Fe-Ni-P-S Melt Pockets in Elga IIE Iron Meteorite: Evidence for the Origin at High-Pressures Up to 20 GPa †
Abstract
:1. Introduction
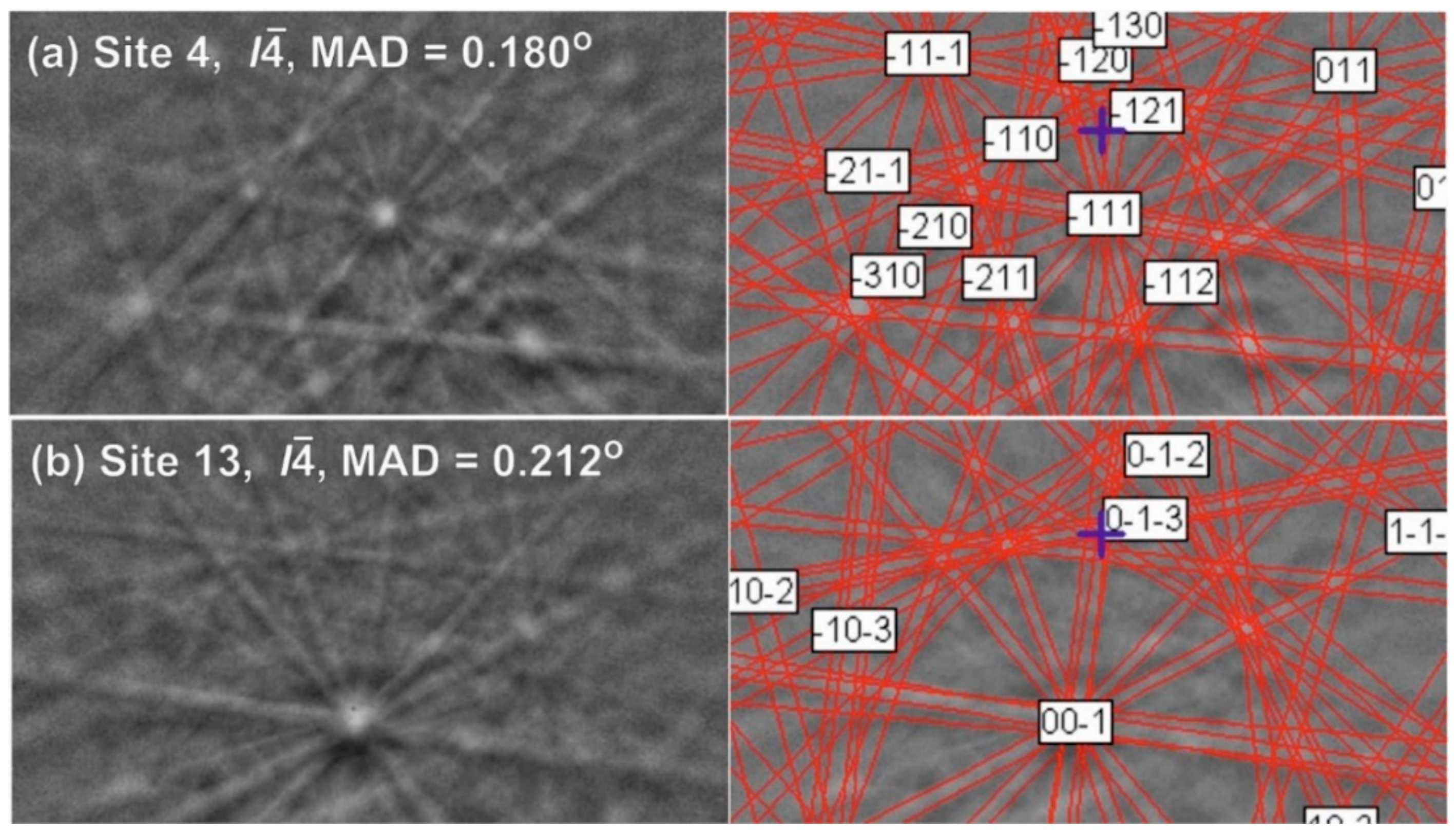
2. Materials and Methods
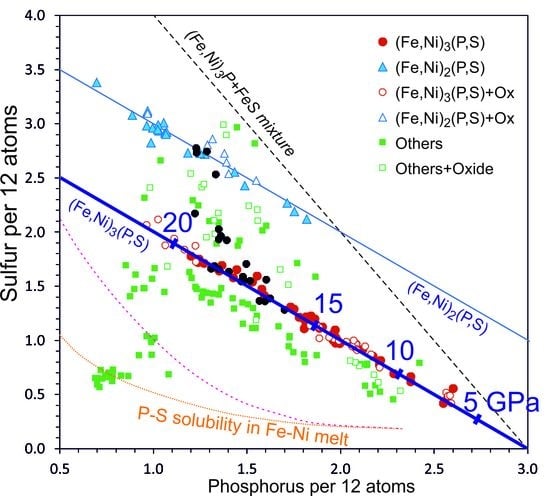
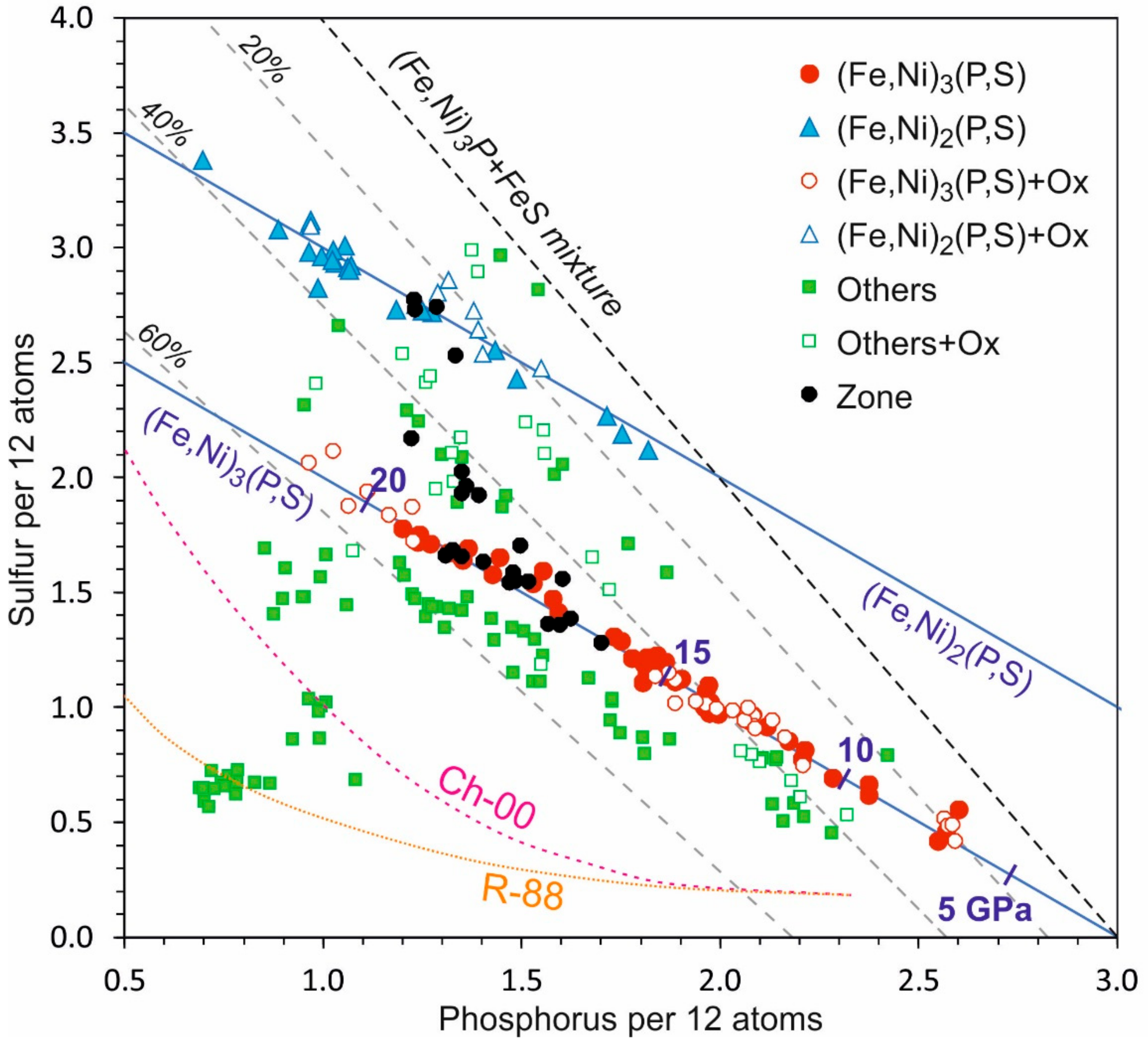
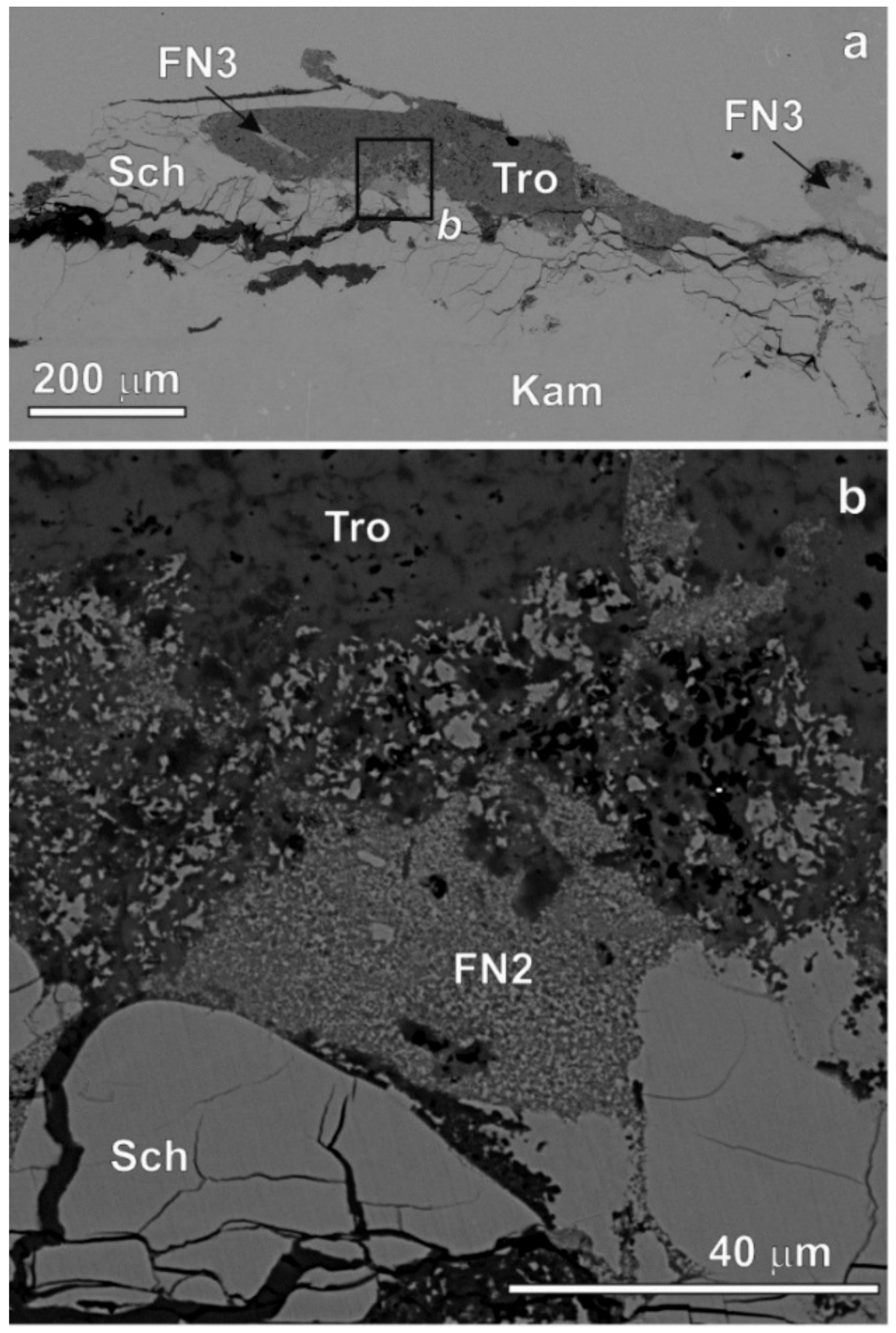
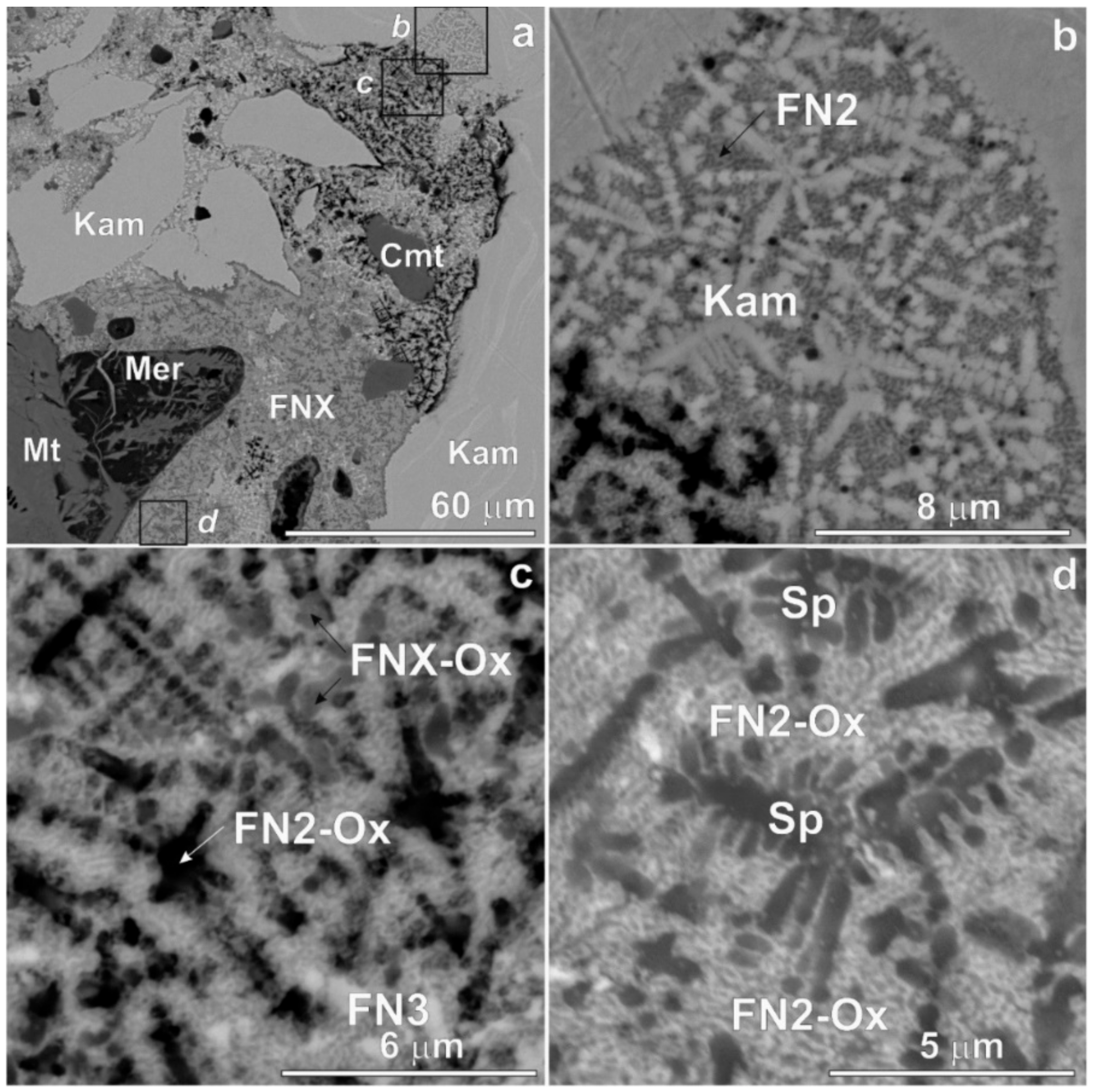
3. Results
4. Discussion
4.1. High-Pressure Origin of (Fe,Ni)3(P,S)
4.2. The Implication of the Results for Putative Impact Events during the Formation of IIE Iron Meteorites and Ureilites
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gillet, P.; El Goresy, A. Shock events in the Solar System: The message from minerals in terrestrial planets and asteroids. Annu. Rev. Earth Planet. Sci. 2013, 41, 257–285. [Google Scholar] [CrossRef]
- Tomioka, N.; Miyahara, M. High-pressure minerals in shocked meteorites. Meteorit. Planet. Sci. 2017, 52, 2017–2039. [Google Scholar] [CrossRef]
- Bevan, A.W.R.; Kinder, J.; Axon, H.J. A metallographic study of the iron meteorite Verkhne Dnieprovsk (BM 51183). Mineral. Mag. 1979, 43, 149–154. [Google Scholar] [CrossRef]
- Buchwald, V.F. Handbook of Iron Meteorites: Their History, Distribution, Composition, and Structure; University of California Press: Berkeley, CA, USA, 1975. [Google Scholar]
- Olsen, E.; Davis, A.; Clarke, R.S., Jr.; Schultz, L.; Weber, H.W.; Clayton, R.; Mayeda, T.; Jarosewich, E.; Sylvester, P.; Grossman, L. Watson: A new link in the HE iron chain. Meteoritics 1994, 29, 200–213. [Google Scholar] [CrossRef]
- Holtstam, D.; Broman, C.; Söderhielm, J.; Zetterqvist, A. First discovery of stishovite in an iron meteorite. Meteorit. Planet. Sci. 2003, 38, 1579–1583. [Google Scholar] [CrossRef]
- Britvin, S.N.; Rudashevsky, N.S.; Krivovichev, S.V.; Burns, P.C.; Polekhovsky, Y.S. Allabogdanite, (Fe,Ni)2P, a new mineral from the Onello meteorite: The occurrence and crystal structure. Am. Mineral. 2002, 87, 1245–1249. [Google Scholar] [CrossRef]
- Britvin, S.N.; Shilovskikh, V.V.; Pagano, R.; Vlasenko, N.S.; Zaitsev, A.N.; Krzhizhanovskaya, M.G.; Lozhkin, M.S.; Zolotarev, A.A.; Gurzhiy, V.V. Allabogdanite, the high-pressure polymorph of (Fe,Ni)2P, a stishovite-grade indicator of impact processes in the Fe–Ni–P system. Sci. Rep. 2019, 9, 1047. [Google Scholar] [CrossRef]
- Litasov, K.D.; Ishikawa, A.; Kopylova, A.G.; Podgornykh, N.M.; Pokhilenko, N.P. Mineralogy, trace element composition, and classification of Onello high-Ni ataxite. Dokl. Earth Sci. 2019, 485, 381–385. [Google Scholar] [CrossRef]
- Litasov, K.D.; Podgornykh, N.M. Raman spectroscopy of various phosphate minerals and occurrence of tuite in the Elga IIE iron meteorite. J. Raman Spectrosc. 2017, 48, 1518–1527. [Google Scholar] [CrossRef]
- Plyashkevich, L.N. Some data regarding the composition and structure of the iron meteorite Elga. Meteoritika 1962, 22, 51–60. (In Russian) [Google Scholar]
- Vronskii, V.I. On the find of the Elga iron meteorite. Meteoritika 1962, 22, 47–50. (In Russian) [Google Scholar]
- Kvasha, L.G.; Lavrent’ev, Y.G.; Sobolev, N.V. Silicate inclusions and evidence of impact metamorphism in the Elga octahedrite. Meteoritika 1974, 33, 143–147. (In Russian) [Google Scholar]
- Osadchii, E.G.; Baryshnikova, G.V.; Novikov, G.V. The Elga meteorite-Silicate inclusions and shock metamorphism. In Proceedings of the 12th Lunar and Planetary Science Conference, Houston, TX, USA, 16–20 March 1981; pp. 1049–1068. [Google Scholar]
- Wasson, J.T.; Wang, J. A non-magmatic origin of group-IIE iron meteorites. Geochim. Cosmochim. Acta 1986, 50, 725–732. [Google Scholar] [CrossRef]
- Teplyakova, S.N. Evolution of molten material in iron cores of small planets. Solar Syst. Res. 2011, 45, 515–522. [Google Scholar] [CrossRef]
- Khisina, N.R.; Teplyakova, S.N.; Wirth, R.; Senin, V.G.; Averin, A.A.; Shiryaev, A.A. Carbon-bearing phases in shock-induced melt zones of the Elga meteorite. Geochem. Int. 2017, 55, 317–329. [Google Scholar] [CrossRef]
- Teplyakova, S.N.; Lorenz, C.A.; Ivanova, M.A.; Kononkova, N.N.; Anosova, M.O.; Ryazantsev, K.M.; Kostitsyn, Y.A. Mineralogy of silicate inclusions in the Elga IIE iron meteorite. Geochem. Int. 2018, 56, 1–23. [Google Scholar] [CrossRef]
- Stewart, A.J.; Schmidt, M.W. Sulfur and phosphorus in the Earth’s core: The Fe-P-S system at 23 GPa. Geophys. Res. Lett. 2007, 34, L13201. [Google Scholar] [CrossRef]
- Gu, T.; Fei, Y.; Wu, X.; Qin, S. Phase stabilities and spin transitions of Fe3(S1-xPx) at high pressure and its implications in meteorites. Am. Mineral. 2016, 101, 205–210. [Google Scholar] [CrossRef]
- Lavrent’ev, Y.G.; Karmanov, N.S.; Usova, L.V. Electron probe microanalysis of minerals: Microanalyzer or scanning electron microscope? Russ. Geol. Geophys. 2015, 56, 1154–1161. [Google Scholar] [CrossRef]
- Shatskiy, A.; Gavryushkin, P.N.; Sharygin, I.S.; Litasov, K.D.; Kupriyanov, I.N.; Higo, Y.; Borzdov, Y.M.; Funakoshi, K.; Palyanov, Y.N.; Ohtani, E. Melting and subsolidus phase relations in the system Na2CO3-MgCO3±H2O at 6 GPa and the stability of Na2Mg(CO3)2 in the upper mantle. Am. Mineral. 2013, 98, 2172–2182. [Google Scholar] [CrossRef]
- Arefiev, A.V.; Shatskiy, A.; Podborodnikov, I.V.; Bekhtenova, A.; Litasov, K.D. The system K2CO3-CaCO3-MgCO3 at 3 GPa: Implications for carbonatite melt compositions in the shallow continental lithosphere. Minerals 2019, 9, 296. [Google Scholar] [CrossRef]
- Skála, R.; Císařová, I. Crystal structure of meteoritic schreibersites: Determination of absolute structure. Phys. Chem. Miner. 2005, 31, 721–732. [Google Scholar] [CrossRef]
- Raghavan, V. The Fe-Ni-P (Iron-Nickel-Phosphorus) system. In Phase Diagrams of Ternary Iron Alloys, Indian Institute of Metals; ASM International: Calcutta, India, 1988; pp. 121–137. [Google Scholar]
- Chabot, N.L.; Drake, M.J. Crystallization of magmatic iron meteorites: The effects of phosphorus and liquid immiscibility. Meteorit. Planet. Sci. 2000, 35, 807–816. [Google Scholar] [CrossRef]
- Urakawa, S.; Matsubara, R.; Katsura, T.; Watanabe, T.; Kikegawa, T. Stability and bulk modulus of Ni3S, a new nickel sulfur compound, and the melting relations of the system Ni-NiS up to 10 GPa. Am. Mineral. 2011, 96, 558–565. [Google Scholar] [CrossRef]
- Nabiei, F.; Badro, J.; Dennenwaldt, T.; Oveisi, E.; Cantoni, M.; Hébert, C.; El Goresy, A.; Barrat, J.-A.; Gillet, P. A large planetary body inferred from diamond inclusions in a ureilite meteorite. Nat. Commun. 2018, 9, 1327. [Google Scholar] [CrossRef] [PubMed]
- Murayama, J.K.; Nakai, S.; Kato, M.; Kumazawa, M. A dense polymorph of Ca3(PO4)2: A high pressure phase of apatite decomposition and its geochemical significance. Phys. Earth Planet. Inter. 1986, 44, 293–303. [Google Scholar] [CrossRef]
- Xie, X.D.; Minitti, M.E.; Chen, M.; Mao, H.K.; Wang, D.Q.; Shu, J.F.; Fei, Y.W. Natural high-pressure polymorph of merrillite in the shock veins of the Suizhou meteorite. Geochim. Cosmochim. Acta 2002, 66, 2439–2444. [Google Scholar] [CrossRef]
- Fei, Y.; Li, J.; Bertka, C.M.; Prewitt, C.T. Structure type and bulk modulus of Fe3S, a new iron-sulfur compound. Am. Mineral. 2000, 85, 1830–1833. [Google Scholar] [CrossRef]
- Kamada, S.; Terasaki, H.; Ohtani, E.; Sakai, T.; Kikegawa, T.; Ohishi, Y.; Hirao, N.; Sata, N.; Kondo, T. Phase relationships of the Fe–FeS system in conditions up to the Earth’s outer core. Earth Planet. Sci. Lett. 2010, 294, 94–100. [Google Scholar] [CrossRef]
- Krot, A.N.; Keil, K.; Scott, E.R.D.; Goodrich, C.A.; Weisberg, M.K. Classification of meteorites and their genetic relationships. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; Volume 1, pp. 1–63. [Google Scholar] [CrossRef]
- Ruzicka, A. Silicate-bearing iron meteorites and their implications for the evolution of asteroidal parent bodies. Chem. der Erde 2014, 74, 3–48. [Google Scholar] [CrossRef]
- Miyahara, M.; Ohtani, E.; El Goresy, A.; Lin, Y.; Feng, L.; Zhang, J.-C.; Gillet, P.; Nagase, T.; Muto, J.; Nishijima, M. Unique large diamonds in a ureilite from Almahata Sitta 2008 TC3 asteroid. Geochim. Cosmochim. Acta 2015, 163, 14–26. [Google Scholar] [CrossRef]

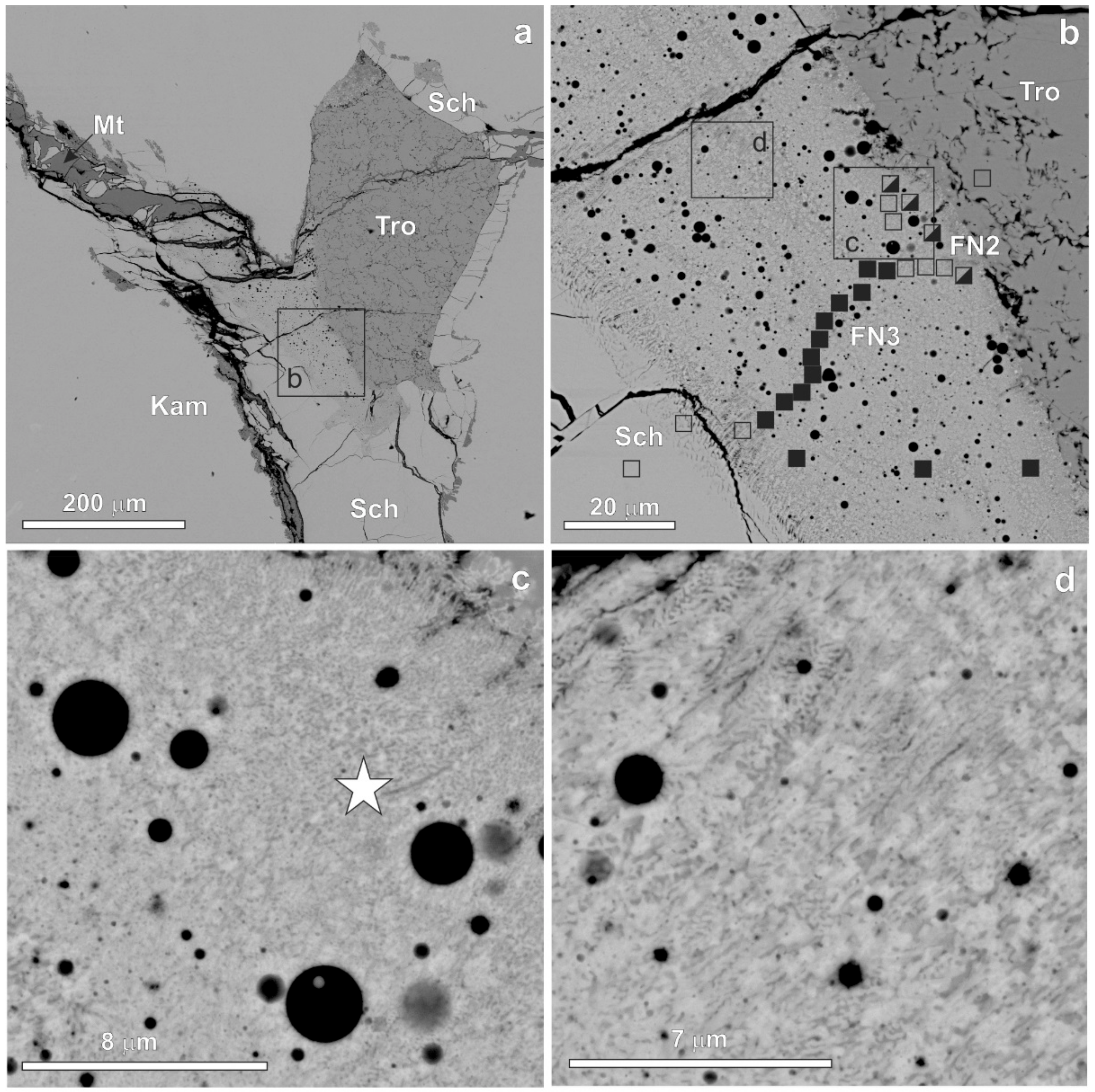
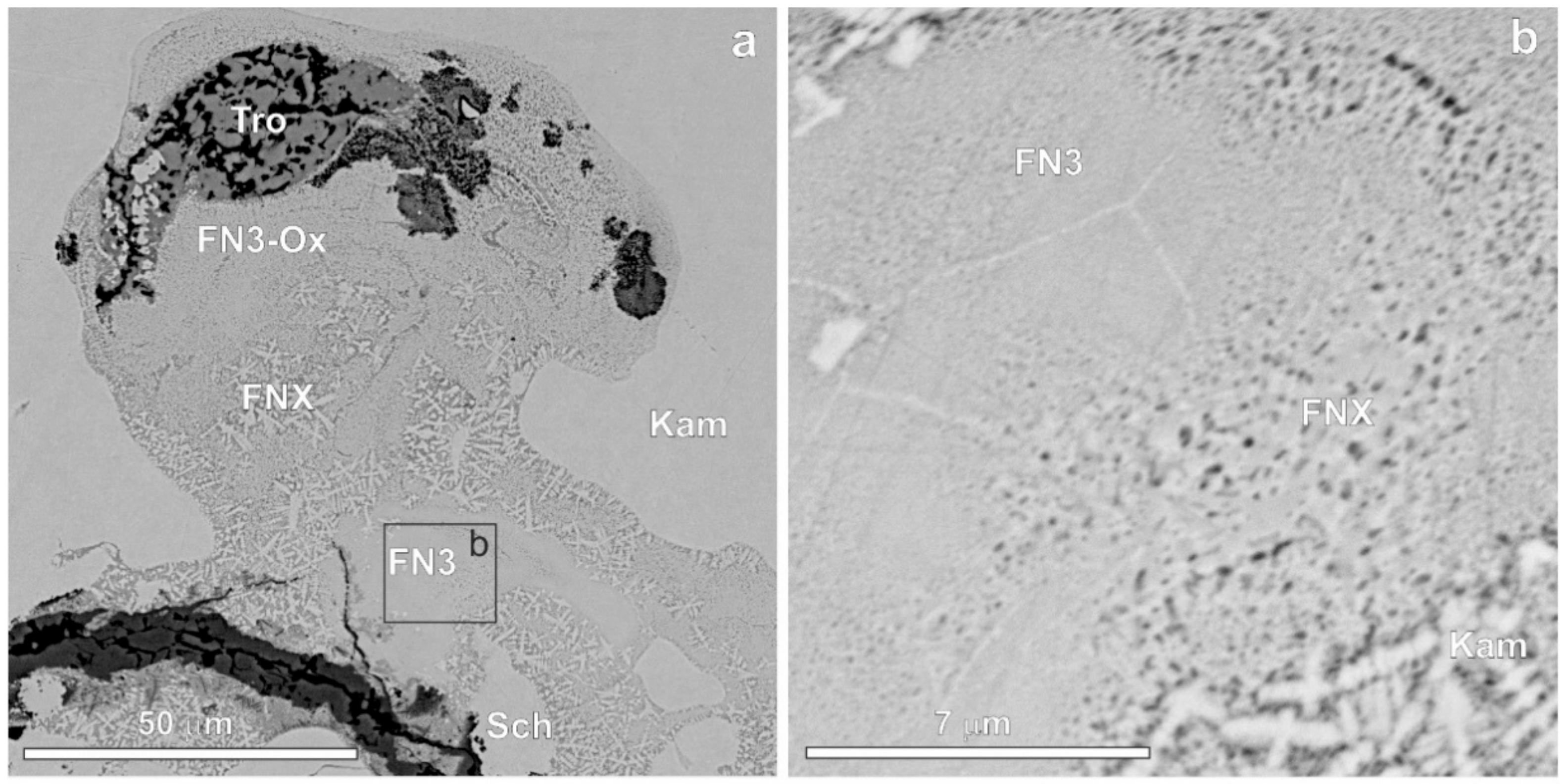

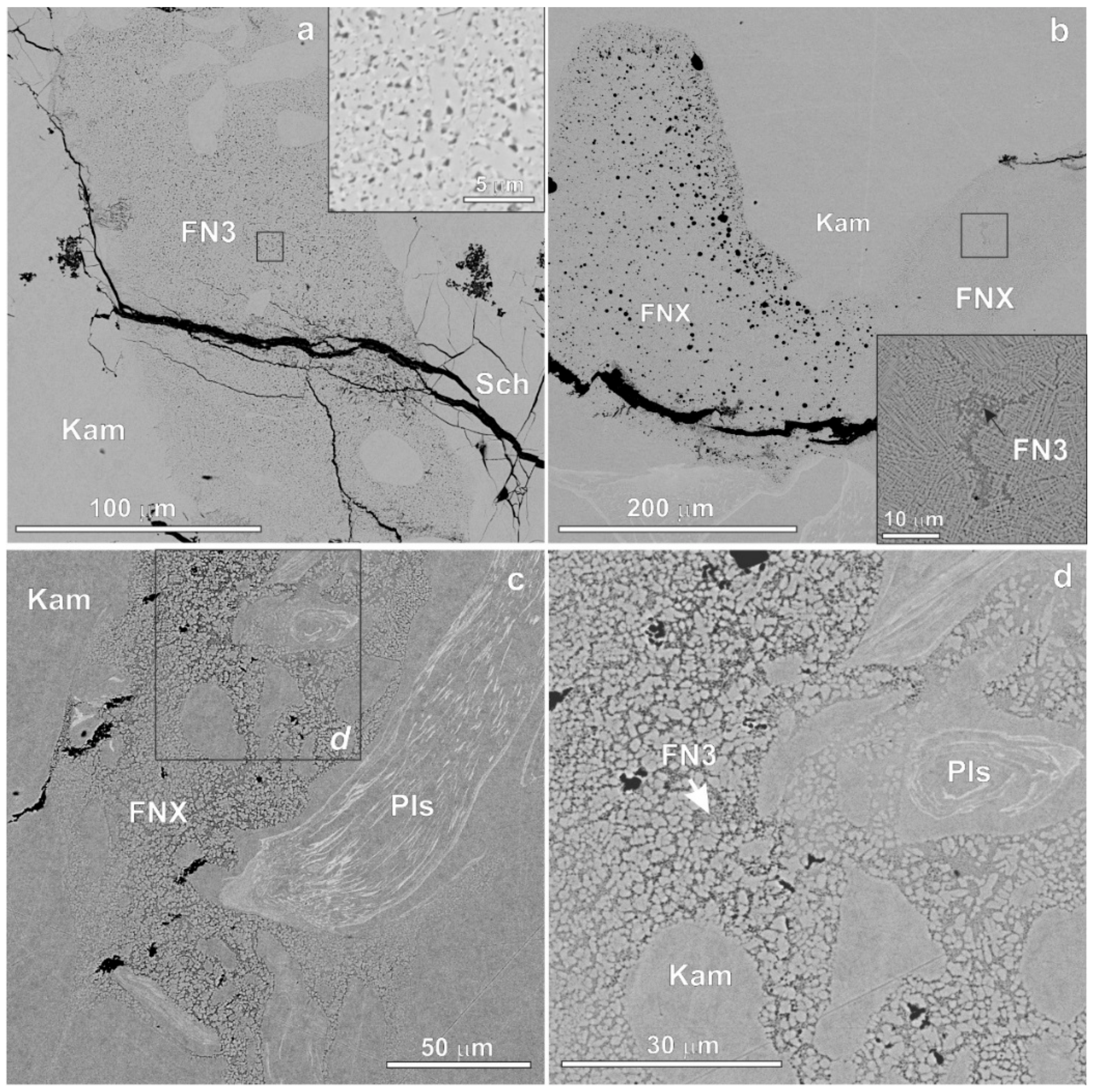
| Mineral | n | Cr | Fe | Co | Ni | P | S | O | Total |
|---|---|---|---|---|---|---|---|---|---|
| Kamacite | 124 | 91.91 | 0.77 | 7.08 | 0.17 | 99.93 | |||
| SD | 2.58 | 0.14 | 0.62 | 0.13 | |||||
| Taenite | 60 | 76.22 | 0.48 | 23.10 | 0.05 | 99.85 | |||
| SD | 3.25 | 0.15 | 3.31 | 0.05 | |||||
| Schreibersite | 56 | 61.16 | 0.41 | 22.66 | 15.35 | 0.02 | 0.22 | 99.82 | |
| SD | 2.49 | 0.12 | 2.45 | 0.73 | 0.01 | 0.21 | |||
| Troilite | 60 | 0.13 | 63.08 | 0.01 | 0.07 | 36.25 | 99.54 | ||
| SD | 0.07 | 1.17 | 0.01 | 0.05 | 1.24 |
| Type | FN3 | FN3 | FN3-Ox | FN3-Cryst | FN2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | 14-15 | 23-24 | 13 | 13 | 15,19 | ||||||
| Fe | 68.79 | 0.63 | 62.78 | 2.88 | 62.78 | 2.31 | 66.85 | 0.96 | 66.19 | 0.94 | |
| Co | 0.55 | 0.07 | 0.49 | 0.13 | 0.66 | 0.16 | 0.50 | 0.09 | 0.45 | 0.07 | |
| Ni | 13.79 | 0.39 | 19.60 | 2.61 | 15.85 | 0.55 | 15.04 | 0.79 | 11.16 | 0.48 | |
| P | 10.31 | 0.50 | 9.26 | 0.10 | 10.15 | 0.39 | 11.06 | 0.11 | 5.44 | 0.20 | |
| S | 4.88 | 0.55 | 6.15 | 0.18 | 4.69 | 0.21 | 4.36 | 0.15 | 16.07 | 0.32 | |
| O | 1.13 | 0.14 | 1.18 | 0.11 | 5.65 | 2.38 | 1.53 | 0.06 | 0.44 | 0.49 | |
| Total | 99.45 | 0.55 | 99.46 | 0.72 | 99.77 | 0.71 | 99.33 | 0.23 | 99.74 | 0.23 | |
| Atomic ratios per 12 atoms. M = Fe + Ni + Co | |||||||||||
| Fe | 7.537 | 6.892 | 7.178 | 7.349 | 6.905 | ||||||
| Ni | 1.438 | 2.048 | 1.725 | 1.573 | 1.107 | ||||||
| Co | 0.057 | 0.051 | 0.071 | 0.052 | 0.045 | ||||||
| P | 2.037 | 1.832 | 2.093 | 2.192 | 1.023 | ||||||
| S | 0.931 | 1.177 | 0.933 | 0.834 | 2.919 | ||||||
| M | 9.032 | 8.991 | 8.974 | 8.974 | 8.057 | ||||||
| P+S | 2.968 | 3.009 | 3.026 | 3.026 | 3.943 | ||||||
| Type | FN3 | FNX | Fe-mix | Fe-mix | |||||||
| Site | 9,23a | 8-9,23a | 27 | 28 | |||||||
| Fe | 68.18 | 0.41 | 81.44 | 0.74 | 68.85 | 1.33 | 77.79 | 1.02 | |||
| Co | 0.56 | 0.11 | 0.77 | 0.09 | 0.54 | 0.11 | 0.70 | 0.07 | |||
| Ni | 14.88 | 0.06 | 10.64 | 0.45 | 15.47 | 1.65 | 11.49 | 0.43 | |||
| P | 6.53 | 0.30 | 3.60 | 0.20 | 8.18 | 1.40 | 4.71 | 0.41 | |||
| S | 9.00 | 0.27 | 3.29 | 0.24 | 5.50 | 1.71 | 4.08 | 0.69 | |||
| O | 0.91 | 0.21 | 0.59 | 0.13 | 0.98 | 0.08 | 0.72 | 0.11 | |||
| Total | 100.06 | 0.48 | 100.33 | 0.36 | 99.53 | 0.56 | 99.49 | 0.10 | |||
| Atomic ratios per 12 atoms. M = Fe + Ni + Co | |||||||||||
| Fe | 7.415 | 9.351 | 7.620 | 8.891 | |||||||
| Ni | 1.540 | 1.162 | 1.630 | 1.249 | |||||||
| Co | 0.058 | 0.084 | 0.056 | 0.076 | |||||||
| P | 1.281 | 0.744 | 1.633 | 0.970 | |||||||
| S | 1.706 | 0.658 | 1.061 | 0.813 | |||||||
| M | 9.013 | 10.597 | 9.306 | 10.216 | |||||||
| P+S | 2.987 | 1.403 | 2.694 | 1.784 | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litasov, K.D.; Teplyakova, S.N.; Shatskiy, A.; Kuper, K.E. Fe-Ni-P-S Melt Pockets in Elga IIE Iron Meteorite: Evidence for the Origin at High-Pressures Up to 20 GPa. Minerals 2019, 9, 616. https://doi.org/10.3390/min9100616
Litasov KD, Teplyakova SN, Shatskiy A, Kuper KE. Fe-Ni-P-S Melt Pockets in Elga IIE Iron Meteorite: Evidence for the Origin at High-Pressures Up to 20 GPa. Minerals. 2019; 9(10):616. https://doi.org/10.3390/min9100616
Chicago/Turabian StyleLitasov, Konstantin D., Svetlana N. Teplyakova, Anton Shatskiy, and Konstantin E. Kuper. 2019. "Fe-Ni-P-S Melt Pockets in Elga IIE Iron Meteorite: Evidence for the Origin at High-Pressures Up to 20 GPa" Minerals 9, no. 10: 616. https://doi.org/10.3390/min9100616
APA StyleLitasov, K. D., Teplyakova, S. N., Shatskiy, A., & Kuper, K. E. (2019). Fe-Ni-P-S Melt Pockets in Elga IIE Iron Meteorite: Evidence for the Origin at High-Pressures Up to 20 GPa. Minerals, 9(10), 616. https://doi.org/10.3390/min9100616