Factors Controlling the Gallium Preference in High-Al Chromitites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineral Analysis
2.2. Whole Rock Analysis
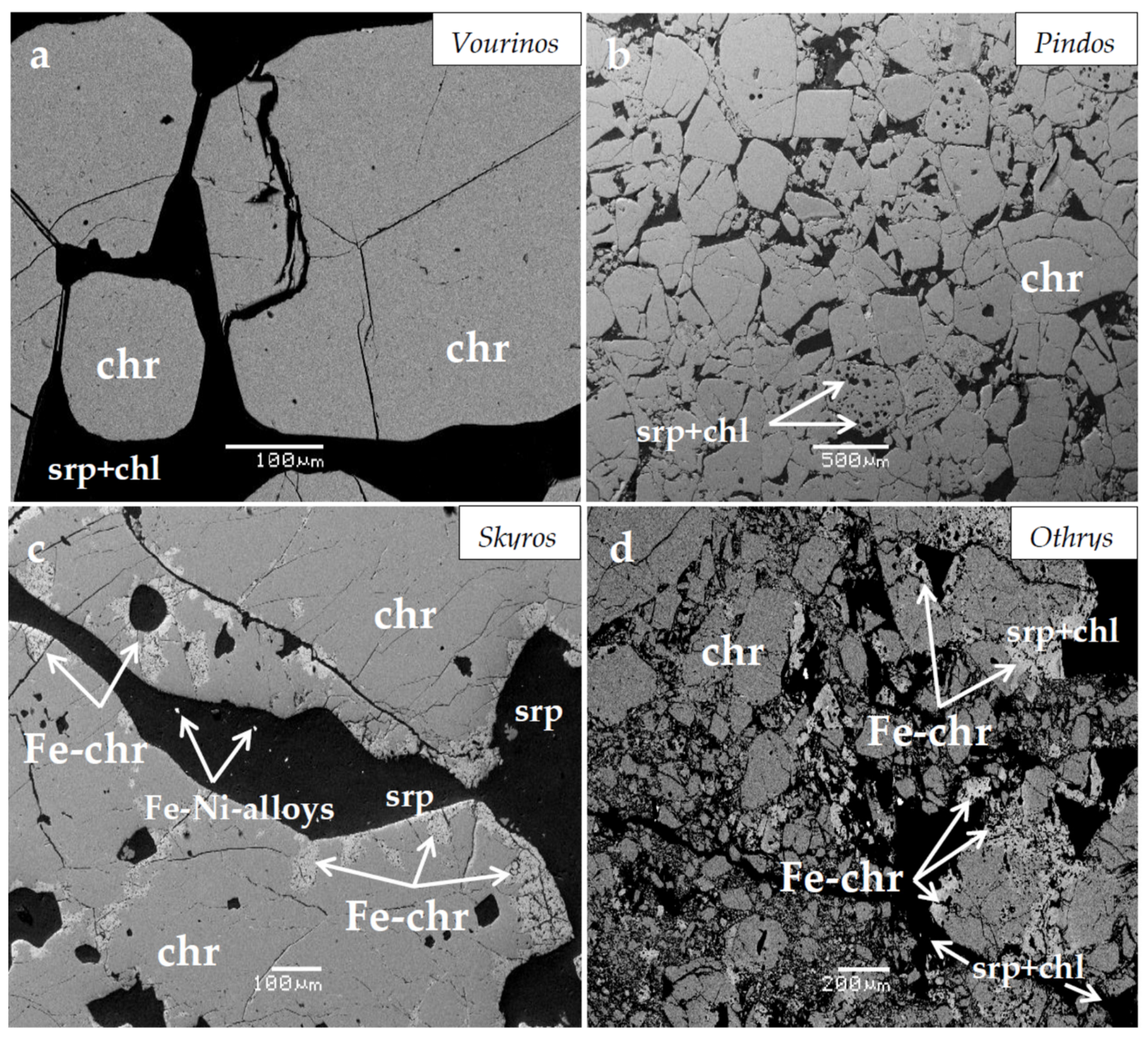
3. A Brief Outline of Characteristics for the Studied Chromitites
4. Results
4.1. Compositional Variations in Chromite
4.2. Distribution of Trace Elements in Chromitites
5. Discussion
5.1. Factors Controlling the Spinel Chemistry
5.1.1. Magmatic Versus Post-Magmatic Processes
5.1.2. Spinel Structure
5.1.3. Applications to Natural Spinels
6. Conclusions
- (1)
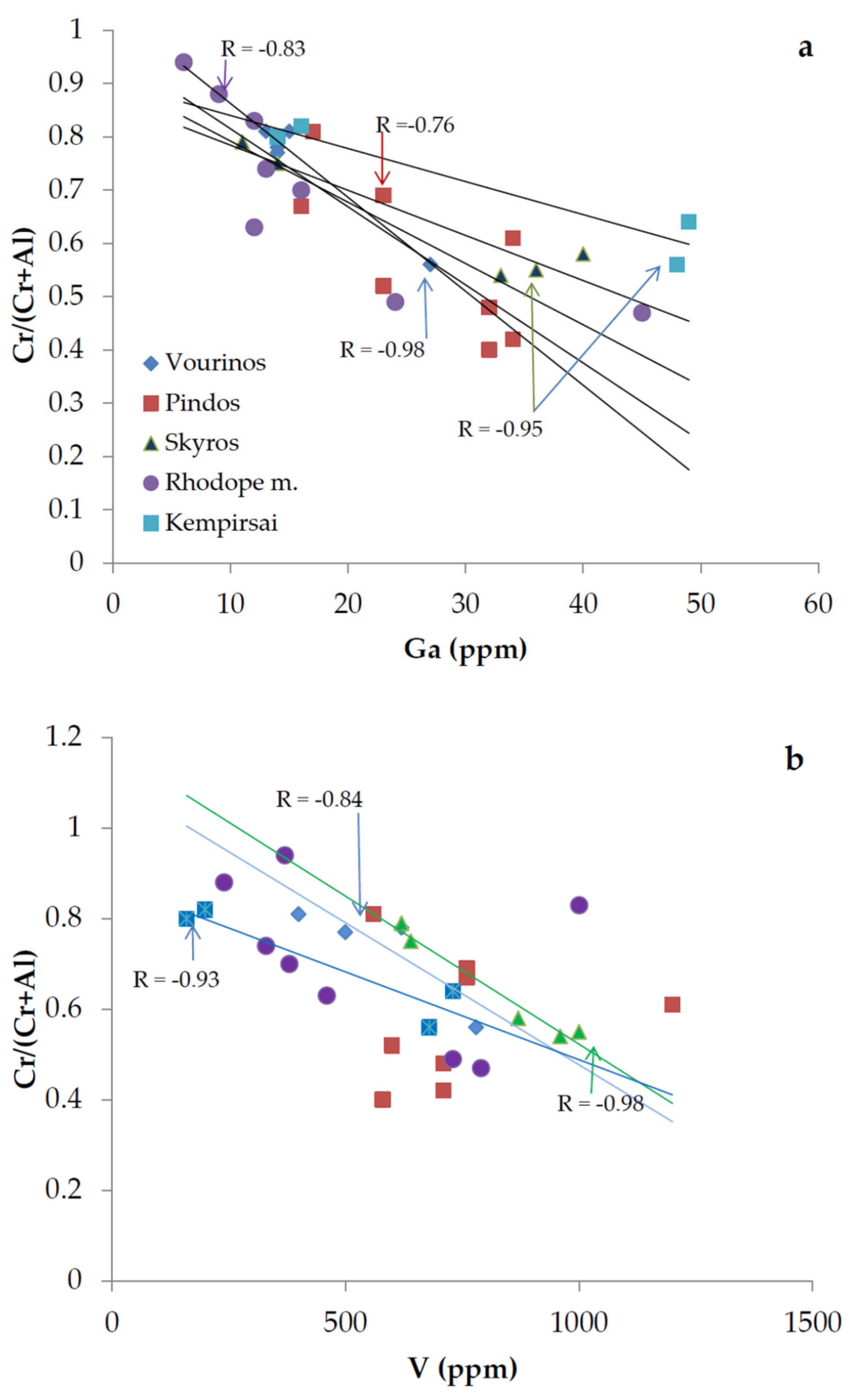
- The lower Ga contents in high-Cr chromitites (11 to 23 ppm) compared to high-Al ones (27–49 ppm) suggest that the composition of the parent magma may be a major factor controlling the preference of Ga in high-Al chromitites.
- (2)
- The positive trend between the Fe3+/(Al + Cr + Fe3+) atomic ratio and Ga content for large chromite deposits may suggest the effect of the redox conditions on the Ga distribution in chromitites.
- (3)
- Plot of the Cr/(Cr + Al) atomic ratios versus Ga content exhibits differences in terms of the slope of correlation lines for the different occurrences, suggesting that, in addition to the composition of parent magmas, other factors such as temperature, pressure or redox conditions may affect the observed deviation from linearity for small metamorphosed chromitite bodies.
- (4)
- The depletion of Ga and Al, and elevated Mn, Co, Zn and Fe contents in certain small chromitite occurrences, transformed during post-magmatic metamorphism, suggest potential change of the Ga content in Cr-spinel during sub-solidus reactions.
- (5)
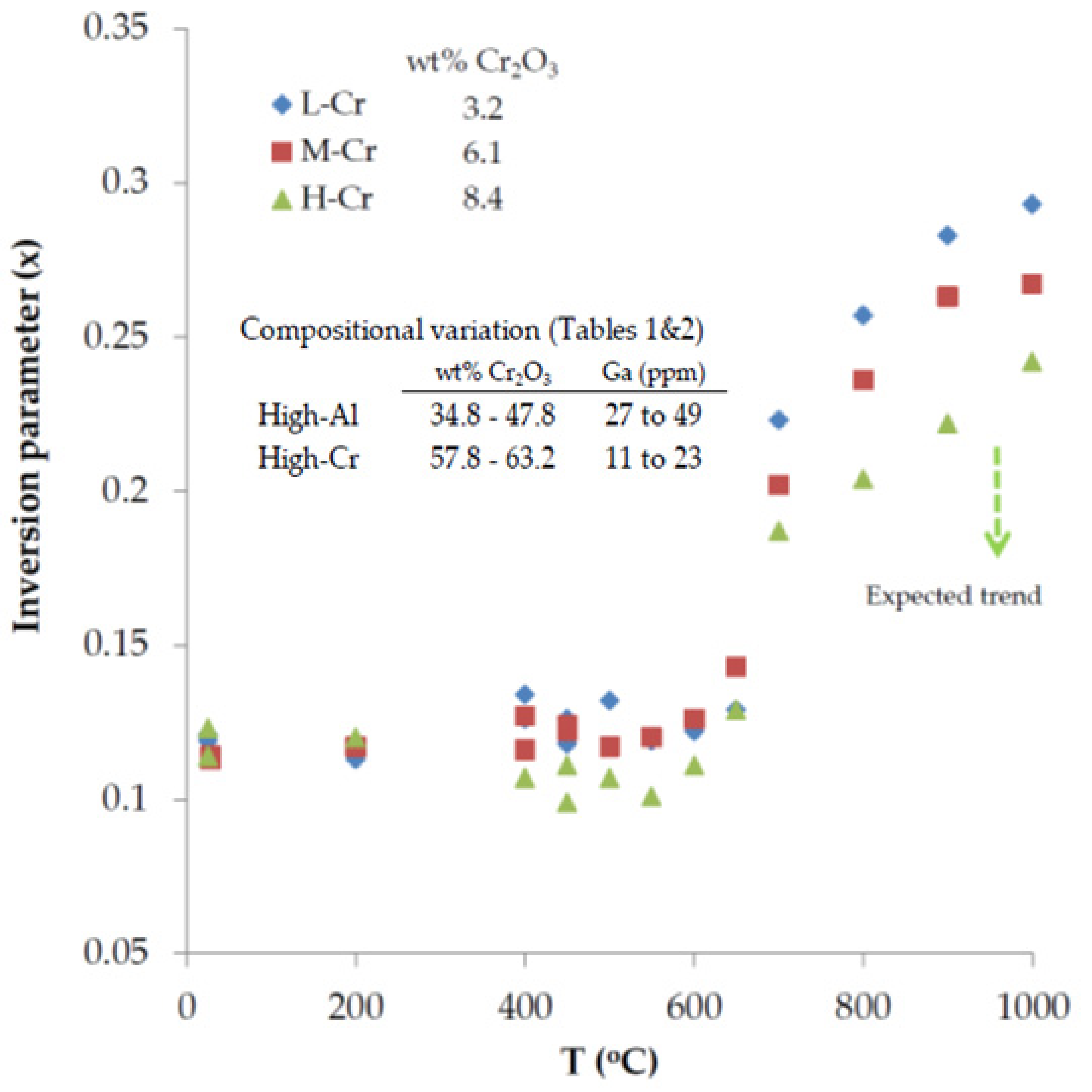
- Assuming that low-Cr spinel is characterized by the highest value of the inversion parameter (x) at higher than 650 °C, then the high Al content in spinels may be a driving force for the degree of inversion in the structure that facilitate the substitution of Al3+ for Ga3+ at magmatic conditions.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Navrotsky, A.; Kleppa, O.J. The thermodynamics of cation distributions in simple spinels. J. Inorg. Nucl. Chem. 1967, 29, 2701–2714. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Navrotsky, A. Simple spinels: Crystallographic parameters, cation radii, lattice energies, and cation distribution. Am. Mineral. 1983, 68, 181–194. [Google Scholar]
- Sickafus, K.E.; Wills, J.M.; Grimes, N.W. Structure of spinel. J. Am. Ceram. Soc. 1999, 82, 3279–3292. [Google Scholar] [CrossRef]
- Atkins, P.; Overton, T.; Rourke, J.; Weller, M.; Hagerman, M. Inorganic Chemistry, 5th ed.; W.H. Freeman and Company: New York, NY, USA, 2013. [Google Scholar]
- Princivalle, F.; Della Giusta, A.; Carbonin, S. Comparative crystal chemistry of spinels from some suites of ultramafic rocks. Mineral. Petrol. 1989, 40, 117–126. [Google Scholar] [CrossRef]
- Bosi, F.; Andreozzi, G.B.; Hålenius, U.; Skogby, H. Zn-O tetrahedral bond length variations in normal spinel oxides. Am. Mineral. 2011, 96, 594–598. [Google Scholar] [CrossRef]
- Fregola, R.A.; Bosi, F.; Skogby, S.; Hålenius, U. Cation ordering over short-range and long-range scales in the MgAl2O4-CuAl2O4 series. Am. Mineral. 2012, 97, 1821–1827. [Google Scholar] [CrossRef]
- Bosi, F. Chemical and structural variability in cubic spinel Oxides. Acta Crystallogr. 2019, B75, 279–285. [Google Scholar] [CrossRef]
- Lenaz, D.; Musco, M.E.; Petrelli, M.; Caldeira, R.; De Min, A.; Marzoli, A.; Mata, J.; Perugini, D.; Princivalle, F.; Boumehdi, M.A.; et al. Restitic or not? Insights from trace element content and crystal—Structure of spinels in African mantle xenoliths. Lithos 2017, 278, 464–476. [Google Scholar] [CrossRef]
- Wei, C.; Feng, Z.; Scherer, G.G.; Barber, J.; Shao-Horn, Y.; Xu, Z.J. Cations in Octahedral Sites: A Descriptor for Oxygen Electrocatalysis on Transition-Metal Spinels. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.D.; Culkin, F.; Riley, J.P. The abundances of gallium and germanium in terrestrial materials. Geochim. Cosmochim. Acta 1959, 16, 151–180. [Google Scholar] [CrossRef]
- Paktunc, A.D.; Cabri, L.J. A proton- and electron-microprobe study of gallium, nickel and zinc distribution in chromian spinel. Lithos 1995, 35, 261–282. [Google Scholar] [CrossRef]
- Wijbrans, C.H.; Klemme, S.; Berndt, J.; Vollmer, C. Experimental determination of trace element partition coefficients between spinel and silicate melt: The influence of chemical composition and oxygen fugacity. Contrib. Mineral. Petrol. 2015, 169, 1–33. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Pearce, J.A.; McDonald, I.; Styles, M.T. Tectonic discrimination of peridotites using fO2-Cr# and Ga–Ti–FeIII systematic in chrome-spinel. Chem. Geol. 2009, 261, 199–216. [Google Scholar]
- Gervilla, F.; Padrón-Navarta, J.A.; Kerestedjian, T.; Sergeeva, I.; González-Jiménez, J.M.; Fanlo, I. Formation of ferrian chromite in podiform chromitites from the Golyamo Kamenyane serpentinte, Eastern Rhodopes, SE Bulgaria: A two-stage process. Contrib. Mineral. Petrol. 2012, 164, 643–657. [Google Scholar] [CrossRef]
- Colás, V.; González-Jiménez, J.M.; Griffin, W.L.; Fanlo, I.; Gervilla, F.; O’Reilly, S.Y.; Pearson, N.J.; Kerestedjian, T.; Proenza, J.A. Fingerprints of metamorphism in chromite: New insights from minor and trace elements. Chem. Geol. 2014, 389, 137–152. [Google Scholar] [CrossRef]
- Scowen, P.; Roeder, P.L.; Helz, R. Re-equilibration of chromite within Kilauea Iki lava lake, Hawaii. Contrib. Mineral. Petrol. 1991, 107, 8–20. [Google Scholar] [CrossRef]
- Prasad, S.R.M.; Prasad, B.B.V.S.V.; Rajesh, B.; Rao, K.H.; Ramesh, K.V. Structural and dielectric studies of Mg2+ substituted Ni–Zn ferrite. Mater. Sci. Pol. 2015, 33, 806–815. [Google Scholar] [CrossRef]
- Zhou, M.F.; Robinson, P.T.; Su, B.X.; Gao, J.F.; Li, J.W.; Yang, J.S.; Malpas, J. Compositions of chromite, associated minerals, and parental magmas of podiform chromite deposits: The role of slab contamination of asthenospheric melts in suprasubduction zone environments. Gondwana Res. 2014, 26, 262–283. [Google Scholar] [CrossRef]
- Uysal, İ.; Tarkian, M.; Sadiklar, M.B.; Zaccarini, F.; Meisel, T.; Garuti, G.; Heidrich, S. Petrology of Al- and Cr-rich ophiolitic chromitites from the Muğla, SW Turkey: Implications from composition of chromite, solid inclusions of platinum-group mineral, silicate, and base-metal mineral, and Os-isotope geochemistry. Contrib. Mineral. Petrol. 2009, 158, 659–674. [Google Scholar] [CrossRef]
- Proenza, J.; Gervilla, F.; Melgarejo, J.; Bodinier, J.L. Al- and Cr-rich chromitites from the Mayarí-Baracoa ophiolitic belt (eastern Cuba); consequence of interaction between volatile-rich melts and peridotites in suprasubduction mantle. Econ. Geol. 1999, 94, 547–566. [Google Scholar] [CrossRef]
- González-Jiménez, J.M.; Locmelis, M.; Belousova, E.; Griffin, W.L.; Gervilla, F.; Kerestedjian, T.N.; Pearson, N.J.; Sergeeva, I. Genesis and tectonic implications of podiform chromitites in the metamorphosed Ultramafic Massif of Dobromirtsi (Bulgaria). Gondwana Res. 2015, 27, 555–574. [Google Scholar] [CrossRef]
- Brough, C.P.; Prichard, H.M.; Neary, C.R.; Fisher, P.C.; McDonald, I. Geochemical variations within podiform chromitite deposits in the Shetland Ophiolite: Implications for petrogenesis and PGE concentration. Econ. Geol. 2015, 110, 187–208. [Google Scholar] [CrossRef]
- Konstantopoulou, G.; Economou-Eliopoulos, M. Distribution of Platinum-group Elements and Gold in the Vourinos Chromitite Ores, Greece. Econ. Geol. 1991, 86, 1672–1682. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M.; Parry, S.J.; Christidis, G. Platinum-group element (PGE) content of chromite ores from the Othrys ophiolite complex, Greece. In Mineral Deposits: Research and Exploration. Where Do They Meet? Papunen, H., Ed.; Balkema: Rotterdam, The Netherlands, 1997; pp. 414–441. [Google Scholar]
- Economou-Eliopoulos, M.; Vacondios, I. Geochemistry of chromitites and host rocks from the Pindos ophiolite complex, northwestern Greece. Chem. Geol. 1995, 122, 99–108. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M.; Sambanis, G.; Karkanas, P. Trace element distribution in chromitites from the Pindos ophiolite complex, Greece: Implications for the chromite exploration. In Mineral Deposits; Stanley, Ed.; Balkema: Rotterdam, The Netherlands, 1999; pp. 713–716. [Google Scholar]
- Economou-Eliopoulos, M. On the origin of the PGE-enrichment in chromitites associated with ophiolite complexes: The case of Skyros island, Greece. In 9th SGA Meeting; Andrew, C.J., Borg, G., Eds.; Digging Deeper: Dublin, Ireland, 2007; pp. 1611–1614. [Google Scholar]
- Economou-Eliopoulos, M. Platinum-group element distribution in chromite ores from ophiolite complexes: Implications for their exploration. Ore Geol. Rev. 1996, 11, 363–381. [Google Scholar] [CrossRef]
- Zhelyaskova-Panayiotova, M.; Economou-Eliopoulos, M. Platinum-group element (PGE) and gold concentrations in oxide and sulfide mineralizations from ultmmafic rocks of Bulgaria. Ann. Univ. Sofia Geol. Congr. 1994, 86, 196–218. [Google Scholar]
- Rassios, A.; Dilek, Y. Rotational deformation in the Jurassic Meohellenic ophiolites, Greece, and its tectonic significance. Lithos 2009, 108, 192–206. [Google Scholar] [CrossRef]
- Economou, M.; Dimou, E.; Economou, G.; Migiros, G.; Vacondios, I.; Grivas, E.; Rassios, A.; Dabitzias, S. Chromite Deposits of Greece: Athens; Theophrastus Publications: Athens, Greece, 1986; pp. 129–159. [Google Scholar]
- Kapsiotis, A.; Grammatikopoulos, T.A.; Tsikouras, B.; Hatzipanagiotou, K. Platinum-group mineral characterization in concentrates from high-grade PGE Al-rich chromitites of Korydallos area in the Pindos ophiolite complex (NW Greece). SGS Miner. Serv. 2009, 60, 178–191. [Google Scholar] [CrossRef]
- Bonev, N.; Moritz, R.; Borisova, M.; Filipov, P. Therma–Volvi–Gomati complex of the Serbo-Macedonian Massif, northern Greece: A Middle Triassic continental margin ophiolite of Neotethyan origin. J. Geol. Soc. 2018. [Google Scholar] [CrossRef]
- Melcher, F.; Grum, W.; Simon, G.; Thalhammer, T.V.; Stumpfl, E. Petrogenesis of the ophiolitic giant chromite deposit of Kempirsai, Kazakhstan: A study of solid and fluid inclusions in chromite. J. Petrol. 1997, 10, 1419–1458. [Google Scholar] [CrossRef]
- Barnes, S.-L.; Naldrett, A.J.; Gorton, M.P. The origin of the fractionation of the platinum-group elements in terrestrial magmas. Chem. Geol. 1985, 53, 203–323. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M. Apatite and Mn, Zn, Co-enriched chromite in Ni-laterites of northern Greece and their genetic significance. J. Geochem. Exp. 2003, 80, 41–54. [Google Scholar] [CrossRef]
- Sack, R.O.; Ghiorso, M.S. Chromian spinels as petrogenetic indicators: Thermodynamic and petrological applications. Am. Mineral. 1991, 76, 827–847. [Google Scholar]
- Pagé, P.; Barnes, S.-J. Using trace elements in chromites to constrain the origin of podiform chromitites in the Thetford Mines Ophiolite, Québec, Canada. Econ. Geol. 2009, 104, 997–1018. [Google Scholar] [CrossRef]
- Uysal, I.; Sadiklar, M.; Tarkian, M.; Karsli, O.; Aydin, F. Mineralogy and composition of the chromitites and their platinum-group minerals from Ortaca (Muğla-SW Turkey): Evidence for ophiolitic chromitite genesis. Mineral. Petrol. 2005, 83, 219–242. [Google Scholar] [CrossRef]
- Leblanc, M.; Violette, J.F. Distribution of aluminum-rich and chromium-rich chromite pods in ophiolite peridotites. Econ. Geol. 1983, 78, 293–301. [Google Scholar] [CrossRef]
- Zhou, M.F.; Sun, M.; Keays, R.R.; Kerrich, R.W. Controls on platinum-group elemental distributions of podiform chromitites: A case study of high-Cr and high-Al chromitites from Chinese orogenic belts. Geochim. Cosmochim. Acta 1998, 62, 677–688. [Google Scholar] [CrossRef]
- Zaccarini, F.; Garuti, G.; Proenza, J.A.; Campos, L.; Thalhammer, O.A.R.; Aiglsperger, T.; Lewis, J.F. Chromite and platinum group elements mineralization in the Santa Elena ultramafic nappe (Costa Rica): Geodynamic implications. Geol. Acta 2011, 9, 407–423. [Google Scholar]
- Colás, V.; Padrón-Navarta, J.A.; González-Jiménez, J.M.; Griffin, W.L.; Fanlo, I.; O’reilly, S.Y.; Gervilla, F.; Proenza, J.A.; Pearson, N.J.; Escayola, M.P. Compositional effects on the solubility of minor and trace elements in oxide spinel minerals: Insights from crystal-crystal partition coefficients in chromite exsolution. Am. Mineral. 2016, 101, 1360–1372. [Google Scholar] [CrossRef]
- Barnes, S.J.; Roeder, P.L. The range of spinel compositions in terrestrial mafic and ultramafic rocks. J. Petrol. 2001, 42, 2279–2302. [Google Scholar] [CrossRef]
- Andreozzi, G.B.; Princivalle, F.; Skogby, H.; Della Giusta, A. Cation ordering and structural variations with temperature in MgAl2O4 spinel: An X-ray single-crystal study. Am. Mineral. 2000, 85, 1164–1171. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, X. Kinetics and Thermodynamics of Mg-Al Disorder in MgAl2O4-spinel: A Review. Molecules 2019, 24, 1704. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.F. Kinetics and thermodynamics of intracrystalline distribution. Mineral. Soc. Am. Spec. Pap. 1969, 2, 83–93. [Google Scholar]
- Mueller, R.F. Model for order-disorder kinetics in certain quasi-binary crystals of continuously variable composition. J. Phys. Chem. Solids 1967, 28, 2239–2243. [Google Scholar] [CrossRef]
- Martignago, F.; Dal Negro, A.; Carbonin, S. How Cr3+ and Fe3+ affect Mg-Al order disorder transformation at high temperature in natural spinels. Phys. Chem. Miner. 2003, 30, 401–408. [Google Scholar] [CrossRef]
- Princivalle, F.; Martignago, F.; Dal Negro, A. Kinetics of cation ordering in natural Mg (Al, Cr3+)2O4 spinels. Am. Mineral. 2006, 91, 313–318. [Google Scholar] [CrossRef]
- Lavina, B.; Reznitskii, L.Z.; Bosi, F. Crystal chemistry of some Mg, Cr, V normal spinels from Sludyanka (Lake Baikal, Russia): The influence of V3+ on structural stability. Phys. Chem. Miner. 2003, 30, 599–605. [Google Scholar] [CrossRef]

| Vourinos | Pindos | Skyros | |||||||||||||
| wt% | Vour. 1 | Vour. 2 | Vour. 3 | Vour. 4 | Vour. 5 | Pi.1. | Pi.2 | Pi. 3 | Pi. 4 | Pi. 5 | Pi.6 | Pi.7 | Sky. 1 | Sky. 2 | Sky. 3 |
| TiO2 | 0.3 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 |
| Al2O3 | 11.4 | 9.4 | 9.8 | 23.9 | 11.3 | 26.2 | 27.2 | 32.9 | 34.5 | 16.5 | 15.5 | 8.2 | 20.11 | 24.5 | 22.1 |
| Cr2O3 | 59.7 | 63.2 | 61.3 | 44.8 | 60.4 | 41.5 | 39.5 | 35.9 | 34.8 | 52.8 | 52.1 | 61.4 | 48.8 | 45.3 | 47.1 |
| MgO | 13.6 | 12.1 | 13.6 | 16.2 | 12.4 | 14.5 | 13.6 | 16.4 | 16.7 | 12.4 | 10.1 | 11.7 | 13.9 | 15.7 | 10.2 |
| FeO | 13.1 | 14.1 | 12.7 | 11.1 | 14.6 | 13.5 | 15.7 | 12.3 | 12.5 | 15.6 | 18.7 | 14.9 | 13.6 | 11.8 | 19.7 |
| Fe2O3 | 1.7 | 0.1 | 2.1 | 4.2 | .5 | 3.4 | 3.1 | 2.3 | 2.6 | 2.9 | 2.7 | 2.8 | 2.2 | 2.3 | 0.3 |
| MnO | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | n.d. | 0.1 | n.d. | 0.2 | 0.3 | 0.3 | 0.1 | 0.1 | 0.2 |
| NiO | 0.2 | 0.1 | n.d. | n.d. | 0.2 | 0.3 | n.d. | 0.2 | n.d. | 0.2 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 |
| Total | 100.3 | 99.5 | 99.9 | 100.5 | 99.8 | 99.9 | 99.4 | 100.1 | 101.2 | 100.7 | 99.7 | 99.6 | 99.01 | 100.1 | 100 |
| Cr/(Cr+Al) | 0.77 | 0.81 | 0.81 | 0.56 | 0.78 | 0.52 | 0.49 | 0.42 | 0.4 | 0.70 | 0.69 | 0.81 | 0.62 | 0.55 | 0.58 |
| Mg/(Mg+Fe2+) | 0.65 | 0.62 | 0.66 | 0.72 | 0.60 | 0.65 | 0.61 | 0.71 | 0.72 | 0.59 | 0.49 | 0.58 | 0.64 | 0.69 | 0.51 |
| Fe3+/(Cr+Al+Fe3+) | 0.031 | 0.000 | 0.025 | 0.036 | 0.0055 | 0.038 | 0.036 | 0.025 | 0.027 | 0.0413 | 0.028 | 0.036 | 0.027 | 0.026 | 0.0033 |
| Numbers of Cations on the Basis of 32 Oxygens | |||||||||||||||
| Ti | 0.019 | 0.039 | 0.040 | 0.018 | 0.039 | 0.036 | 0.054 | 0.035 | 0.014 | 0.019 | 0.039 | 0.020 | 0.037 | 0.036 | 0.038 |
| Al | 3.426 | 2.889 | 2.997 | 6.736 | 3.451 | 7.415 | 7.758 | 8.966 | 9.241 | 4.650 | 4.728 | 2.555 | 5.896 | 6.922 | 6.532 |
| Cr | 12.037 | 13.031 | 12.523 | 8.471 | 12.383 | 7.891 | 7.558 | 6.563 | 6.254 | 10.645 | 10.662 | 12.835 | 9.602 | 8.586 | 9.340 |
| Mg | 5.170 | 4.703 | 5.250 | 5.774 | 4.790 | 5.210 | 4.906 | 5.652 | 5.658 | 4.712 | 3.896 | 4.611 | 5.156 | 5.610 | 3.813 |
| Fe2+ | 2.744 | 3.271 | 2.744 | 2.203 | 3.164 | 2.706 | 3.149 | 2.326 | 2.377 | 3.222 | 4.056 | 3.300 | 2.840 | 2.367 | 4.142 |
| Fe3+ | 0.498 | 0.001 | 0.400 | 0.757 | 0.087 | 0.620 | 0.575 | 0.401 | 0.436 | 0.659 | 0.533 | 0.570 | 0.427 | 0.420 | 0.053 |
| Mn | 0.065 | 0.044 | 0.045 | 0.040 | 0.044 | 0.061 | 0.000 | 0.020 | 0.000 | 0.043 | 0.065 | 0.067 | 0.021 | 0.020 | 0.042 |
| Ni | 0.157 | 0.021 | 0.000 | 0.000 | 0.042 | 0.058 | 0.000 | 0.037 | 0.000 | 0.041 | 0.020 | 0.042 | 0.020 | 0.039 | 0.040 |
| Rhodope Massif | Urals Kempirsai | ||||||||||||||
| Skyros | Greece | Bulgaria | |||||||||||||
| Goliamo | Northern Part | Southern Part | |||||||||||||
| wt% | Sky. 4 | Sky. 5 | Soufli 1 | Soufli 2 | Gomati | Broucevci | Jacovitsa | Pletena | Kamenyane 1 | Kamenyane 2 | Batamshinsk | Main Ore Field | |||
| TiO2 | 0.1 | 0.2 | n.d. | 0.2 | 0.2 | 0.4 | 0.2 | 0.5 | 0.4 | 0.3 | 0.3 | 0.3 | 0.1 | 0.2 | |
| Al2O3 | 11.2 | 12.6 | 15.8 | 19.6 | 30.8 | 27.9 | 5.5 | 10.4 | 4.5 | 1.2 | 24.4 | 23.3 | 9.3 | 9.8 | |
| Cr2O3 | 59.1 | 58.1 | 53.9 | 47.8 | 35.5 | 37.3 | 58.8 | 49.8 | 33.2 | 24.9 | 46.6 | 47.1 | 61.3 | 60.2 | |
| MgO | 13.8 | 14.4 | 13.4 | 14.1 | 15.3 | 16.6 | 10.2 | 8.2 | 11.9 | 7.2 | 14.3 | 14.2 | 15.5 | 15.2 | |
| FeO | 12.1 | 11.5 | 13.4 | 13.5 | 13.9 | 10.4 | 16.2 | 20.7 | 13.6 | 20.2 | 14.1 | 14.1 | 9.9 | 10.3 | |
| Fe2O3 | 2.4 | 2.2 | 3.1 | 5.0 | 5.1 | 6.0 | 7.2 | 9.5 | 35.1 | 45.6 | 0.3 | 0.8 | 4.5 | 4.3 | |
| MnO | 0.2 | 0.2 | 0.2 | 0.2 | n.d. | 0.2 | 0.6 | 0.5 | n.d. | 0.2 | n.d. | n.d. | n.d. | n.d. | |
| NiO | 0.2 | 0.2 | n.d. | 0.2 | n.d. | 0.2 | 0.2 | 0.2 | 0.3 | 0.4 | 0.2 | 0.2 | 0.1 | 0.3 | |
| Total | 99.1 | 99.7 | 100.2 | 99.8 | 100.3 | 99.1 | 99.1 | 100 | 99.2 | 99.9 | 100.4 | 99.9 | 100.8 | 100.3 | |
| Cr/(Cr+Al) | 0.75 | 0.79 | 0.70 | 0.63 | 0.44 | 0.47 | 0.88 | 0.74 | 0.83 | 0.94 | 0.56 | 0.58 | 0.82 | 0.80 | |
| Mg/(Mg+Fe2+) | 0.68 | 0.69 | 0.62 | 0.65 | 0.66 | 0.72 | 0.52 | 0.37 | 0.52 | 0.38 | 0.64 | 0.65 | 0.74 | 0.73 | |
| Fe3+/(Cr+Al+Fe3+) | 0.03 | 0.026 | 0.036 | 0.059 | 0.057 | 0.068 | 0.093 | 0.123 | 0.455 | 0.62 | 0.0015 | 0.009 | 0.053 | 0.052 | |
| Numbers of cations on the basis of 32 oxygens | |||||||||||||||
| Ti | 0.019 | 0.038 | 0.019 | 0.036 | 0.035 | 0.035 | 0.041 | 0.102 | 0.082 | 0.062 | 0.054 | 0.054 | 0.019 | 0.038 | |
| Al | 3.414 | 3.813 | 4.636 | 5.591 | 8.475 | 7.828 | 1.767 | 3.277 | 1.452 | 0.386 | 6.987 | 6.,675 | 2.787 | 2.947 | |
| Cr | 12.066 | 11.695 | 10.745 | 9.396 | 6.553 | 7.021 | 12.670 | 10.582 | 7.168 | 5.648 | 8.880 | 9.070 | 12.323 | 12.143 | |
| Mg | 5.321 | 5.512 | 5.036 | 5.231 | 5.324 | 5.890 | 4.143 | 3.291 | 4.858 | 3.088 | 5.137 | 5.151 | 5.874 | 5.780 | |
| Fe2+ | 2.613 | 2.442 | 2.940 | 2.805 | 2.711 | 2.067 | 3.715 | 4.653 | 3.114 | 4.839 | 2.879 | 2.864 | 2.125 | 2.197 | |
| Fe3+ | 0.480 | 0.414 | 0.580 | 0.940 | 0.901 | 1.079 | 1.481 | 1.936 | 7.215 | 9,844 | 0.024 | 0.146 | 0.852 | 0.833 | |
| Mn | 0.044 | 0.044 | 0.043 | 0.000 | 0.000 | 0.040 | 0.138 | 0.115 | 0.000 | 0.046 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Ni | 0.041 | 0.041 | 0.000 | 0.000 | 0.000 | 0.038 | 0.044 | 0.044 | 0.114 | 0.088 | 0.039 | 0.038 | 0.020 | 0.062 | |
| Location | SEM/EDS | Trace Element (ppm) | wt% | ppb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cr/(Cr+Al) | Mg/(Mg+Fe2+) | Ni | Co | V | Zn | Ga | Fe | ΣPGE * | Pd/Ir * | |
| Vourinos 1 | 0.77 | 0.65 | 2000 | 240 | 500 | 260 | 14 | 8.1 | 140 | 0.06 |
| Vourinos 2 | 0.81 | 0.62 | 1580 | 210 | 560 | 550 | 15 | 7.9 | 135 | 0.35 |
| Vourinos 3 | 0.81 | 0.66 | 1900 | 200 | 400 | 300 | 13 | 8.72 | 92 | 0.55 |
| Vourinos 4 | 0.56 | 0.72 | 1800 | 170 | 780 | 360 | 27 | 9.2 | 30 | 0.54 |
| Vourinos 5 | 0.78 | 0.61 | 1800 | 180 | 620 | 280 | 14 | 7.76 | 109 | 0.63 |
| Pindos 1 | 0.52 | 0.63 | 1300 | 140 | 600 | 400 | 23 | 7.84 | 51 | 1.0 |
| Pindos 2 | 0.48 | 0.6 | 1500 | 260 | 710 | 410 | 32 | 9.77 | 143 | 1.0 |
| Pindos 3 | 0.42 | 0.71 | 1500 | 260 | 710 | 520 | 34 | 7.8 | 117 | 6.33 |
| Pindos 4 | 0.4 | 0.72 | 1630 | 240 | 580 | 460 | 32 | 7.3 | 6123 | 34.2 |
| Pindos5 | 0.67 | 0.57 | 750 | 270 | 760 | 520 | 16 | 9.7 | 3875 | 12.2 |
| Pindos 6 | 0.69 | 0.49 | 720 | 240 | 760 | 620 | 23 | 10.1 | 2098 | 7.2 |
| Pindos 7 | 0.81 | 0.58 | 1450 | 290 | 560 | 490 | 17 | 10.2 | 181 | 0.22 |
| Skyros 1 | 0.61 | 0.64 | 1300 | 250 | 1200 | 540 | 34 | 10.9 | 2300 | 0.08 |
| Skyros 2 | 0.55 | 0.69 | 1600 | 200 | 1000 | 400 | 36 | 9.45 | 464 | 0.7 |
| Skyros 3 | 0.58 | 0.51 | 1500 | 240 | 870 | 450 | 40 | 9.6 | 251 | 0.67 |
| Skyros 4 | 0.75 | 0.69 | 1250 | 220 | 640 | 420 | 14 | 10.7 | 145 | 0.33 |
| Skyros 5 | 0.79 | 0.69 | 1200 | 200 | 620 | 400 | 11 | 10.1 | 145 | 0.1 |
| Othrys (n = 4) | 0.54 | 0.69 | 1400 | 210 | 960 | 370 | 33 | 9.8 | 91 | 0.36 |
| Rhodope Massif | ||||||||||
| Greece | ||||||||||
| Soufli1 | 0.70 | 0.62 | 1700 | 230 | 380 | 580 | 16 | 13.2 | 150 | 0.2 |
| Soufli2 | 0.63 | 0.65 | 1150 | 220 | 460 | 280 | 12 | 10.6 | 82 | 1.0 |
| Gomati | 0.49 | 0.67 | 1030 | 130 | 730 | 280 | 24 | 9.6 | 104 | 0.25 |
| Bulgaria | ||||||||||
| Broucevci | 0.47 | 0.72 | 1300 | 230 | 790 | 420 | 45 | 11 | 60 | 0.92 |
| Jacovitsa | 0.88 | 0.52 | 2250 | 310 | 240 | 760 | 9 | 13.9 | 197 | 0.46 |
| Pletena | 0.74 | 0.37 | 890 | 290 | 330 | 1030 | 13 | 17.9 | 563 | 0.07 |
| Goliamo Kamenyane 1 | 0.83 | 0.52 | 1550 | 80 | 1000 | 450 | 12 | 11.6 | 87 | 0.14 |
| Goliamo Kamenyane 2 | 0.94 | 0.38 | 2260 | 970 | 370 | 4030 | 6 | 64.3 | 40 | 1.93 |
| Kemprsai (Urals) | ||||||||||
| Northern | 0.80 | 0.73 | 1600 | 210 | 160 | 160 | 14 | 9.1 | ||
| Batamshinsk | 0.82 | 0.74 | 1600 | 230 | 200 | 190 | 16 | 9.5 | ||
| Southern | 0.56 | 0.58 | 1500 | 240 | 680 | 480 | 48 | 12.6 | ||
| XL Let Kazakhstan | 0.64 | 0.65 | 1700 | 230 | 730 | 340 | 49 | 10.1 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eliopoulos, I.-P.D.; Eliopoulos, G.D. Factors Controlling the Gallium Preference in High-Al Chromitites. Minerals 2019, 9, 623. https://doi.org/10.3390/min9100623
Eliopoulos I-PD, Eliopoulos GD. Factors Controlling the Gallium Preference in High-Al Chromitites. Minerals. 2019; 9(10):623. https://doi.org/10.3390/min9100623
Chicago/Turabian StyleEliopoulos, Ioannis-Porfyrios D., and George D. Eliopoulos. 2019. "Factors Controlling the Gallium Preference in High-Al Chromitites" Minerals 9, no. 10: 623. https://doi.org/10.3390/min9100623
APA StyleEliopoulos, I.-P. D., & Eliopoulos, G. D. (2019). Factors Controlling the Gallium Preference in High-Al Chromitites. Minerals, 9(10), 623. https://doi.org/10.3390/min9100623




