Patynite, NaKCa4[Si9O23], a New Mineral from the Patynskiy Massif, Southern Siberia, Russia
Abstract
:1. Introduction
2. Occurrence, Geological Settings, and Mineral Association
3. Methods
4. Results
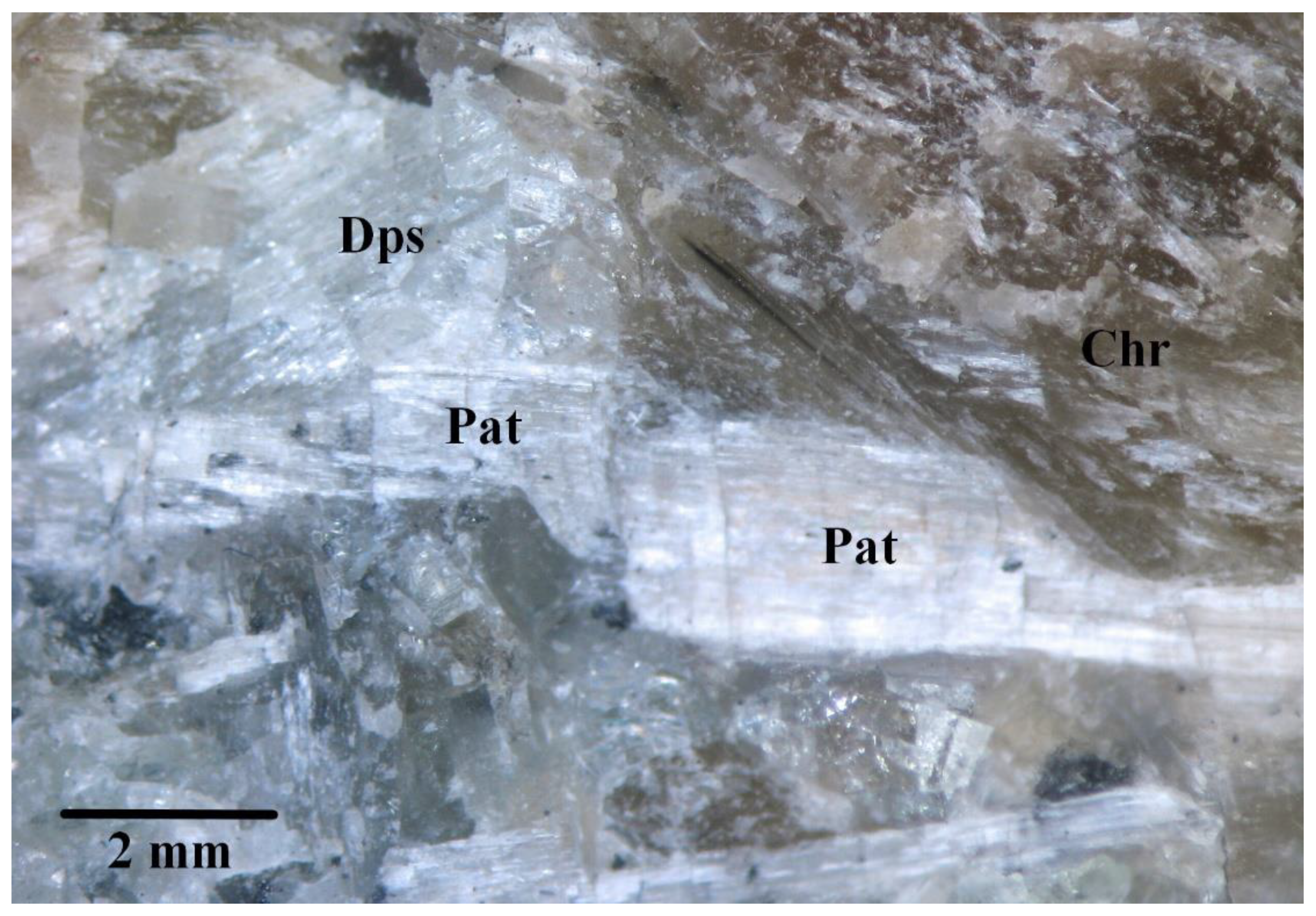
4.1. General Appearance, Physical, Chemical, and Optical Properties
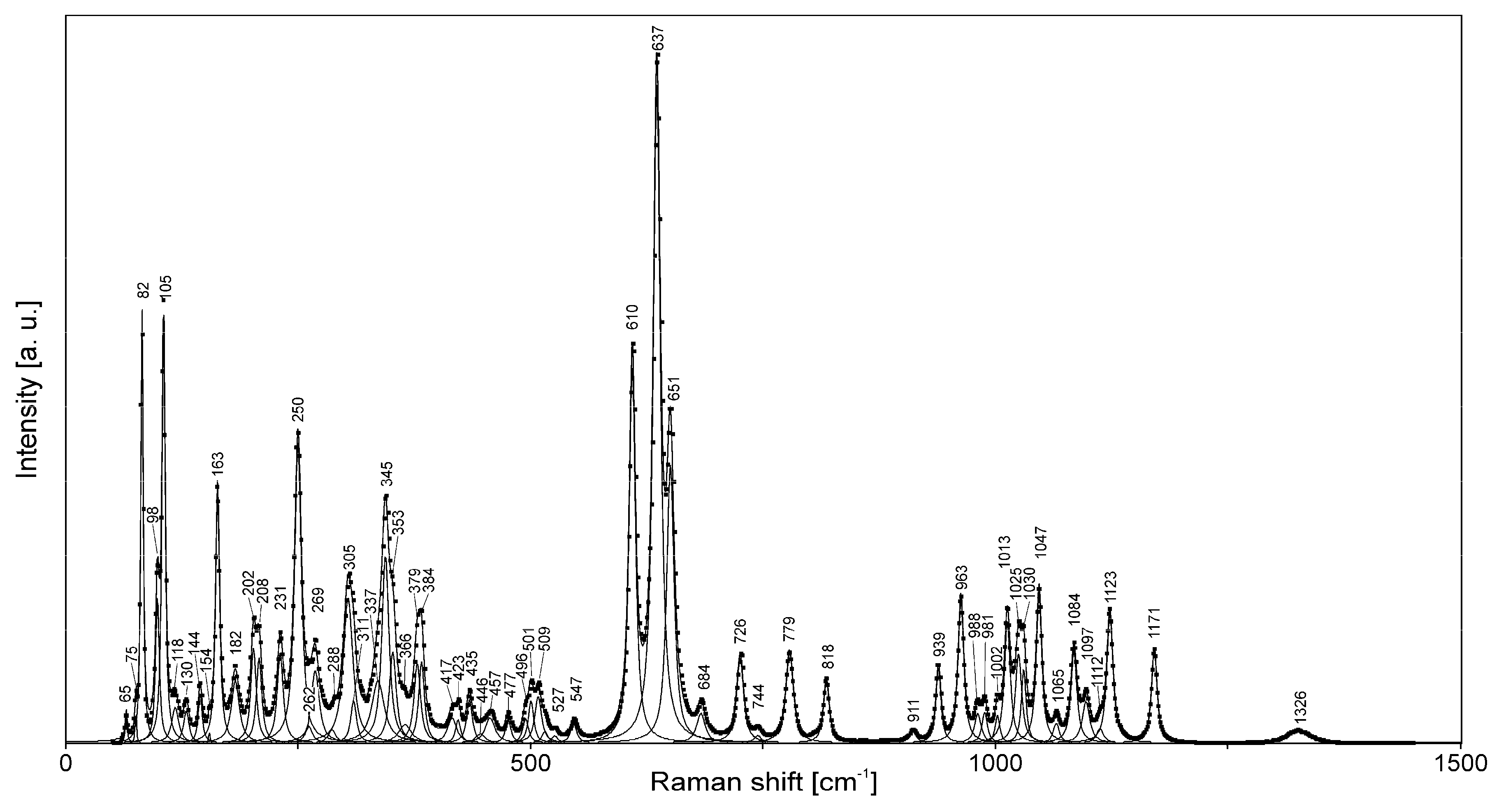
4.2. Raman Spectroscopy
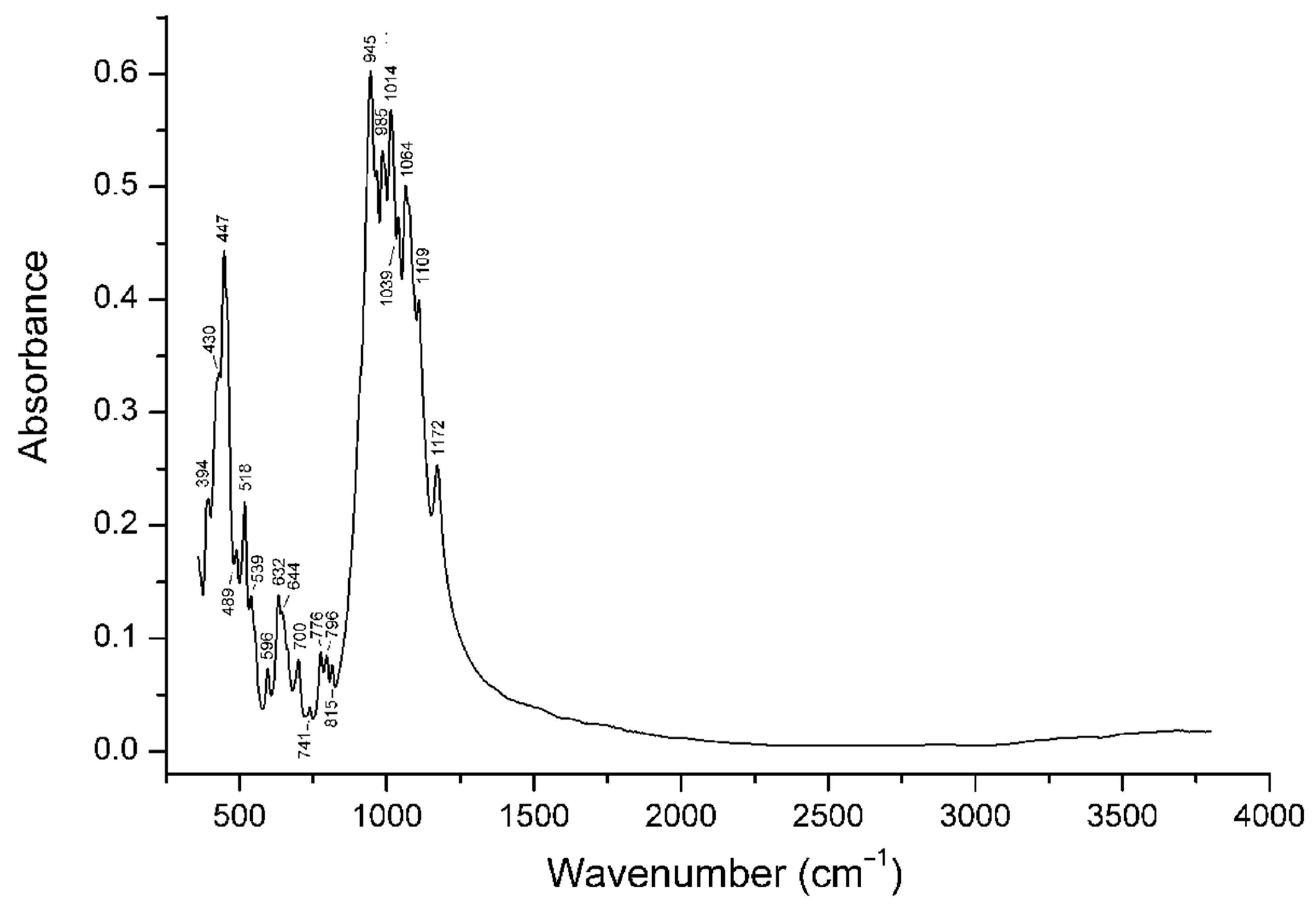
4.3. Infrared Spectroscopy
4.4. Chemical Composition
4.5. X-ray Diffraction Data
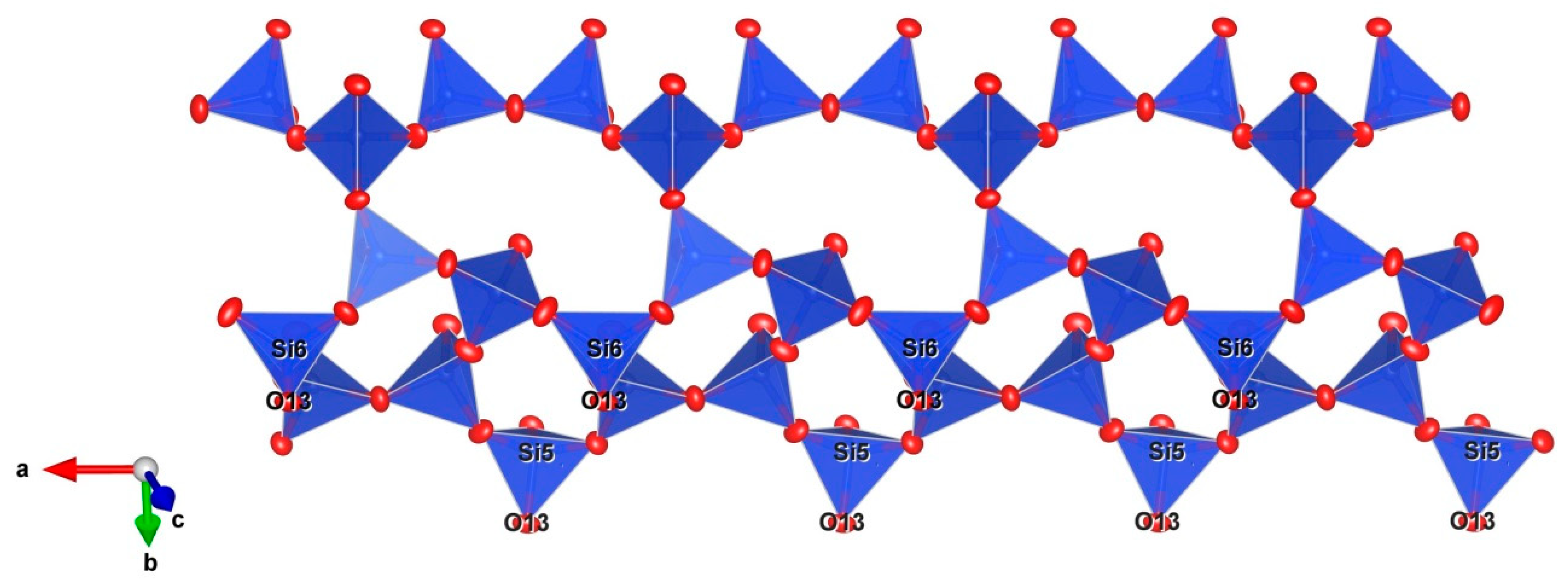
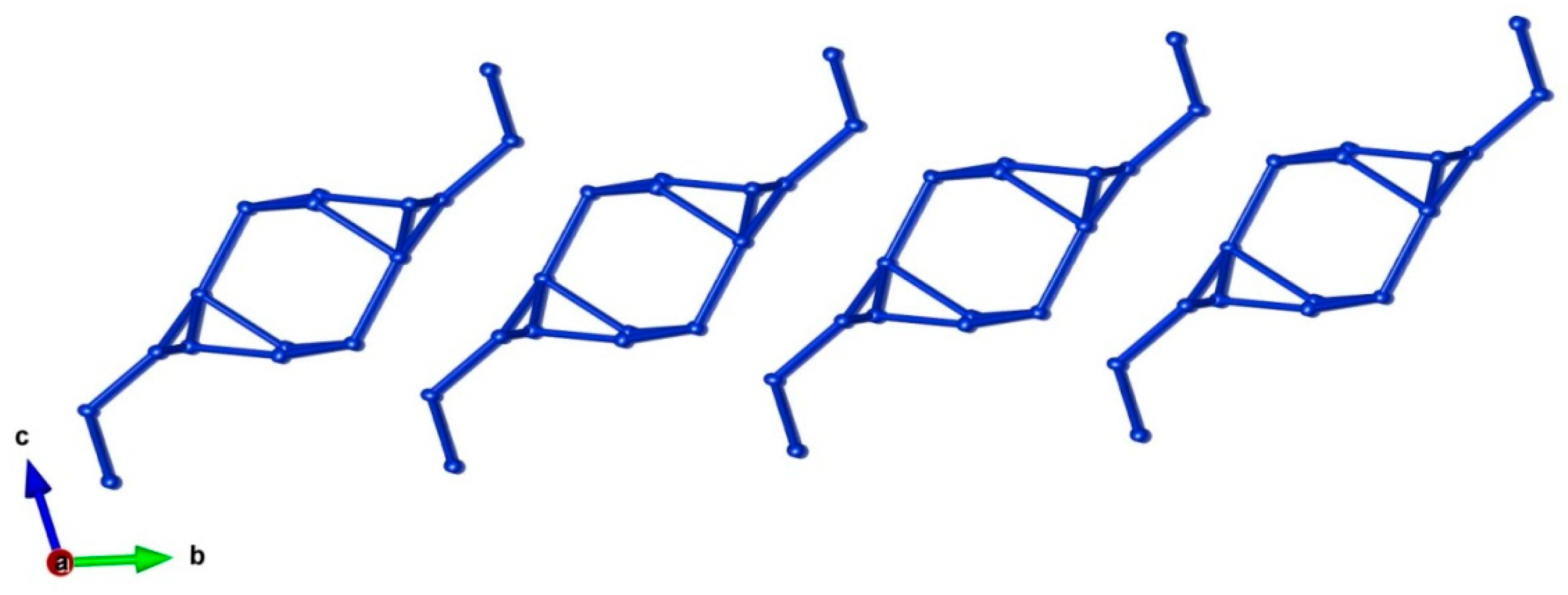
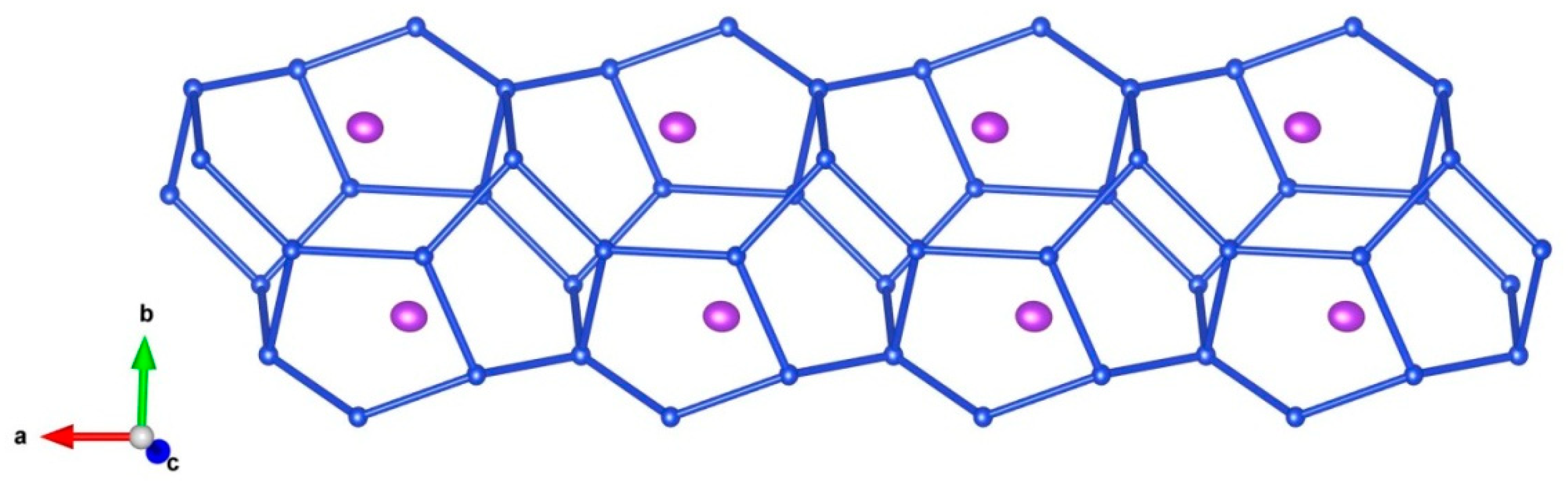
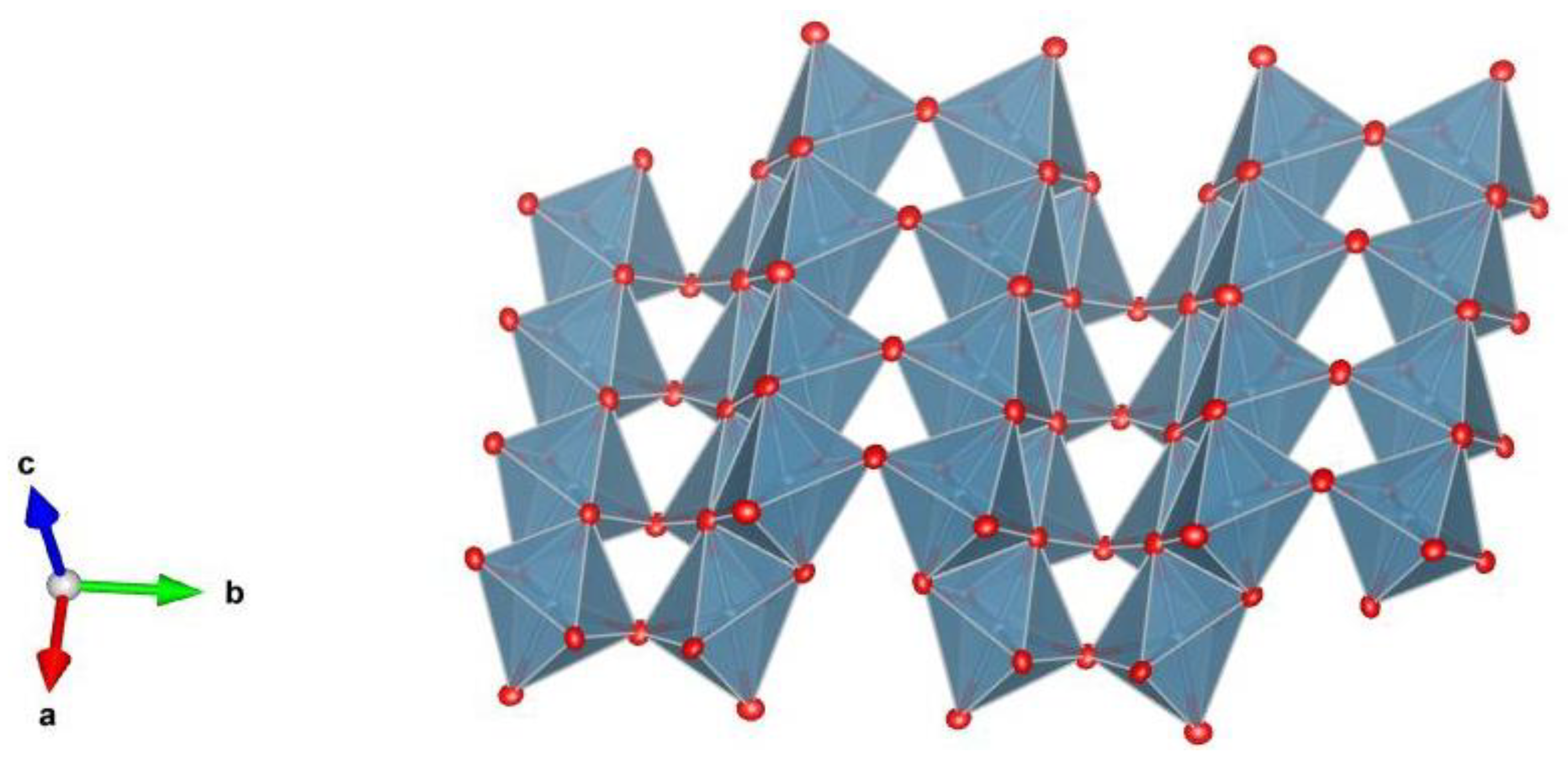
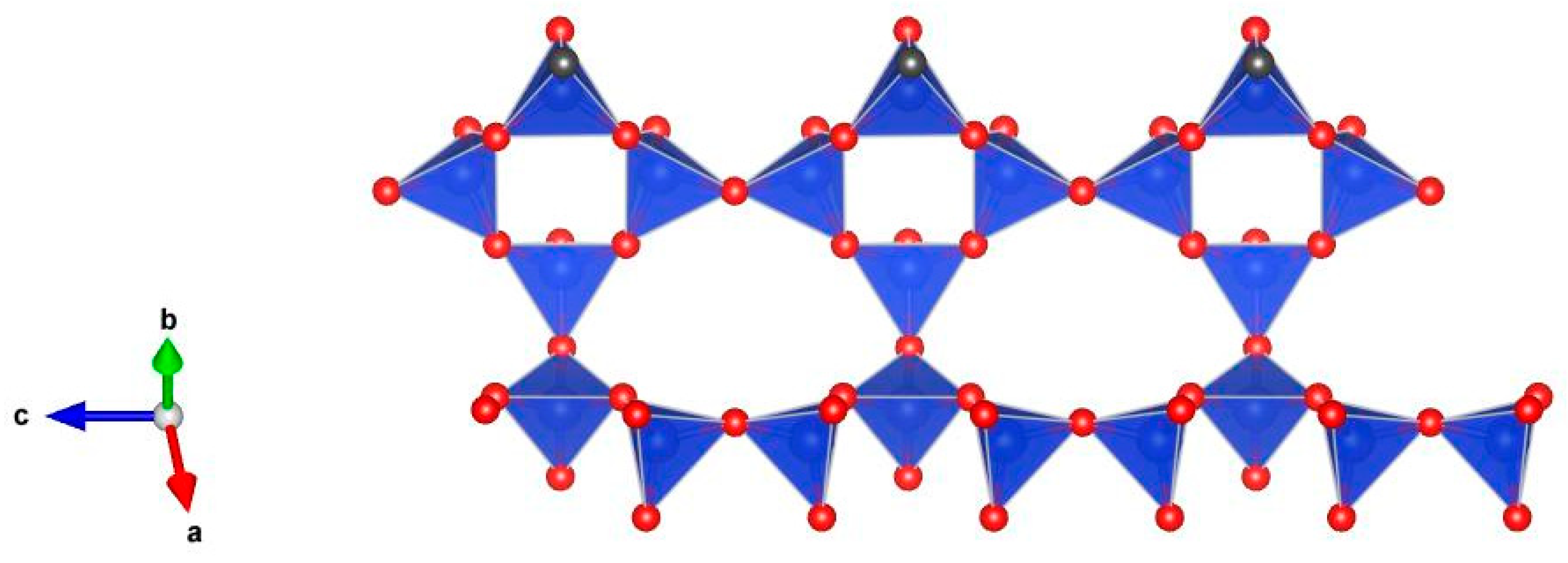
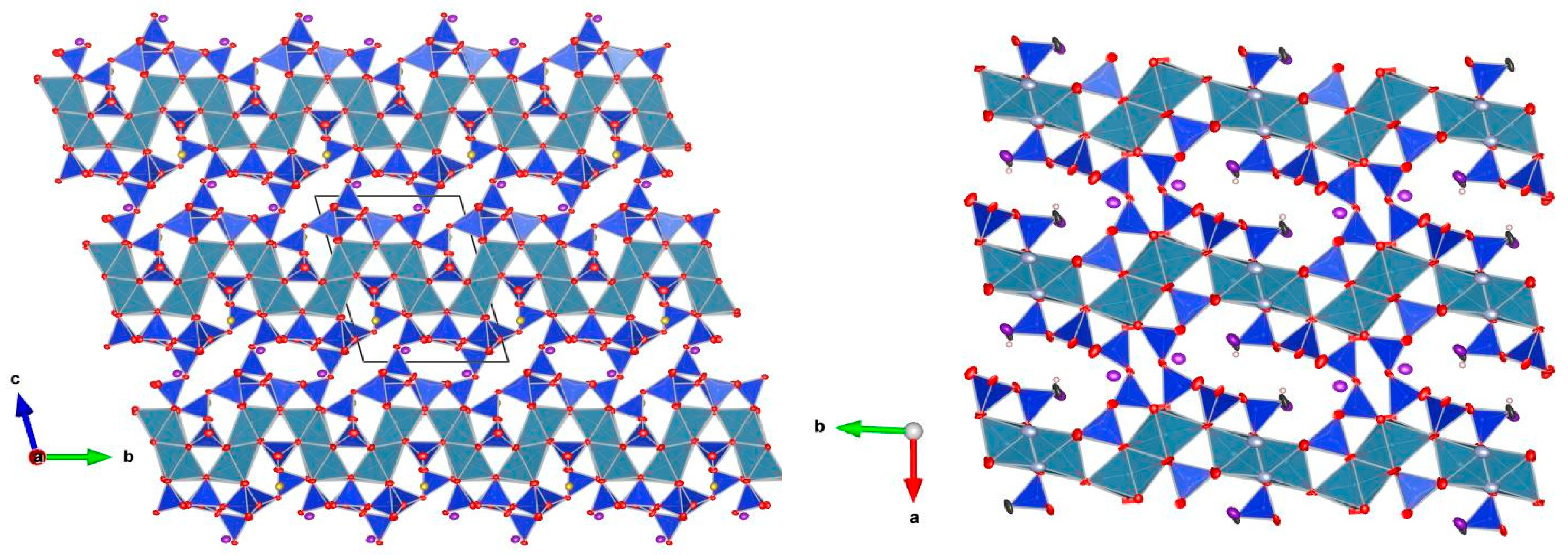
5. Description of Crystal Structure and Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chukanov, N.V.; Aksenov, S.M.; Rastsvetaeva, R.K.; Blass, G.; Varlamov, D.A.; Pekov, I.V.; Belakovskiy, D.I.; Gurzhiy, V.V. Calcinaksite, KNaCa(Si4O10)·H2O, a new mineral from the Eifel volcanic area, Germany. Mineral. Petrol. 2015, 109, 397–404. [Google Scholar] [CrossRef]
- Dorfman, M.D.; Rogachev, D.D.; Goroshchenko, Z.I.; Uspenskaya, E.I. Canasite, a new mineral. Trudy Mineral. Muzeya Akad. Nauk SSSR 1959, 9, 158–166. (In Russian) [Google Scholar]
- Rozhdestvenskaya, I.V.; Nikishova, L.V.; Bannova, I.I.; Lasebnik, Y.D. Canasite: The refinement of crystal structure and comparison with that of miserite. Acta Crystallogr. 1987, 43, C159. [Google Scholar] [CrossRef]
- Khomyakov, A.P.; Nechelyustov, G.N.; Krivokoneva, G.K.; Rastsvetaeva, R.K.; Rozenberg, K.A.; Rozhdestvenskaya, I.V. Fluorcanasite, K3Na3Ca5Si12O30(F,OH)4∙H2O, a new mineral from Khibiny Alkaline Massif (Kola Peninsula, Russia) and new data on canasite. Zap. Ross. Mineral. Obsh. 2009, 138, 52–66. (In Russian) [Google Scholar]
- Nikishova, L.V.; Lazebnik, K.A.; Rozhdestvenskaya, I.V.; Emelyanova, N.N.; Lazebnik, Y.D. Frankamenite K3Na3Ca5(Si12O30)F3(OH)∙H2O—A new mineral, triclinic variety of canasite from charoitites. Zap. Vser. Mineral. Obsh. 1996, 125, 106–108. (In Russian) [Google Scholar]
- Rozhdestvenskaya, I.V.; Nikishova, L.V.; Lazebnik, K.A. The crystal structure of frankamenite. Mineral. Mag. 1996, 60, 897–905. [Google Scholar] [CrossRef]
- Rogov, Y.G.; Rogova, V.P.; Voronkov, A.A.; Moleva, V.A. Tinaksite NaK2Ca2TiSi7O19(OH), a new mineral. Dokl. Akad. Nauk SSSR 1965, 162, 658–661. (In Russian) [Google Scholar]
- Rozhdestvenskaya, I.V.; Nikishova, L.V.; Lazebnik, Y.D.; Lazebnik, K.A. The crystal structure of tokkoite and its relation to the structure of tinaksite. Z. Kristallogr. 1989, 189, 195–204. [Google Scholar]
- Lacalamita, M.; Mesto, E.; Kaneva, E.; Scordari, F.; Pedrazzi, G.; Vladykin, N.; Schingaro, E. Structure refinement and crystal chemistry of tokkoite and tinaksite from the Murun massif (Russia). Mineral. Mag. 2017, 81, 251–272. [Google Scholar] [CrossRef]
- Ilyenok, S.S. Osnovnye cherty petrologii Patynskogo massiva (Main features of the Petrology of Patynskiy massif). Geologiya I geofizika (Geol. Geophys.) SO AN SSSR 1960, 4, 76–91. (In Russian) [Google Scholar]
- Ilyenok, S.S. Petrologiya gabbro-siyenitovogo complexa Gornoy Shorii (Petrology of Gabbro-Syenite Complex of Gornaya Shoriya); Tomsk University: Tomsk, Russia, 1964; p. 130. (In Russian) [Google Scholar]
- Dovgal, V.N. Rannepaleozoyskaya gabbro-siyenitovaya formatsiya tsentralnoy tchasti Altae-Sayanskoy skladtchatoy oblasti (Early Paleozoic Gabbro Syenite Formation of the Central Part of Altai-Sayany Folded Area); Nauka: Moscow, Russia, 1968; p. 207. (In Russian) [Google Scholar]
- Belyaev, E.V. The Polygenous Nature of the Patyn Ore-Magmatic Cluster (Mountainous Shoriya). Dokl. Earth Sci. 2010, 435, 1420–1422. [Google Scholar] [CrossRef]
- Rogova, V.P.; Rogov, Y.G.; Drits, V.A.; Kuznetsova, N.N. Charoite, a new mineral, and a new jewelry stone. Zap. Vses. Mineral. Obsh. 1978, 107, 94–100. [Google Scholar] [CrossRef]
- Nikishova, L.V.; Lazebnik, K.A.; Lazebnik, Y.D. About the crystallochemical formula of charoite. In Crystal Chemistry and Structure of Minerals; Nauka: Leningrad, Russia, 1985; pp. 100–105. [Google Scholar]
- Rozhdestvenskaya, I.; Mugnaioli, E.; Czank, M.; Depmeier, W.; Kolb, U.; Reinholdt, A.; Weirich, T. The structure of charoite, (K,Sr,Ba,Mn)15-16(Ca,Na)32[(Si70(O,OH)180)](OH,F)4.0·nH2O, solved by conventional and automated electron diffraction. Mineral. Mag. 2010, 74, 159–177. [Google Scholar] [CrossRef]
- Lazebnik, K.A.; Nikishova, L.V.; Lazebnik, Y.D. Tokkoite—A new mineral of charoitites. Mineralog. Zhurnal 1986, 8, 85–89. (In Russian) [Google Scholar]
- Mandarino, J.A. The Gladstone-Dale relationship. IV. The compatibility concept and its 209 application. Can. Mineral. 1981, 41, 989–1002. [Google Scholar]
- Sitarz, M.; Mozgawa, W.; Handke, M. Vibrational spectra of complex ring silicate anions—Method of recognition. J. Mol. Struct. 1997, 404, 193–197. [Google Scholar] [CrossRef]
- Chukanov, N.V. Infrared Spectra of Mineral Species: Extended Library; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2014; p. 1716. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Redfern, S.A.T. Unit cell refinement from powder diffraction data: The use of regression diagnostics. Mineral. Mag. 1995, 61, 65–77. [Google Scholar] [CrossRef]
- Westrip, S.P. publCIF: Software for editing, validating and formatting crystallographic information files. J. Appl. Crystallogr. 2010, 43, 920–925. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Crystallogr. 2015, B71, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Rozhdestvenskaya, I.V.; Krivovichev, S.V. Tubular chains in the structures of natural and synthetic silicates. Crystallogr. Rep. 2011, 56, 1007–1018. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Rastsvetaeva, R.K.; Chukanov, N.V.; Kolitsch, U. Structure of calcinaksite KNa[Ca(H2O)][Si4Ol0], the first hydrous member of the litidionite group of silicates with [Si8O20]8− tubes. Acta Crystallogr. B 2014, 70, 768–775. [Google Scholar] [CrossRef] [PubMed]





| Constituent | wt.% | Range | Standard Deviation |
|---|---|---|---|
| Na2O | 3.68 | 3.25–4.04 | 0.25 |
| K2O | 5.62 | 5.48–5.98 | 0.17 |
| CaO | 26.82 | 26.63–27.12 | 0.15 |
| SiO2 | 64.27 | 64.03–64.49 | 0.17 |
| Total | 100.39 |
| Formula: Ca4KNaSi9O23 | R[F2 > 2σ(F2)] = 0.032 |
| Symmetry: triclinic | wR(F2) = 0.088 |
| Space group: P1 | S = 1.38 |
| Mr = 843.22 | 12,417 reflections, 349 parameters |
| a = 7.27420(10) Å | F(000) = 840 |
| b = 10.5516(2) Å | Mo Kα radiation, λ = 0.71073 Å |
| c = 13.9851(3) Å | μ = 1.97 mm−1 |
| Α = 104.20° | T = 293 K |
| β = 104.302(2)° | Rint = 0.074 |
| γ = 92.0280(10)° | θmax = 40.3°, θmin = 1.6° |
| V = 1003.07(3) Å3 | h = −13→13 |
| Z = 2 | k = −19→19 |
| Dx = 2.792 g cm−3 | l = −25→25 |
| Radiation source: fine-focus sealed tube | Secondary atom site location: difference Fourier map |
| Graphite monochromator | [0.00000 + 1.00000exp(1.30(sinθ/λ)2)]/[σ2(Fo2) + 0.0000 + 0.0448 × P + (0.0377P)2 + 0.0000sinθ/λ] where P = 0.33333Fo2 + 0.66667Fc2 |
| 64,993 measured reflections | (Δ/σ)max = 0.002 |
| 12,417 independent reflections | Δρmax = 0.89 e Å−3 |
| 10,758 reflections with I > 2σ(I) | Δρmin = −0.84 e Å−3 |
| Site | x | y | z | Ueq |
|---|---|---|---|---|
| Ca1 | 0.82421 (3) | 0.51657 (2) | 0.381141 (17) | 0.00783 (5) |
| Ca2 | 1.31382 (3) | 0.90730 (2) | 0.382301 (17) | 0.00894 (6) |
| Ca3 | 0.81839 (3) | 0.89891 (2) | 0.366450 (17) | 0.00896 (6) |
| Ca4 | 1.32069 (3) | 0.50733 (2) | 0.383805 (17) | 0.00822 (5) |
| Na1 | 0.49261 (10) | 0.16604 (6) | 0.24880 (5) | 0.01881 (18) |
| K1 | 0.95296 (5) | 0.31141 (3) | 0.06920 (2) | 0.01780 (8) |
| Si1 | 0.81655 (4) | 0.23437 (3) | 0.46046 (2) | 0.00626 (5) |
| Si2 | 0.61746 (4) | 0.76796 (3) | 0.53758 (2) | 0.00604 (5) |
| Si3 | 1.01727 (4) | 0.23158 (3) | 0.29416 (2) | 0.00660 (5) |
| Si4 | 0.65171 (4) | 0.80298 (3) | 0.03303 (2) | 0.00661 (5) |
| Si5 | 1.00983 (4) | 0.97746 (3) | 0.16364 (2) | 0.00648 (5) |
| Si6 | 1.37874 (4) | 0.88172 (3) | 0.15444 (2) | 0.00695 (5) |
| Si7 | 0.78112 (4) | 0.60955 (3) | 0.16865 (2) | 0.00716 (5) |
| Si8 | 1.21164 (4) | 0.62856 (3) | 0.18476 (2) | 0.00689 (5) |
| Si9 | 0.47777 (4) | 0.40913 (3) | 0.16444 (2) | 0.00644 (5) |
| O1 | 0.88140 (12) | 0.38775 (8) | 0.51075 (7) | 0.00927 (13) |
| O2 | 1.21329 (12) | 0.97974 (9) | 0.13360 (7) | 0.01035 (13) |
| O3 | 1.50826 (12) | 0.93270 (9) | 0.26821 (7) | 0.01100 (14) |
| O4 | 0.58346 (12) | 0.21518 (10) | 0.43054 (7) | 0.01196 (14) |
| O5 | 0.89613 (12) | 0.12808 (9) | 0.51932 (7) | 0.01073 (13) |
| O6 | 0.79457 (14) | 0.67329 (9) | 0.28513 (7) | 0.01174 (14) |
| O7 | 1.02215 (13) | 0.92523 (9) | 0.26198 (7) | 0.01069 (13) |
| O8 | 1.02637 (13) | 0.38183 (9) | 0.29368 (7) | 0.01073 (13) |
| O9 | 0.52356 (12) | 0.40238 (9) | 0.28020 (6) | 0.00956 (13) |
| O10 | 0.66264 (12) | 0.46186 (9) | 0.13305 (7) | 0.01013 (13) |
| O11 | 1.26701 (13) | 0.73514 (9) | 0.12639 (7) | 0.01143 (14) |
| O12 | 0.62744 (12) | 0.61611 (8) | 0.48453 (7) | 0.00912 (13) |
| O13 | 0.40927 (13) | 0.25908 (8) | 0.09035 (6) | 0.00955 (13) |
| O14 | 0.63569 (12) | 0.88002 (9) | 0.48068 (7) | 0.00999 (13) |
| O15 | 0.77274 (12) | 0.80587 (9) | 0.65113 (7) | 0.01171 (14) |
| O16 | 1.28126 (13) | 0.67805 (9) | 0.30517 (7) | 0.01079 (13) |
| O17 | 0.48846 (13) | 0.88853 (9) | 0.06690 (7) | 0.01199 (14) |
| O18 | 0.85399 (13) | 0.19139 (10) | 0.34567 (7) | 0.01196 (14) |
| O19 | 0.66731 (13) | 0.68766 (10) | 0.08966 (8) | 0.01312 (15) |
| O20 | 1.29865 (13) | 0.49326 (9) | 0.13704 (7) | 0.01187 (14) |
| O21 | 0.85363 (12) | 0.88962 (9) | 0.06076 (7) | 0.01119 (14) |
| O22 | 0.98268 (12) | 0.58570 (9) | 0.13860 (7) | 0.01107 (14) |
| O23 | 0.95847 (12) | 0.13084 (8) | 0.17922 (6) | 0.00831 (12) |
| Site | U11 | U22 | U33 | U12 | U13 | U23 |
|---|---|---|---|---|---|---|
| Ca1 | 0.00678 (8) | 0.00948 (9) | 0.00662 (8) | 0.00074 (6) | 0.00049 (6) | 0.00229 (6) |
| Ca2 | 0.00806 (8) | 0.01095 (10) | 0.00734 (9) | 0.00075 (6) | 0.00132 (6) | 0.00231 (6) |
| Ca3 | 0.00750 (8) | 0.01223 (10) | 0.00764 (9) | 0.00178 (6) | 0.00151 (6) | 0.00388 (6) |
| Ca4 | 0.00752 (8) | 0.00955 (9) | 0.00720 (8) | 0.00052 (6) | 0.00068 (6) | 0.00280 (6) |
| Na1 | 0.0272 (3) | 0.0130 (3) | 0.0141 (3) | −0.0012 (2) | 0.0011 (2) | 0.0044 (2) |
| K1 | 0.02204 (14) | 0.01743 (13) | 0.01207 (12) | 0.00197 (10) | 0.00066 (9) | 0.00438 (9) |
| Si1 | 0.00569 (10) | 0.00692 (11) | 0.00614 (11) | 0.00074 (8) | 0.00104 (9) | 0.00213 (9) |
| Si2 | 0.00521 (10) | 0.00682 (11) | 0.00554 (11) | 0.00059 (8) | 0.00022 (8) | 0.00184 (9) |
| Si3 | 0.00593 (11) | 0.00792 (12) | 0.00498 (11) | 0.00061 (9) | 0.00043 (9) | 0.00091 (9) |
| Si4 | 0.00647 (11) | 0.00782 (12) | 0.00514 (11) | 0.00075 (9) | 0.00078 (9) | 0.00172 (9) |
| Si5 | 0.00611 (11) | 0.00756 (12) | 0.00531 (11) | 0.00052 (8) | 0.00048 (9) | 0.00191 (9) |
| Si6 | 0.00643 (11) | 0.00826 (12) | 0.00620 (11) | 0.00074 (9) | 0.00126 (9) | 0.00239 (9) |
| Si7 | 0.00645 (11) | 0.00827 (12) | 0.00705 (12) | 0.00078 (9) | 0.00102 (9) | 0.00333 (9) |
| Si8 | 0.00629 (11) | 0.00738 (12) | 0.00679 (11) | 0.00070 (9) | 0.00056 (9) | 0.00265 (9) |
| Si9 | 0.00594 (11) | 0.00694 (11) | 0.00558 (11) | 0.00027 (8) | 0.00032 (9) | 0.00134 (9) |
| O1 | 0.0103 (3) | 0.0074 (3) | 0.0089 (3) | −0.0004 (2) | 0.0014 (2) | 0.0014 (2) |
| O2 | 0.0079 (3) | 0.0126 (3) | 0.0130 (3) | 0.0037 (2) | 0.0039 (3) | 0.0062 (3) |
| O3 | 0.0088 (3) | 0.0142 (4) | 0.0081 (3) | 0.0004 (3) | −0.0005 (2) | 0.0022 (3) |
| O4 | 0.0062 (3) | 0.0164 (4) | 0.0129 (4) | 0.0017 (3) | 0.0024 (3) | 0.0031 (3) |
| O5 | 0.0107 (3) | 0.0113 (3) | 0.0114 (3) | 0.0029 (3) | 0.0015 (3) | 0.0065 (3) |
| O6 | 0.0162 (4) | 0.0109 (3) | 0.0081 (3) | 0.0008 (3) | 0.0032 (3) | 0.0023 (3) |
| O7 | 0.0110 (3) | 0.0134 (3) | 0.0089 (3) | 0.0016 (3) | 0.0019 (3) | 0.0059 (3) |
| O8 | 0.0114 (3) | 0.0083 (3) | 0.0111 (3) | 0.0005 (2) | 0.0011 (3) | 0.0020 (3) |
| O9 | 0.0101 (3) | 0.0116 (3) | 0.0059 (3) | 0.0002 (2) | 0.0004 (2) | 0.0022 (2) |
| O10 | 0.0089 (3) | 0.0093 (3) | 0.0125 (3) | −0.0006 (2) | 0.0036 (3) | 0.0029 (3) |
| O11 | 0.0137 (3) | 0.0103 (3) | 0.0102 (3) | −0.0021 (3) | 0.0017 (3) | 0.0043 (3) |
| O12 | 0.0103 (3) | 0.0072 (3) | 0.0085 (3) | 0.0012 (2) | 0.0005 (2) | 0.0014 (2) |
| O13 | 0.0130 (3) | 0.0081 (3) | 0.0059 (3) | −0.0007 (2) | 0.0009 (2) | 0.0005 (2) |
| O14 | 0.0120 (3) | 0.0100 (3) | 0.0094 (3) | 0.0012 (2) | 0.0032 (3) | 0.0048 (3) |
| O15 | 0.0081 (3) | 0.0157 (4) | 0.0076 (3) | 0.0027 (3) | −0.0025 (2) | 0.0006 (3) |
| O16 | 0.0133 (3) | 0.0108 (3) | 0.0074 (3) | 0.0008 (3) | 0.0004 (3) | 0.0032 (3) |
| O17 | 0.0141 (3) | 0.0133 (4) | 0.0139 (4) | 0.0062 (3) | 0.0088 (3) | 0.0074 (3) |
| O18 | 0.0110 (3) | 0.0165 (4) | 0.0080 (3) | −0.0011 (3) | 0.0049 (3) | 0.0005 (3) |
| O19 | 0.0124 (3) | 0.0138 (4) | 0.0148 (4) | 0.0026 (3) | 0.0005 (3) | 0.0097 (3) |
| O20 | 0.0102 (3) | 0.0109 (3) | 0.0129 (3) | 0.0048 (3) | 0.0010 (3) | 0.0016 (3) |
| O21 | 0.0096 (3) | 0.0128 (3) | 0.0087 (3) | −0.0025 (3) | 0.0002 (3) | 0.0011 (3) |
| O22 | 0.0066 (3) | 0.0136 (4) | 0.0119 (3) | 0.0002 (2) | 0.0017 (3) | 0.0021 (3) |
| O23 | 0.0104 (3) | 0.0078 (3) | 0.0055 (3) | 0.0027 (2) | 0.0003 (2) | 0.0010 (2) |
| Ca1–O1i | 2.3214 (9) | Ca2–O5i | 2.3696 (9) |
| Ca1–O6 | 2.3571 (9) | Ca2–O16 | 2.3715 (9) |
| Ca1–O12 | 2.3720 (9) | Ca2–O7 | 2.4062 (9) |
| Ca1–O9 | 2.3834 (9) | Ca2–O3 | 2.4324 (9) |
| Ca1–O8 | 2.4214 (9) | Ca2–O14ii | 2.4715 (9) |
| Ca1–O1 | 2.4865 (9) | Ca2–O14iii | 2.5153 (9) |
| <Ca1–O> | 2.390 | <Ca2–O> | 2.428 |
| Ca3–O6 | 2.3528 (10) | Ca4–O16 | 2.3215 (9) |
| Ca3–O14 | 2.3546 (9) | Ca4–O8 | 2.3607 (9) |
| Ca3–O5i | 2.3605 (9) | Ca4–O12ii | 2.3985 (9) |
| Ca3–O7 | 2.3767 (9) | Ca4–O9ii | 2.4265 (9) |
| Ca3–O3iv | 2.4228 (9) | Ca4–O1i | 2.4385 (9) |
| Ca3–O5v | 2.7398 (10) | Ca4–O12i | 2.4694 (9) |
| <Ca3–O> | 2.435 | <Ca4–O> | 2.403 |
| Na1–O4 | 2.3800 (12) | K1–O11viii | 2.7214 (10) |
| Na1–O9 | 2.4159 (11) | K1–O23 | 2.7226 (9) |
| Na1–O3vi | 2.5457 (11) | K1–O22 | 2.7998 (10) |
| Na1–O13 | 2.5860 (11) | K1–O10 | 2.8724 (9) |
| Na1–O18 | 2.6134 (12) | K1–O20 | 2.9141 (10) |
| Na1–O15vii | 2.6351 (12) | K1–O8 | 2.9483 (10) |
| Na1–O2vi | 2.6709 (11) | K1–O21viii | 3.0601 (10) |
| <Na1–O> | 2.550 | K1–O13ii | 3.3369 (10) |
| <K1–O> | 2.922 | ||
| Si1–O5 | 1.5919 (9) | Si2–O14 | 1.5974 (9) |
| Si1–O1 | 1.6000 (9) | Si2–O12 | 1.6044 (9) |
| Si1–O4 | 1.6355 (9) | Si2–O4vii | 1.6334 (9) |
| Si1–O18 | 1.6505 (9) | Si2–O15 | 1.6515 (9) |
| <Si1–O> | 1.619 | <Si2–O> | 1.622 |
| Si3–O8 | 1.5861 (9) | Si4–O19 | 1.5996 (9) |
| Si3–O18 | 1.6268 (9) | Si4–O21 | 1.6103 (9) |
| Si3–O23 | 1.6390 (9) | Si4–O17 | 1.6104 (9) |
| Si3–O15i | 1.6397 (9) | Si4–O13x | 1.6278 (9) |
| <Si3–O> | 1.623 | <Si4–O> | 1.612 |
| Si5–O7 | 1.5868 (9) | Si6–O3 | 1.5849 (9) |
| Si5–O2 | 1.6361 (9) | Si6–O11 | 1.6317 (9) |
| Si5–O21 | 1.6382 (9) | Si6–O17ii | 1.6317 (9) |
| Si5–O23v | 1.6481 (9) | Si6–O2 | 1.6327 (9) |
| <Si5–O> | 1.627 | <Si6–O> | 1.620 |
| Si7–O6 | 1.5794 (10) | Si8–O16 | 1.5791 (9) |
| Si7–O19 | 1.6212 (9) | Si8–O22 | 1.6332 (9) |
| Si7–O22 | 1.6335 (9) | Si8–O11 | 1.6353 (9) |
| Si7–O10 | 1.6527 (9) | Si8–O20 | 1.6399 (9) |
| <Si7–O> | 1.622 | <Si8–O> | 1.622 |
| Si9–O9 | 1.5902 (9) | ||
| Si9–O20iv | 1.6232 (9) | ||
| Si9–O10 | 1.6339 (9) | ||
| Si9–O13 | 1.6470 (9) | ||
| <Si9–O> | 1.624 |
| K | Ca1 | Ca2 | Ca3 | Ca4 | Na1 | Si1 | Si2 | Si3 | Si4 | Si5 | Si6 | Si7 | Si8 | Si9 | Σ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O1 | - | 0.363 0.242 | - | - | 0.273 | - | 1.064 | - | - | - | - | - | - | - | - | 1.942 |
| O2 | - | - | - | - | - | 0.098 | - | - | - | - | 0.969 | 0.934 | - | - | - | 2.001 |
| O3 | - | - | 0.277 | 0.283 | - | 0.132 | - | - | - | - | - | 1.086 | - | - | - | 1.778 |
| O4 | - | - | - | - | - | 0.196 | 0.971 | 0.976 | - | - | - | - | - | - | - | 2.143 |
| O5 | - | 0.323 | 0.330 0.131 | - | - | 1.086 | - | - | - | - | - | - | - | - | 1.739 | |
| O6 | - | 0.331 | - | 0.336 | - | - | - | - | - | - | - | - | 1.071 | - | - | 1.740 |
| O7 | - | - | 0.295 | 0.317 | - | - | - | - | - | - | 1.100 | - | - | - | - | 1.713 |
| O8 | 0.104 | 0.284 | - | - | 0.330 | - | - | - | 1.102 | - | - | - | - | - | - | 1.820 |
| O9 | - | 0.312 | - | - | 0.281 | 0.180 | - | - | - | - | - | - | - | - | 1.065 | 1.837 |
| O10 | 0.126 | - | - | - | - | - | - | - | - | - | - | - | 0.932 | - | 1.036 | 2.093 |
| O11 | 0.184 | - | - | - | - | - | - | - | - | - | - | 1.064 | - | 0.962 | - | 2.210 |
| O12 | - | 0.321 | - | - | 0.301 0.253 | - | - | 1.052 | - | - | - | - | - | - | - | 1.926 |
| O13 | 0.039 | - | - | - | - | 0.120 | - | - | - | 0.990 | - | - | - | - | 0.990 | 2.140 |
| O14 | - | - | 0.252 0.226 | 0.335 | - | - | - | 1.071 | - | - | - | - | - | - | - | 1.883 |
| O15 | - | - | - | - | - | 0.107 | - | 0.932 | 0.960 | - | - | - | - | - | - | 1.999 |
| O16 | - | - | 0.321 | - | 0.363 | - | - | - | - | - | - | - | - | 1.102 | - | 1.786 |
| O17 | - | - | - | - | - | - | - | - | - | 1.036 | 0.971 | - | - | - | 2.006 | |
| O18 | - | - | - | - | - | 0.112 | 0.934 | - | 0.993 | - | - | - | - | - | - | 2.039 |
| O19 | - | - | - | - | - | - | - | - | - | 1.065 | - | - | 1.052 | - | - | 2.116 |
| O20 | 0.113 | - | - | - | - | - | - | - | - | - | - | - | - | 0.960 | 1.036 | 2.109 |
| O21 | 0.078 | - | - | - | - | - | - | - | - | 1.036 | 0.964 | - | - | - | - | 2.078 |
| O22 | 0.151 | - | - | - | - | - | - | - | - | - | - | - | 0.976 | 0.993 | - | 2.120 |
| O23 | 0.183 | - | - | - | - | - | - | - | 0.962 | - | 0.940 | - | - | - | - | 2.085 |
| Σ | 0.978 | 1.855 | 1.693 | 1.732 | 1.800 | 0.944 | 4.055 | 4.030 | 4.018 | 4.126 | 3.974 | 4.055 | 4.030 | 4.018 | 4.126 | 45.725 |
| dobs | Iobs | dcalc | Icalc | hkl |
|---|---|---|---|---|
| 13.141 | 12 | 13.077 | 86 | 0 0 1 |
| 10.175 | 8 | 10.176 | 5 | 0 1 0 |
| 9.364 | 7 | 9.300 | 7 | 0 1 −1 |
| 7.030 | 11 | 7.171 | 2 | 0 1 1 |
| 6.332 | 7 | 6.304 | 18 | 0 1 −2 |
| 5.636 | 10 | 5.664 | 10 | 1 −1 −1 |
| 4.947 | 13 | 4.943 | 24 | 0 1 2 |
| 4.618 | 9 | 4.650 | 29 | 0 2 −2 |
| 4.220 | 8 | 4.211 | 12 | 1 1 −3 |
| 3.844 | 6 | 3.846 | 42 | 0 2 −3 |
| 3.454 | 100 | 3.459 | 35 | 2 −1 −1 |
| 3.262 | 66 | 3.270 | 92 | 2 −1 −2 |
| 3.103 | 64 | 3.152 | 100 | 0 2 −4 |
| 2.931 | 16 | 2.954 | 22 | 1 1 3 |
| 2.801 | 21 | 2.786 | 37 | 2 0 −4 |
| 2.592 | 18 | 2.630 | 40 | 0 4 −1 |
| 2.392 | 10 | 2.398 | 14 | 0 3 −5 |
| 2.161 | 8 | 2.158 | 12 | 2 3 1 |
| 2.124 | 6 | 2.127 | 12 | 2 0 4 |
| 2.024 | 8 | 2.027 | 25 | 2 −3 4 |
| 1.985 | 8 | 1.989 | 14 | 2 −4 3 |
| 1.820 | 28 | 1.817 | 77 | 4 0 −2 |
| 1.747 | 3 | 1.743 | 5 | 0 6 −3 |
| 1.692 | 4 | 1.693 | 4 | 3 0 −7 |
| 1.640 | 3 | 1.639 | 6 | 2 3 4 |
| 1.590 | 4 | 1.601 | 19 | 0 5 −7 |
| 1.553 | 6 | 1.558 | 10 | 4 2 −6 |
| 1.496 | 5 | 1.496 | 11 | 4 3 0 |
| 1.455 | 4 | 1.456 | 2 | 1 5 4 |
| 1.354 | 5 | 1.361 | 8 | 4 −5 2 |
| 1.250 | 2 | 1.251 | 8 | 0 8 −6 |
| 1.169 | 3 | 1.169 | 5 | 6 0 0 |
| 1.134 | 3 | 1.134 | 2 | 3 −1 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasatkin, A.V.; Cámara, F.; Chukanov, N.V.; Škoda, R.; Nestola, F.; Agakhanov, A.A.; Belakovskiy, D.I.; Lednyov, V.S. Patynite, NaKCa4[Si9O23], a New Mineral from the Patynskiy Massif, Southern Siberia, Russia. Minerals 2019, 9, 611. https://doi.org/10.3390/min9100611
Kasatkin AV, Cámara F, Chukanov NV, Škoda R, Nestola F, Agakhanov AA, Belakovskiy DI, Lednyov VS. Patynite, NaKCa4[Si9O23], a New Mineral from the Patynskiy Massif, Southern Siberia, Russia. Minerals. 2019; 9(10):611. https://doi.org/10.3390/min9100611
Chicago/Turabian StyleKasatkin, Anatoly V., Fernando Cámara, Nikita V. Chukanov, Radek Škoda, Fabrizio Nestola, Atali A. Agakhanov, Dmitriy I. Belakovskiy, and Vladimir S. Lednyov. 2019. "Patynite, NaKCa4[Si9O23], a New Mineral from the Patynskiy Massif, Southern Siberia, Russia" Minerals 9, no. 10: 611. https://doi.org/10.3390/min9100611
APA StyleKasatkin, A. V., Cámara, F., Chukanov, N. V., Škoda, R., Nestola, F., Agakhanov, A. A., Belakovskiy, D. I., & Lednyov, V. S. (2019). Patynite, NaKCa4[Si9O23], a New Mineral from the Patynskiy Massif, Southern Siberia, Russia. Minerals, 9(10), 611. https://doi.org/10.3390/min9100611





