1. Introduction
Grinding is a sophisticated process composed of complex physical and chemical reactions. Theoretical studies and industrial practices indicate that the reactions that occur in the grinding process are directly related to mineral surface properties and pulp characteristics. These were found to have effect in the mineral froth-flotation process [
1,
2,
3]. Spodumene and beryl are typical crystalline silicate minerals and are important industrial raw materials [
4]. It is of great theoretical and practical significance to investigate the flotation behavior of spodumene and beryl to help in improving their recoveries.
The research work on the influences of grinding media on mineral flotation behavior has been conducted previously by various scholars. In the early 1960s, Rey and Formanek pointed out that the floatability of iron-ball milling of galena and sphalerite was worse than the porcelain ball milling of galena [
5]. The work by Forssberg, Yuan and their collaborators found that the grinding of minerals with non-ferrous medium, the pulp potential, conductivity and total sulfur concentration of sulfide minerals were higher than those ground with ferrous medium [
6,
7,
8,
9,
10]. Moreover, Peng et al. [
11,
12,
13], and Huang et al. [
14] showed the influence of grinding medium and grinding environment on mineral floatability. It was found that the surface properties and flotation of sulfide minerals, such as galena and chalcopyrite, are obviously affected by the fluctuation of physical or chemical factors in grinding process. In addition, the galvanization had adverse effects on the sulfide mineral flotation, and grinding with high chromium medium was beneficial to fine-grained mineral flotation. Iwasaki et al. [
15] studied the influence of a traditional ball mill and self-grinding machine on the flotation of copper-nickel ores. It was found that the copper flotation recovery after using a self-grinding machine was higher under the same flotation feed size. Recently, Li et al. [
16] investigated the surface properties and flotation behavior of scheelite particles produced by ball and rod mills. The results showed that the rod mill particles had a lower critical surface tension and a smaller specific surface area. The study by He [
17], showed that the effect of the grinding medium on the flotation of sulfide minerals was better when grinding with ceramic medium than with iron medium. The research work by Song et al. [
18] on the influence of grinding medium (Iron and zirconia) on the surface properties of calcite and the recovery of calcite in dodecylamine flotation system, the recovery of calcite by zirconia ball grinding was higher than that of steel ball grinding.
In this paper, the effect of two different grinding medium, iron ball and zirconium ball, under two different collectors (dodecylamine or sodium oleate) on spodumene and beryl in the flotation experiments were studied. The mechanism of the effect of grinding media on the flotation of silicate minerals was uncovered by conducting X-ray photoelectron spectroscopy, deep molecular simulation and infrared spectroscopy.
2. Materials and Methods
2.1. Raw Material
The bulk beryl and spodumene ore were obtained from a mine in Xinjiang area. All the samples were separately prepared into refined sample with purity greater than 99% and particle size in the range of −0.335 to +0.045 mm. More details on sample preparation and purity test could be found in previous literature published by the author [
19]. In the wet-grinding process, the zirconia ball and iron ball were used, while both dodecylamine anion and sodium oleate cations collectors were used in the flotation tests.
The tank body of the grinder is made of 1010 nylon bar with 90 mm outer diameter, 60 mm inner diameter, 53 mm depth and 150 mL volume. The medium change ratio is between 30–40%.
2.2. Experimental Procedure
In the mono sized-mineral wet-grinding experiments, the solid–liquid ratio used was 1:3 (Mineral 10 g and deionized water 30 mL). The ground mineral was washed with 120 mL of deionized water and divided equally into five parts. The flotation tests were carried out in a hanging trough flotation machine (Model: XFG-76, Jilin Exploration Machinery Plant, Changchun, China), the volume of the flotation cell was 30 mL and the rotation speed was 1750 revolutions per minute, and pulp temperature was 20–30 °C.
2.3. Molecular Simulation Calculation
The models of mineral crystal and flotation reagent molecule are established by Material Studio (MS) software, and the lattice parameters of mineral crystal are optimized by the generalized gradient approximation (GGA) method based on density functional theory (DFT) [
20].
The adsorption energies of the collectors are computed from Equation (1):
where E
system is the energy of the surface slab with collector, E
slab is the energy of the surface slab and E
collector is the energy of the isolated collector molecule. A negative value of E
ads shows a strong interaction between the adsorbate and the surface, whereas a positive value reveals the opposite.
2.4. Test Method
2.4.1. X-Ray Photoelectron Spectroscopy
The X-ray photoelectron spectroscopy (Thermo Scientific K-Alpha, Thermo Electron Corporation, Waltham, MA, USA) of minerals is mainly used to determine the types of elements on the surface of minerals and analyze the states (valence states) of various elements on the surface of minerals. In this study, the composition, relative content and valence state of the surface elements of the test ore samples were determined, and the properties of the surface elements of the minerals under different grinding factors and before and after the chemical action were determined.
2.4.2. Determination of Zeta Potential
The zeta potentials of minerals under different grinding factors were measured. The zeta potentials of the mineral surface were measured by the potential analyzer (NANO-ZS90, Malvern Instruments Ltd., Malvern, UK). Measurement steps: weighed 10 g of ore sample each time, grind the ore sample to −0.074 mm by different grinding methods, accounting for 80%. The ore samples after grinding are divided into 5 parts, and then placed it in a 50 mL beaker, washed with 30 mL fixed amount of deionized water, adjusted the pH value of ore pulp with HCl or NaOH, mixed it with a magnetic stirrer for 5 min, and then measured by the zeta potential analyzer.
2.4.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX)
A scanning electron microscope (SEM) was developed by an analytical scanning electron microscope after being equipped with X-ray energy spectrometer. It is not only faster than X-ray spectrometer in analysis speed and sensitivity but can also be used for qualitative and non-standard quantitative analysis. In this study, the surface morphology and composition of minerals after grinding were analyzed and detected by Hitachi S-3500N Scanning Electron Microscope (Chiyoda-ku, Japan) and Oxford Inca Energy Spectrometer (Oxfordshire, UK).
2.4.4. Infrared Spectrum Analysis
Infrared spectrum analysis referred to the analysis and identification of substance molecules by using the infrared spectrum. In this study, Equinox 55 FTIR (Bruker, Billerica, MA, USA) was used to analyze the form of iron adsorbed on the mineral surface after iron grinding.
3. Results
3.1. Cationic Collectors System
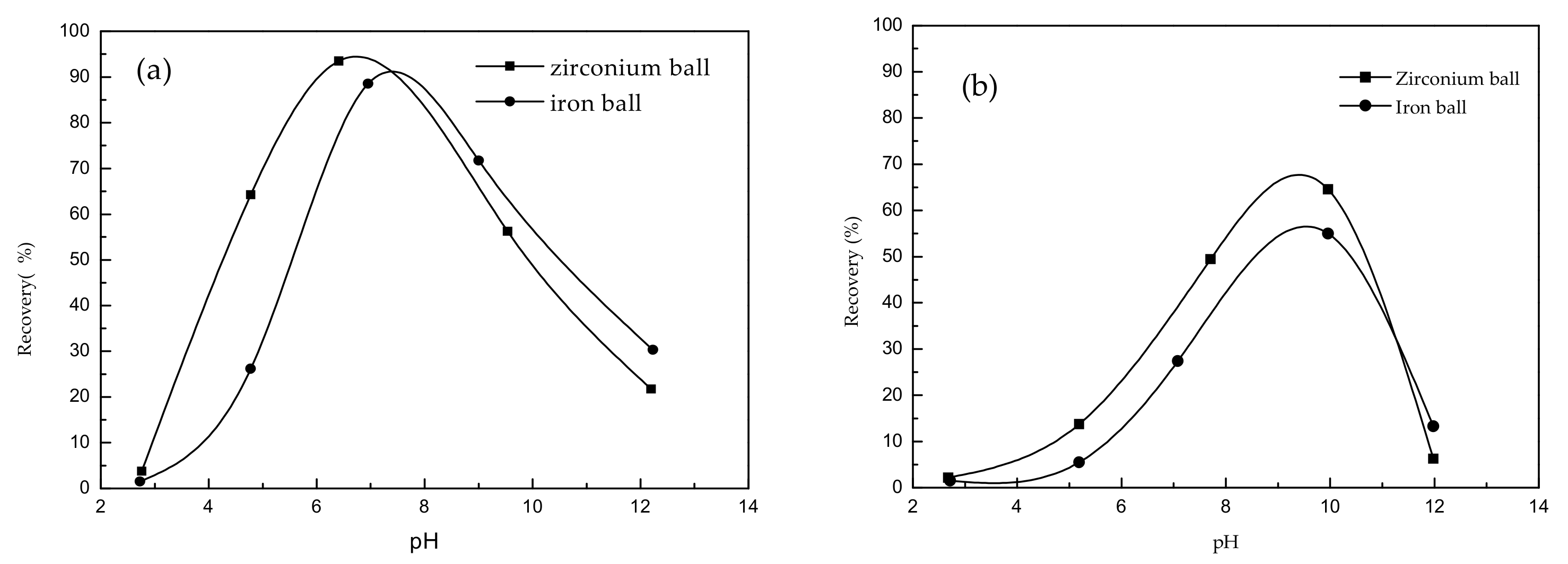
Dodecylamine was used as a cationic collector with a dosage of 60 mg·L
−1. The flotation recoveries based on the influence of different grinding media (zirconium and iron balls) of beryl and spodumene are shown in
Figure 1. Through fine operation, all flotation experiment errors are controlled within 3%.
The experimental results of
Figure 1 showed that, when dodecylamine was used as a collector: (1) under both zirconium and iron ball grinding, the influence of pH on the recovery of beryl and spodumene was similar. With the increase of pH, the flotation recovery shows an ascending trend followed by a descending trend. (2) The recovery of beryl and spodumene under zirconium wet-grinding was higher than that of iron ball wet-grinding when pH was lower than optimum pH. While when the pH was higher than the optimum pH, the flotation recovery under both grinding conditions was similar.
3.2. Anionic Collector System
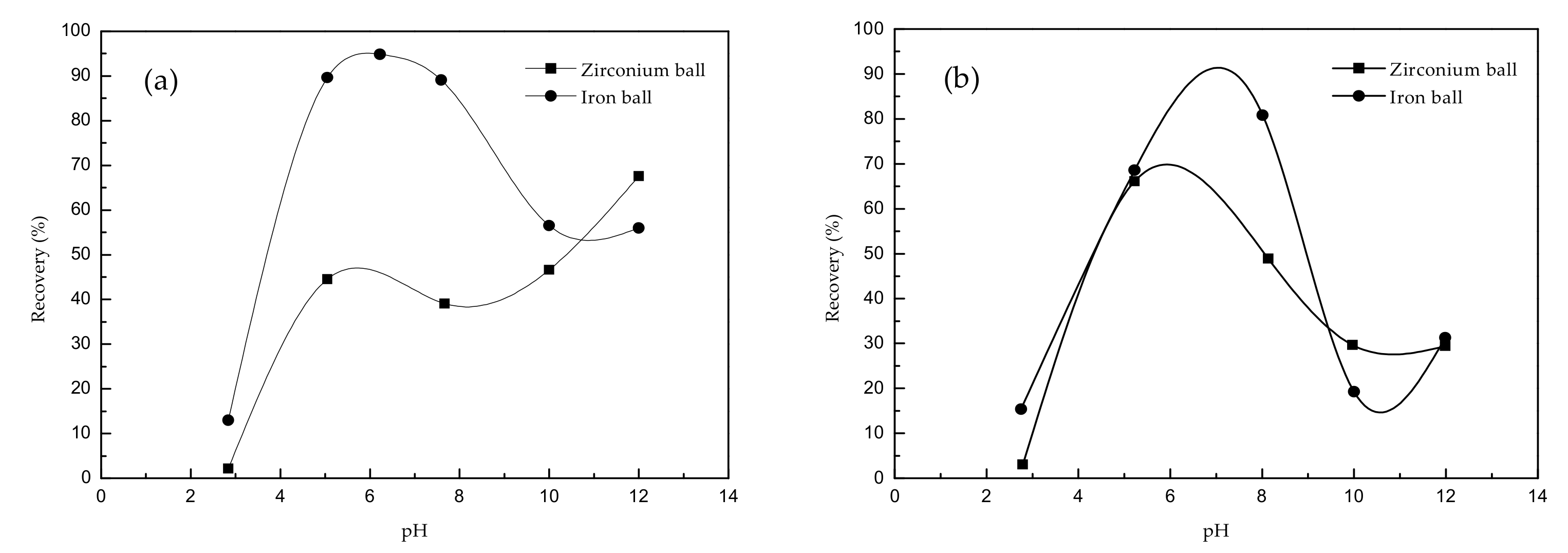
The effect of different grinding media on the flotation behavior of silicate minerals when using sodium oleate as a collector with a 160 mg·L
−1 dosage is shown in
Figure 2.
The experimental results shown in
Figure 2 indicated that when using sodium oleate as a collector in the flotation at different pH values, the effect of wet-grinding using both zirconium and iron balls showed different the flotation recoveries.
Figure 2 shows results for zirconium ball wet-grinding, and the flotation recovery of beryl was observed to increases gradually with the increase of pH value (
Figure 2a), while that of spodumene increases first and then decreases (
Figure 2b). As the pH increased, the flotation recovery of beryl (
Figure 2a) and spodumene (
Figure 2b) increases first and then decreases under iron ball wet-grinding. The flotation recovery of beryl and spodumene in iron ball grinding was generally higher than that of zirconium ball in wet-grinding at the same pH value.
4. Mechanism Analysis
4.1. Effect of Grinding Factors of Potentiodynamic
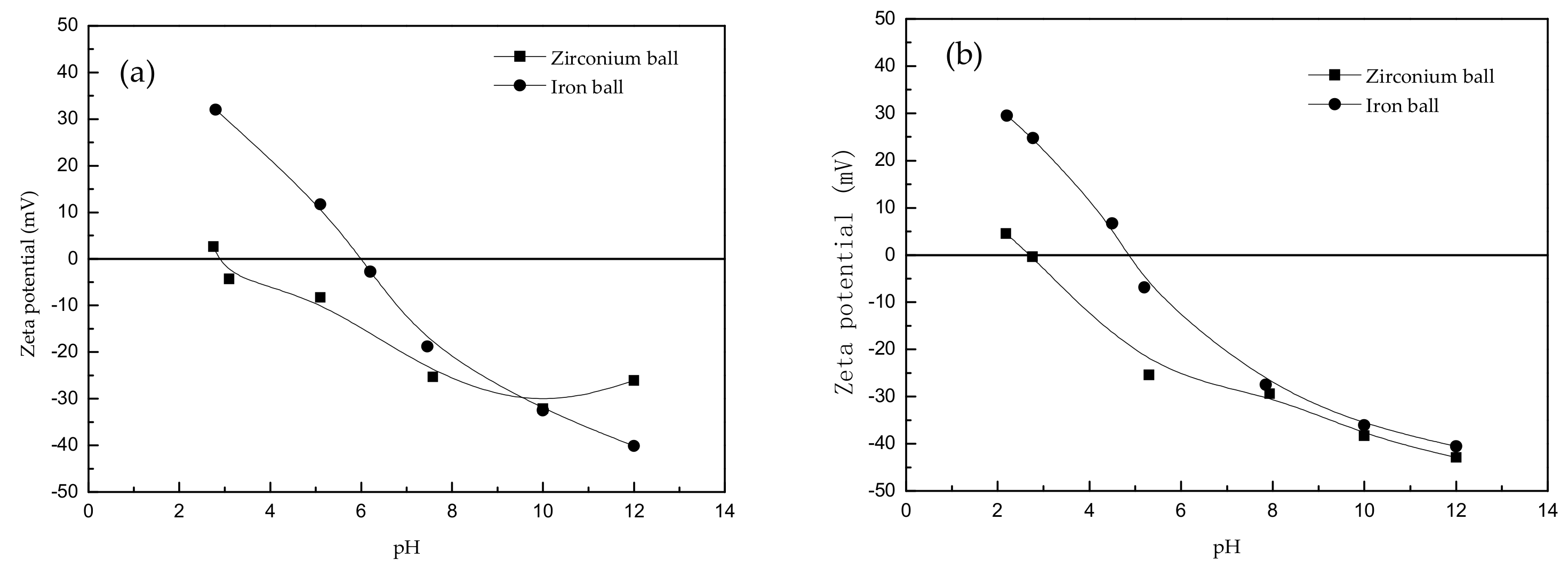
Zetasizer (model: Nano-Zs) tester was used to detect the surface potential of minerals. The effects of different grinding media on the surface potential of beryl and spodumene minerals were investigated as shown in
Figure 3.
The results in
Figure 3 show that: (1) under different grinding media, the trend for potentiodynamic of beryl and spodumene was similar. We observed that as the pH increased, the potentiodynamic decreased gradually. (2) Under zirconium ball wet-grinding, the corresponding pH values of the zero-electric point of beryl and spodumene were 2.9 and 2.6, respectively. Under iron ball wet-grinding, the corresponding pH values of the zero-electric point of beryl and spodumene were 6.0 and 5.0, respectively. (3) The corresponding pH values of the zero-electric point of beryl and spodumene in wet-grinding of zirconium ball were lower than those in wet-grinding of iron ball.
When dodecylamine was used as a collector, the interaction between dodecylamine and the mineral surface was mainly formed by the cation RH
3+ or RH
2·RH
3+ adsorbed on the mineral surface through electrostatic attraction on the negatively charged mineral surface [
21]. When the flotation pH was lower than optimum, the surface potential of beryl and spodumene under zirconium wet-grinding is lower than that under iron wet-grinding. The interaction between dodecylamine and mineral surface was stronger, hence the flotation recovery of these two minerals after zirconium wet-grinding was comparably higher than that of iron ball wet-grinding. Furthermore, as the pH increased, more negative charges were contacted on the surface of beryl and spodumene, and the flotation recovery under both grinding conditions was similar.
4.2. Mineral Surface Elements Analysis
The mineral surface property was closely allied to its flotation behavior. X-ray photoelectron spectroscopy (XPS) was used to unveil the changing pattern of the elements on the beryl and spodumene surface after wet-grinding in different grinding media. The results are shown in
Table 1 and
Table 2 below.
We have found that: (1) After wet zirconium ball milling, the percentage content of Al, Be, Fe, O and Si on the surface of beryl was little different from that of raw ore, after wet iron ball milling, the content of Be on the surface of beryl decreased from 24.67% to 21.02%, while the content of Fe increased from 0.24% to 2.06%. (2) The content of Al, Fe, Li, Si and O on the surface of spodumene had a slightly different effect from that of raw ore after wet milling using zirconium ball. Furthermore, after wet milling using iron ball, the content of Li on the surface of spodumene increased from 9.87% to 19.98%, and the content of Fe from 0.42% to 2.10%.
The presence of iron was detected on the surface of silicate minerals after wet-grinding using the iron ball. We observed that when metal cations were adsorbed on the surface of silicate minerals, the electrical properties of the mineral surface improved, and the electrostatic adsorption force of cationic collectors correspondingly weakened. The concentration of cationic collectors in the mineral interfacial layer was reduced, thus the collection effect of collectors on minerals was weakened [
21]. Therefore, when using dodecylamine as collector and iron ball as grinding medium, the iron existing on the surface of beryl and spodumene minerals inhibited mineral flotation. In addition, when the flotation pH was lower than the optimum flotation pH, the flotation recovery of beryl and spodumene was lower than when using zirconium ball wet milling.
In the case of using sodium oleate as a collector, we observed that after wet milling using zirconium ball, Be and Li were exposed on the surface of beryl and spodumene and this can be used as active spots to bind with sodium oleate, resulting in partial floatation of beryl and spodumene. After wet iron-grinding, the content of Fe on the surface of beryl and spodumene increased obviously, which indicated that some of the Fe medium wears and was fixed on the mineral surface. The binding energy of Fe2p peak at around 711 eV, was similar to the binding energy of iron hydroxyl complex, and thus the interaction between beryl and spodumene surface and anion collector was enhanced by the existing Fe on the surface. We have found that the wet-grinding using iron ball, gave an increase in the flotation recovery of beryl and spodumene which was higher than that of zirconium ball wet milling.
4.3. Mineral Surface Product Analysis under Iron Ball Grinding Environment
The results of scanning electron microscopy (SEM) and energy dispersive spectrum analysis (EDS) showed that there were flocs and abrasion zones on the surface of the minerals after iron ball grinding, and these flocs were detected to contain iron. The XPS analysis showed that the binding energy of Fe2p peaks on the surface of beryl and spodumene was near 711 eV, which was similar to the binding energy of hydroxyl complex on iron.
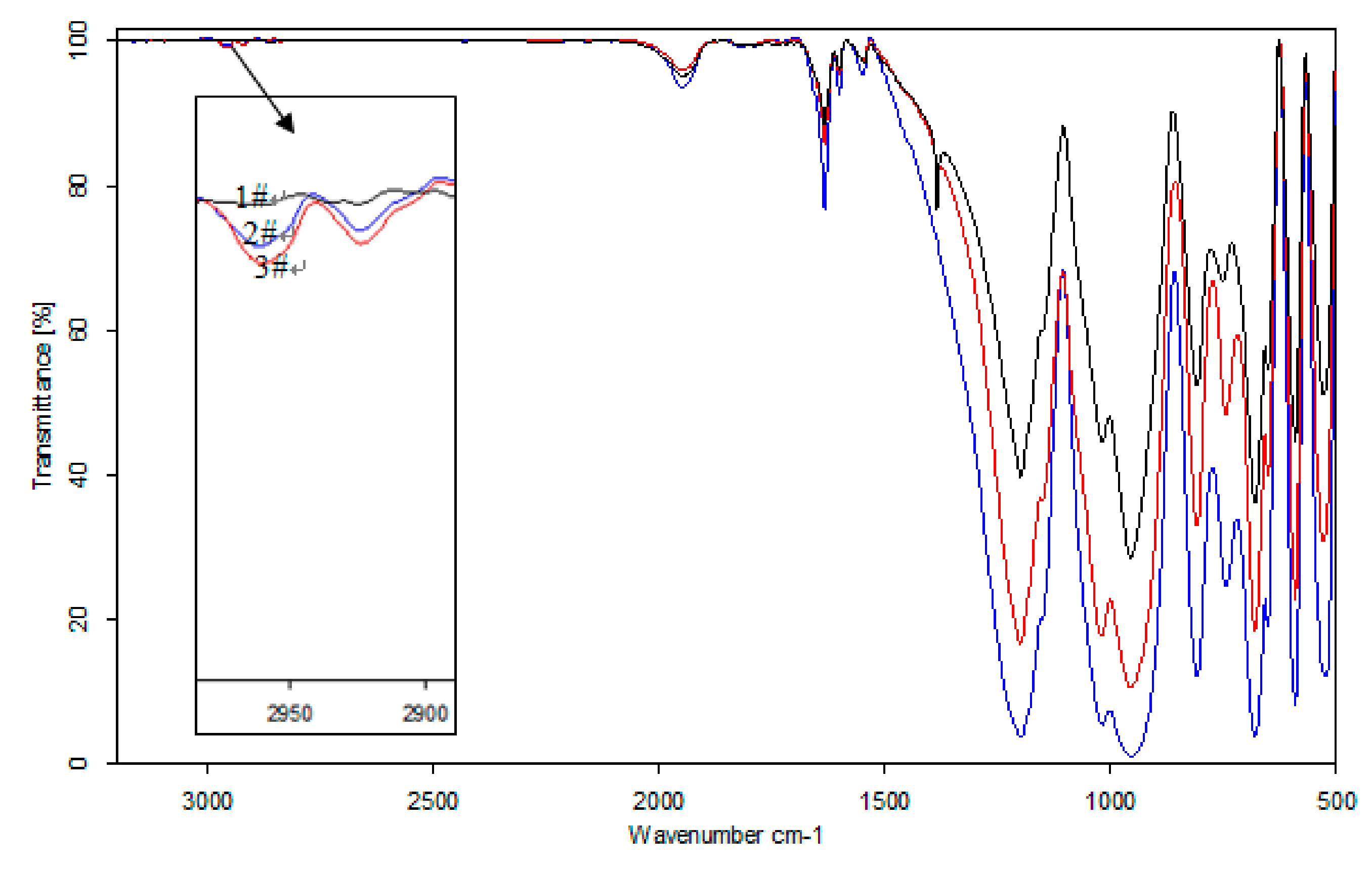
We have conducted infrared spectroscopy on the mineral surface of beryl raw ore after iron dry grinding and wet grinding. And the results are shown in
Figure 4. This illustrated that the characteristic peaks of iron hydroxyl complexes have been detected on the surface of beryl by both dry and wet grinding at the wavelength of 2950 cm
−1. This further indicated that iron hydroxyl complexes have been formed on the surface of silicate minerals after iron grinding.
We noted that after the iron ball grinding, the existing iron ions on the surface of beryl and spodumene were the activation spots, which enhanced the interaction of minerals with an anionic collector. Therefore, under the same pH value, using sodium oleate as a collector, the flotation recovery of these two minerals after iron ball milling was significantly higher than after zirconium ball milling.
In summary, iron ions were mainly adsorbed on the surface of minerals in the form of hydroxyl complexes after grinding, and collectors were adsorbed on the surface of silicate minerals through the hydroxyl complexes of iron.
4.4. Molecular Simulation of Interaction between Collector and Mineral Surface before and after Iron Media Grinding
4.4.1. Adsorption Model of Reagent Molecules on Minerals
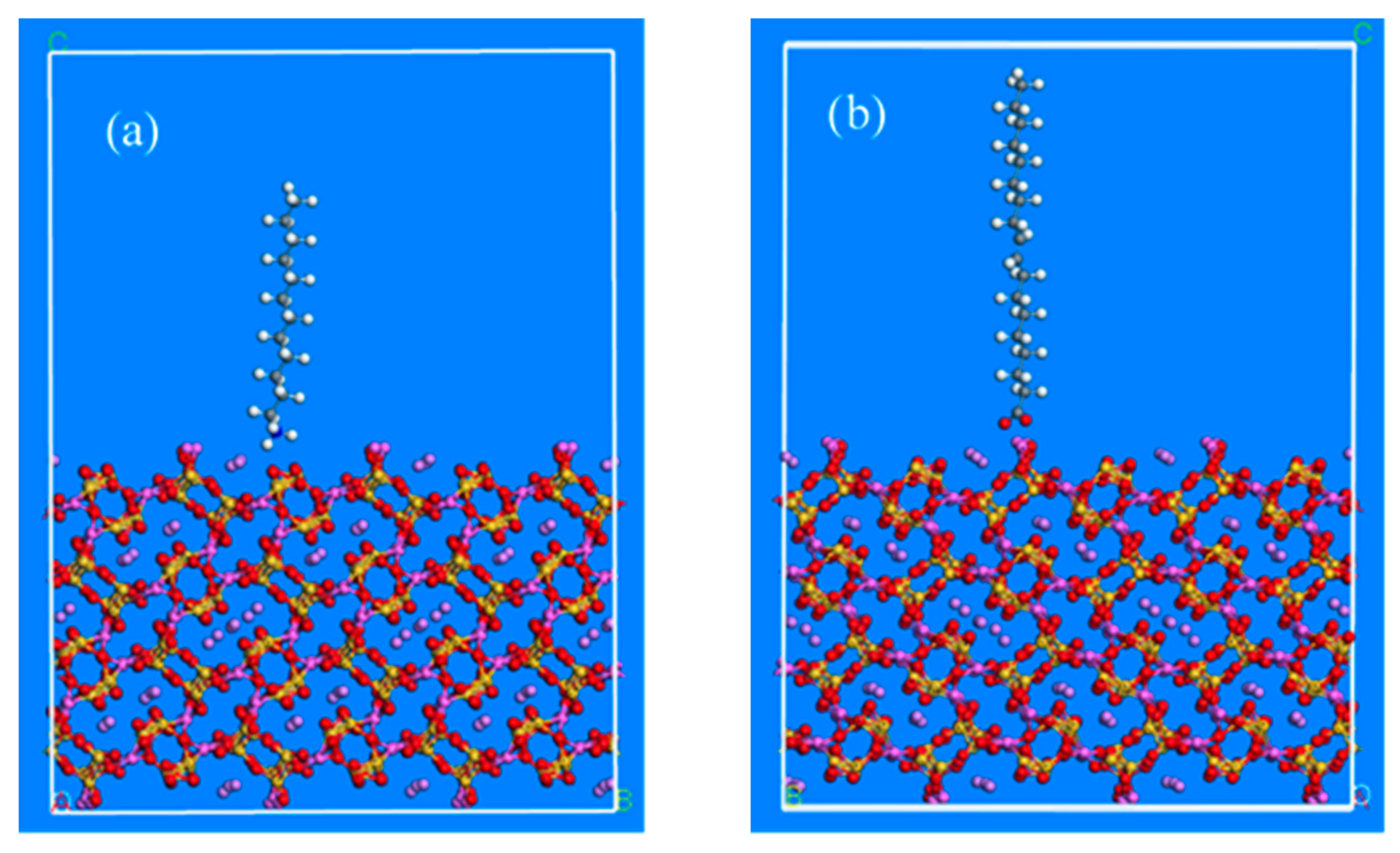
The adsorption models between beryl, spodumene mineral crystalloid and dodecylamine and sodium oleate were established based on Material Studio (
Figure 5 and
Figure 6). The interaction change trend of collector molecules on the surface of beryl and spodumene after iron media grinding was studied.
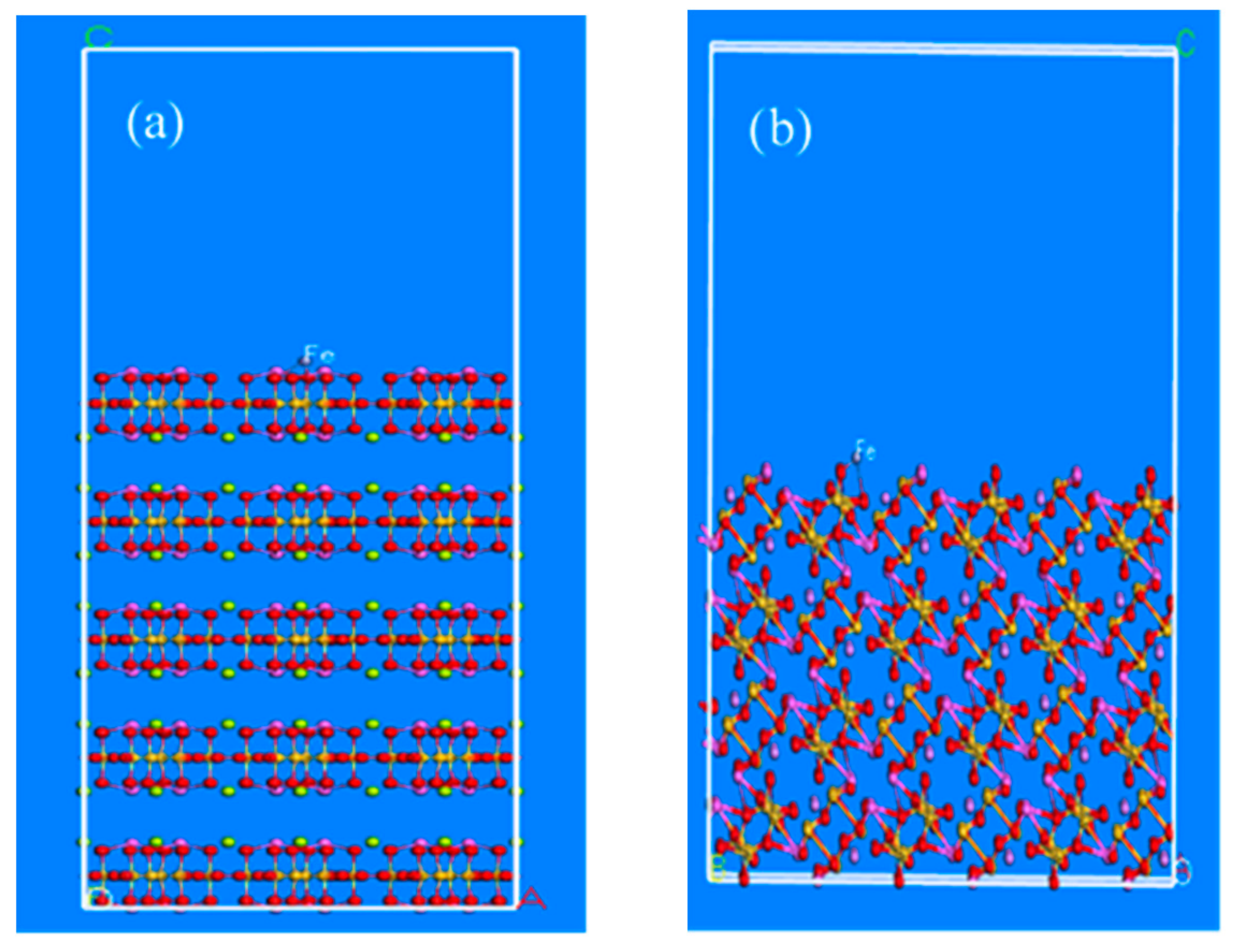
4.4.2. Deduction of Adsorption Model and Adsorption Energy Calculation after Iron Ball Grinding
After iron ball grinding, we found that iron existed in the complicated pulp system in the form of various hydroxyl complexes. However, its stable range was limited, mainly at a pH of 1.5–3.0 [
22]. Generally, most of the flotation experiments were conducted under the pH varying from weak acidity to alkalinity and within this range, iron existed in the form of Fe(OH)
3. In order to illustrate the problem intuitively, the corresponding metal ion Fe
3+ was introduced into the cleavage surface of the mineral after grinding. This formed the theoretical cleavage surface of beryl and spodumene minerals that is activated by metal ions, as shown in
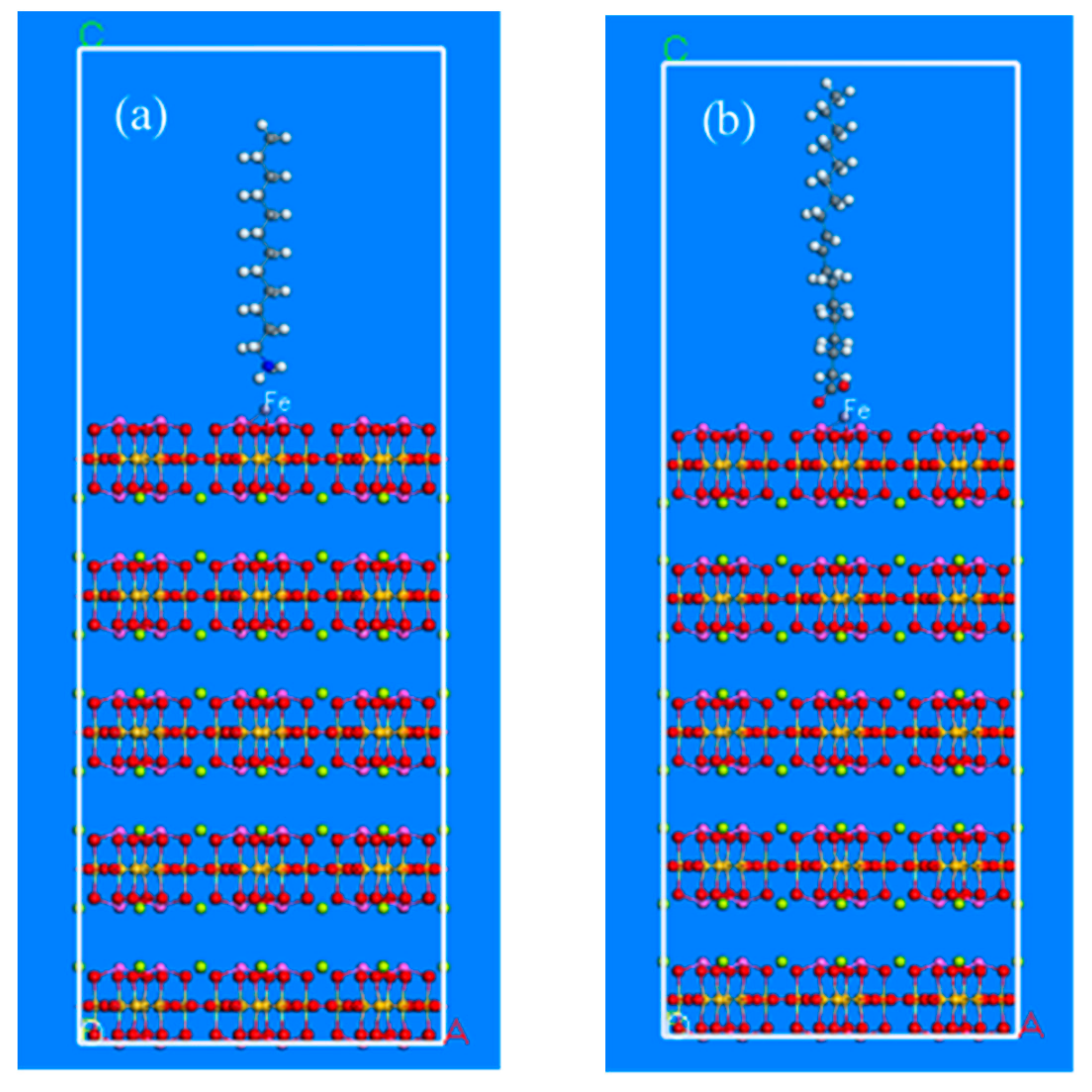
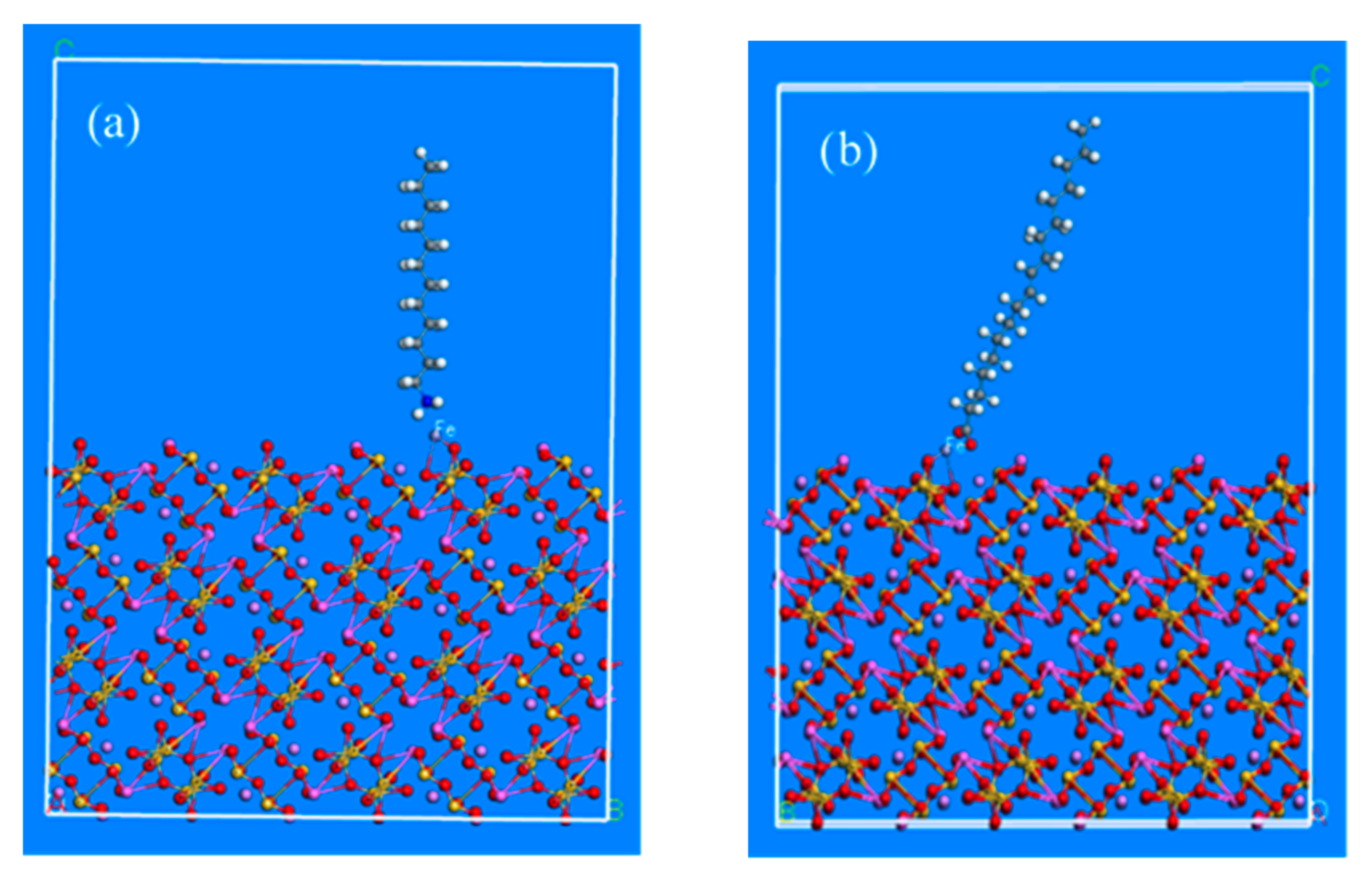
Figure 7a,b. Based on the above-mentioned adsorption models of beryl and spodumene surfaces the collector molecules were introduced after iron-grinding, as shown in
Figure 8 and
Figure 9.
Figure 8a diagrams represent the adsorption models of dodecylamine cations, while
Figure 8b shows the adsorption models of oleate anions on the beryl surface after iron balls grinding. The adsorption models of spodumene are shown in
Figure 9, where
Figure 9a diagrams represent the adsorption models of dodecylamine cations, while
Figure 9b shows the adsorption models of oleate anions on the spodumene surface after iron balls grinding.
4.4.3. Adsorption Energy Calculation
According to the calculation method described in
Section 2.3, the adsorption energy between dodecylamine/sodium oleate molecules with beryl and spodumene before and after iron media grinding is calculated and listed in
Table 3 and
Table 4, respectively.
The calculated results in
Table 3 revealed that: (1) The adsorption energy between beryl and dodecylamine before iron grinding was −1931.87 kJ/mol, while after grinding was −1592.55 kJ/mol. The adsorption energy increased 339.32 kJ/mol. This indicated that the adsorption energy will increase with iron grinding, which showed a weakening of the interaction between collector and beryl. (2) The adsorption energy between beryl and sodium oleate before iron grinding was −1596.48 kJ/mol, after grinding we found −2320.48 kJ/mol. The change value of adsorption energy was −724.00 kJ/mol. The adsorption energy decreased after grinding, which indicated that iron grinding strengthened the interaction between sodium oleate and beryl.
The results in
Table 4 showed that: (1) The adsorption energy between spodumene and dodecylamine was −43.10 kJ/mol before grinding and −34.94 kJ/mol after grinding. The increased adsorption energy was 8.16 kJ/mol. This showed an increase after iron media grinding, which potentially weakened the interaction between collectors and spodumene. (2) The adsorption energy between spodumene and sodium oleate was −54.84 kJ/mol before grinding, and −57.12 kJ/mol after grinding, the change value of adsorption energy was −2.28 kJ/mol. The adsorption energy was weakened after iron media grinding, which strengthened the interaction between collectors and spodumene.
In terms of adsorption of reagents on the mineral surface, theoretically, the more negative the adsorption force, the adsorption will be exothermic and more stable. The calculations showed that the adsorption energy between oleate anion and beryl before iron grinding was larger than that between dodecylamine cation and beryl, and the adsorption energy between oleate anion and spodumene was smaller than that between dodecylamine cation and spodumene. As such the dodecylamine could easier adsorb on the surface of beryl than oleate anion, while oleate anion was comparably easier to adsorb on the surface of spodumene. Based on the calculation, after iron media grinding, the adsorption energy of oleate anion between beryl and spodumene after iron grinding was less than that between dodecylamine cation and beryl and spodumene, which indicates that oleate anion after iron grinding could easier adsorb on the surface of beryl and spodumene than dodecylamine anion.
By calculating the adsorption energy between beryl/spodumene and reagent molecular, it is reasonable to infer that, after iron ball grinding, the iron wear were adsorbed on the mineral surface and changed the adsorption energy. The adsorption energy between dodecylamine cationic collector and the mineral surface was increased, while the adsorption energy between sodium oleate anionic collector and the mineral surface was decreased, hence the interaction between reagent and mineral was improved.
5. Conclusions
(1) When dodecylamine was used as a collector, with the increase of pH, the flotation recovery of beryl and spodumene shows an ascending trend followed by a descending trend. We observed that when the flotation pH was lower than optimum, the flotation recovery after using zirconium ball wet-grinding of beryl and spodumene was higher than that of iron ball wet-grinding. As the pH value continued to rise, the flotation recovery of zirconium ball wet-grinding and iron ball wet-grinding were similar.
(2) When sodium oleate was used as collector, under the same pH, the recovery of beryl and spodumene under zirconium ball grinding was generally lower than that of iron ball wet-grinding.
(3) The results of X-ray photoelectron spectroscopy (XPS) on the surface of minerals showed that under iron ball wet-grinding, the iron content on the surface of beryl and spodumene increased obviously, which indicated that part of iron medium abrasion occurred and attached on the surface of the mineral.
(4) Infrared spectroscopy further indicated that, with iron ball grinding, iron ions were mainly bound on the surface of beryl and spodumene minerals in the form of hydroxyl complexes. Moreover, the collector molecular was adsorbed on the surface of the silicate minerals through the hydroxyl complexes compound of iron.
(5) The adsorption energy of reagent molecules with the mineral surface of beryl and spodumene before and after iron medium grinding were analyzed. The results illustrated that: after iron medium grinding, the adsorption energy between dodecylamine cationic collector and the surface of the minerals increased, while the adsorption energy between sodium oleate anionic collector and the surface of the minerals decreased. This further affects the interaction between collector molecules and minerals.
(6) For silicate minerals, after iron ball grinding, the adsorption of dodecylamine cationic collector on the mineral surface was generally prevented. On the other hand, for oleate anion collector, the adsorption of oleate ion on minerals surface was enhanced.
Author Contributions
Z.H. conceived and designed experiments and wrote the paper; C.S. provided guidance for the design, test and data analysis.
Funding
This research was funded by the BGRIMM Technology Group Scientific Research Fund (Project No. JTKJ1821); the National Natural Science Foundation of China (Grant No. 51634009); BGRIMM Technology Group Science and Technology Industry Development Fund (Project No.04602019-125).
Acknowledgments
We would like to thank the BGRIMM Technology Group for providing us with the experimental platform.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, D.Z. Lastest Development of Flotation Theory; Science Press: Beijing, China, 1992. [Google Scholar]
- Fuerstenau, D.W. The froth flotation century. In Advances in Flotation Technology; Parekh, B.K., Miller, J.D., Eds.; Society for Mining Metallurgy: Littleton, CO, USA, 1999; p. 3. [Google Scholar]
- Hu, Z.F.; Sun, C.Y. Effects and mechanism of different grinding media on the flotation behaviors of typical silicate minerals. Chin. J. Eng. 2016, 38, 1221. [Google Scholar]
- Information Center of Ministry of Land and Resources of People’s Republic of China. World Mineral Resources Annual Review; Geological Publishing House: Beijing, China, 2013; pp. 234–237.
- Rey, M.; Formanek, V. Some factors affecting selectivity in the differential flotation of lead-zinc ores, particularly in the presence of oxidised lead minerals. In Proceedings of the 5th International Mineral Processing Congress, London, UK, 5–9 April 1960; p. 343. [Google Scholar]
- Forssberg, E.; Sundberg, S.; Zhai, H.X. Influence of Different Grinding Methods on Floatability. Int. J. Miner. Process. 1988, 22, 183–192. [Google Scholar] [CrossRef]
- Subrahmanyam, T.V.; Forssberg, K.S.E. Grinding and flotation pulp chemistry of a low grade copper ore. Miner. Eng. 1995, 8, 913. [Google Scholar] [CrossRef]
- Forssberg, E.; Subrahmanyam, T.V. Floatability of grinding, pulp chemistry and particles. Met. Ore Dress. Abroad 1994, 31, 31. [Google Scholar]
- Yuan, X.M.; Palsson, B.I.; Forssberg, K.S.E. Flotation of a complex sulphide ore II. Influence of grinding environments on Cu/Fe sulphide selectivity and pulp chemistry. Int. J. Miner. Process. 1996, 46, 181. [Google Scholar] [CrossRef]
- Yuan, X.M.; Palsson, B.I.; Forssberg, K.S.E. Flotation of a complex sulphide ore I. Cu/Zn selectivity control by adjusting pulp potential with different gases. Int. J. Miner. Process. 1996, 46, 155. [Google Scholar] [CrossRef]
- Peng, Y.; Grano, S.; Fornasiero, D.; Ralston, J. Control of grinding conditions in the flotation of galena and its separation from pyrite. Int. J. Miner. Process. 2003, 70, 67. [Google Scholar] [CrossRef]
- Peng, Y.; Grano, S.; Fornasiero, D.; Ralston, J. Control of grinding conditions in the flotation of chalcopyrite and its separation from pyrite. Int. J. Miner. Process. 2003, 69, 87. [Google Scholar] [CrossRef]
- Peng, Y.; Grano, S.; Ralston, J.; Fornasiero, D. Towards prediction of oxidation during grinding I. galena flotation. Miner. Eng. 2002, 15, 493. [Google Scholar] [CrossRef]
- Huang, G.; Grano, S.; Skinner, W. Galvanic interaction between grinding media and arsenopyrite and its effect on flotation: Part II. Effect of grinding on flotation. Int. J. Miner. Process. 2006, 78, 198. [Google Scholar]
- Iwasaki, I.; Reid, K.J.; Lex, H.A.; Smith, K.A. Effect of autogenous and ball mill grinding on sulfide flotation. Min. Eng. 1983, 35, 1184–1190. [Google Scholar]
- Li, C.W.; Gao, Z.Y. Effect of grinding media on the surface property and flotation behavior of scheelite particles. Powder Technol. 2017, 322, 386–392. [Google Scholar] [CrossRef]
- He, F.Y. Effects of Grinding Environment on Flotation of Sulfides Minerals; Northeastern University: Shenyang, China, 2006. [Google Scholar]
- Song, Z.G.; Sun, C.Y. Effects of grinding medium on the flotation of calcite with laurylamine. Met. Mine 2009, 2, 91. [Google Scholar]
- Hu, Z.F. Effects of Physical and Chemical Factors on the Flotation Behaviors of Typical Silicates in Grinding Processes; University of Science and Technology: Beijing, China, 2016. [Google Scholar]
- Zhengfeng, H.; Chuanyao, S. Research on effect of iron grinding media on the flotation behaviors of spodumene and beryl by molecular simulation. Nonferrous Met. 2016, 6, 1. [Google Scholar]
- Yin, W.Z.; Sun, C.Y. Flotation Principle of Silicate Minerals; Science Press: Beijing, China, 2001. [Google Scholar]
- Dianzuo, W.; Yuehua, H. Solution Chemistry of Flotation; Hunan Science and Technology Press: Changsha, China, 1988. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).