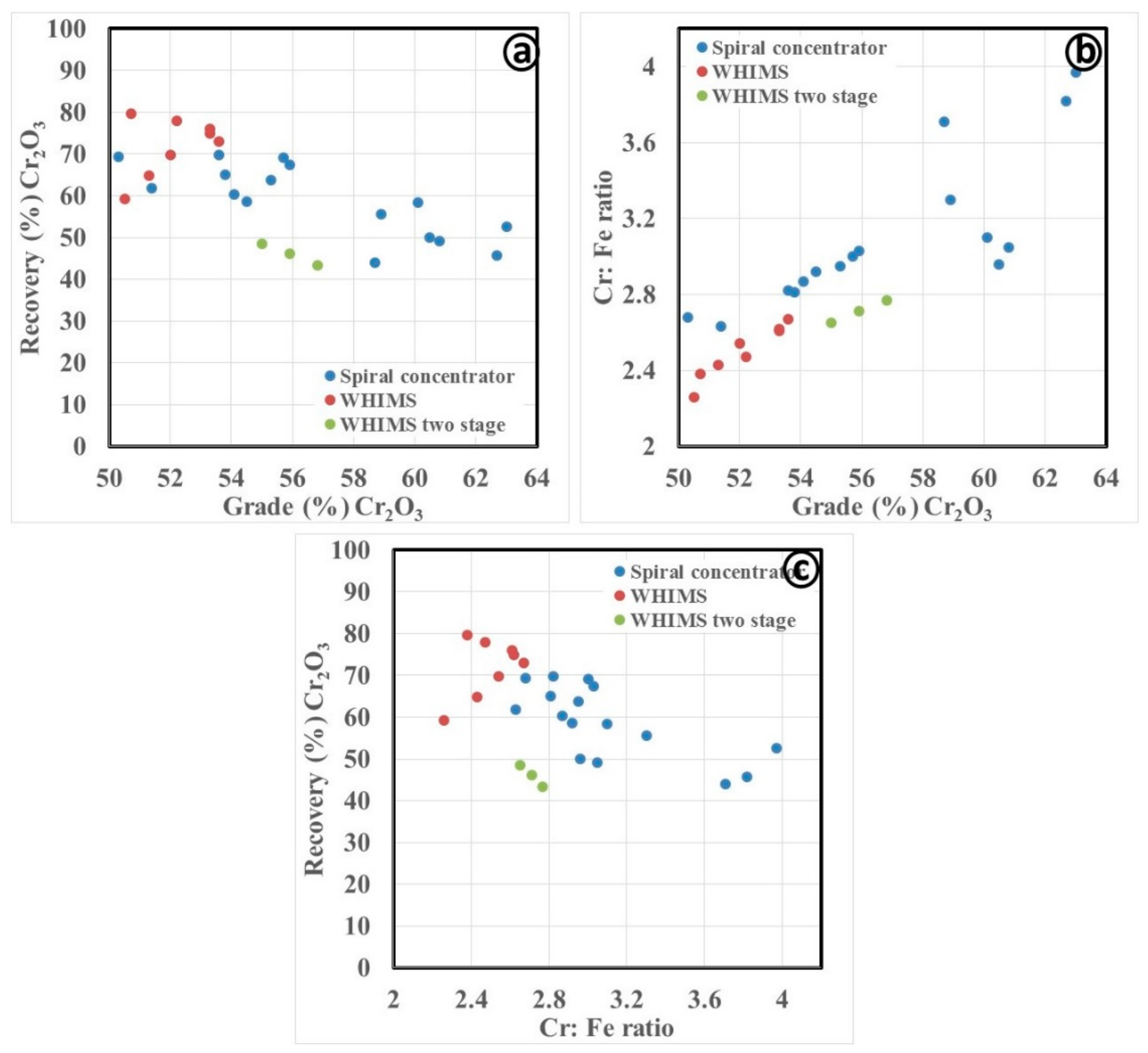
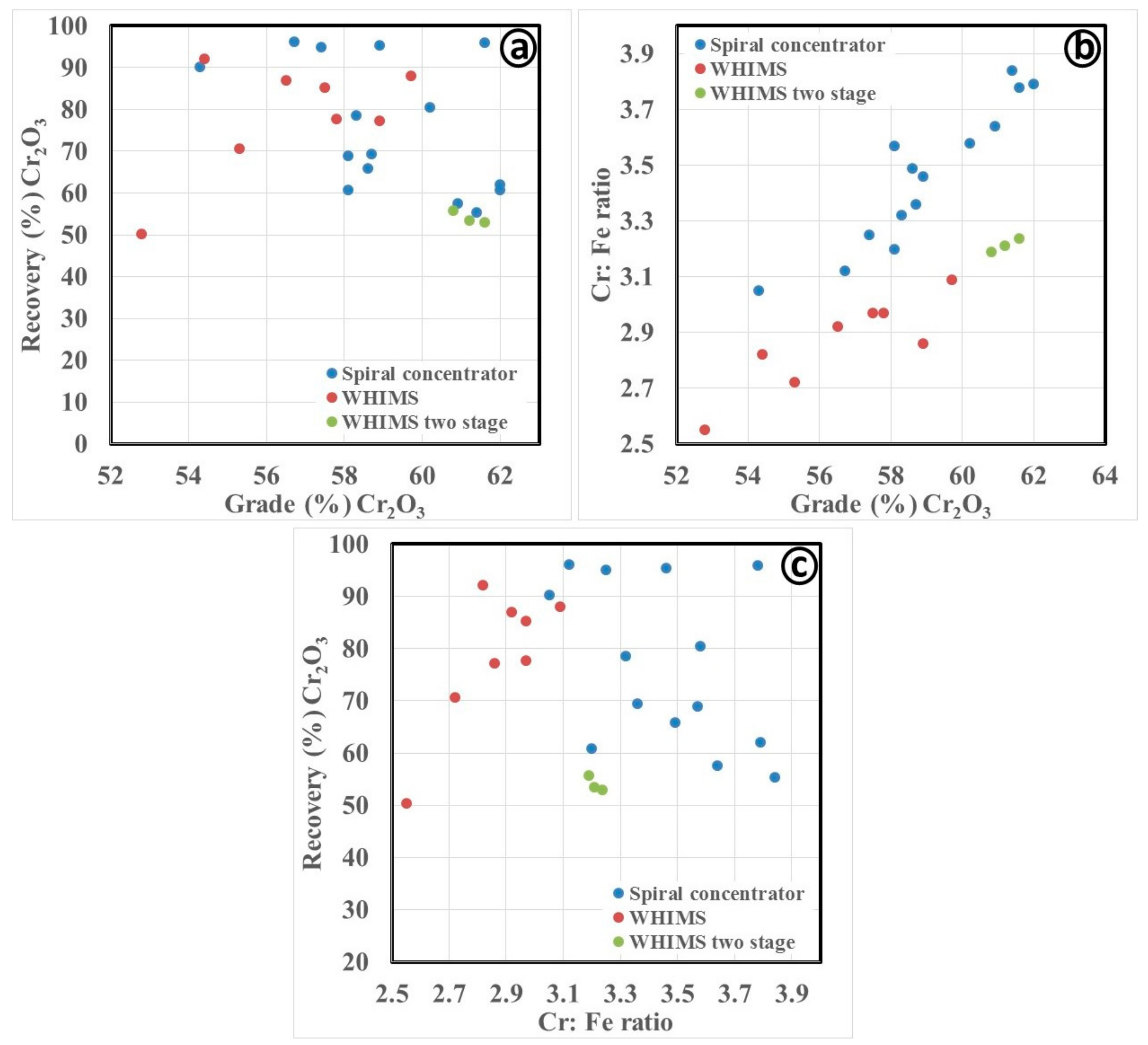
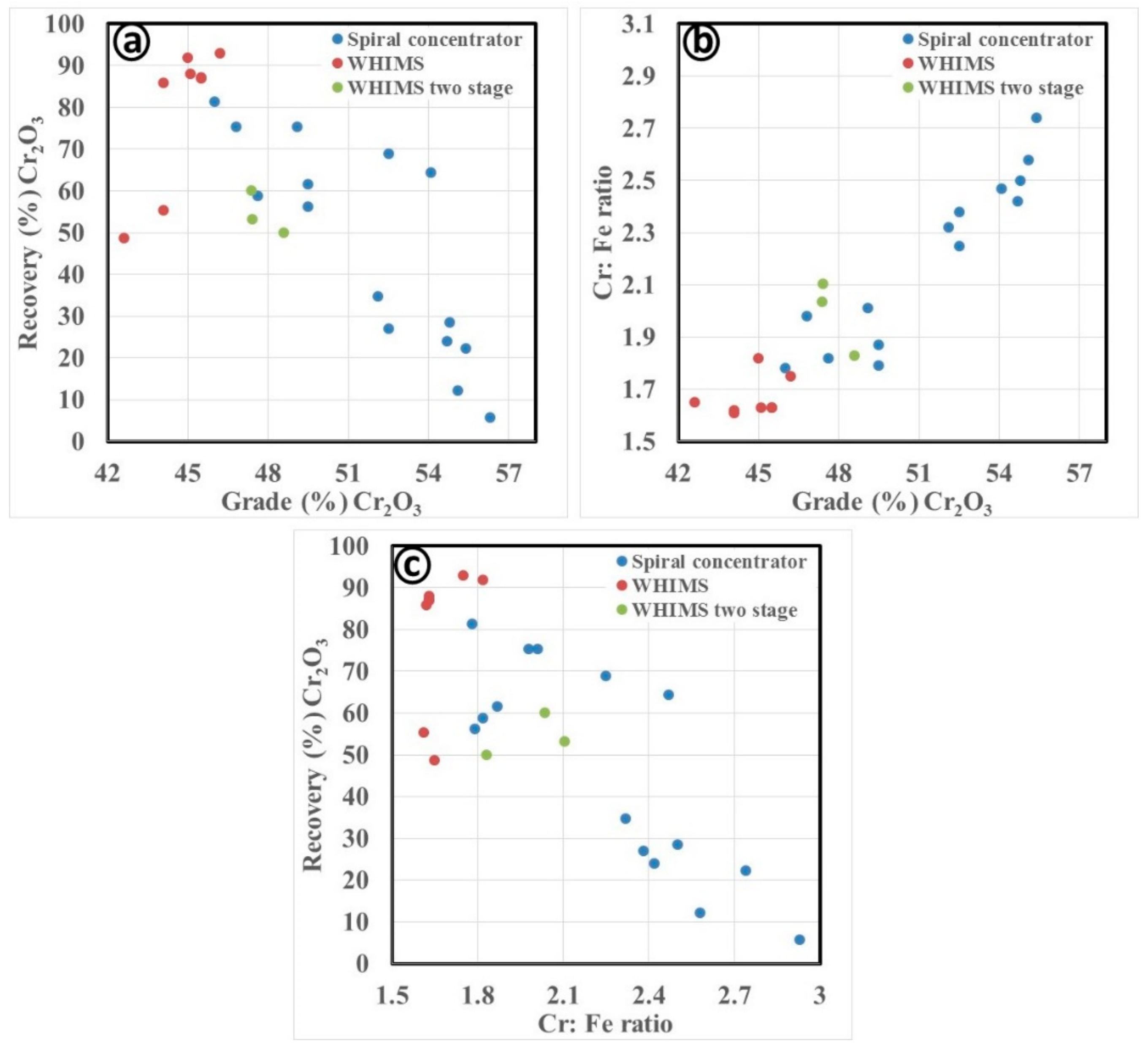
3.2.1. Gravity Separation by Spiral Concentrator
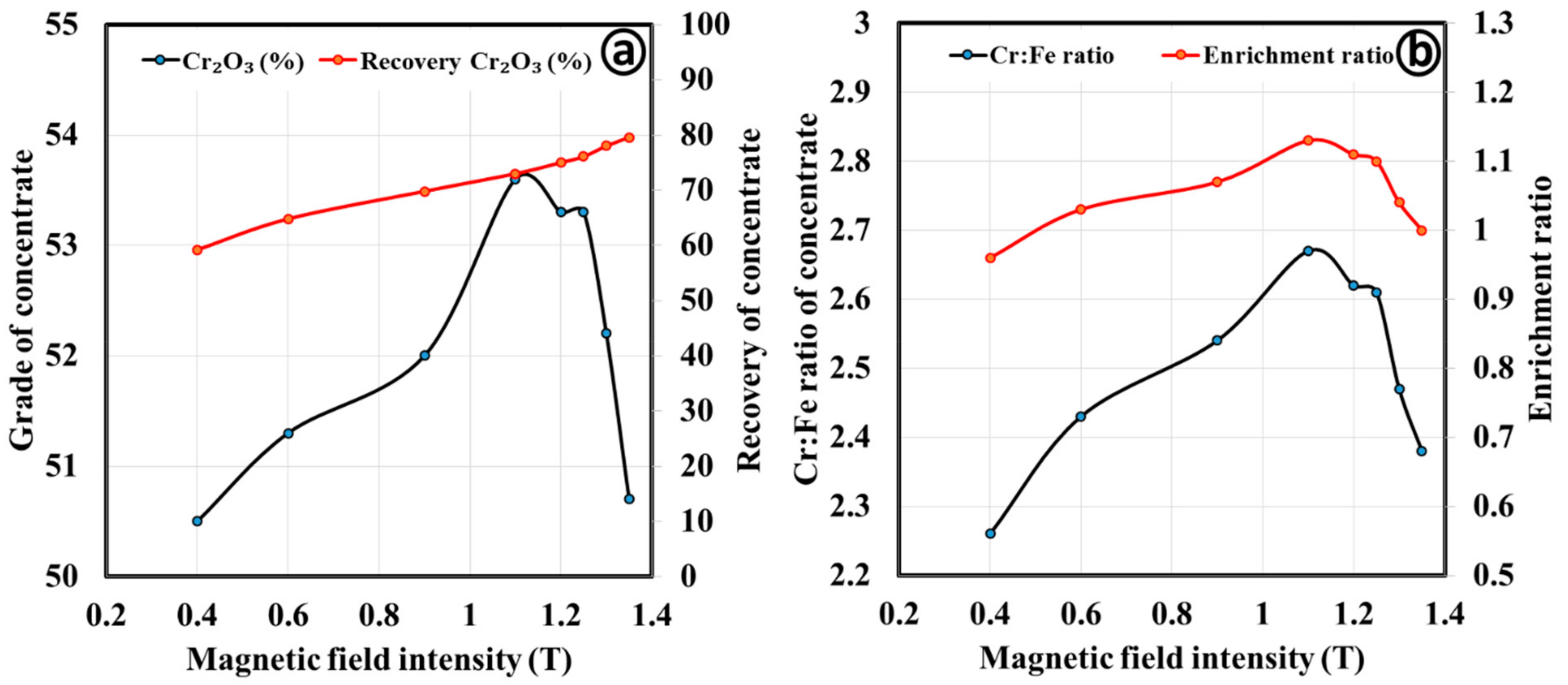
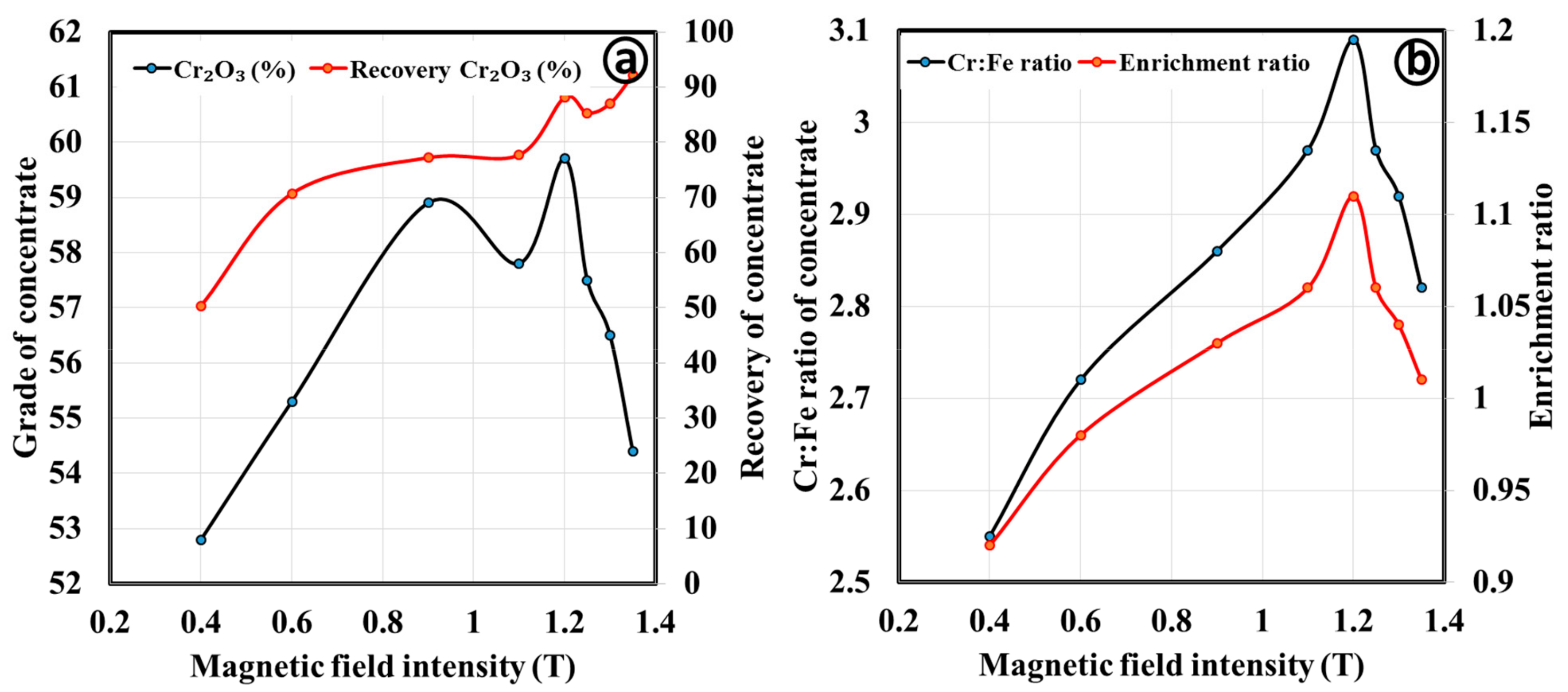
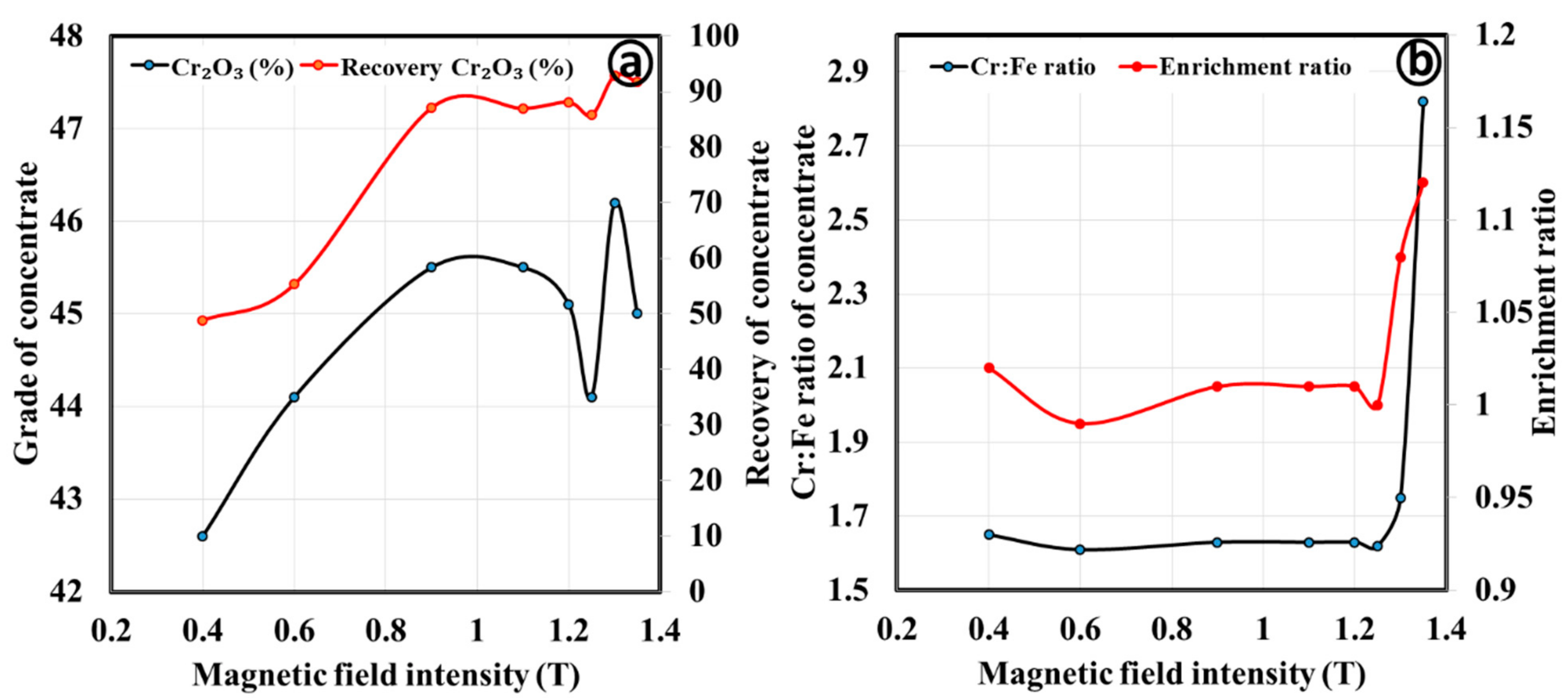
3.2.2. Magnetic Separation by Wet High Intensity Magnetic Separator
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azari, J. Effect of Chrome ore Qulaity on Ferrochrome Production Efficiency. In Proceedings of the Tenth International Ferroalloys Congress, Cape Town, South Africa, 1—4 February 2004. [Google Scholar]
- Dwarapudi, S.; Tathavadkar, V.; Rao, B.C.; Kumar, T.K.S.; Ghosh, T.K.; Denys, M. Development of Cold Bonded Chromite Pellets for Ferrochrome Production in Submerged Arc Furnace. ISIJ Int. 2013, 53, 9–17. [Google Scholar] [CrossRef]
- Nurjaman, F.; Subandrio, S.; Ferdian, D.; Suharno, B. Effect of basicity on beneficiated chromite sand smelting process using submerged arc furnace. AIP Conf. Proc. 2018, 1964, 020009. [Google Scholar]
- Murthy, Y.R.; Tripathy, S.K.; Kumar, C.R. Chrome ore beneficiation challenges & opportunities–A review. Miner. Eng. 2011, 24, 375–380. [Google Scholar]
- Nafziger, R.H. A review of the deposits and beneficiation of lower-grade chromite. J. S. Afr. Inst. Min. Metall. 1982, 82, 205–226. [Google Scholar]
- Atalay, U.; Özbayoğlu, G. Beneficiation and agglomeration of chromite—It’s application in Turkey. Miner. Process. Extr. Metall. Rev. 1992, 9, 185–194. [Google Scholar] [CrossRef]
- Das, A.; Sarkar, B. Advanced gravity concentration of fine particles: A review. Miner. Process. Extr. Metall. Rev. 2018, 39, 359–394. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Murthy, Y.R.; Singh, V. Characterisation and separation studies of Indian chromite beneficiation plant tailing. Int. J. Miner. Process. 2013, 122, 47–53. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Banerjee, P.; Suresh, N. Magnetic separation studies on ferruginous chromite fine to enhance Cr: Fe ratio. Int. J. Miner. Metall. Mater. 2015, 22, 217–224. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Murthy, Y.R. Modeling and optimization of spiral concentrator for separation of ultrafine chromite. Powder Technol. 2012, 221, 387–394. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Murthy, Y.R. Multiobjective optimisation of spiral concentrator for separation of ultrafine chromite. Int. J. Min. Miner. Eng. 2012, 4, 151–162. [Google Scholar] [CrossRef]
- Sunil, K.T.; Rama, M.Y.; Tathavadkar, V.; Mark, B.D. Efficacy of multi gravity separator for concentrating ferruginous chromite fines. J. Min. Metall. Min. 2012, 48, 39–49. [Google Scholar]
- Tripathy, S.K.; Murthy, Y.R.; Singh, V.; Suresh, N. Processing of Ferruginous Chromite Ore by Dry High-Intensity Magnetic Separation. Miner. Process. Extr. Metall. Rev. 2016, 37, 196–210. [Google Scholar] [CrossRef]
- Özgen, S. Modelling and optimization of clean chromite production from fine chromite tailings by a combination of multigravity separator and hydrocyclone. J. S. Afr. Inst. Min. Metall. 2012, 112, 387–394. [Google Scholar]
- Öztürk, F.D.; Temel, H.A. Beneficiation of Konya-Beyşehir Chromite for Producing Concentrates Suitable for Industry. JOM 2016, 68, 2449–2454. [Google Scholar] [CrossRef]
- Çiçek, T.; Cengizler, H.; Cöcen, İ. An efficient process for the beneficiation of a low grade chromite ore. Miner. Process. Extr. Metall. 2010, 119, 142–146. [Google Scholar] [CrossRef]
- Özgen, S. Clean chromite production from fine chromite tailings by combination of Multi Gravity Separator and Hydrocyclone. Sep. Sci. Technol. 2012, 47, 1948–1956. [Google Scholar] [CrossRef]
- Singh, R.K.; Dey, S.; Mohanta, M.K.; Das, A. Enhancing the Utilization Potential of a Low Grade Chromite Ore through Extensive Physical Separation. Sep. Sci. Technol. 2014, 49, 1937–1945. [Google Scholar] [CrossRef]
- Can, İ.B.; Özsoy, B.; Ergün, Ş.L. Developing an optimum beneficiation route for a low-grade chromite ore. Physicochem. Probl. Miner. Process. 2019, 55, 865–878. [Google Scholar]
- Tripathy, S.K.; Ramamurthy, Y.; Singh, V. Recovery of chromite values from plant tailings by gravity concentration. J. Miner. Mater. Charact. Eng. 2011, 10, 13. [Google Scholar] [CrossRef]
- Akar Sen, G. Application of Full Factorial Experimental Design and Response Surface Methodology for Chromite Beneficiation by Knelson Concentrator. Minerals 2016, 6, 5. [Google Scholar] [CrossRef]
- Çiçek, T.; Cöcen, I.; Engin, V.T.; Cengizler, H.; Şen, S. Technical and economical applicability study of centrifugal force gravity separator (MGS) to Kef chromite concentration plant. Miner. Process. Extr. Metall. 2008, 117, 248–255. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Bhoja, S.K.; Murthy, Y.R. Processing of chromite ultra-fines in a water only cyclone. Int. J. Min. Sci. Technol. 2017, 27, 1057–1063. [Google Scholar] [CrossRef]
- Aslan, N. Application of response surface methodology and central composite rotatable design for modeling and optimization of a multi-gravity separator for chromite concentration. Powder Technol. 2008, 185, 80–86. [Google Scholar] [CrossRef]
- Foucaud, Y.; Dehaine, Q.; Filippov, L.O.; Filippova, I.V. Application of Falcon Centrifuge as a Cleaner Alternative for Complex Tungsten Ore Processing. Minerals 2019, 9, 448. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Filippov, L.; Fornasiero, D. Pre-concentration of nickel in laterite ores using physical separation methods. Miner. Eng. 2019, 141, 105892. [Google Scholar] [CrossRef]
- Altın, G.; İnal, S.; Alp, İ. Recovery of Chromite from Processing Plant Tailing by Vertical Ring and Pulsating High-Gradient Magnetic Separation. MT Bilimsel 2018, 13, 23–35. [Google Scholar]
- Ayinla, K.I.; Baba, A.A.; Tripathy, B.C.; Ghosh, M.K.; Dwari, R.K.; Padhy, S.K. Enrichment of a Nigerian chromite ore for metallurgical application by dense medium flotation and magnetic separation. Metall. Res. Technol. 2019, 116, 324. [Google Scholar] [CrossRef]
- Subandrio, S.; Dahani, W.; Alghifar, M.; Purwiyono, T.T. Enrichment Chromite Sand Grade Using Magnetic Separator. IOP Conf. Ser. Mater. Sci. Eng. 2019, 588, 012033. [Google Scholar] [CrossRef]
- Gupta, P.; Bhandary, A.K.; Chaudhuri, M.G.; Mukherjee, S.; Dey, R. Kinetic Studies on the Reduction of Iron Oxides in Low-Grade Chromite Ore by Coke Fines for Its Beneficiation. Arab. J. Sci. Eng. 2018, 43, 6143–6154. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Singh, V.; Ramamurthy, Y. Improvement in Cr:Fe Ratio of Indian Chromite Ore for Ferro Chrome Production. Int. J. Min. Eng. Miner. Process. 2012, 1, 101–106. [Google Scholar]
- Güney, A.; Onal, G.; Çelik, M.S. A new flowsheet for processing chromite fines by column flotation and the collector adsorption mechanism. Miner. Eng. 1999, 12, 1041–1049. [Google Scholar] [CrossRef]
- Ucbas, Y.; Bozkurt, V.; Bilir, K.; Ipek, H. Concentration of chromite by means of magnetic carrier using sodium oleate and other reagents. Physicochem. Probl. Miner. Process. 2014, 50, 767–782. [Google Scholar]
- Ucbas, Y.; Bozkurt, V.; Bilir, K.; Ipek, H. Separation of Chromite from Serpentine in Fine Sizes using Magnetic Carrier. Sep. Sci. Technol. 2014, 49, 946–956. [Google Scholar] [CrossRef]
- Gallios, G.P.; Deliyanni, E.A.; Peleka, E.N.; Matis, K.A. Flotation of chromite and serpentine. Sep. Purif. Technol. 2007, 55, 232–237. [Google Scholar] [CrossRef]
- Seifelnasr, A.A.; Tammam, T. Flotation behavior of Sudanese chromite ores. J. Eng. Sci. Fac. Eng. Assiut Univ. 2011, 39, 649–661. [Google Scholar]
- Alesse, V.; Belardi, G.; Freund, J.; Piga, L.; Shehu, N. Acidic medium flotation separation of chromite from olivine and serpentine. Min. Metall. Explor. 1997, 14, 26–35. [Google Scholar] [CrossRef]
- Güney, A.; Atak, S. Separation of chromite from olivine by anionic collectors. Fizykochem. Probl. Miner. 1997, 31, 99–106. [Google Scholar]
- Panda, L.; Banerjee, P.K.; Biswal, S.K.; Venugopal, R.; Mandre, N.R. Modelling and optimization of process parameters for beneficiation of ultrafine chromite particles by selective flocculation. Sep. Purif. Technol. 2014, 132, 666–673. [Google Scholar] [CrossRef]
- Devasahayam, S. Predicting the liberation of sulfide minerals using the breakage distribution function. Miner. Process. Extr. Metall. 2015, 36, 136–144. [Google Scholar] [CrossRef]
- Das, S.K. Quantitative mineralogical characterization of chrome ore beneficiation plant tailing and its beneficiated products. Int. J. Miner. Metall. Mater. 2015, 22, 335–345. [Google Scholar] [CrossRef]
- Dixit, P.; Tiwari, R.; Mukherjee, A.K.; Banerjee, P.K. Application of response surface methodology for modeling and optimization of spiral separator for processing of iron ore slime. Powder Technol. 2015, 275, 105–112. [Google Scholar] [CrossRef]
- Dehaine, Q.; Filippov, L.O. Modelling heavy and gangue mineral size recovery curves using the spiral concentration of heavy minerals from kaolin residues. Powder Technol. 2016, 292, 331–341. [Google Scholar] [CrossRef]
- Sadeghi, M.; Bazin, C.; Renaud, M. Effect of wash water on the mineral size recovery curves in a spiral concentrator used for iron ore processing. Int. J. Miner. Process. 2014, 129, 22–26. [Google Scholar] [CrossRef]

| Parameters | Spiral Design Types | |
|---|---|---|
| HG10i | FM1 | |
| Spiral length (m) | 2.84 | 3.12 |
| Trough diameter (mm) | 580 | 685 |
| Trough height/pitch (mm) | 350 | 360 |
| Number of turns | 8 | 8 |
| Slurry flow rate (m3/h) | 0.9–2.3 | 0.9 and 2.3 |
| Slurry pulp density (wt.%) | 20 and 25 | 15, 20, 25, 30 |
| Sample (Concentrates) | Assay (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe(T) | CaO | SiO2 | MgO | Al2O3 | Cr2O3 | LOI | Cr:Fe Ratio | |
| Coarse | 14.4 | 0.3 | 7.9 | 9.3 | 9.5 | 49.9 | 3.8 | 2.4 |
| Fine | 13.2 | 0.3 | 3.9 | 10.8 | 9.2 | 53.7 | 3.7 | 2.8 |
| Ultrafine | 18.1 | 0.2 | 8.8 | 7.9 | 9.5 | 42.9 | 4.3 | 1.6 |
| Size Fractions (µm) | Cr2O3 | Fe(T) | Cr:Fe Ratio |
|---|---|---|---|
| Coarse concentrate | |||
| +710 | 51 | 14.6 | 2.4 |
| −710 + 500 | 47.9 | 15.3 | 2.1 |
| −500 + 250 | 48.3 | 15.1 | 2.2 |
| −250 + 150 | 51.4 | 14.2 | 2.5 |
| −150 + 75 | 51.8 | 12.1 | 2.9 |
| −75 | 50.4 | 14.4 | 2.4 |
| Fine concentrate | |||
| +150 | 51.6 | 13.6 | 2.6 |
| −150 + 75 | 54.6 | 13.1 | 2.9 |
| −75 + 45 | 56.4 | 12.1 | 3.2 |
| −45 + 25 | 55.8 | 13.4 | 2.8 |
| −25 | 48.3 | 22.9 | 1.4 |
| Ultrafine concentrate | |||
| +150 | 25.6 | 19.8 | 0.9 |
| −150 + 100 | 32.3 | 18.7 | 1.2 |
| −100 + 75 | 33.1 | 18.6 | 1.2 |
| −75 + 45 | 42.6 | 16.8 | 1.7 |
| −45 + 25 | 51.8 | 14.4 | 2.5 |
| −25 | 36.2 | 25.6 | 1.0 |
| Product | Mass Split (%) | Assay (%) | Cr:Fe Ratio | Distribution (%) | ||
|---|---|---|---|---|---|---|
| Cr2O3 | Fe(T) | Cr2O3 | Fe(T) | |||
| Coarse concentrate | ||||||
| Density (2.8 g/cm3) | ||||||
| Float | 7.5 | 11.6 | 11.3 | 0.7 | 1.7 | 5.9 |
| Sink | 92.5 | 53.0 | 14.7 | 2.5 | 98.3 | 94.1 |
| Density (3.3 g/ cm3) | ||||||
| Float | 12.4 | 17.8 | 18.7 | 0.7 | 4.4 | 16.1 |
| Sink | 87.6 | 54.4 | 13.8 | 2.7 | 95.6 | 83.9 |
| Fine concentrate | ||||||
| Density (2.8 g/ cm3) | ||||||
| Float | 3.2 | 22.9 | 49.4 | 0.3 | 1.4 | 11.9 |
| Sink | 96.8 | 54.7 | 12.1 | 3.1 | 98.6 | 88.1 |
| Density (3.3 g/ cm3) | ||||||
| Float | 6.1 | 10.7 | 33.3 | 0.2 | 1.2 | 15.3 |
| Sink | 93.9 | 56.5 | 12.0 | 3.2 | 98.8 | 84.7 |
| Ultrafine concentrate | ||||||
| Density (2.8 g/ cm3) | ||||||
| Float | 5.6 | 9.5 | 41.5 | 0.2 | 1.2 | 12.9 |
| Sink | 94.4 | 44.9 | 16.7 | 1.8 | 98.8 | 87.1 |
| Density (3.3 g/ cm3) | ||||||
| Float | 14.3 | 10.5 | 48.9 | 0.1 | 3.5 | 38.7 |
| Sink | 85.7 | 48.3 | 13.0 | 2.5 | 96.5 | 61.3 |
| Minerals | Mineral Mass (%) | Grain Size (µm) | ||||
|---|---|---|---|---|---|---|
| Coarse Concentrate | Fine Concentrate | Ultrafine Concentrate | Coarse Concentrate | Fine Concentrate | Ultrafine Concentrate | |
| Chromite | 81.0 | 89.4 | 76.1 | 110.7 | 58.5 | 42.1 |
| Goethite | 4.0 | 2.5 | 7.5 | 25.0 | 24.9 | 18.1 |
| Hematite | 4.1 | 2.3 | 4.0 | 26.7 | 35.9 | 20.8 |
| Fe-silicate | 4.7 | 1.8 | 5.1 | 19.9 | 21.0 | 14.4 |
| Kaolinite | 0.6 | 0.4 | 1.1 | 28.7 | 27.0 | 21.3 |
| Silicate | 1.0 | 0.8 | 1.6 | 28.6 | 36.7 | 24.3 |
| Gibbsite | 0.5 | 0.2 | 0.7 | 28.2 | 37.5 | 21.2 |
| Others | 4.1 | 2.7 | 4.0 | 17.5 | 25.7 | 15.7 |
| Process Parameters | Products | Mass Split (%) | Cr2O3 (%) | Cr:Fe Ratio | Cr2O3 (%) Recovery | Enrichment Ratio |
|---|---|---|---|---|---|---|
| Coarse concentrate | ||||||
| Spiral type: HG10i; Slurry density (wt.%): 25; Slurry flow rate (m3/h); 0.9; Splitter position: 20 cm | Concentrate | 41.6 | 63.0 | 3.97 | 52.5 | 1.67 |
| Middling | 15.9 | 49.4 | 2.34 | 15.7 | 0.98 | |
| Tailing | 42.6 | 37.3 | 1.43 | 31.8 | 0.60 | |
| Fine concentrate | ||||||
| Spiral type: HG10i; Slurry density (wt.%): 25; Slurry flow rate (m3/h); 2.3; Splitter position: 20 cm | Concentrate | 52.6 | 62.0 | 4.06 | 60.8 | 1.46 |
| Middling | 29.2 | 57.6 | 3.17 | 31.3 | 1.14 | |
| Tailing | 18.2 | 23.2 | 0.72 | 7.8 | 0.26 | |
| Ultrafine concentrate | ||||||
| Spiral type: FM1; Slurry density (wt.%): 15; Slurry flow rate (m3/h); 0.9; Splitter position: 18 cm | Concentrate | 51.1 | 54.1 | 2.47 | 64.4 | 1.52 |
| Middling | 24.1 | 27.5 | 0.94 | 15.5 | 0.58 | |
| Tailing | 24.8 | 34.9 | 1.05 | 20.2 | 0.65 | |
| Magnetic Field Intensity | Products | Mass Split (%) | Cr2O3 (%) | Cr:Fe Ratio | Cr2O3 (%) Recovery | Enrichment Ratio |
|---|---|---|---|---|---|---|
| 1.2 | Magnetic | 70.3 | 53.3 | 2.6 | 75.0 | 1.11 |
| Middling | 8.3 | 46.4 | 2.1 | 7.7 | 0.89 | |
| Nonmagnetic | 21.4 | 40.2 | 1.8 | 17.3 | 0.74 | |
| 0.4 | Magnetic | 38.1 | 56.8 | 2.8 | 43.4 | 1.17 |
| Middling | 12.5 | 52.74 | 2.7 | 13.2 | 1.12 | |
| Nonmagnetic | 19.7 | 46.76 | 2.3 | 18.5 | 0.98 |
| Magnetic Field Intensity | Products | Mass Split (%) | Cr2O3 (%) | Cr:Fe Ratio | Cr2O3 (%) Recovery | Enrichment Ratio |
|---|---|---|---|---|---|---|
| 1.2 | Magnetic | 79.3 | 59.7 | 3.1 | 88.1 | 1.11 |
| Middling | 4.0 | 48.3 | 2.6 | 3.6 | 0.92 | |
| Nonmagnetic | 16.6 | 26.4 | 1.4 | 8.2 | 0.50 | |
| 0.4 | Magnetic | 46.1 | 61.6 | 3.2 | 52.9 | 1.16 |
| Middling | 11.4 | 57.6 | 3.2 | 12.2 | 1.14 | |
| Nonmagnetic | 21.8 | 56.6 | 2.7 | 23.0 | 0.98 |
| Magnetic Field Intensity | Products | Mass Split (%) | Cr2O3 (%) | Cr:Fe Ratio | Cr2O3 (%) Recovery | Enrichment Ratio |
|---|---|---|---|---|---|---|
| 1.3 | Magnetic | 87.4 | 45.0 | 1.8 | 91.8 | 1.12 |
| Middling | 4.1 | 37.3 | 2.0 | 3.6 | 1.21 | |
| Nonmagnetic | 8.5 | 20.2 | 1.1 | 4.0 | 0.70 | |
| 0.4 | Magnetic | 48.1 | 47.4 | 2.1 | 53.2 | 1.30 |
| Middling | 15.0 | 45.3 | 1.6 | 15.8 | 0.99 | |
| Nonmagnetic | 24.3 | 40.3 | 1.5 | 22.8 | 0.91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathy, S.K.; Murthy, Y.R.; Singh, V.; Farrokhpay, S.; Filippov, L.O. Improving the Quality of Ferruginous Chromite Concentrates Via Physical Separation Methods. Minerals 2019, 9, 667. https://doi.org/10.3390/min9110667
Tripathy SK, Murthy YR, Singh V, Farrokhpay S, Filippov LO. Improving the Quality of Ferruginous Chromite Concentrates Via Physical Separation Methods. Minerals. 2019; 9(11):667. https://doi.org/10.3390/min9110667
Chicago/Turabian StyleTripathy, Sunil Kumar, Y Rama Murthy, Veerendra Singh, Saeed Farrokhpay, and Lev O. Filippov. 2019. "Improving the Quality of Ferruginous Chromite Concentrates Via Physical Separation Methods" Minerals 9, no. 11: 667. https://doi.org/10.3390/min9110667
APA StyleTripathy, S. K., Murthy, Y. R., Singh, V., Farrokhpay, S., & Filippov, L. O. (2019). Improving the Quality of Ferruginous Chromite Concentrates Via Physical Separation Methods. Minerals, 9(11), 667. https://doi.org/10.3390/min9110667








